The Effect of Sodium Butyrate on Adventitious Shoot Formation Varies among the Plant Species and the Explant Types
Abstract
:1. Introduction
2. Results
2.1. Sodium Butyrate Significantly Enhanced the Growth and Adventitious Shoot Formation of Tobacco Calli
2.2. NaB Does Not Have a Positive Role in Enhancing Callus Growth in Tomato
2.3. Adventitious Shoot Formation in Cotyledon Explants of Tobacco is Significantly Affected by the NaB Concentration
2.4. NaB Inhibits New Shoot Formation, but Improves Normal Shoot Growth in Cotyledon Explants of Tomato
2.5. Gene Expression of NbCYCD3-1 and NbWUS in Tobacco Calli after NaB Treatment
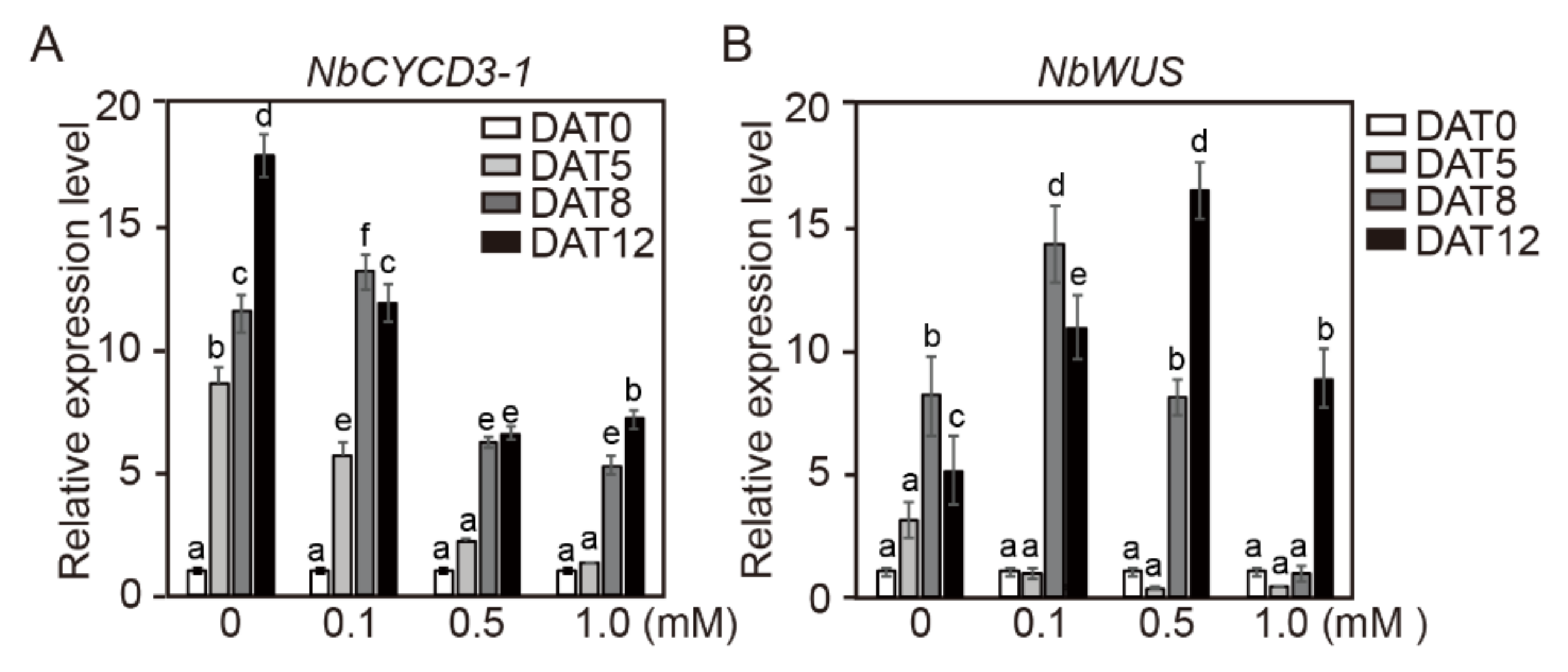
2.6. Gene Expression of NbCYCD3-1 and NbWUS in Cotyledon Explants of Tobacco
2.7. Effect of NaB on the Expression of SlCYCD3-1 and SlWUS Genes in Cotyledon Explants of Tomato
2.8. NaB Promotes Histone Acetylation in Tobacco Protoplast-Derived Calli
3. Discussion
4. Materials and Methods
4.1. Plant Materials
4.2. Protoplasts Isolation and Culture Condition
4.3. The Effect of Sodium Butyrate in Protoplast-Derived Calli of Tobacco
4.4. The Effect of Sodium Butyrate in Protoplast-Derived Calli of Tomato
4.5. Culture Conditions for Adventitious Shoot Formation in Cotyledon Explants of Tobacco and Tomato in the NaB Containing SIM
4.6. Histone Extraction and Western Blotting
4.7. RNA Isolation and Quantitative Reverse Transcription PCR (qRT-PCR) Analysis
4.8. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
References
- Skoog, F.; Miller, C.O. Chemical regulation of growth and organ formation in plant tissues cultured in vitro. Symp. Soc. Exp. Biol. 1957, 54, 118–130. [Google Scholar]
- Murashige, T. Plant propagation through tissue cultures. Annu. Rev. Plant Physiol. 1974, 25, 135–166. [Google Scholar] [CrossRef]
- Valvekens, D.; Montagu, M.V.; Van Lijsebettens, M. Agrobacterium tumefaciens-mediated transformation of Arabidopsis thaliana root explants by using kanamycin selection. Proc. Natl. Acad. Sci. USA 1988, 85, 5536–5540. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Duclercq, J.; Sangwan-Norreel, B.; Catterou, M.; Sangwan, R.S. De novo shoot organogenesis: From art to science. Trends Plant Sci. 2011, 16, 597–606. [Google Scholar] [CrossRef]
- Barton, M.K.; Poethig, R.S. Formation of the shoot apical meristem in Arabidopsis thaliana: An analysis of development in the wild type and in the shoot meristemless mutant. Development 1993, 119, 823–831. [Google Scholar]
- Aida, M.; Ishida, T.; Fukaki, H.; Fujisawa, H.; Tasaka, M. Genes involved in organ separation in Arabidopsis: An analysis of the cup-shaped cotyledon mutant. Plant Cell 1997, 9, 841–857. [Google Scholar] [CrossRef] [Green Version]
- Mayer, K.F.; Schoof, H.; Haecker, A.; Lenhard, M.; Jurgens, G.; Laux, T. Role of WUSCHEL in regulating stem cell fate in the Arabidopsis shoot meristem. Cell 1998, 95, 805–815. [Google Scholar] [CrossRef] [Green Version]
- Takada, S.; Hibara, K.; Ishida, T.; Tasaka, M. The CUP-SHAPED COTYLEDON1 gene of Arabidopsis thaliana regulates shoot apical meristem formation. Development 2001, 128, 1127–1135. [Google Scholar]
- Hibara, K.; Takada, S.; Tasaka, M. CUC1 gene activates the expression of SAM-related genes to induce adventitious shoot formation. Plant J. 2003, 36, 687–696. [Google Scholar] [CrossRef]
- Lenhard, M.; Jürgens, G.; Laux, T. The WUSCHEL and SHOOT MERISTEMLESS genes fulfill complementary roles in Arabidopsis shoot meristem regulation. Development 2002, 129, 3195–3206. [Google Scholar]
- Su, Y.H.; Zhao, X.Y.; Liu, Y.B.; Zhang, C.L.; O’Neill, S.D.; Zhang, X.S. Auxin-induced WUS expression is essential for embryonic stem cell renewal during somatic embryogenesis in Arabidopsis. Plant J. 2009, 59, 448–460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Lian, H.; Zhou, C.; Xu, L.; Jiao, Y.; Wang, J. A two-step model for de novo activation of WUSCHEL during plant shoot regeneration. Plant Cell 2007, 29, 1073–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, W.J.; Cheng, Z.J.; Sang, Y.L.; Zhang, M.M.; Rong, X.F.; Wang, Z.W.; Tang, Y.Y.; Zhang, X.S. Type-B ARABIDOPSIS RESPONSE REGULATORs specify the shoot stem cell niche by dual regulation of WUSCHEL. Plant Cell 2017, 29, 1357–1372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunter, R.H. Components of oviduct physiology in eutherian mammals. Biol. Rev. Camb. Philos. Soc. 2012, 287, 244–255. [Google Scholar] [CrossRef] [PubMed]
- Dewitte, W.; Scofield, S.; Alcasabas, A.A.; Maughan, S.C.; Menges, M.; Braun, N.; Collins, C.; Nieuwland, J.; Prinsen, E.; Sundaresan, V.; et al. Arabidopsis CYCD3 D-type cyclins link cell proliferation and endocycles and are rate-limiting for cytokinin responses. Proc. Natl. Acad. Sci. USA 2017, 104, 14537–14542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, H.; Ogas, J. An epigenetic perspective on developmental regulation of seed genes. Mol. Plant 2009, 2, 610–627. [Google Scholar] [CrossRef] [PubMed]
- Jiang, C.; Pugh, B.F. Nucleosome positioning and gene regulation: Advances through genomics. Nat. Rev. Genet. 2009, 10, 161–172. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Henikoff, S.; Shilatifard, A. Histone modification: Cause or cog? Trends Genet. 2011, 27, 389–396. [Google Scholar] [CrossRef]
- Kornberg, R.D.; Lorch, Y. Twenty-five years of the nucleosome, fundamental particle of the eukaryote chromosome. Cell 1999, 98, 285–294. [Google Scholar] [CrossRef] [Green Version]
- Seto, E.; Yoshida, M. Erasers of Histone Acetylation: The Histone Deacetylase Enzymes. Cold Spring Harb. Perspect. Biol. 2014, 6, a018713. [Google Scholar] [CrossRef] [Green Version]
- Abel, T.; Zukin, R.S. Epigenetic targets of HDAC inhibition in neurodegenerative and psychiatric disorders. Curr. Opin. Pharm. 2008, 8, 57–64. [Google Scholar] [CrossRef] [Green Version]
- Chuang, D.M.; Leng, Y.; Marinova, Z.; Kim, H.J.; Chiu, C.T. Multiple roles of HDAC inhibition in neurodegenerative conditions. Trends Neurosci. 2009, 32, 591–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanhaecke, T.; Papeleu, P.; Elaut, G.; Rogiers, V. Trichostatin A-like hydroxamate histone deacetylase inhibitors as therapeutic agents: Toxicological point of view. Curr. Med. Chem. 2004, 11, 1629–1643. [Google Scholar] [CrossRef] [PubMed]
- Wang, J.; Tian, C.; Zhang, C.; Shi, B.; Cao, X.; Zhang, T.; Zhao, Z.; Wang, J.; Jiao, Y. Cytokinin Signaling Activates WUSCHEL Expression during Axillary Meristem Initiation. Plant Cell 2017, 29, 1373–1387. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, H.; Soriano, M.; Cordewener, J.; Muino, J.M.; Riksen, T.; Fukuoka, H.; Angenent, G.C.; Boutilier, K. The Histone Deacetylase Inhibitor Trichostatin A Promotes Totipotency in the Male Gametophyte. Plant Cell 2014, 26, 195–209. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Johnstone, R.W. Histone-deacetylase inhibitors: Novel drugs for the treatment of cancer. Nat. Rev. Drug Discov. 2002, 1, 287–299. [Google Scholar] [CrossRef] [PubMed]
- Boffa, L.C.; Vidali, G.; Mann, R.S.; Allfrey, V.G. Suppression of histone deacetylation in vivo and in vitro by sodium butyrate. J. Biol. Chem. 1978, 253, 3364–3366. [Google Scholar] [PubMed]
- Sealy, L.; Chalkley, R. The effect of sodium butyrate on histone modification. Cell 1978, 14, 115–121. [Google Scholar] [CrossRef]
- Monneret, C. Inhibition of histone deacetylase activity by butyrate. Eur. J. Med. Chem. 2005, 40, 1–13. [Google Scholar] [CrossRef]
- Tanaka, M.; Akikuchi, A.; Kamada, H. The Arabidopsis histone deacetylases HDA6 and HDA19 contribute to the repression of embryonic properties after germination. Plant Physiol. 2008, 146, 149–161. [Google Scholar] [CrossRef] [Green Version]
- Uddenberg, D.; Valladares, S.; Abrahamsson, M.; Sundstrom, J.F.; Sundas-Larsson, A.; von Arnold, S. Embryogenic potential and expression of embryogenesis-related genes in conifers are affected by treatment with a histone deacetylase inhibitor. Plant 2011, 234, 527–539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, X.; Zhang, C.; Zhang, B.; Yang, C.; Li, S. Identification of genes regulated by histone acetylation during root development in Populus trichocarpa. BMC Genom. 2016, 17, 96. [Google Scholar] [CrossRef] [Green Version]
- Jiang, F.; Ryabova, D.; Diedhiou, J.; Hucl, P.; Randhawa, H.; Marillia, E.-F.; Foroud, N.A.; Eudes, F.; Kathiria, P. Trichostatin A increases embryo and green plant regeneration in wheat. Plant Cell Rep. 2017, 36, 1701–1706. [Google Scholar] [CrossRef]
- Pagano, A.; de Sousa Araujo, S.; Macovei, A.; Dondi, D.; Lazzaroni, S.; Balestrazzi, A. Metabolic and gene expression hallmarks of seed germination uncovered by sodium butyrate in Medicago truncatula. Plant Cell Environ. 2019, 42, 259–269. [Google Scholar] [CrossRef] [Green Version]
- Pedersen, T.J.; Minocha, S.C. Effects of n-sodium butyrate on cell division in Jerusalem Artichoke (Helianthus tuberosus L.) tube explants cultured in vitro. J. Plant Physiol. 1988, 132, 623–630. [Google Scholar] [CrossRef]
- Lochmanova, G.; Ihnatova, I.; Kucharikova, H.; Brabencova, S.; Zachova, D.; Fajkus, J.; Zdrahal, Z.; Fojtova, M. Different Modes of Action of Genetic and Chemical Downregulation of Histone Deacetylases with Respect to Plant Development and Histone Modifications. Int. J. Mol. Sci. 2019, 20, 5093. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Furuta, K.; Kubo, M.; Sano, K.; Demura, T.; Fukuda, H.; Liu, Y.G.; Shibata, D.; Kakimoto, T. The CKH2/PKL chromatin-remodeling factor negatively regulates cytokinin responses in Arabidopsis calli. Plant Cell Physiol. 2011, 52, 618–628. [Google Scholar] [CrossRef] [Green Version]
- Lee, M.H.; Lee, J.; Jie, E.Y.; Choi, S.H.; Jiang, L.; Ahn, W.S.; Kim, C.Y.; Kim, S.W. Temporal and spatial expression analysis of shoot-regeneration regulatory genes during the adventitious shoot formation in hypocotyl and cotyledon explants of tomato (cv. Micro-Tom). Int. J. Mol. Sci. 2020, 21, 5309. [Google Scholar] [CrossRef]
- Van Lint, C.; Emiliani, S.; Verdin, E. The expression of a small fraction of cellular genes is changed in response to histone hyperacetylation. Gene Expr. 1996, 5, 245–253. [Google Scholar]
- Chambers, A.E.; Banerjee, S.; Chaplin, T.; Dunne, J.; Debernardi, S.; Joel, S.P.; Young, B.D. Histone acetylation-mediated regulation of genes in leukaemic cells. Eur. J. Cancer 2003, 39, 1165–1175. [Google Scholar] [CrossRef]
- Glaser, K.B.; Staver, M.J.; Waring, J.F.; Stender, J.; Ulrich, R.G.; Davidsen, S.K. Gene expression profiling of multiple histone deacetylase (HDAC) inhibitors: Defining a common gene set produced by HDAC inhibition in T24 and MDA carcinoma cell lines. Mol. Cancer 2003, 2, 151–163. [Google Scholar]
- Mitsiades, C.S.; Mitsiades, N.S.; McMullan, C.J.; Poulaki, V.; Shringarpure, R.; Hideshima, T.; Akiyama, M.; Chauhan, D.; Munshi, N.; Gu, X.; et al. Transcriptional signature of histone deacetylase inhibition in multiple myeloma: Biological and clinical implications. Proc. Natl. Acad. Sci. USA 2004, 101, 540–545. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chang, S.; Pikaard, C.S. Transcript profiling in Arabidopsis reveals complex responses to global inhibition of DNA methylation and histone deacetylation. J. Biol. Chem. 2005, 280, 796–804. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peart, M.J.; Smyth, G.K.; van Laar, R.K.; Bowtell, D.D.; Richon, V.M.; Marks, P.A.; Holloway, A.J.; Johnstone, R.W. Identification and functional significance of genes regulated by structurally different histone deacetylase inhibitors. Proc. Natl. Acad. Sci. USA 2005, 102, 3697–3702. [Google Scholar] [CrossRef] [Green Version]
- Sasakawa, Y.; Naoe, Y.; Sogo, N.; Inoue, T.; Sasakawa, T.; Matsuo, M.; Manda, T.; Mutoh, S. Marker genes to predict sensitivity to FK228, a histone deacetylase inhibitor. Biochem. Pharm. 2005, 69, 603–616. [Google Scholar] [CrossRef]
- Halsall, J.; Gupta, V.; O’Neill, L.P.; Turner, B.M.; Nightingale, K.P. Genes are often sheltered from the global histone hyperacetylation induced by HDAC inhibitors. PLoS ONE 2012, 7, e33453. [Google Scholar] [CrossRef]
- Inoue, K.; Oikawa, M.; Kamimura, S.; Ogonuki, N.; Nakamura, T.; Nakano, T.; Abe, K.; Ogura, A. Trichostatin A specifically improves the aberrant expression of transcription factor genes in embryos produced by somatic cell nuclear transfer. Sci. Rep. 2015, 5, 10127. [Google Scholar] [CrossRef] [Green Version]
- Zhao, L.; Wang, P.; Hou, H.; Zhang, H.; Wang, Y.; Yan, S.; Huang, Y.; Li, H.; Tan, J.; Hu, A.; et al. Transcriptional Regulation of Cell Cycle Genes in Response to Abiotic Stresses Correlates with Dynamic Changes in Histone Modifications in Maize. PLoS ONE 2014, 9, e106070. [Google Scholar] [CrossRef]
- Kim, J.; Yang, W.; Forner, J.; Lohmann, J.U.; Noh, B.; Noh, Y. Epigenetic reprogramming by histone acetyltransferase HAG1/AtGCN5 is required for pluripotency acquisition in Arabidopsis. EMBO 2018, 37, e98726. [Google Scholar] [CrossRef]
- Lee, K.; Seo, P.J. Dynamic Epigenetic Changes during Plant Regeneration. Trends Plant Sci. 2018, 23, 235–247. [Google Scholar] [CrossRef]
- Sako, K.; Kim, J.-M.; Matsui, A.; Nakamura, K.; Tanaka, M.; Kobayashi, M.; Saito, K.; Nishino, N.; Kusano, N.; Taji, T.; et al. Ky-2, a Histone Deacetylase Inhibitor, Enhances High-Salinity Stress Tolerance in Arabidopsis thaliana. Plant Cell Physiol. 2016, 57, 776–783. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ueda, M.; Matsui, A.; Tanaka, M.; Nakamura, T.; Abe, T.; Sako, K.; Sasaki, K.; Kim, J.-M.; Ito, A.; Nishino, N.; et al. The distinct roles of class I and II RPD3-like histone deacetylases in salinity stress response. Plant Physiol. 2017, 175, 1760–1773. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, H.M.; Sako, K.; Matsui, A.; Ueda, M.; Tanaka, M.; Ito, A.; Nishino, N.; Yoshida, M.; Seki, M. Transcriptomic analysis of Arabidopsis thaliana plants treated with the Ky-9 and Ky-72 histone deacetylase inhibitors. Plant Signal. Behav. 2018, 13, e1448333. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patanun, O.; Ueda, M.; Itouga, M.; Kato, Y.; Utsumi, Y.; Matsui, A.; Tanaka, M.; Utsumi, C.; Sakakibara, H.; Yoshida, M.; et al. The Histone Deacetylase Inhibitor Suberoylanilide Hydroxamic Acid Alleviates Salinity Stress in Cassava. Front. Plant Sci. 2016, 7, 2039. [Google Scholar] [CrossRef] [Green Version]
- Lee, K.; Park, O.-S.; Jung, S.-J.; Seo, P.J. Histone deacetylation-mediated cellular dedifferentiation in Arabidopsis. J. Plant Physiol. 2016, 191, 95–100. [Google Scholar] [CrossRef]
- Weidle, U.H.; Grossmann, A. Inhibition of histone deacetylases: A new strategy to target epigenetic modifications for anticancer treatment. Anticancer Res 2000, 20, 1471–1485. [Google Scholar]
- Marks, P.A.; Richon, V.M.; Breslow, R.; Rifkind, R.A. Histone deacetylase inhibitors as new cancer drugs. Curr. Opin. Oncol. 2001, 13, 477–483. [Google Scholar] [CrossRef]
- Gao, Y.; Zhao, M.; Wu, X.-H.; Li, D.; Borthakur, D.; Ye, J.-H.; Zheng, X.-Q.; Lu, J.-L. Analysis of Differentially expressed genes in tissues of Camellia sinensis during dedifferentiation and root redifferentiation. Sci. Rep. 2019, 9, 2935. [Google Scholar] [CrossRef]
- Kruh, J. Effects of sodium butyrate, a new pharmacological agent, on cells in culture. Mol. Cell. Biochem. 1982, 42, 65–82. [Google Scholar] [CrossRef]
- Chen, Z.J.; Pikaard, C.S. Epigenetic silencing of RNA polymerase I transcription: A role for DNA methylation and histone modification in nucleolar dominance. Genes Dev. 1997, 16, 2124–2136. [Google Scholar] [CrossRef] [Green Version]
- Chua, Y.L.; Watson, L.A.; Gray, J.C. The transcriptional enhancer of the pea plastocyanin gene associates with the nuclear matrix and regulates gene expression through histone acetylation. Plant Cell 2003, 15, 1468–1479. [Google Scholar] [CrossRef] [PubMed]
- Waterborg, J.H.; Kapros, T.B. Kinetic analysis of histone acetylation turnover and trichostatin A induced hyper- and hypoacetylation in alfalfa. Biochem. Cell Biol. 2002, 80, 279–293. [Google Scholar] [CrossRef] [PubMed]
- Jie, E.Y.; Kim, S.W.; Jang, H.R.; In, D.S.; Liu, J.R. Myo-inositol increases the plating efficiency of protoplast derived from cotyledon of cabbage (Brassica oleracea var. capitata). J. Plant Biotechnol. 2011, 38, 69–76. [Google Scholar] [CrossRef] [Green Version]
- Frearson, E.M.; Power, J.B.; Cocking, E.C. The isolation and regeneration of Petunia leaf protoplasts. Dev. Biol. 1973, 33, 130–137. [Google Scholar] [CrossRef]
- Mlotshwa, S.; Yan, Z.; Kim, Y.; Chen, X. Floral patterning defects induced by Arabidopsis APETALA2 and microRNA172 expression in Nicotiana Benthamiana. Plant Mol. Biol. 2006, 61, 781–793. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Lee, M.H.; Lee, J.; Choi, S.H.; Jie, E.Y.; Jeong, J.C.; Kim, C.Y.; Kim, S.W. The Effect of Sodium Butyrate on Adventitious Shoot Formation Varies among the Plant Species and the Explant Types. Int. J. Mol. Sci. 2020, 21, 8451. https://doi.org/10.3390/ijms21228451
Lee MH, Lee J, Choi SH, Jie EY, Jeong JC, Kim CY, Kim SW. The Effect of Sodium Butyrate on Adventitious Shoot Formation Varies among the Plant Species and the Explant Types. International Journal of Molecular Sciences. 2020; 21(22):8451. https://doi.org/10.3390/ijms21228451
Chicago/Turabian StyleLee, Myoung Hui, Jiyoung Lee, Seung Hee Choi, Eun Yee Jie, Jae Cheol Jeong, Cha Young Kim, and Suk Weon Kim. 2020. "The Effect of Sodium Butyrate on Adventitious Shoot Formation Varies among the Plant Species and the Explant Types" International Journal of Molecular Sciences 21, no. 22: 8451. https://doi.org/10.3390/ijms21228451




