Sleep Apnoea Adverse Effects on Cancer: True, False, or Too Many Confounders?
Abstract
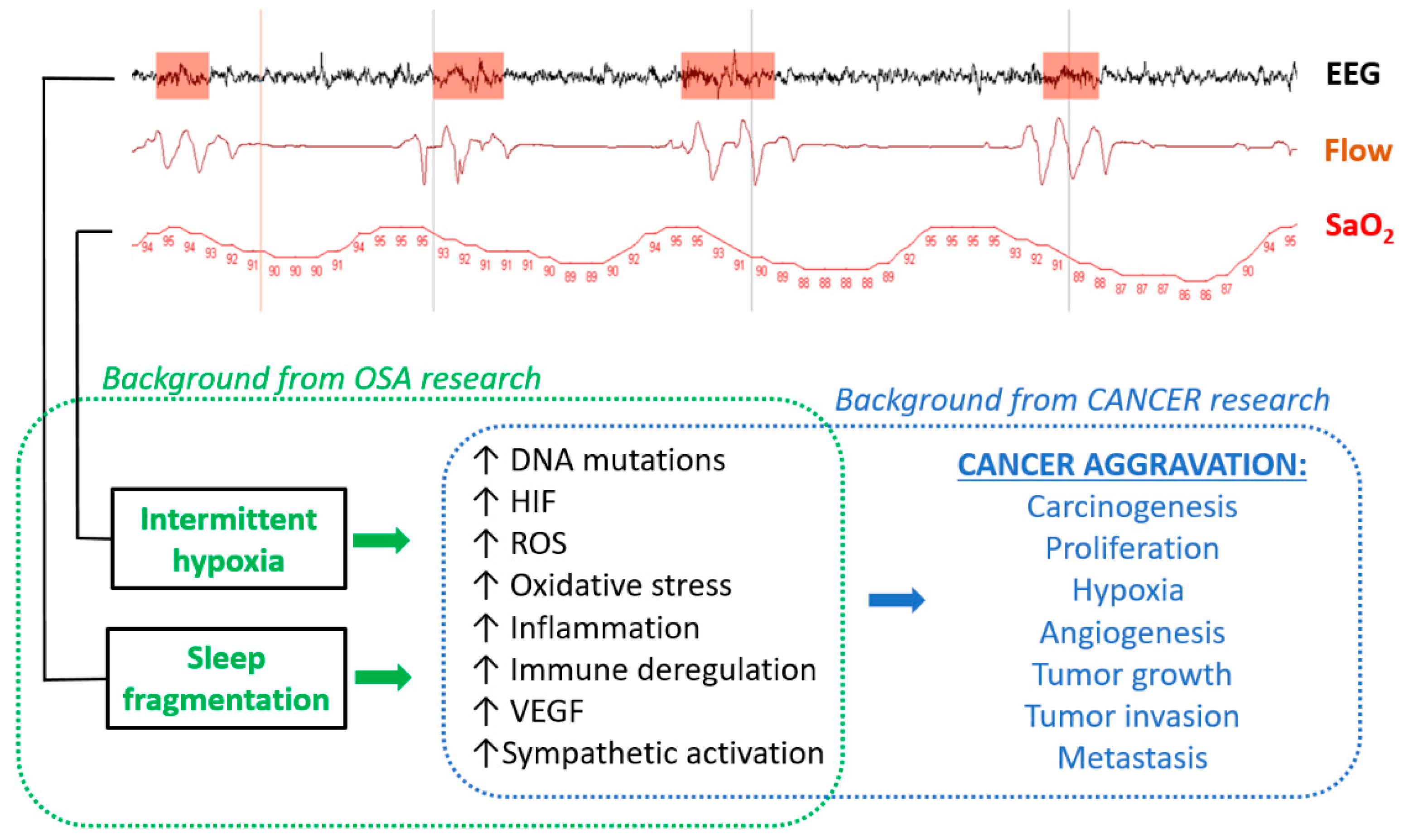
:1. Introduction
2. Biological Plausibility—Cellular and Animal Models
3. Not All Tumours are Born Equal—Differential Susceptibility to Intermittent Hypoxia
4. Potential Mechanisms and Immunological Considerations—Effects of Obesity and Aging
5. Melanoma as the Prototype
6. What about Other Specific Cancers?
7. Summary of Epidemiological Evidence—An Ocean of Confusion
8. Where do We Go from Here?
Funding
Conflicts of Interest
References
- Gozal, D.; Almendros, I.; Hakim, F. Sleep apnea awakens cancer. OncoImmunology 2014, 3, e28326. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Barbe, F. Cancer and OSA: Current evidence from human studies. Chest 2016, 150, 451–463. [Google Scholar] [CrossRef] [PubMed]
- Gozal, D.; Farré, R.; Nieto, F.J. Obstructive sleep apnea and cancer: Epidemiologic links and theoretical biological constructs. Sleep Med. Rev. 2016, 27, 43–55. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Samanta, D.; Semenza, G.L. Metabolic adaptation of cancer and immune cells mediated by hypoxia-inducible factors. Biochim. Biophys. Acta (BBA) Bioenerg. 2018, 1870, 15–22. [Google Scholar] [CrossRef] [PubMed]
- Saxena, K.; Jolly, M.K. Acute vs. Chronic vs. Cyclic Hypoxia: Their differential dynamics, molecular mechanisms, and effects on tumor progression. Biomolecules 2019, 9, 339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonsignore, M.R.; Baiamonte, P.; Mazzuca, E.; Castrogiovanni, A.; Marrone, O. Obstructive sleep apnea and comorbidities: A dangerous liaison. Multidiscip. Respir. Med. 2019, 14, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hunyor, I.; Cook, K.M. Models of intermittent hypoxia and obstructive sleep apnea: Molecular pathways and their contribution to cancer. Am. J. Physiol. Integr. Comp. Physiol. 2018, 315, R669–R687. [Google Scholar] [CrossRef] [Green Version]
- Gozal, D.; Farré, R.; Nieto, F.J. Putative links between sleep apnea and cancer. Chest 2015, 148, 1140–1147. [Google Scholar] [CrossRef] [Green Version]
- Almendros, I.; Farré, R.; Planas, A.M.; Torres, M.; Bonsignore, M.R.; Navajas, D.; Montserrat-Capdevila, J. Tissue oxygenation in brain, muscle, and fat in a rat model of sleep apnea: Differential effect of obstructive apneas and intermittent hypoxia. Sleep 2011, 34, 1127–1133. [Google Scholar] [CrossRef] [Green Version]
- Briançon-Marjollet, A.; Pépin, J.-L.; Weiss, J.W.; Lévy, P.; Tamisier, R. Intermittent hypoxia upregulates serum VEGF. Sleep Med. 2014, 15, 1425–1426. [Google Scholar] [CrossRef]
- Lacedonia, D.; Carpagnano, G.E.; Crisetti, E.; Cotugno, G.; Palladino, G.P.; Patricelli, G.; Sabato, R.; Barbaro, M.P.F. Mitochondrial DNA alteration in obstructive sleep apnea. Respir. Res. 2015, 16, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ehakim, F.; Egozal, D.; Kheirandish-Gozal, L. Sympathetic and catecholaminergic alterations in sleep apnea with particular emphasis on children. Front. Neurol. 2012, 3, 7. [Google Scholar] [CrossRef] [Green Version]
- Akbarpour, M.; Khalyfa, A.; Qiao, Z.; Gileles-Hillel, A.; Almendros, I.; Farré, R.; Gozal, D. Altered CD8+ T-Cell Lymphocyte function and tc1 cell stemness contribute to enhanced malignant tumor properties in murine models of sleep apnea. Sleep 2016, 40, 40. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Almendros, I.; Gozal, D. Intermittent hypoxia and cancer: Undesirable bed partners? Respir. Physiol. Neurobiol. 2018, 256, 79–86. [Google Scholar] [CrossRef] [PubMed]
- Martinez, C.-A.; Kerr, B.; Jin, C.; Cistulli, P.A.; Cook, K.M. Obstructive sleep apnea activates hif-1 in a hypoxia dose-dependent manner in hct116 colorectal carcinoma cells. Int. J. Mol. Sci. 2019, 20, 445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hakim, F.; Wang, Y.; Zhang, S.X.; Zheng, J.; Yolcu, E.S.; Carreras, A.; Khalyfa, A.; Shirwan, H.; Almendros, I.; Gozal, D. Fragmented sleep accelerates tumor growth and progression through recruitment of tumor-associated macrophages and tlr4 signaling. Cancer Res. 2014, 74, 1329–1337. [Google Scholar] [CrossRef] [Green Version]
- Almendros, I.; Wang, Y.; Becker, L.; Lennon, F.E.; Zheng, J.; Coats, B.R.; Schoenfelt, K.S.; Carreras, A.; Hakim, F.; Zhang, S.X.; et al. Intermittent hypoxia-induced changes in tumor-associated macrophages and tumor malignancy in a mouse model of sleep apnea. Am. J. Respir. Crit. Care Med. 2014, 189, 593–601. [Google Scholar] [CrossRef]
- Farré, R.; Montserrat, J.M.; Gozal, D.; Almendros, I.; Navajas, D. Intermittent hypoxia severity in animal models of sleep apnea. Front. Physiol. 2018, 9, 1556. [Google Scholar] [CrossRef]
- Nair, D.; Zhang, S.X.L.; Ramesh, V.; Hakim, F.; Kaushal, N.; Wang, Y.; Gozal, D. Sleep fragmentation induces cognitive deficits via nicotinamide adenine dinucleotide phosphate oxidase–dependent pathways in mouse. Am. J. Respir. Crit. Care Med. 2011, 184, 1305–1312. [Google Scholar] [CrossRef]
- Gallego-Martin, T.; Farré, R.; Almendros, I.; Gonzalez-Obeso, E.; Obeso, A. Chronic intermittent hypoxia mimicking sleep apnoea increases spontaneous tumorigenesis in mice. Eur. Respir. J. 2017, 49, 1602111. [Google Scholar] [CrossRef]
- Almendros, I.; Montserrat, J.M.; Torres, M.; Bonsignore, M.R.; Chimenti, L.; Navajas, D.; Farré, R. Obesity and intermittent hypoxia increase tumor growth in a mouse model of sleep apnea. Sleep Med. 2012, 13, 1254–1260. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Campillo, N.; Nonaka, P.N.; Montserrat, J.M.; Gozal, D.; Martínez-García, M.Á.; Campos-Rodriguez, F.; Navajas, D.; Farré, R.; Almendros, I. Aging reduces intermittent hypoxia–induced lung carcinoma growth in a mouse model of sleep apnea. Am. J. Respir. Crit. Care Med. 2018, 198, 1234–1236. [Google Scholar] [CrossRef] [PubMed]
- Torres, M.; Martinez-Garcia, M.; Ángel; Campos-Rodriguez, F.; Gozal, D.; Montserrat, J.M.; Navajas, D.; Farré, R.; Almendros, I. Lung cancer aggressiveness in an intermittent hypoxia murine model of postmenopausal sleep apnea. Menopause 2020, 27, 706–713. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Almendros, I.; Wang, Y.; Zhang, S.X.; Carreras, A.; Qiao, Z.; Gozal, D. Reduced NADPH oxidase type 2 activity mediates sleep fragmentation-induced effects on TC1 tumors in mice. OncoImmunology 2015, 4, e976057. [Google Scholar] [CrossRef] [PubMed]
- Almendros, I.; Gileles-Hillel, A.; Khalyfa, A.; Wang, Y.; Zhang, S.X.; Carreras, A.; Farré, R.; Gozal, D. Adipose tissue macrophage polarization by intermittent hypoxia in a mouse model of OSA: Effect of tumor microenvironment. Cancer Lett. 2015, 361, 233–239. [Google Scholar] [CrossRef]
- Cortese, R.; Almendros, I.; Wang, Y.; Gozal, D. Tumor circulating DNA profiling in xenografted mice exposed to intermittent hypoxia. Oncotarget 2014, 6, 556–569. [Google Scholar] [CrossRef] [Green Version]
- Almendros, I.; Khalyfa, A.; Trzepizur, W.; Gileles-Hillel, A.; Huang, L.; Akbarpour, M.; Andrade, J.; Farré, R.; Gozal, D. Tumor Cell Malignant Properties Are Enhanced by Circulating Exosomes in Sleep Apnea. Chest 2016, 150, 1030–1041. [Google Scholar] [CrossRef]
- Khalyfa, A.; Almendros, I.; Gileles-Hillel, A.; Akbarpour, M.; Trzepizur, W.; Mokhlesi, B.; Huang, L.; Andrade, J.; Farré, R.; Gozal, D. Circulating exosomes potentiate tumor malignant properties in a mouse model of chronic sleep fragmentation. Oncotarget 2016, 7, 54676–54690. [Google Scholar] [CrossRef] [Green Version]
- Campillo, N.; Torres, M.; Vilaseca, A.; Nonaka, P.N.; Gozal, D.; Roca-Ferrer, J.; Picado, C.; Montserrat, J.M.; Farré, R.; Navajas, D.; et al. Role of Cyclooxygenase-2 on Intermittent Hypoxia-Induced Lung Tumor Malignancy in a Mouse Model of Sleep Apnea. Sci. Rep. 2017, 7, 44693. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Yang, Y.-Y.; Zeng, Y.-M.; Zeng, H.-Q.; Fu, B.-B.; Ko, C.-Y.; Luo, X.; Du, Y.-P.; Chen, L.-D.; Lai, Y.-T.; et al. Anti-tumor effect of endostatin in a sleep-apnea mouse model with tumor. Clin. Transl. Oncol. 2018, 21, 572–581. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.; Liu, Y.; Kim, J.L.; Kim, E.Y.; Kim, E.Q.; Jansen, A.; Li, K.; Chan, M.; Keenan, B.T.; Conejo-Garcia, J.; et al. Effect of cyclical intermittent hypoxia on Ad5CMVCre induced solitary lung cancer progression and spontaneous metastases in the KrasG12D+; p53fl/fl; myristolated p110fl/fl ROSA-gfp mouse. PLoS ONE 2019, 14, e0212930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, M.-H.; Zhang, X.-B.; Wang, H.-L.; Li, L.-X.; Zeng, Y.-M.; Wang, M.; Zeng, H.-Q. Intermittent hypoxia enhances the tumor programmed death ligand 1 expression in a mouse model of sleep apnea. Ann. Transl. Med. 2019, 7, 97. [Google Scholar] [CrossRef]
- Almendros, I.; Montserrat-Capdevila, J.; Ruz, J.R.; Torres, M.; Durán-Cantolla, J.; Navajas, D.; Farré, R. Intermittent hypoxia enhances cancer progression in a mouse model of sleep apnoea. Eur. Respir. J. 2011, 39, 215–217. [Google Scholar] [CrossRef] [PubMed]
- Almendros, I.; Montserrat, J.M.; Torres, M.; Dalmases, M.; Cabañas, M.L.; Campos-Rodríguez, F.; Navajas, D.; Farré, R. Intermittent hypoxia increases melanoma metastasis to the lung in a mouse model of sleep apnea. Respir. Physiol. Neurobiol. 2013, 186, 303–307. [Google Scholar] [CrossRef] [PubMed]
- Perini, S.; Martinez, D.; Montanari, C.C.; Fiori, C. Enhanced expression of melanoma progression markers in mouse model of sleep apnea. Rev. Port. Pneumol. 2016, 22, 209–213. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yoon, D.W.; So, D.; Min, S.; Kim, J.; Lee, M.; Khalmuratova, R.; Cho, C.H.; Park, J.W.; Shin, H.W. Accelerated tumor growth under intermittent hypoxia is associated with hypoxia-inducible factor-1-dependent adaptive responses to hypoxia. Oncotarget 2017, 8, 61592–61603. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, L.; Ren, F.; Qi, C.; Xu, L.; Fang, Y.; Liang, M.; Feng, J.; Chen, B.; Ning, W.; Cao, J. Intermittent hypoxia promotes melanoma lung metastasis via oxidative stress and inflammation responses in a mouse model of obstructive sleep apnea. Respir. Res. 2018, 19, 28. [Google Scholar] [CrossRef] [Green Version]
- Vilaseca, A.; Campillo, N.; Torres, M.; Musquera, M.; Gozal, D.; Montserrat, J.M.; Alcaraz, A.; Touijer, K.A.; Farré, R.; Almendros, I. Intermittent hypoxia increases kidney tumor vascularization in a murine model of sleep apnea. PLoS ONE 2017, 12, e0179444. [Google Scholar] [CrossRef] [Green Version]
- Chen, A.; Sceneay, J.; Gödde, N.; Kinwel, T.; Ham, S.; Thompson, E.W.; Humbert, P.O.; Möller, A. Intermittent hypoxia induces a metastatic phenotype in breast cancer. Oncogene 2018, 37, 4214–4225. [Google Scholar] [CrossRef]
- Liu, L.; Liu, W.; Wang, L.; Zhu, T.; Zhong, J.; Xie, N. Hypoxia-inducible factor 1 mediates intermittent hypoxia-induced migration of human breast cancer MDA-MB-231 cells. Oncol. Lett. 2017, 14, 7715–7722. [Google Scholar] [CrossRef] [Green Version]
- Yoon, D.W.; Kim, Y.; Hwang, S.; Khalmuratova, R.; Lee, M.; Kim, J.H.; Lee, G.Y.; Koh, S.; Park, J.; Shin, H.-W. Intermittent hypoxia promotes carcinogenesis in azoxymethane and dextran sodium sulfate-induced colon cancer model. Mol. Carcinog. 2019, 58, 654–665. [Google Scholar] [CrossRef]
- Ali, M.; Kowkuntla, S.; Delloro, D.J.; Galambos, C.; Hathi, D.; Janz, S.; Shokeen, M.; Tripathi, C.; Xu, H.; Yuk, J.; et al. Chronic intermittent hypoxia enhances disease progression in myeloma-resistant mice. Am. J. Physiol. Integr. Comp. Physiol. 2019, 316, R678–R686. [Google Scholar] [CrossRef]
- Kang, H.S.; Kwon, H.Y.; Kim, I.K.; Ban, W.H.; Kim, S.W.; Kang, H.H.; Yeo, C.D.; Lee, S.H. Intermittent hypoxia exacerbates tumor progression in a mouse model of lung cancer. Sci. Rep. 2020, 10, 1854. [Google Scholar] [CrossRef]
- Chao, Y.; Shang, J.; Ji, W. ALKBH5-m6A-FOXM1 signaling axis promotes proliferation and invasion of lung adenocarcinoma cells under intermittent hypoxia. Biochem. Biophys. Res. Commun. 2020, 521, 499–506. [Google Scholar] [CrossRef]
- Marhuenda, E.; Campillo, N.; Gabasa, M.; Martínez-García, M.A.; Campos-Rodríguez, F.; Gozal, D.; Navajas, D.; Alcaraz, J.; Farré, R.; Almendros, I. Effects of sustained and intermittent hypoxia on human lung cancer cells. Am. J. Respir. Cell Mol. Biol. 2019, 61, 540–544. [Google Scholar] [CrossRef]
- Roerink, S.F.; Sasaki, N.; Lee-Six, H.; Young, M.D.; Alexandrov, L.B.; Behjati, S.; Mitchell, T.J.; Grossmann, S.; Lightfoot, H.; Egan, D.A.; et al. Intra-tumour diversification in colorectal cancer at the single-cell level. Nat. Cell Biol. 2018, 556, 457–462. [Google Scholar] [CrossRef]
- Campos-Rodriguez, F.; Martinez-Garcia, M.A.; Martinez, M.; Duran-Cantolla, J.; De La Peña, M.; Masdeu, M.J.; Gonzalez, M.; Del Campo, F.; Gallego, I.; Marin, J.M.; et al. Association between obstructive sleep apnea and cancer incidence in a large multicenter spanish cohort. Am. J. Respir. Crit. Care Med. 2013, 187, 99–105. [Google Scholar] [CrossRef] [Green Version]
- Nieto, F.J.; Peppard, P.E.; Young, T.; Finn, L.; Hla, K.M.; Farré, R. Sleep-disordered breathing and cancer mortality. Am. J. Respir. Crit. Care Med. 2012, 186, 190–194. [Google Scholar] [CrossRef]
- Vaupel, P.; Mayer, A. Hypoxia in cancer: Significance and impact on clinical outcome. Cancer Metastasis Rev. 2007, 26, 225–239. [Google Scholar] [CrossRef]
- Cubillos-Zapata, C.; Avendano-Ortiz, J.; Hernandez-Jimenez, E.; Toledano, V.; Casas-Martin, J.; Varela-Serrano, A.; Torres, M.; Almendros, I.; Casitas, R.; Fernandez-Navarro, I.; et al. Hypoxia-induced PD-L1/PD-1 crosstalk impairs T-cell function in sleep apnoea. Eur. Respir. J. 2017, 50, 1700833. [Google Scholar] [CrossRef]
- Gaoatswe, G.; Kent, B.D.; Corrigan, M.A.; Nolan, G.; Hogan, A.E.; McNicholas, W.T.; O’Shea, D. Invariant natural killer t cell deficiency and functional impairment in sleep apnea: Links to cancer comorbidity. Sleep 2015, 38, 1629–1634. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gharib, S.A.; Seiger, A.N.; Hayes, A.L.; Mehra, R.; Patel, S.R. Treatment of obstructive sleep apnea alters cancer-associated transcriptional signatures in circulating leukocytes. Sleep 2014, 37, 709–714. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gaines, J.; Vgontzas, A.N.; Fernandez-Mendoza, J.; Bixler, E.O. Obstructive sleep apnea and the metabolic syndrome: The road to clinically-meaningful phenotyping, improved prognosis, and personalized treatment. Sleep Med. Rev. 2018, 42, 211–219. [Google Scholar] [CrossRef] [PubMed]
- Jarrar, Y.; Zihlif, M.; Al Bawab, A.Q.; Sharab, A. Effects of intermittent hypoxia on expression of glucose metabolism genes in mcf7 breast cancer cell line. Curr. Cancer Drug Targets 2020, 20, 216–222. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Cao, H.; Zhang, Q.; Wang, B. The effect of intermittent hypoxia and fecal microbiota of OSAS on genes associated with colorectal cancer. Sleep Breath. 2020, 1–13. [Google Scholar] [CrossRef]
- Cubillos-Zapata, C.; Balbás-García, C.; Avendaño-Ortiz, J.; Toledano, V.; Torres, M.; Almendros, I.; Casitas, R.; Zamarrón, E.; García-Sánchez, A.; Feliu, J.; et al. Age-dependent hypoxia-induced PD-L1 upregulation in patients with obstructive sleep apnoea. Respirology 2019, 24, 684–692. [Google Scholar] [CrossRef]
- Dimitriou, F.; Krattinger, R.; Ramelyte, E.; Barysch, M.J.; Micaletto, S.; Dummer, R.; Goldinger, S.M. The world of melanoma: Epidemiologic, genetic, and anatomic differences of melanoma across the globe. Curr. Oncol. Rep. 2018, 20, 87. [Google Scholar] [CrossRef]
- Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Almendros, I.; Garcia-Rio, F.; Sánchez-De-La-Torre, M.; Farre, R.; Gozal, D. Cancer and sleep apnea: Cutaneous melanoma as a case study. Am. J. Respir. Crit. Care Med. 2019, 200, 1345–1353. [Google Scholar] [CrossRef]
- Cohen, J.M.; Li, Y.T.; Wu, S.; Han, J.; Qureshi, A.A.; Cho, E. Sleep duration and sleep-disordered breathing and the risk of melanoma among US women and men. Int. J. Dermatol. 2015, 54, e492–e495. [Google Scholar] [CrossRef] [Green Version]
- Gozal, D.; Ham, S.A.; Mokhlesi, B. Sleep apnea and cancer: Analysis of a nationwide population sample. Sleep 2016, 39, 1493–1500. [Google Scholar] [CrossRef]
- Sillah, A.; Watson, N.F.; Schwartz, S.M.; Gozal, D.; Phipps, A.I. Sleep apnea and subsequent cancer incidence. Cancer Causes Control. 2018, 29, 987–994. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; Martorell-Calatayud, A.; Nagore, E.; Valero, I.; Selma, M.J.; Chiner, E.; Landete, P.; Montserrat, J.-M.; Carrera, C.; Pérez-Gil, A.; et al. Association between sleep disordered breathing and aggressiveness markers of malignant cutaneous melanoma. Eur. Respir. J. 2014, 43, 1661–1668. [Google Scholar] [CrossRef] [PubMed]
- Martinez-Garcia, M.A.; Campos-Rodriguez, F.; Nagore, E.; Martorell, A.; Rodriguez-Peralto, J.L.; Riveiro-Falkenbach, E.; Hernandez, L.; Bañuls, J.; Arias, E.; Ortiz, P.; et al. Sleep-disordered breathing is independently associated with increased aggressiveness of cutaneous melanoma. Chest 2018, 154, 1348–1358. [Google Scholar] [CrossRef]
- Martínez-García, M.A.; Campos-Rodriguez, F.; Durán-Cantolla, J.; De La Peña, M.; Masdeu, M.J.; González, M.; Del Campo, F.; Serra, P.C.; Valero-Sánchez, I.; Ferrer, M.S.; et al. Obstructive sleep apnea is associated with cancer mortality in younger patients. Sleep Med. 2014, 15, 742–748. [Google Scholar] [CrossRef] [PubMed]
- Cubillos-Zapata, C.; Martínez-García, M.Á.; Campos-Rodriguez, F.; De La Torre, M.S.; Nagore, E.; Martorell-Calatayud, A.; Blasco, L.H.; Vives, E.C.; Abad-Capa, J.; Montserrat, J.M.; et al. Soluble PD-L1 is a potential biomarker of cutaneous melanoma aggressiveness and metastasis in obstructive sleep apnoea patients. Eur. Respir. J. 2019, 53, 1801298. [Google Scholar] [CrossRef]
- Chang, W.-C.; Liu, M.-E.; Yang, A.C.; Ku, Y.-C.; Pai, J.-T.; Lin, Y.-W.; Tsai, S.-J. Sleep apnea and the subsequent risk of breast cancer in women: A nationwide population-based cohort study. Sleep Med. 2014, 15, 1016–1020. [Google Scholar] [CrossRef]
- Phipps, A.I.; Bhatti, P.; Neuhouser, M.L.; Chen, C.; Crane, T.E.; Kroenke, C.H.; Ochs-Balcom, H.; Rissling, M.; Snively, B.M.; Stefanick, M.L.; et al. Pre-diagnostic sleep duration and sleep quality in relation to subsequent cancer survival. J. Clin. Sleep Med. 2016, 12, 495–503. [Google Scholar] [CrossRef] [Green Version]
- Campos-Rodriguez, F.; Cruz-Medina, A.; Selma, M.J.; Rodriguez-De-La-Borbolla-Artacho, M.; Sanchez-Vega, A.; Ripoll-Orts, F.; Almeida-Gonzalez, C.V.; Martinez-Garcia, M.A. Association between sleep-disordered breathing and breast cancer aggressiveness. PLoS ONE 2018, 13, e0207591. [Google Scholar] [CrossRef]
- Choi, J.H.; Lee, J.Y.; Han, K.D.; Lim, Y.C.; Cho, J.H. Association between obstructive sleep apnoea and breast cancer: The Korean National Health Insurance Service Data 2007–2014. Sci. Rep. 2019, 9, 1–6. [Google Scholar] [CrossRef] [Green Version]
- Seijo, L.; Pérez-Warnisher, M.T.; Giraldo-Cadavid, L.F.; Oliveros, H.; Cabezas, E.; Troncoso, M.F.; Gómez, T.; Melchor, R.; Pinillos, E.J.; El Hachem, A.; et al. Obstructive sleep apnea and nocturnal hypoxemia are associated with an increased risk of lung cancer. Sleep Med. 2019, 63, 41–45. [Google Scholar] [CrossRef]
- Dreher, M.; Krüger, S.; Schulze-Olden, S.; Keszei, A.; Storre, J.H.; Woehrle, H.; Arzt, M.; Müller, T. Sleep-disordered breathing in patients with newly diagnosed lung cancer. BMC Pulm. Med. 2018, 18, 1–6. [Google Scholar] [CrossRef]
- Li, L.; Lu, J.; Xue, W.; Wang, L.; Zhai, Y.; Fan, Z.; Wu, G.; Fan, F.; Li, J.; Zhang, C.; et al. Target of obstructive sleep apnea syndrome merge lung cancer: Based on big data platform. Oncotarget 2017, 8, 21567–21578. [Google Scholar] [CrossRef] [Green Version]
- Liu, W.; Luo, M.; Fang, Y.-Y.; Wei, S.; Zhou, L.; Liu, K. Relationship between Occurrence and Progression of Lung Cancer and Nocturnal Intermittent Hypoxia, Apnea and Daytime Sleepiness. Curr. Med. Sci. 2019, 39, 568–575. [Google Scholar] [CrossRef]
- Zhang, X.; Giovannucci, E.L.; Wu, K.; Gao, X.; Hu, F.; Ogino, S.; Schernhammer, E.S.; Fuchs, C.S.; Redline, S.; Willett, W.C.; et al. Associations of self-reported sleep duration and snoring with colorectal cancer risk in men and women. Sleep 2013, 36, 681–688. [Google Scholar] [CrossRef]
- Lee, S.; Kim, B.G.; Kim, J.W.; Lee, K.L.; Koo, D.L.; Nam, H.; Im, J.P.; Kim, J.S.; Koh, S.-J. Obstructive sleep apnea is associated with an increased risk of colorectal neoplasia. Gastrointest. Endosc. 2017, 85, 568–573.e1. [Google Scholar] [CrossRef]
- Chung, S.-D.; Hung, S.-H.; Lin, H.-C.; Tsai, M.-C.; Kao, L.-T. Obstructive sleep apnea and urological comorbidities in males: A population-based study. Sleep Breath. 2016, 20, 1203–1208. [Google Scholar] [CrossRef]
- Vilaseca, A.; Nguyen, D.P.; Vertosick, E.A.; Corradi, R.B.; Musquera, M.; Pérez, M.; Fossati, N.; Sjoberg, D.D.; Farré, R.; Almendros, I.; et al. Obstructive sleep apnea and Fuhrman grade in patients with clear cell renal cell carcinoma treated surgically. World J. Urol. 2017, 35, 51–56. [Google Scholar] [CrossRef] [Green Version]
- Molin, M.D.; Brant, A.; Blackford, A.L.; Griffin, J.F.; Shindo, K.; Barkley, T.; Rezaee, N.; Hruban, R.H.; Wolfgang, C.L.; Goggins, M. Obstructive sleep apnea and pathological characteristics of resected pancreatic ductal adenocarcinoma. PLoS ONE 2016, 11, e0164195. [Google Scholar] [CrossRef]
- Chen, J.-C.; Hwang, J.-H. Sleep apnea increased incidence of primary central nervous system cancers: A nationwide cohort study. Sleep Med. 2014, 15, 749–754. [Google Scholar] [CrossRef]
- Choi, J.H.; Kim, S.-Y.; Han, K.D.; Cho, J.H. The incidence of non-Hodgkin lymphoma is increased in patients with obstructive sleep apnea. Leuk. Res. 2020, 98, 106455. [Google Scholar] [CrossRef]
- Marshall, N.S.; Wong, K.K.; Cullen, S.R.; Knuiman, M.W.; Grunstein, R.R. Sleep apnea and 20-year follow-up for all-cause mortality, stroke, and cancer incidence and mortality in the busselton health study cohort. J. Clin. Sleep Med. 2014, 10, 355–362. [Google Scholar] [CrossRef] [Green Version]
- Fang, H.-F.; Miao, N.-F.; Chen, C.-D.; Sithole, T.; Chung, M.-H. Risk of cancer in patients with insomnia, parasomnia, and obstructive sleep apnea: A nationwide nested case-control study. J. Cancer 2015, 6, 1140–1147. [Google Scholar] [CrossRef]
- Huang, T.; Lin, B.M.; Stampfer, M.J.; Schernhammer, E.S.; Saxena, R.; Tworoger, S.S.; Redline, S. Associations of self-reported obstructive sleep apnea with total and site-specific cancer risk in older women: A prospective study. Sleep 2020. [Google Scholar] [CrossRef]
- Jara, S.M.; Phipps, A.I.; Maynard, C.; Weaver, E.M. The association of sleep apnea and cancer in veterans. Otolaryngol. Neck Surg. 2020, 162, 581–588. [Google Scholar] [CrossRef]
- Zhang, X.-B.; Peng, L.-H.; Lyu, Z.; Jiang, X.-T.; Du, Y.-P. Obstructive sleep apnoea and the incidence and mortality of cancer: A meta-analysis. Eur. J. Cancer Care 2015, 26, e12427. [Google Scholar] [CrossRef]
- Kendzerska, T.; Leung, R.S.; Hawker, G.; Tomlinson, G.; Gershon, A.S. Obstructive sleep apnea and the prevalence and incidence of cancer. Can. Med. Assoc. J. 2014, 186, 985–992. [Google Scholar] [CrossRef] [Green Version]
- Young, T.; Evans, L.; Finn, L.; Palta, M. Estimation of the clinically diagnosed proportion of sleep apnea syndrome in middle-aged men and women. Sleep 1997, 20, 705–706. [Google Scholar] [CrossRef]



| Cancer Type | IH N Pub (References) | SF N Pub (References) | Tumour Growth | Invasion/Metastasis | Angiogenesis | Carcinogenesis |
|---|---|---|---|---|---|---|
| Melanoma | 8 [17,21,23,33,34,35,36,37] | 0 | ↑ 8/8 | ↑ 3/3 | ↑ 2/2 | ― |
| Lung | 13 [13,17,20,22,25,26,27,29,30,31,32,43,44] | 3 [16,24,28] | ↑ 13/13 | ↑ 13/13 | ↑ 1/1 | ↑ 2/2 |
| Kidney | 1 [38] | 0 | ↑ 0/1 | ― | ↑ 1/1 | ― |
| Breast | 2 [39,40] | 0 | ↑ 2/2 | ↑ 2/2 | ― | ― |
| Colon | 2 [36,41] | 0 | ― | ― | ― | ↑ 2/2 |
| Myeloma | 1 [42] | 0 | ↑ 1/1 | ― | ― | ― |
| Author (Year) (Reference) | Design | Patients | Cancer Outcome | Main Results |
|---|---|---|---|---|
| Epidemiological-based studies | ||||
| Cohen (2015) [59] | Epidemiological study | Three prospective cohorts (178,633 subjects with 880 patients with melanoma) | Incidence | Incidence: No association between snoring, sleep duration or OSA and risk of incident melanoma |
| Gozal (2016) [60] | Epidemiological Case-control study | 5.6 million subjects (with 1.7 million of OSA patients and 19,927 of melanoma patients) Health insurance database | Incidence Aggressiveness | Incidence: Higher in OSA patients Aggressiveness. No relationship between OSA and risk of metastasis |
| Sillah (2018) [61] | Epidemiological Retrospective study | 34,402 patients with sleep apnoea (129 patients with melanoma) | Incidence | Incidence: Higher in OSA patients Association more prominent in patients <60 years and men |
| Clinical-based studies | ||||
| Martinez-Garcia (2014) [62] | Multicentric, prospective | 56 patients with melanoma | Prevalence Aggressiveness | Prevalence: AHI ≥ 15: 30.3%. Aggressiveness. AHI and Tsat90% were predictors of fast-growing melanoma |
| Martinez-Garcia (2018) [63] | Multicentric, Prospective | 443 patients with melanoma | Aggressiveness | Aggressiveness: AHI or DI4% values were associated with some markers of melanoma aggressiveness. Association more prominent in patients < 56 years and Breslow index > 2 mm |
| Author (Year) (Reference) | Design and SDB Assessment | Patients | Main Outcomes |
|---|---|---|---|
| Breast cancer | |||
| Chang (2014) [66] | Retrospective study. Data were retrieved from a national health insurance database. SDB was diagnosed based on ICD-9 codes Subjects were followed for 5 years. | 846 women with SDB and 4230 age-matched control women without ICD-9 codes corresponding to SDB | -A diagnosis of SDB was associated with increased risk of having a diagnosis of breast cancer, after a 5 years follow-up, compared to the control group (adjusted HR 2.09; 95% CI 1.06–4.12). |
| Phipps (2016) [67] | Longitudinal study. SDB was not assessed by objective methods. Sleep characteristics were recorded at the time of enrolment. | 6825 postmenopausal women with diagnosis of primary breast cancer during follow-up from the Women’s Health Initiative cohort. | -Women who reported at enrolment a sleep duration ≤6 h/night combined with frequent snoring (≥5 nights/week) had substantially poorer breast cancer survival than those reporting neither (HR 2.14, 95% CI 1.47–3.13). |
| Campos-Rodriguez (2018) [68] | Cross-sectional study specifically designed to address the association between SDB and breast cancer. Women underwent a home respiratory polygraphy. SDB was defined as an AHI ≥ 5. | 83 consecutive women <65 years with a diagnosis of primary breast cancer. | -The prevalence of SDB was 51·8%, with a median (IQR) AHI of 5.1 (2–9.4) and ODI4 of 1.5 (0.5–5.8). -No associations were found between AHI or oximetric parameters and different markers of severity of breast cancer, including Ki67, Nottingham histologic grade, tumour stage, absence of hormone receptors, and molecular subtypes of breast cancer. |
| Choi (2019) [69] | Retrospective study. Data were retrieved from a national health insurance database. SDB was diagnosed based on ICD-10 codes Subjects were followed for 3.7 ± 2.3 years. | 45,699 women >20 years with SDB and 228,502 age-matched control women without ICD-10 codes corresponding to SDB | -The incidence of breast cancer among women with OSA was significantly higher than that among the controls (HR 1.20, 95%CI 1.04–1.39). -The incidence of breast cancer was higher among patients aged ≥65 years (HR 1.72; 95%CI 1.10–2.71). |
| Lung cancer | |||
| Seijo (2019) [70] | Cross-sectional study. Participants underwent a home respiratory polygraphy. SDB was defined as an AHI ≥ 15. | 302 individuals from 2 cohorts: SAIL cohort, which investigated SDB prevalence in lung cancer patients, and SAILS cohort, which assessed SDB in subjects participating in a lung cancer screening program | -The prevalence of SDB was 42%. -Lung cancer was 8% more prevalent in patients with an AHI ≥ 15, compared to those with an AHI < 15. -After adjustment for potential confounders, AHI, nocturnal hypoxemia, including time spent below 90% oxyhaemoglobin saturation, and 3% oxygen desaturation index were significantly associated with lung cancer. |
| Dreher (2018) [71] | Cross-sectional study. SDB was assessed by means of a type IV device (two-channel screening system). SDB was defined as an AHI ≥ 5. | 100 patients with newly diagnosed lung cancer from 3 German centres. | -The prevalence of SDB was 49%, with 32% showing mild SDB (AHI5-14.9) and 17% showing moderate-to-severe SDB (AHI ≥ 15). -The median (IQR) AHI of 7.7/h (5.4–10.4) and oxygen desaturation index of 8.5 (4.2–13.4). |
| Li (2017) [72] | Retrospective study. The method to investigate SDB is not clarified. SDB was defined as an AHI ≥ 5. | 43 consecutive patients >18 years with concurrent SDB and lung cancer. Patients were obtained from an electronic hospital database. Patients treated with CPAP were excluded. | -Patients with moderate or severe SDB (AHI 15–29.9 and ≥30, respectively) and lung cancer had lower survival than those with only mild SDB (AHI5–14.9). |
| Liu (2019) [73] | Case-control study. 1-year follow-up SDB was assessed by respiratory polygraphy, although the threshold to define SDB is not provided. | 45 patients with primary lung cancer suitable for surgical resection and 45 age and sex-matched controls. | -Patients with lung cancer had statistically significant greater risk of SDB, as well as more hypoxemia, time spent with O2 saturation <90% and higher ODI compared to the matched control group. -The SDB and control groups had similar risk of recurrence, metastasis or mortality after a 1-year follow-up. |
| Colorectal cancer | |||
| Zhang (2013) [74] | Prospective cohort study. SDB was not assessed by objective methods. Sleep duration and snoring was recorded at the time of enrolment. Cancer diagnosis were obtained from medical records. | 30,121 men in the Health Professionals Follow-up Study and 76,368 women in the Nurses’ Health Study | -The presence of long sleep duration (≥9 h/day) associated with regular snoring (defined as snoring at least few days per week) was associated with incident colorectal tumours in both men and women, compared to individuals who slept an average of 7 h (men: HR 1.80, 95% CI 1.14–2.84; women HR 2.32, 95% CI 1.24–4.36). -Short sleep duration (<5 h/day) was not associated with increased risk of colorectal cancer even in the snoring group. |
| Lee (2017) [75] | Retrospective study plus case-control study. SDB was assessed by polysomnography. SDB was defined as an AHI ≥ 5. | 163 consecutive patients who underwent overnight polysomnography and subsequent colonoscopy. For the case-control study, 195 age-, sex-, BMI-, and smoking-matched subjects without clinical symptoms of SDB who underwent colonoscopy were included. | -SDB was associated with an increased risk of having an advanced colorectal cancer for both, mild (AHI 5–14·9) and moderate-to-severe (AHI ≥ 15) SDB after adjusting for confounders (OR 14.09; 95% CI 1.55–127.83; and OR 14.12; 95% CI 1.52–131.25, respectively). -In the case-control study, patients with SDB had a three-fold greater risk of having an advanced colorectal tumor compared to the control group (OR 3.03; 95% CI 1.44–6.34). |
| Prostate cancer | |||
| Chung (2016) [76] | Retrospective case-control study. Data were retrieved from a national health insurance database. SDB was diagnosed based on ICD-9 codes | 1236 men with SDB and 4944 age-matched men without ICD-9 codes corresponding to SDB. | -The prevalence of prostate cancer was higher in the SDB than in the control group (0.97% vs. 0.40%, p = 0.013). -Men with SDB were more likely to have prostate cancer compared to the non-SDB men (adjusted OR 2.14, 95% CI 1.03-4.43). |
| Kidney cancer | |||
| Vilaseca (2016) [77] | Retrospective study. Patients data, including Information on SDB, were retrieved from medical records and databases. SDB was based on self-report. | 2579 patients who underwent radical or partial nephrectomy for clear cell renal cell carcinoma. | -The prevalence of self-reported SDB was 7%. - Self-reported SDB was associated with higher Fuhrman grade compared to those without SDB (adjusted OR 1.41, 95% CI 1.00–1.99). - Self-reported SDB was not associated with tumour size, metastasis-free survival or cancer survival. |
| Pancreatic cancer | |||
| Dal Molin (2016) [78] | Retrospective study. Data were retrieved from medical records. SDB was diagnosed based on a positive polysomnography that was either directly available for review or reported in the medical records (the threshold to define SDB is not provided). | 1031 patients who underwent surgical resection without neoadjuvant therapy for pancreatic ductal adenocarcinoma. | -Patients with SDB were significantly more likely to have lymph node-negative tumours than non-OSA cases (37.7% vs. 21.8%, p = 0.004). -SDB was an independent predictor of lymph node status (OR 0.51, 95%CI 0.27–0.96). -SDB, however, was not associated with greater cancer mortality (OR 0.89, 95% CI 0.65–1.24). |
| Central nervous system cancers | |||
| Chen (2014) [79] | Retrospective case-control study. Data were retrieved from a National health institute database. SDB was diagnosed based on ICD-9 codes. 10-year follow-up period. | 23,055 newly diagnosed cases of SDB and 69,165 age- and gender-matched individuals without ICD-9 codes corresponding to SDB. | -SDB was associated with higher incidence of primary central nervous system cancers compared to non-SDB individuals (adjusted HR 1.54, 95% CI 1.01–2.37). -SDB without surgical treatment was associated with higher incidence risk for primary CNS cancers than the comparison group (adjusted HR 1.83, 95% CI 1.23–2.74). -SDB patients who underwent surgical treatment for their sleep disorder had a similar risk of incident primary CNS cancers than the comparison group (adjusted HR 0.98, 95% CI 0.31–3.09). |
| Lymphoma (2020)[80] | Retrospective case-control study. Data were retrieved from a National health institute database. SDB was diagnosed based on ICD-10 codes. 4.8 ± 2.3 follow-up period. | 198,574 newly diagnosed cases of SDB and 992,870 age- and gender-matched individuals without ICD-10 codes corresponding to SDB | -The incidence of non-Hodgkin lymphoma among patients with OSA was significantly higher than that among the controls (HR 1.40, 95%CI 1.16-1.69). - This incidence was higher in women than that in men (1.62 vs. 1.28), but difference by age were not observed. |
| Prospective longitudinal, multicentred studies focused on a single organ cancer type that incorporate polysomnographic assessments at the time of cancer diagnosis, track adherence to treatment of OSA, and include multi-omics and tumour heterogeneity characterization. |
| Longitudinal studies of large cohorts of patients diagnosed with OSA without cancer and matched controls and long-term surveillance for cancer. |
| Prospective cancer treatment outcomes of patients with and without OSA with site- and type-specific cancers. |
| Search for OSA-related specific cancer biomarkers of aggressiveness, treatment selection, and personalized outcomes. |
| In vitro studies of differential effects of variable sustained and intermittent hypoxia profiles on specific tumours and on a large repertoire of specific tumour variants that incorporate multicellular matrices and organoid-based cellular interactions. |
| Exploration of animal models of OSA and their effect on cancer immunosurveillance and formulation of novel preventive strategies. |
| Identification and development of novel cancer therapeutic targets related to intrinsically driven pathways related to sleep-disordered breathing. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gozal, D.; Almendros, I.; Phipps, A.I.; Campos-Rodriguez, F.; Martínez-García, M.A.; Farré, R. Sleep Apnoea Adverse Effects on Cancer: True, False, or Too Many Confounders? Int. J. Mol. Sci. 2020, 21, 8779. https://doi.org/10.3390/ijms21228779
Gozal D, Almendros I, Phipps AI, Campos-Rodriguez F, Martínez-García MA, Farré R. Sleep Apnoea Adverse Effects on Cancer: True, False, or Too Many Confounders? International Journal of Molecular Sciences. 2020; 21(22):8779. https://doi.org/10.3390/ijms21228779
Chicago/Turabian StyleGozal, David, Isaac Almendros, Amanda I. Phipps, Francisco Campos-Rodriguez, Miguel A. Martínez-García, and Ramon Farré. 2020. "Sleep Apnoea Adverse Effects on Cancer: True, False, or Too Many Confounders?" International Journal of Molecular Sciences 21, no. 22: 8779. https://doi.org/10.3390/ijms21228779
APA StyleGozal, D., Almendros, I., Phipps, A. I., Campos-Rodriguez, F., Martínez-García, M. A., & Farré, R. (2020). Sleep Apnoea Adverse Effects on Cancer: True, False, or Too Many Confounders? International Journal of Molecular Sciences, 21(22), 8779. https://doi.org/10.3390/ijms21228779






