Primary Cilia and Calcium Signaling Interactions
Abstract
:1. Introduction
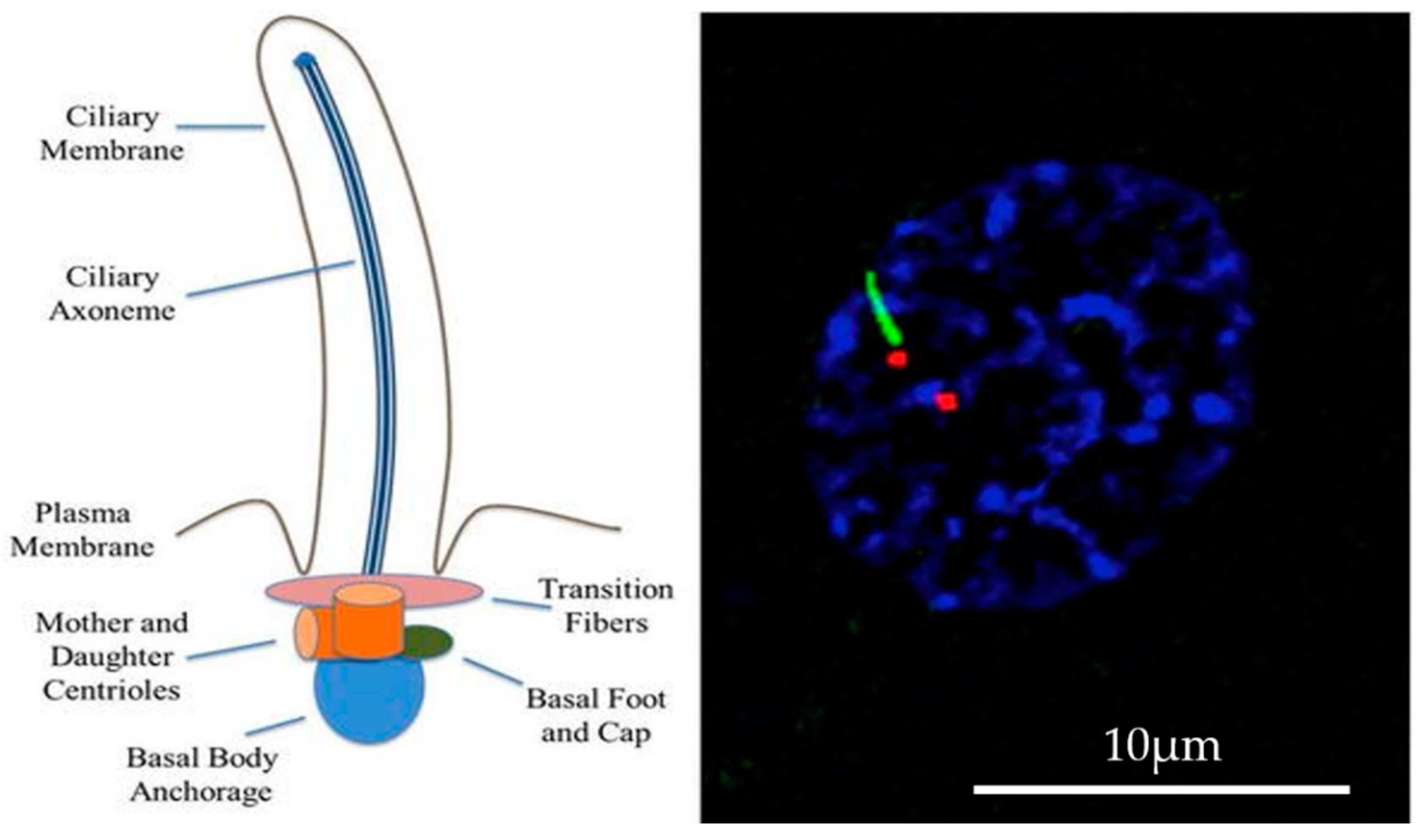
2. Calcium and Ciliary Signal Transduction: Sensory Function and Cilia Structure
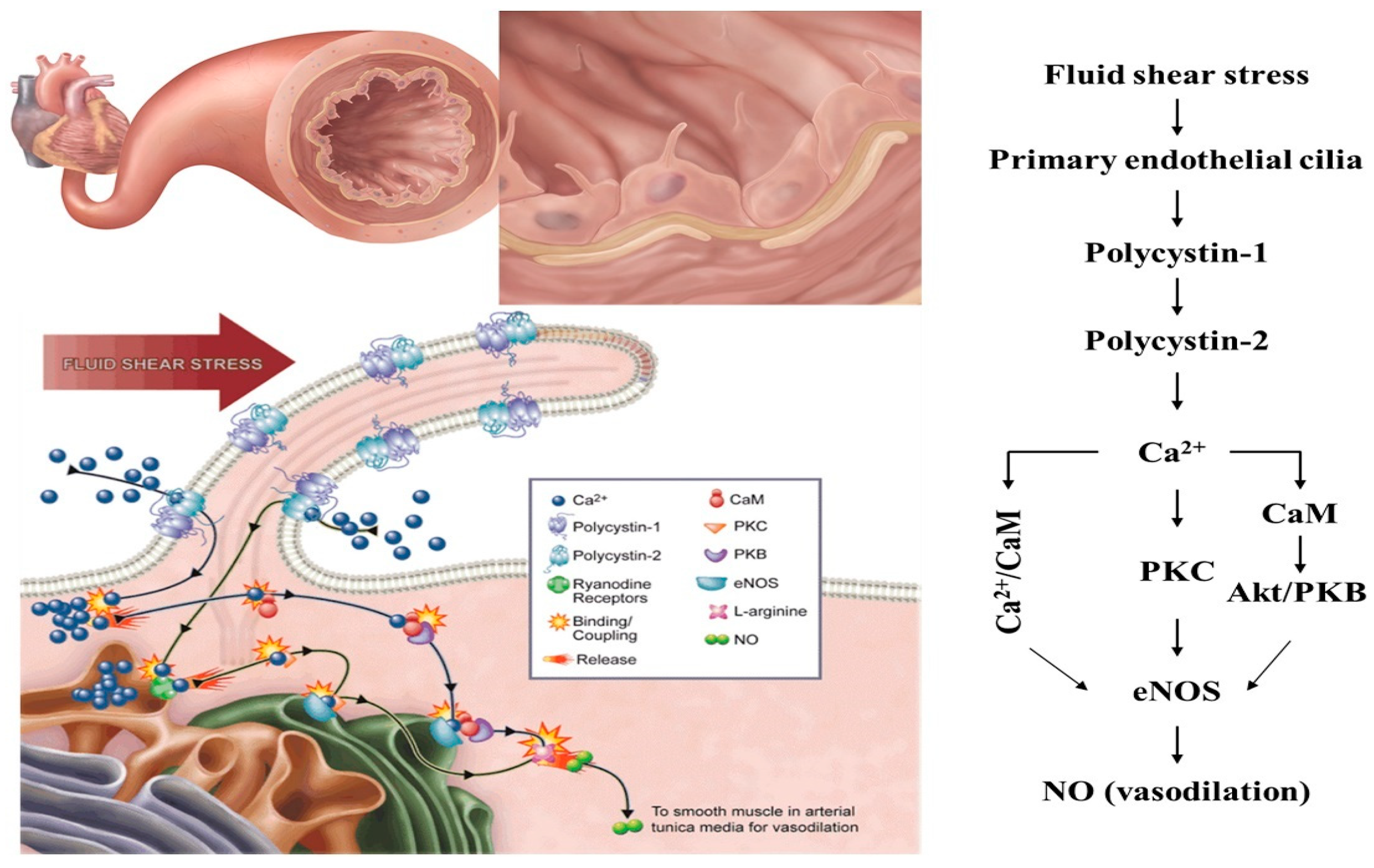
2.1. Mechanosensation
2.2. Chemosensation
2.3. Electrosensation
3. Cilia-Mediated and Calcium-Dependent Biological Processes
3.1. Cell Cycle
3.2. Cell Polarity and Migration
3.3. Neuronal Patterning
3.4. Glucose-Mediated Insulin Secretion
3.5. Biliary Regulation
3.6. Bone Formation
4. Conclusions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| Ca2+ | Calcium cation |
| PC-2 | Polycystin 2 |
| TRPV4 | Transient receptor potential cation channel subfamily V member 4 |
| Wnt | Wingless-related integration site |
| PDGF | Platelet-derived growth factor |
| Hh | Hedgehog |
| PKD | Polycystic kidney disease |
| PC-1 | Polycystin 1 |
| NO | Nitric oxide |
| Ca2+/CaM | Calcium/calmodulin complex |
| eNOS | Endothelial nitric oxide synthase |
| CaM | Calmodulin |
| PKC | Protein kinase C |
| AKT/PKB | Protein kinase B |
| OSN | Olfactory sensory neurons |
| GPCR | G-protein-coupled receptor |
| CNG | Cyclic nucleotide-gated channel |
| CaMKII | Calmodulin-dependent protein kinase II |
| CP110 | Centriolar coiled-coil protein of 110 kDa |
| TRPV2 | Transient receptor potential cation channel subfamily V member 2 |
| TRPP2 | Transient receptor potential polycystin 2 |
| TRPC1 | Transient receptor potential cation channel subfamily C member 1 |
| TGFβ | Transforming growth factor beta |
| CSF | Cerebrospinal fluid |
| AMPA | α-amino-3-hydroxy-5-methyl-4-isoxazolepropionic acid |
| NMDA | N-Methyl-d-aspartate |
| GABA | Gamma aminobutyric acid |
| Cl− | Chlorine ion |
| Ni2+ | Nickel (II) ion |
| SK | Small conductance calcium-activated potassium channel |
| βCKO | Cilia-less β-cell line |
| TRPV6 | Transient receptor potential cation channel subfamily V member 6 |
| Kif3a | Kinesin family member 3A |
References
- Rizzuto, R.; Pozzan, T. Microdomains of intracellular Ca2+: Molecular determinants and functional consequences. Physiol. Rev. 2006, 86, 369–408. [Google Scholar] [CrossRef] [PubMed]
- Clapham, D.E. Calcium signaling. Cell 2007, 131, 1047–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mitchison, H.M.; Valente, E.M. Motile and non-motile cilia in human pathology: From function to phenotypes. J. Pathol. 2017, 241, 294–309. [Google Scholar] [CrossRef] [PubMed]
- Brooks, E.R.; Wallingford, J.B. Multiciliated cells. Curr. Biol. 2014, 24, R973–R982. [Google Scholar] [CrossRef] [Green Version]
- Bayless, B.A.; Navarro, F.M.; Winey, M. Motile cilia: Innovation and insight from ciliate model organisms. Front. Cell Dev. Biol. 2019, 7, 265. [Google Scholar] [CrossRef] [Green Version]
- Omran, A.J.A.; Saternos, H.C.; Althobaiti, Y.S.; Wisner, A.; Sari, Y.; Nauli, S.M.; AbouAlaiwi, W.A. Alcohol consumption impairs the ependymal cilia motility in the brain ventricles. Sci. Rep. 2017, 7, 13652. [Google Scholar] [CrossRef] [Green Version]
- Nonaka, S.; Shiratori, H.; Saijoh, Y.; Hamada, H. Determination of left–right patterning of the mouse embryo by artificial nodal flow. Nature 2002, 418, 96. [Google Scholar] [CrossRef]
- Pazour, G.J.; Witman, G.B. The vertebrate primary cilium is a sensory organelle. Curr. Opin. Cell Biol. 2003, 15, 105–110. [Google Scholar] [CrossRef]
- Rohatgi, R.; Snell, W.J. The ciliary membrane. Curr. Opin. Cell Biol. 2010, 22, 541–546. [Google Scholar] [CrossRef]
- Sonkusare, S.K.; Bonev, A.D.; Ledoux, J.; Liedtke, W.; Kotlikoff, M.I.; Heppner, T.J.; Hill-Eubanks, D.C.; Nelson, M.T. Elementary Ca2+ signals through endothelial TRPV4 channels regulate vascular function. Science 2012, 336, 597–601. [Google Scholar] [CrossRef] [Green Version]
- Nauli, S.M.; Pala, R.; Kleene, S.J. Calcium channels in primary cilia. Curr. Opin. Nephrol. Hypertens. 2016, 25, 452. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Spasic, M.; Jacobs, C.R. Primary cilia: Cell and molecular mechanosensors directing whole tissue function. Semin. Cell Dev. Biol. 2017, 71, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Yuan, S.; Zhao, L.; Brueckner, M.; Sun, Z. Intraciliary calcium oscillations initiate vertebrate left-right asymmetry. Curr. Biol. 2015, 25, 556–567. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, W.; Murcia, N.S.; Duan, Y.; Weinbaum, S.; Yoder, B.K.; Schwiebert, E.; Satlin, L.M. Mechanoregulation of intracellular Ca2+ concentration is attenuated in collecting duct of monocilium-impaired orpk mice. Am. J. Physiol. Ren. Physiol. 2005, 289, F978–F988. [Google Scholar] [CrossRef] [Green Version]
- Nauli, S.M.; Alenghat, F.J.; Luo, Y.; Williams, E.; Vassilev, P.; Li, X.; Elia, A.E.; Lu, W.; Brown, E.M.; Quinn, S.J. Polycystins 1 and 2 mediate mechanosensation in the primary cilium of kidney cells. Nat. Genet. 2003, 33, 129–137. [Google Scholar] [CrossRef]
- Praetorius, H.A. The primary cilium as sensor of fluid flow: New building blocks to the model. A review in the theme: Cell signaling: Proteins, pathways and mechanisms. Am. J. Physiol. Cell. Physiol. 2015, 308, C198–C208. [Google Scholar] [CrossRef] [Green Version]
- Spasic, M.; Jacobs, C.R. Lengthening primary cilia enhances cellular mechanosensitivity. Eur. Cells Mater. 2017, 33, 158–168. [Google Scholar] [CrossRef]
- Collingridge, G.L.; Volianskis, A.; Bannister, N.; France, G.; Hanna, L.; Mercier, M.; Tidball, P.; Fang, G.; Irvine, M.W.; Costa, B.M.; et al. The NMDA receptor as a target for cognitive enhancement. Neuropharmacology 2013, 64, 13–26. [Google Scholar] [CrossRef] [Green Version]
- AbouAlaiwi, W.A.; Takahashi, M.; Mell, B.R.; Jones, T.J.; Ratnam, S.; Kolb, R.J.; Nauli, S.M. Ciliary polycystin-2 is a mechanosensitive calcium channel involved in nitric oxide signaling cascades. Circ. Res. 2009, 104, 860–869. [Google Scholar] [CrossRef]
- Lee, S.R.; Adams, P.J.; Yue, D.T. Large Ca2+-dependent facilitation of CaV2. 1 channels revealed by Ca2+ photo-uncaging. J. Physiol. 2015, 593, 2753–2778. [Google Scholar] [CrossRef] [Green Version]
- Cai, X.; Akber, M.; Spirov, A.; Baumgartner, S. Cortical movement of Bicoid in early Drosophila embryos is actin- and microtubule-dependent and disagrees with the SDD diffusion model. PLoS ONE 2017, 12, e0185443. [Google Scholar] [CrossRef] [PubMed]
- Cantero, M.D.R.; Perez, P.L.; Smoler, M.; Villa Etchegoyen, C.; Cantiello, H.F. Electrical oscillations in two-dimensional microtubular structures. Sci. Rep. 2016, 6, 27143. [Google Scholar] [CrossRef] [PubMed]
- Kirschen, G.W.; Xiong, Q. Primary cilia as a novel horizon between neuron and environment. Neural Regen. Res. 2017, 12, 1225–1230. [Google Scholar] [CrossRef] [PubMed]
- Khayyeri, H.; Barreto, S.; Lacroix, D. Primary cilia mechanics affects cell mechanosensation: A computational study. J. Theor. Biol. 2015, 379, 38–46. [Google Scholar] [CrossRef]
- Sun, S.; Fisher, R.L.; Bowser, S.S.; Pentecost, B.T.; Sui, H. Three-dimensional architecture of epithelial primary cilia. Proc. Natl. Acad. Sci. USA 2019, 116, 9370–9379. [Google Scholar] [CrossRef] [Green Version]
- Battle, C.; Ott, C.M.; Burnette, D.T.; Lippincott-Schwartz, J.; Schmidt, C.F. Intracellular and extracellular forces drive primary cilia movement. Proc. Natl. Acad. Sci. USA 2015, 112, 1410–1415. [Google Scholar] [CrossRef] [Green Version]
- Chapman, A.B.; Stepniakowski, K.; Rahbari-Oskoui, F. Hypertension in autosomal dominant polycystic kidney disease. Adv. Chronic Kidney Dis. 2010, 17, 153–163. [Google Scholar] [CrossRef]
- Besschetnova, T.Y.; Kolpakova-Hart, E.; Guan, Y.; Zhou, J.; Olsen, B.R.; Shah, J.V. Identification of signaling pathways regulating primary cilium length and flow-mediated adaptation. Curr. Biol. 2010, 20, 182–187. [Google Scholar] [CrossRef] [Green Version]
- Gardner, K.; Arnoczky, S.P.; Lavagnino, M. Effect of in vitro stress-deprivation and cyclic loading on the length of tendon cell cilia in situ. J. Orthop. Res. 2011, 29, 582–587. [Google Scholar] [CrossRef]
- Saternos, H.C.; AbouAlaiwi, W.A. Signaling interplay between primary cilia and nitric oxide: A mini review. Nitric Oxide Biol. Chem. 2018, 80, 108–112. [Google Scholar] [CrossRef]
- Leyssac, P.P. Changes in single nephron renin release are mediated by tubular fluid flow rate. Kidney Int. 1986, 30, 332–339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Staruschenko, A. Regulation of transport in the connecting tubule and cortical collecting duct. Compr. Physiol. 2012, 2, 1541–1584. [Google Scholar] [PubMed] [Green Version]
- Piperi, C.; Basdra, E.K. Polycystins and mechanotransduction: From physiology to disease. World J. Exp. Med. 2015, 5, 200. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, H.; Spring, K.R. Bending the MDCK cell primary cilium increases intracellular calcium. J. Membr. Biol. 2001, 184, 71–79. [Google Scholar] [CrossRef] [PubMed]
- Praetorius, H.A.; Spring, K.R. The renal cell primary cilium functions as a flow sensor. Curr. Opin. Nephrol. Hypertens. 2003, 12, 517–520. [Google Scholar] [CrossRef] [PubMed]
- Yoder, B.K.; Hou, X.; Guay-Woodford, L.M. The polycystic kidney disease proteins, polycystin-1, polycystin-2, polaris, and cystin, are co-localized in renal cilia. J. Am. Soc. Nephrol. 2002, 13, 2508–2516. [Google Scholar] [CrossRef] [Green Version]
- Yoder, B.K.; Richards, W.G.; Sweeney, W.E.; Wilkinson, J.E.; Avener, E.D.; Woychik, R.P. Insertional mutagenesis and molecular analysis of a new gene associated with polycystic kidney disease. Proc. Assoc. Am. Physicians 1995, 107, 314–323. [Google Scholar]
- Dell, K.M. The role of cilia in the pathogenesis of cystic kidney disease. Curr. Opin. Pediatr. 2015, 27, 212–218. [Google Scholar] [CrossRef] [Green Version]
- Boo, Y.C.; Jo, H. Flow-dependent regulation of endothelial nitric oxide synthase: Role of protein kinases. Am. J. Physiol. Cell Physiol. 2003, 285, C499–C508. [Google Scholar] [CrossRef] [Green Version]
- AbouAlaiwi, W.A.; Nauli, S.M. Roles of ciliary polycystin and survivin in cardiovascular abnormality in PKD. FASEB J. 2009, 23, 594–598. [Google Scholar] [CrossRef]
- Nauli, S.M.; Kawanabe, Y.; Kaminski, J.J.; Pearce, W.J.; Ingber, D.E.; Zhou, J. Endothelial cilia are fluid shear sensors that regulate calcium signaling and nitric oxide production through polycystin-1. Circulation 2008, 117, 1161–1171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeCaen, P.G.; Delling, M.; Vien, T.N.; Clapham, D.E. Direct recording and molecular identification of the calcium channel of primary cilia. Nature 2013, 504, 315–318. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delling, M.; Indzhykulian, A.; Liu, X.; Li, Y.; Xie, T.; Corey, D.; Clapham, D. Primary cilia are not calcium-responsive mechanosensors. Nature 2016, 531, 656. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sigg, M.A.; Menchen, T.; Lee, C.; Johnson, J.; Jungnickel, M.K.; Choksi, S.P.; Garcia, G., 3rd; Busengdal, H.; Dougherty, G.W.; Pennekamp, P. Evolutionary proteomics uncovers ancient associations of cilia with signaling pathways. Dev. Cell 2017, 43, 744–762.e11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, J.; Mathur, J.; Vessières, E.; Hammack, S.; Nonomura, K.; Favre, J.; Grimaud, L.; Petrus, M.; Francisco, A.; Li, J. GPR68 senses flow and is essential for vascular physiology. Cell 2018, 173, 762–775.e16. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abdul-Majeed, S.; Nauli, S.M. Dopamine receptor type 5 in the primary cilia has dual chemo-and mechano-sensory roles. Hypertension 2011, 58, 325–331. [Google Scholar] [CrossRef] [PubMed]
- Martín-Guerrero, E.; Tirado-Cabrera, I.; Buendía, I.; Alonso, V.; Gortázar, A.R.; Ardura, J.A. Primary cilia mediate parathyroid hormone receptor type 1 osteogenic actions in osteocytes and osteoblasts via Gli activation. J. Cell. Physiol. 2020, 235, 7356–7369. [Google Scholar] [CrossRef]
- Zamparo, I.; Francia, S.; Franchi, S.A.; Redolfi, N.; Costanzi, E.; Kerstens, A.; Fukutani, Y.; Battistutta, R.; Polverino de Laureto, P.; Munck, S.; et al. Axonal odorant receptors mediate axon targeting. Cell Rep. 2019, 29, 4334–4348.e7. [Google Scholar] [CrossRef] [Green Version]
- Kuhlmann, K.; Tschapek, A.; Wiese, H.; Eisenacher, M.; Meyer, H.E.; Hatt, H.H.; Oeljeklaus, S.; Warscheid, B. The membrane proteome of sensory cilia to the depth of olfactory receptors. Mol. Cell. Proteom. 2014, 13, 1828–1843. [Google Scholar] [CrossRef] [Green Version]
- Castillo, K.; Restrepo, D.; Bacigalupo, J. Cellular and molecular Ca2+ microdomains in olfactory cilia support low signaling amplification of odor transduction. Eur. J. Neurosci. 2010, 32, 932–938. [Google Scholar] [CrossRef]
- Falk, N.; Losl, M.; Schroder, N.; Giessl, A. Specialized cilia in mammalian sensory systems. Cells 2015, 4, 500–519. [Google Scholar] [CrossRef] [Green Version]
- Jenkins, P.M.; McEwen, D.P.; Martens, J.R. Olfactory cilia: Linking sensory cilia function and human disease. Chem. Senses 2009, 34, 451–464. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hronik-Tupaj, M.; Kaplan, D.L. A review of the responses of two- and three-dimensional engineered tissues to electric fields. Tissue Eng. Part B Rev. 2012, 18, 167–180. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, M.; Kang, E.T.; Neoh, K.G. Electrical stimulation of adipose-derived mesenchymal stem cells in conductive scaffolds and the roles of voltage-gated ion channels. Acta Biomater. 2016, 32, 46–56. [Google Scholar] [CrossRef] [PubMed]
- Bergh, J.J.; Xu, Y.; Farach-Carson, M.C. Osteoprotegerin expression and secretion are regulated by calcium influx through the L-type voltage-sensitive calcium channel. Endocrinology 2004, 145, 426–436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zayzafoon, M. Calcium/calmodulin signaling controls osteoblast growth and differentiation. J. Cell. Biochem. 2006, 97, 56–70. [Google Scholar] [CrossRef] [PubMed]
- Brownell, W.E.; Qian, F.; Anvari, B. Cell membrane tethers generate mechanical force in response to electrical stimulation. Biophys. J. 2010, 99, 845–852. [Google Scholar] [CrossRef] [Green Version]
- Hart, F.X. Cytoskeletal forces produced by extremely low-frequency electric fields acting on extracellular glycoproteins. Bioelectromagnetics 2010, 31, 77–84. [Google Scholar] [CrossRef]
- Kleene, S.J.; Van Houten, J.L. Electrical signaling in motile and primary cilia. Bioscience 2014, 64, 1092–1102. [Google Scholar] [CrossRef]
- Kahl, C.R.; Means, A.R. Regulation of cell cycle progression by calcium/calmodulin-dependent pathways. Endocr. Rev. 2003, 24, 719–736. [Google Scholar] [CrossRef] [Green Version]
- Corrigan, M.A.; Johnson, G.P.; Stavenschi, E.; Riffault, M.; Labour, M.-N.; Hoey, D.A. TRPV4-mediates oscillatory fluid shear mechanotransduction in mesenchymal stem cells in part via the primary cilium. Sci. Rep. 2018, 8, 3824. [Google Scholar] [CrossRef] [PubMed]
- Yuan, N.; Rezzadeh, K.S.; Lee, J.C. Biomimetic scaffolds for osteogenesis. Recept. Clin. Investig. 2015, 2. [Google Scholar] [CrossRef]
- Yuan, T.F.; Hou, G.; Arias-Carrion, O. Chronic stress impacts on olfactory system. CNS Neurol. Disord. Drug Targets 2015, 14, 486–491. [Google Scholar] [CrossRef] [PubMed]
- Wolfrum, U.; Salisbury, J.L. Expression of centrin isoforms in the mammalian retina. Exp. Cell Res. 1998, 242, 10–17. [Google Scholar] [CrossRef] [Green Version]
- Doerr, N.; Wang, Y.; Kipp, K.R.; Liu, G.; Benza, J.J.; Pletnev, V.; Pavlov, T.S.; Staruschenko, A.; Mohieldin, A.M.; Takahashi, M.; et al. Regulation of polycystin-1 function by calmodulin binding. PLoS ONE 2016, 11, e0161525. [Google Scholar] [CrossRef] [Green Version]
- Merrick, D.; Bertuccio, C.A.; Chapin, H.C.; Lal, M.; Chauvet, V.; Caplan, M.J. Polycystin-1 cleavage and the regulation of transcriptional pathways. Pediatric Nephrol. 2014, 29, 505–511. [Google Scholar] [CrossRef]
- Masyuk, A.I.; Gradilone, S.A.; Banales, J.M.; Huang, B.Q.; Masyuk, T.V.; Lee, S.O.; Splinter, P.L.; Stroope, A.J.; Larusso, N.F. Cholangiocyte primary cilia are chemosensory organelles that detect biliary nucleotides via P2Y12 purinergic receptors. Am. J. Physiol. Liver Physiol. 2008, 295, G725–G734. [Google Scholar] [CrossRef] [Green Version]
- McGrath, J.; Somlo, S.; Makova, S.; Tian, X.; Brueckner, M. Two populations of node monocilia initiate left-right asymmetry in the mouse. Cell 2003, 114, 61–73. [Google Scholar] [CrossRef] [Green Version]
- Yoshiba, S.; Shiratori, H.; Kuo, I.Y.; Kawasumi, A.; Shinohara, K.; Nonaka, S.; Asai, Y.; Sasaki, G.; Belo, J.A.; Sasaki, H.; et al. Cilia at the node of mouse embryos sense fluid flow for left-right determination via Pkd2. Science 2012, 338, 226–231. [Google Scholar] [CrossRef] [Green Version]
- Li, Q.; Montalbetti, N.; Wu, Y.; Ramos, A.; Raychowdhury, M.K.; Chen, X.Z.; Cantiello, H.F. Polycystin-2 cation channel function is under the control of microtubular structures in primary cilia of renal epithelial cells. J. Biol. Chem. 2006, 281, 37566–37575. [Google Scholar] [CrossRef] [Green Version]
- Qiu, N.; Xiao, Z.; Cao, L.; Buechel, M.M.; David, V.; Roan, E.; Quarles, L.D. Disruption of Kif3a in osteoblasts results in defective bone formation and osteopenia. J. Cell Sci. 2012, 125, 1945–1957. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, S.; Tsiokas, L. Cilia and cell cycle re-entry: More than a coincidence. Cell Cycle 2011, 10, 2683–2690. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Katsianou, M.A.; Skondra, F.G.; Gargalionis, A.N.; Piperi, C.; Basdra, E.K. The role of transient receptor potential polycystin channels in bone diseases. Ann. Transl. Med. 2018, 6, 246. [Google Scholar] [CrossRef] [PubMed]
- Masyuk, A.I.; Masyuk, T.V.; Splinter, P.L.; Huang, B.Q.; Stroope, A.J.; LaRusso, N.F. Cholangiocyte cilia detect changes in luminal fluid flow and transmit them into intracellular Ca2+ and cAMP signaling. Gastroenterology 2006, 131, 911–920. [Google Scholar] [CrossRef] [Green Version]
- Delaine-Smith, R.M.; Sittichokechaiwut, A.; Reilly, G.C. Primary cilia respond to fluid shear stress and mediate flow-induced calcium deposition in osteoblasts. FASEB J. 2014, 28, 430–439. [Google Scholar] [CrossRef] [Green Version]
- Basten, S.G.; Giles, R.H. Functional aspects of primary cilia in signaling, cell cycle and tumorigenesis. Cilia 2013, 2, 6. [Google Scholar] [CrossRef] [Green Version]
- Hua, K.; Ferland, R.J. Primary cilia proteins: Ciliary and extraciliary sites and functions. Cell. Mol. Life Sci. 2018, 75, 1521–1540. [Google Scholar] [CrossRef]
- Humeau, J.; Bravo-San Pedro, J.M.; Vitale, I.; Nunez, L.; Villalobos, C.; Kroemer, G.; Senovilla, L. Calcium signaling and cell cycle: Progression or death. Cell Calcium 2018, 70, 3–15. [Google Scholar] [CrossRef]
- Liang, Y.; Meng, D.; Zhu, B.; Pan, J. Mechanism of ciliary disassembly. Cell. Mol. Life Sci. 2016, 73, 1787–1802. [Google Scholar] [CrossRef]
- Seeley, E.S.; Nachury, M.V. The perennial organelle: Assembly and disassembly of the primary cilium. J. Cell Sci. 2010, 123, 511–518. [Google Scholar] [CrossRef] [Green Version]
- Kobayashi, T.; Tsang, W.Y.; Li, J.; Lane, W.; Dynlacht, B.D. Centriolar kinesin Kif24 interacts with CP110 to remodel microtubules and regulate ciliogenesis. Cell 2011, 145, 914–925. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chauvet, V.; Tian, X.; Husson, H.; Grimm, D.H.; Wang, T.; Hiesberger, T.; Igarashi, P.; Bennett, A.M.; Ibraghimov-Beskrovnaya, O.; Somlo, S.; et al. Mechanical stimuli induce cleavage and nuclear translocation of the polycystin-1 C terminus. J. Clin. Investig. 2004, 114, 1433–1443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nikonova, A.S.; Plotnikova, O.V.; Serzhanova, V.; Efimov, A.; Bogush, I.; Cai, K.Q.; Hensley, H.H.; Egleston, B.L.; Klein-Szanto, A.; Seeger-Nukpezah, T.; et al. Nedd9 restrains renal cystogenesis in Pkd1-/- mice. Proc. Natl. Acad. Sci. USA 2014, 111, 12859–12864. [Google Scholar] [CrossRef] [Green Version]
- Gunzer, M. Migration, cell-cell interaction and adhesion in the immune system. Ernst Scher. Found. Symp. Proc. 2007, 2007, 97–137. [Google Scholar] [CrossRef]
- Trepat, X.; Chen, Z.; Jacobson, K. Cell migration. Compr. Physiol. 2012, 2369–2392. [Google Scholar] [CrossRef] [Green Version]
- Ezan, J.; Lasvaux, L.; Gezer, A.; Novakovic, A.; May-Simera, H.; Belotti, E.; Lhoumeau, A.C.; Birnbaumer, L.; Beer-Hammer, S.; Borg, J.P.; et al. Primary cilium migration depends on G-protein signalling control of subapical cytoskeleton. Nat. Cell Biol. 2013, 15, 1107–1115. [Google Scholar] [CrossRef]
- Gencer, S.; Oleinik, N.; Kim, J.; Panneer Selvam, S.; De Palma, R.; Dany, M.; Nganga, R.; Thomas, R.J.; Senkal, C.E.; Howe, P.H.; et al. TGF-β receptor I/II trafficking and signaling at primary cilia are inhibited by ceramide to attenuate cell migration and tumor metastasis. Sci. Signal. 2017, 10. [Google Scholar] [CrossRef] [Green Version]
- Iruzubieta-Agudo, P.; Monzón, M.; Castiella, T.; Ramírez, T.; Junquera, C. Hedgehog signalling pathway activation in gastrointestinal stromal tumours is mediated by primary cilia. Gastric Cancer 2019, 23, 64–72. [Google Scholar] [CrossRef]
- Vladar, E.K.; Königshoff, M. Noncanonical Wnt planar cell polarity signaling in lung development and disease. Biochem. Soc. Trans. 2020, 48, 231–243. [Google Scholar] [CrossRef] [Green Version]
- Doyle, A.; Marganski, W.; Lee, J. Calcium transients induce spatially coordinated increases in traction force during the movement of fish keratocytes. J. Cell Sci. 2004, 117, 2203–2214. [Google Scholar] [CrossRef] [Green Version]
- Schneider, L.; Cammer, M.; Lehman, J.; Nielsen, S.K.; Guerra, C.F.; Veland, I.R.; Stock, C.; Hoffmann, E.K.; Yoder, B.K.; Schwab, A.; et al. Directional cell migration and chemotaxis in wound healing response to PDGF-AA are coordinated by the primary cilium in fibroblasts. Cell. Physiol. Biochem. 2010, 25, 279–292. [Google Scholar] [CrossRef] [PubMed]
- Veland, I.R.; Lindbæk, L.; Christensen, S.T. Linking the primary cilium to cell migration in tissue repair and brain development. Bioscience 2014, 64, 1115–1125. [Google Scholar] [CrossRef] [PubMed]
- Taverna, E.; Götz, M.; Huttner, W.B. The cell biology of neurogenesis: Toward an understanding of the development and evolution of the neocortex. Annu. Rev. Cell Dev. Biol. 2014, 30, 465–502. [Google Scholar] [CrossRef] [PubMed]
- Zolessi, F. Vertebrate neurogenesis: Cell polarity. eLS 2016, 1–14. [Google Scholar] [CrossRef]
- Banda, E.; McKinsey, A.; Germain, N.; Carter, J.; Anderson, N.C.; Grabel, L. Cell polarity and neurogenesis in embryonic stem cell-derived neural rosettes. Stem Cells Dev. 2015, 24, 1022–1033. [Google Scholar] [CrossRef]
- Barakat, B.; Yu, L.; Lo, C.; Vu, D.; De Luca, E.; Cain, J.E.; Martelotto, L.G.; Dedhar, S.; Sadler, A.J.; Wang, D.; et al. Interaction of smoothened with integrin-linked kinase in primary cilia mediates Hedgehog signalling. EMBO Rep. 2013, 14, 837–844. [Google Scholar] [CrossRef] [Green Version]
- Lie, D.C.; Colamarino, S.A.; Song, H.J.; Désiré, L.; Mira, H.; Consiglio, A.; Lein, E.S.; Jessberger, S.; Lansford, H.; Dearie, A.R.; et al. Wnt signalling regulates adult hippocampal neurogenesis. Nature 2005, 437, 1370–1375. [Google Scholar] [CrossRef]
- Ossipova, O.; Sokol, S.Y. Cell polarity, notch signaling and neurogenesis. Cell Cycle 2010, 9. [Google Scholar] [CrossRef] [Green Version]
- Pala, R.; Alomari, N.; Nauli, S.M. Primary cilium-dependent signaling mechanisms. Int. J. Mol. Sci. 2017, 18, 2272. [Google Scholar] [CrossRef] [Green Version]
- Werner, R. Effect of metopirone-ditartrate on thermogenesis in the guinea-pig. Comp. Biochem. Physiol. Part C Comp. Pharmacol. 1988, 90, 445–450. [Google Scholar] [CrossRef]
- Vogel, T.W.; Carter, C.S.; Abode-Iyamah, K.; Zhang, Q.; Robinson, S. The role of primary cilia in the pathophysiology of neural tube defects. Neurosurg. Focus 2012, 33, E2. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trulioff, A.; Ermakov, A.; Malashichev, Y. Primary cilia as a possible link between left-right asymmetry and neurodevelopmental diseases. Genes 2017, 8, 48. [Google Scholar] [CrossRef] [PubMed]
- Omori, T.; Sugai, H.; Imai, Y.; Ishikawa, T. Nodal cilia-driven flow: Development of a computational model of the nodal cilia axoneme. J. Biomech. 2017, 61, 242–249. [Google Scholar] [CrossRef] [PubMed]
- Dasgupta, A.; Amack, J.D. Cilia in vertebrate left-right patterning. Philos. Trans. R. Soc. Lond B Biol. Sci. 2016, 371. [Google Scholar] [CrossRef] [Green Version]
- Norris, D.P. Cilia, calcium and the basis of left-right asymmetry. BMC Biol. 2012, 10, 102. [Google Scholar] [CrossRef] [Green Version]
- Ban, J.; Bonifazi, P.; Pinato, G.; Broccard, F.D.; Studer, L.; Torre, V.; Ruaro, M.E. Embryonic stem cell-derived neurons form functional networks in vitro. Stem Cells 2007, 25, 738–749. [Google Scholar] [CrossRef]
- Blankenship, A.G.; Feller, M.B. Mechanisms underlying spontaneous patterned activity in developing neural circuits. Nat. Rev. Neurosci. 2010, 11, 18–29. [Google Scholar] [CrossRef]
- Komuro, H.; Rakic, P. Intracellular Ca2+ fluctuations modulate the rate of neuronal migration. Neuron 1996, 17, 275–285. [Google Scholar] [CrossRef] [Green Version]
- Spitzer, N.C. Electrical activity in early neuronal development. Nature 2006, 444, 707–712. [Google Scholar] [CrossRef]
- Wong, R.O.; Chernjavsky, A.; Smith, S.J.; Shatz, C.J. Early functional neural networks in the developing retina. Nature 1995, 374, 716–718. [Google Scholar] [CrossRef]
- Yacubova, E.; Komuro, H. Stage-specific control of neuronal migration by somatostatin. Nature 2002, 415, 77–81. [Google Scholar] [CrossRef]
- Malmersjö, S.; Rebellato, P.; Smedler, E.; Planert, H.; Kanatani, S.; Liste, I.; Nanou, E.; Sunner, H.; Abdelhady, S.; Zhang, S.; et al. Neural progenitors organize in small-world networks to promote cell proliferation. Proc. Natl. Acad. Sci. USA 2013, 110, E1524–E1532. [Google Scholar] [CrossRef] [Green Version]
- Fabbro, A.; Pastore, B.; Nistri, A.; Ballerini, L. Activity-independent intracellular Ca2+ oscillations are spontaneously generated by ventral spinal neurons during development in vitro. Cell Calcium 2007, 41, 317–329. [Google Scholar] [CrossRef]
- Berridge, M.J.; Lipp, P.; Bootman, M.D. The versatility and universality of calcium signalling. Nat. Rev. Mol. Cell Biol. 2000, 1, 11–21. [Google Scholar] [CrossRef]
- Verkhratsky, A.J.; Petersen, O.H. Neuronal calcium stores. Cell Calcium 1998, 24, 333–343. [Google Scholar] [CrossRef]
- Sah, P. Ca2+-activated K+ currents in neurones: Types, physiological roles and modulation. Trends Neurosci. 1996, 19, 150–154. [Google Scholar] [CrossRef]
- Khan, D.; Moffet, C.R.; Flatt, P.R.; Kelly, C. Role of islet peptides in beta cell regulation and type 2 diabetes therapy. Peptides 2018, 100, 212–218. [Google Scholar] [CrossRef] [PubMed]
- Hughes, J.W.; Cho, J.H.; Conway, H.E.; DiGruccio, M.R.; Ng, X.W.; Roseman, H.F.; Abreu, D.; Urano, F.; Piston, D.W. Primary cilia control glucose homeostasis via islet paracrine interactions. Proc. Natl. Acad. Sci. USA 2020, 117, 8912–8923. [Google Scholar] [CrossRef] [Green Version]
- Benninger, R.K.P.; Piston, D.W. Cellular communication and heterogeneity in pancreatic islet insulin secretion dynamics. Trends Endocrinol. Metab. 2014, 25, 399–406. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fendler, B.; Zhang, M.; Satin, L.; Bertram, R. Synchronization of pancreatic islet oscillations by intrapancreatic ganglia: A modeling study. Biophys. J. 2009, 97, 722–729. [Google Scholar] [CrossRef] [Green Version]
- Delling, M.; DeCaen, P.G.; Doerner, J.F.; Febvay, S.; Clapham, D.E. Primary cilia are specialized calcium signalling organelles. Nature 2013, 504, 311–314. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gradilone, S.A.; Masyuk, A.I.; Splinter, P.L.; Banales, J.M.; Huang, B.Q.; Tietz, P.S.; Masyuk, T.V.; Larusso, N.F. Cholangiocyte cilia express TRPV4 and detect changes in luminal tonicity inducing bicarbonate secretion. Proc. Natl. Acad. Sci. USA 2007, 104, 19138–19143. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boyer, J.L. Bile formation and secretion. Compr. Physiol. 2013, 3, 1035–1078. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mansini, A.P.; Peixoto, E.; Thelen, K.M.; Gaspari, C.; Jin, S.; Gradilone, S.A. The cholangiocyte primary cilium in health and disease. Biochim. Biophys. Acta Mol. Basis Dis. 2018, 1864, 1245–1253. [Google Scholar] [CrossRef]
- Vaughan, T.J.; Mullen, C.A.; Verbruggen, S.W.; McNamara, L.M. Bone cell mechanosensation of fluid flow stimulation: A fluid-structure interaction model characterising the role integrin attachments and primary cilia. Biomech. Model. Mechanobiol. 2015, 14, 703–718. [Google Scholar] [CrossRef]
- Temiyasathit, S.; Jacobs, C.R. Osteocyte primary cilium and its role in bone mechanotransduction. Ann. N. Y. Acad. Sci. 2010, 1192, 422–428. [Google Scholar] [CrossRef]
- Malone, A.M.; Anderson, C.T.; Tummala, P.; Kwon, R.Y.; Johnston, T.R.; Stearns, T.; Jacobs, C.R. Primary cilia mediate mechanosensing in bone cells by a calcium-independent mechanism. Proc. Natl. Acad. Sci. USA 2007, 104, 13325–13330. [Google Scholar] [CrossRef] [Green Version]
- Uda, Y.; Azab, E.; Sun, N.; Shi, C.; Pajevic, P.D. Osteocyte Mechanobiology. Curr. Osteoporos. Rep. 2017, 15, 318–325. [Google Scholar] [CrossRef]
| Ion Channel, GPCR, or Protein | Property | Functional Response | Citation |
|---|---|---|---|
| PC-1 | Mechanosensative Membrane-Bound Protein, Possible Atypical GPCR | Vascular endothelial cells: activation of PC-1/PC-2 complex triggers CaM, PKC, and AKT/PKB, which in turn trigger eNOS, leading to NO production and subsequent vasodilationCell cycle: initially activates PC-2, which activates Ca2+/CaM-dependent pathways, then tail is cleaved and translocated to the nucleus to regulate DNA transcriptionCholangiocytes: involved in biliary regulation with PC-2, generates signals that modulate bile secretion based on external stimuli | [30,65,66,67] |
| PC-2 | Ca2+-permeable Non-selective TRP Cation Channel | Vascular endothelial cells: activation of PC-1/PC-2 complex triggers CaM, PKC, and AKT/PKB, which in turn trigger eNOS, leading to NO production and subsequent vasodilationCell cycle: allows Ca2+ influx, which activates Ca2+/CaM-dependent pathwaysNeuronal patterning: allows for the asymmetrical Ca2+ distribution needed for left-right patterning Cholangiocytes: involved in biliary regulation with PC-2, generates signals that modulate bile secretion based on external stimuliOsteocytes: involved in osteoblast mechano-functions, possibly along with Kif3a | [30,65,67,68,69,70,71] |
| CaM | Ca2+-binding Messenger Protein | Vascular endothelial cells: activation of PC-1/PC-2 complex triggers CaM, which triggers eNOS, leading to NO production and subsequent vasodilationCell cycle: modulates Ca2+/CaM-dependent kinases I, II, and IV | [30,60] |
| TRPV2 | Ca2+-permeable Non-selective TRP Cation Channel | Cell cycle: allows for an isolated rise in intraciliary Ca2+ | [72] |
| TRPC1 | Ca2+-permeable Non-selective TRP Cation Channel | Cell cycle: allows for an isolated rise in intraciliary Ca2+ | [72] |
| TRPV4 | Ca2+-permeable Non-selective TRP Cation Channel | Osteocytes: modulates Ca2+ levels, possibly in response to mechanical forcesCholangiocytes: osmoregulation of bile | [67,73,74] |
| TRPV6 | Ca2+-permeable Non-selective TRP Cation Channel | Osteocytes: modulates Ca2+ levels, possibly in response to mechanical forces | [73,75] |
| Kif3a | Kinesin-like Protein | Osteocytes: involved in bone formation and osteoblast mechano-functions, possibly along with PC-2 | [70,71] |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Saternos, H.; Ley, S.; AbouAlaiwi, W. Primary Cilia and Calcium Signaling Interactions. Int. J. Mol. Sci. 2020, 21, 7109. https://doi.org/10.3390/ijms21197109
Saternos H, Ley S, AbouAlaiwi W. Primary Cilia and Calcium Signaling Interactions. International Journal of Molecular Sciences. 2020; 21(19):7109. https://doi.org/10.3390/ijms21197109
Chicago/Turabian StyleSaternos, Hannah, Sidney Ley, and Wissam AbouAlaiwi. 2020. "Primary Cilia and Calcium Signaling Interactions" International Journal of Molecular Sciences 21, no. 19: 7109. https://doi.org/10.3390/ijms21197109
APA StyleSaternos, H., Ley, S., & AbouAlaiwi, W. (2020). Primary Cilia and Calcium Signaling Interactions. International Journal of Molecular Sciences, 21(19), 7109. https://doi.org/10.3390/ijms21197109






