Anti-Inflammatory and Anti-Oxidative Activity of Indole-3-Acetic Acid Involves Induction of HO-1 and Neutralization of Free Radicals in RAW264.7 Cells
Abstract
1. Introduction
2. Results
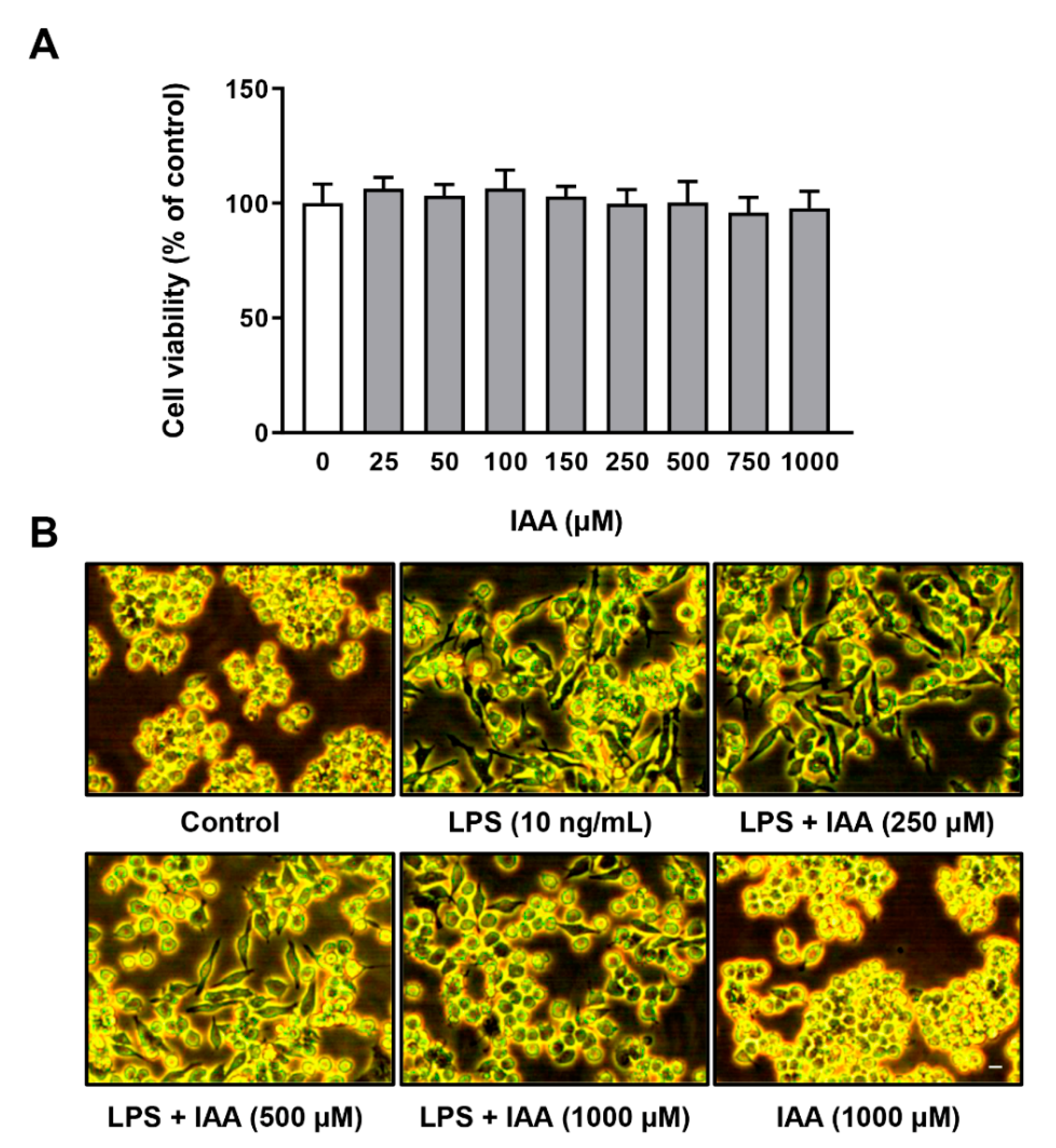
2.1. Effect of IAA on Cell Viability and Morphology of RAW264.7 Cells Treated with LPS
2.2. Alleviation of LPS-Induced Proinflammatory Response by IAA
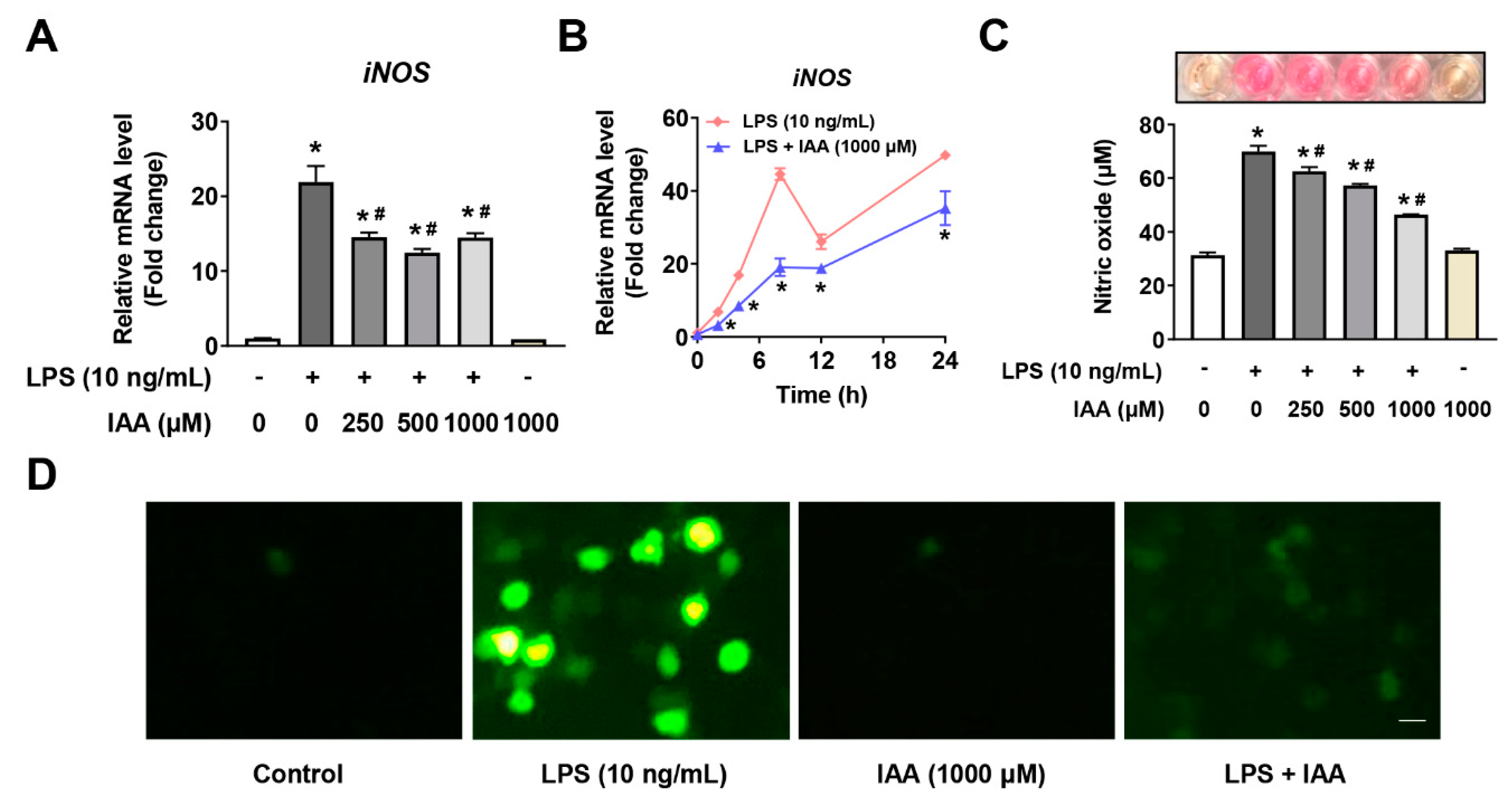
2.3. Suppression of Free Radical Production by IAA
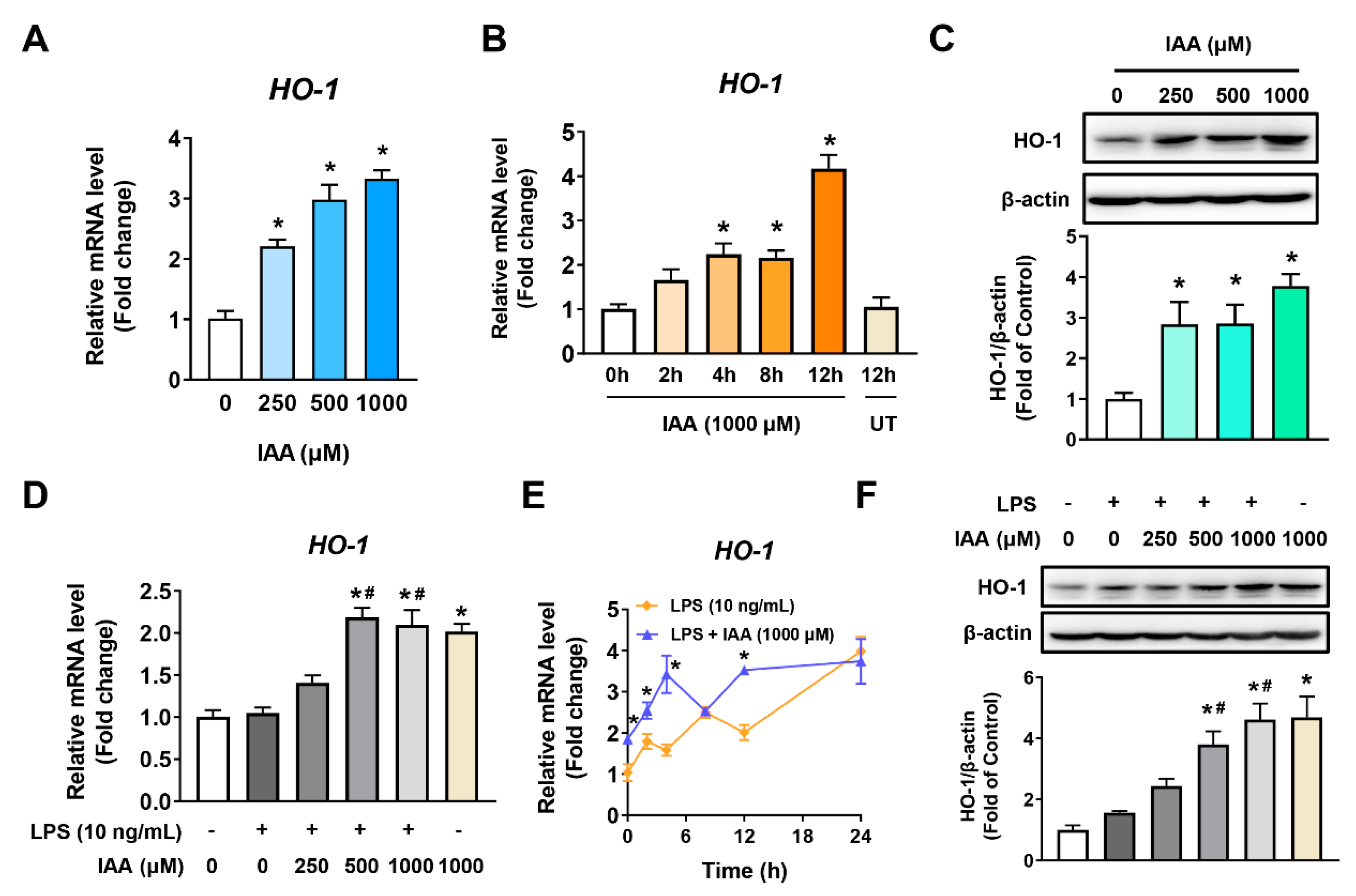
2.4. Increment of HO-1 Levels in RAW 264.7 Cells in the Presence and Absence of LPS
2.5. IAA Attenuates the Generation of IL-1β and IL-6 in HO-1-Dependent Manner
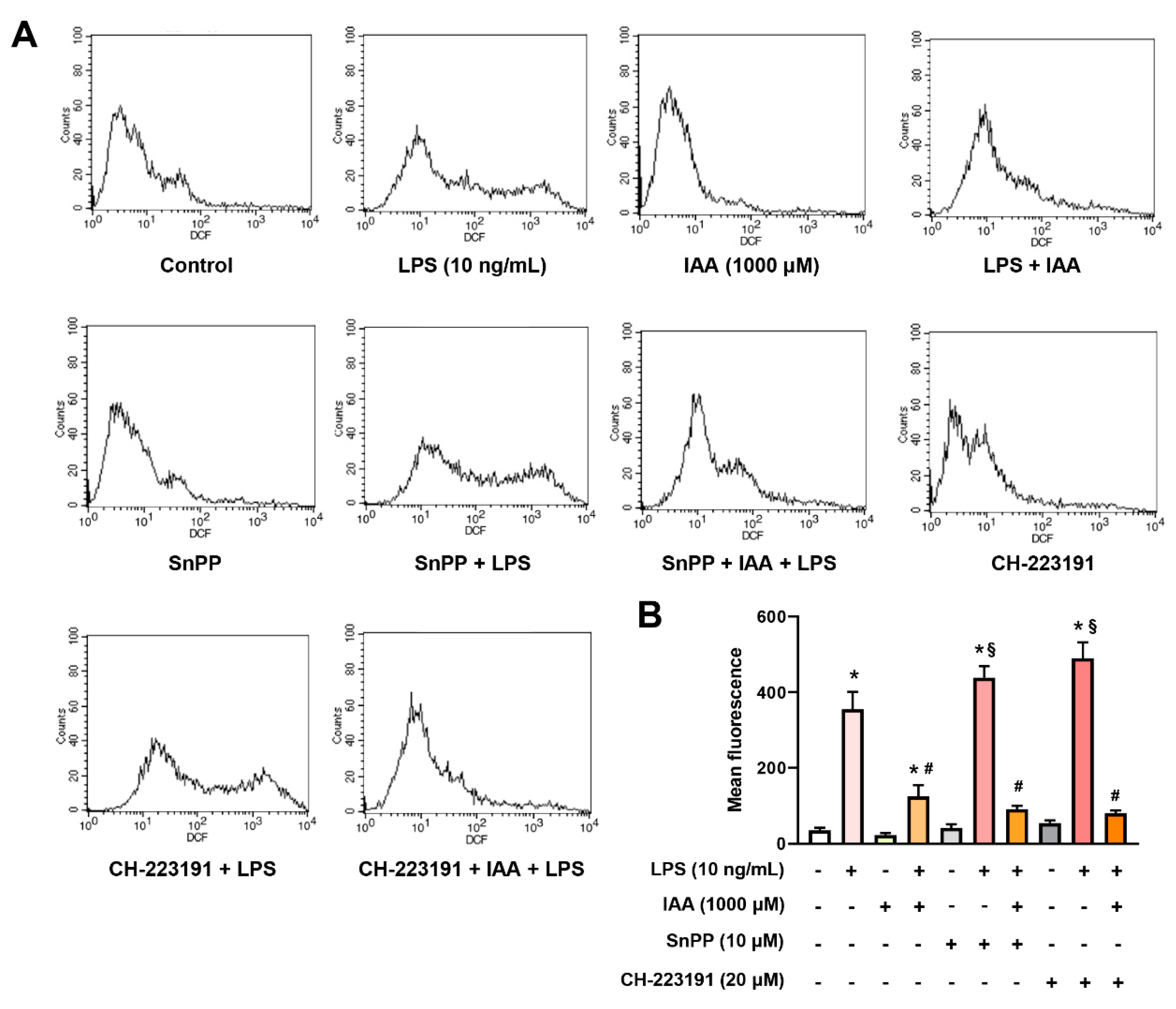
2.6. The Inhibitory Effect of IAA on the Production of ROS and NO is Independent of HO-1 Induction and AhR
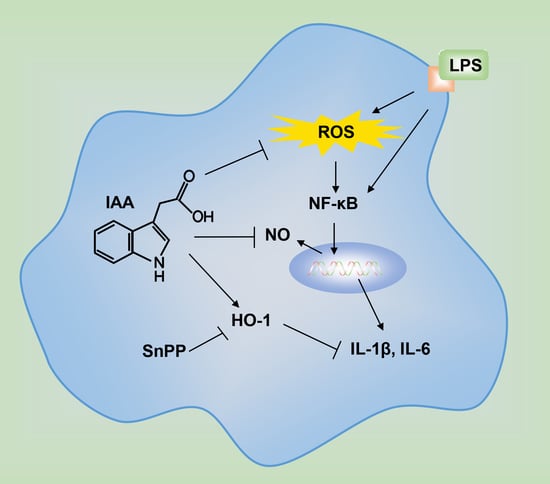
3. Discussion
4. Materials and Methods
4.1. Materials
4.2. Cell Culture and Cell Viability
4.3. RNA Extraction and Real-Time PCR (RT-qPCR)
4.4. Enzyme-Linked Immunosorbent Assay
4.5. Nitric Oxide Measurement
4.6. Reactive Oxygen Species Determination
4.7. Immunofluorescence
4.8. Protein Extraction and Western Blot
4.9. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| AhR | aryl hydrocarbon receptor; |
| DMEM | Dulbecco’s modified Eagle’s medium; |
| FBS | fetal bovine serum; |
| FITC | fluorescein isothiocyanate; |
| IL-1β | interleukin-1β; |
| IL-6 | interleukin-6; |
| HO-1 | heme oxygenase-1; |
| IAA | indole-3-acetic acid; |
| iNOS | inducible nitric oxide synthase; |
| LPS | lipopolysaccharide; |
| MCP-1 | monocyte chemoattractant protein-1; |
| NF-κB | nuclear factor kappa B; |
| NO | nitric oxide; |
| ROS | reactive oxidative species; |
| SnPP | tin protoporphyrin IX; |
| TNF-α | tumor necrosis factor-α. |
References
- Kho, Z.Y.; Lal, S.K. The Human Gut Microbiome—A Potential Controller of Wellness and Disease. Front. Microbiol. 2018, 9, 1835. [Google Scholar] [CrossRef]
- Brown, K.; DeCoffe, D.; Molcan, E.; Gibson, D.L. Diet-induced dysbiosis of the intestinal microbiota and the effects on immunity and disease. Nutrients 2012, 4, 1095–1119. [Google Scholar] [CrossRef]
- Hirayama, D.; Iida, T.; Nakase, H. The Phagocytic Function of Macrophage-Enforcing Innate Immunity and Tissue Homeostasis. Int. J. Mol. Sci. 2017, 19, 92. [Google Scholar] [CrossRef]
- Hsu, H.Y.; Wen, M.H. Lipopolysaccharide-mediated reactive oxygen species and signal transduction in the regulation of interleukin-1 gene expression. J. Biol. Chem. 2002, 277, 22131–22139. [Google Scholar] [CrossRef] [PubMed]
- Murray, R.Z.; Stow, J.L. Cytokine Secretion in Macrophages: SNAREs, Rabs, and Membrane Trafficking. Front. Immunol. 2014, 5, 538. [Google Scholar] [CrossRef] [PubMed]
- Arango Duque, G.; Descoteaux, A. Macrophage cytokines: Involvement in immunity and infectious diseases. Front. Immunol. 2014, 5, 491. [Google Scholar] [CrossRef] [PubMed]
- Chen, L.; Deng, H.; Cui, H.; Fang, J.; Zuo, Z.; Deng, J.; Li, Y.; Wang, X.; Zhao, L. Inflammatory responses and inflammation-associated diseases in organs. Oncotarget 2018, 9, 7204–7218. [Google Scholar] [CrossRef]
- Palego, L.; Betti, L.; Rossi, A.; Giannaccini, G. Tryptophan Biochemistry: Structural, Nutritional, Metabolic, and Medical Aspects in Humans. J. Amino Acids 2016, 2016, 8952520. [Google Scholar] [CrossRef]
- Li, P.; Yin, Y.L.; Li, D.; Kim, S.W.; Wu, G. Amino acids and immune function. Br. J. Nutr. 2007, 98, 237–252. [Google Scholar] [CrossRef]
- Jones, L.H.; Abdalla, D.S.; Freitas, J.C. Effects of indole-3-acetic acid on croton oil- and arachidonic acid-induced mouse ear edema. Inflamm. Res. 1995, 44, 372–375. [Google Scholar] [CrossRef]
- Ji, Y.; Gao, Y.; Chen, H.; Yin, Y.; Zhang, W. Indole-3-Acetic Acid Alleviates Nonalcoholic Fatty Liver Disease in Mice via Attenuation of Hepatic Lipogenesis, and Oxidative and Inflammatory Stress. Nutrients 2019, 11, 62. [Google Scholar] [CrossRef] [PubMed]
- Kazankov, K.; Jorgensen, S.M.D.; Thomsen, K.L.; Moller, H.J.; Vilstrup, H.; George, J.; Schuppan, D.; Gronbaek, H. The role of macrophages in nonalcoholic fatty liver disease and nonalcoholic steatohepatitis. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 145–159. [Google Scholar] [CrossRef] [PubMed]
- Geng, S.; Chen, K.; Yuan, R.; Peng, L.; Maitra, U.; Diao, N.; Chen, C.; Zhang, Y.; Hu, Y.; Qi, C.F.; et al. The persistence of low-grade inflammatory monocytes contributes to aggravated atherosclerosis. Nat. Commun. 2016, 7, 13436. [Google Scholar] [CrossRef] [PubMed]
- Guo, H.; Diao, N.; Yuan, R.; Chen, K.; Geng, S.; Li, M.; Li, L. Subclinical-Dose Endotoxin Sustains Low-Grade Inflammation and Exacerbates Steatohepatitis in High-Fat Diet-Fed Mice. J. Immunol. 2016, 196, 2300–2308. [Google Scholar] [CrossRef]
- Vijayan, V.; Wagener, F.; Immenschuh, S. The macrophage heme-heme oxygenase-1 system and its role in inflammation. Biochem. Pharm. 2018, 153, 159–167. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis and its role in plant development. Annu. Rev. Plant. Biol. 2010, 61, 49–64. [Google Scholar] [CrossRef]
- Zhao, Y. Auxin biosynthesis: A simple two-step pathway converts tryptophan to indole-3-acetic acid in plants. Mol. Plant. 2012, 5, 334–338. [Google Scholar] [CrossRef]
- Gao, J.; Xu, K.; Liu, H.; Liu, G.; Bai, M.; Peng, C.; Li, T.; Yin, Y. Impact of the Gut Microbiota on Intestinal Immunity Mediated by Tryptophan Metabolism. Front. Cell Infect. Microbiol 2018, 8, 13. [Google Scholar] [CrossRef]
- Krishnan, S.; Ding, Y.; Saedi, N.; Choi, M.; Sridharan, G.V.; Sherr, D.H.; Yarmush, M.L.; Alaniz, R.C.; Jayaraman, A.; Lee, K. Gut Microbiota-Derived Tryptophan Metabolites Modulate Inflammatory Response in Hepatocytes and Macrophages. Cell Rep. 2018, 23, 1099–1111. [Google Scholar] [CrossRef]
- Kim, D.; Kim, H.; Kim, K.; Roh, S. The Protective Effect of Indole-3-Acetic Acid (IAA) on H2O2-Damaged Human Dental Pulp Stem Cells Is Mediated by the AKT Pathway and Involves Increased Expression of the Transcription Factor Nuclear Factor-Erythroid 2-Related Factor 2 (Nrf2) and Its Downstream Target Heme Oxygenase 1 (HO-1). Oxid. Med. Cell Longev. 2017, 2017, 8639485. [Google Scholar] [CrossRef]
- Jain, A.; Pasare, C. Innate Control of Adaptive Immunity: Beyond the Three-Signal Paradigm. J. Immunol. 2017, 198, 3791–3800. [Google Scholar] [CrossRef] [PubMed]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Kempe, S.; Kestler, H.; Lasar, A.; Wirth, T. NF-kappaB controls the global pro-inflammatory response in endothelial cells: Evidence for the regulation of a pro-atherogenic program. Nucleic Acids Res. 2005, 33, 5308–5319. [Google Scholar] [CrossRef]
- Kanarek, N.; London, N.; Schueler-Furman, O.; Ben-Neriah, Y. Ubiquitination and degradation of the inhibitors of NF-kappaB. Cold Spring Harb. Perspect. Biol. 2010, 2, a000166. [Google Scholar] [CrossRef] [PubMed]
- Yang, D.; Elner, S.G.; Bian, Z.M.; Till, G.O.; Petty, H.R.; Elner, V.M. Pro-inflammatory cytokines increase reactive oxygen species through mitochondria and NADPH oxidase in cultured RPE cells. Exp. Eye Res. 2007, 85, 462–472. [Google Scholar] [CrossRef] [PubMed]
- Aktan, F. iNOS-mediated nitric oxide production and its regulation. Life Sci. 2004, 75, 639–653. [Google Scholar] [CrossRef] [PubMed]
- Pizzino, G.; Irrera, N.; Cucinotta, M.; Pallio, G.; Mannino, F.; Arcoraci, V.; Squadrito, F.; Altavilla, D.; Bitto, A. Oxidative Stress: Harms and Benefits for Human Health. Oxid. Med. Cell Longev. 2017, 2017, 8416763. [Google Scholar] [CrossRef]
- Castaneda, O.A.; Lee, S.C.; Ho, C.T.; Huang, T.C. Macrophages in oxidative stress and models to evaluate the antioxidant function of dietary natural compounds. J. Food Drug Anal. 2017, 25, 111–118. [Google Scholar] [CrossRef]
- Arnao, M.B.; Sanchez-Bravo, J.; Acosta, M. Indole-3-carbinol as a scavenger of free radicals. Biochem. Mol. Biol. Int. 1996, 39, 1125–1134. [Google Scholar] [CrossRef]
- Mourao, L.R.; Santana, R.S.; Paulo, L.M.; Pugine, S.M.; Chaible, L.M.; Fukumasu, H.; Dagli, M.L.; de Melo, M.P. Protective action of indole-3-acetic acid on induced hepatocarcinoma in mice. Cell Biochem. Funct. 2009, 27, 16–22. [Google Scholar] [CrossRef]
- Araujo, J.A.; Zhang, M.; Yin, F. Heme oxygenase-1, oxidation, inflammation, and atherosclerosis. Front. Pharm. 2012, 3, 119. [Google Scholar] [CrossRef] [PubMed]
- Lecube, M.L.; Noriega, G.O.; Santa Cruz, D.M.; Tomaro, M.L.; Batlle, A.; Balestrasse, K.B. Indole acetic acid is responsible for protection against oxidative stress caused by drought in soybean plants: The role of heme oxygenase induction. Redox Rep. 2014, 19, 242–250. [Google Scholar] [CrossRef] [PubMed]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef] [PubMed]
- Hubbard, T.D.; Murray, I.A.; Perdew, G.H. Indole and Tryptophan Metabolism: Endogenous and Dietary Routes to Ah Receptor Activation. Drug Metab. Dispos. 2015, 43, 1522–1535. [Google Scholar] [CrossRef]
- Heath-Pagliuso, S.; Rogers, W.J.; Tullis, K.; Seidel, S.D.; Cenijn, P.H.; Brouwer, A.; Denison, M.S. Activation of the Ah receptor by tryptophan and tryptophan metabolites. Biochemistry 1998, 37, 11508–11515. [Google Scholar] [CrossRef]
- Hunt, C.; Zhao, X.; Crisp, Z.; Choi, K.; de Figueiredo, P.; Jayaraman, A.; Karpac, J.; Alaniz, R.C. The microbiota-derived metabolite indole is an endogenous regulator of autophagy. Am. Assoc. Immnol. 2017, 198, 62.17. [Google Scholar]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]



| Gene | Accession No. | Forward Primer (5’-3’) | Reverse Primer (5’-3’) |
|---|---|---|---|
| MCP-1 | NM_011333.3 | TCCCAATGAGTAGGCTGGAG | TCTGGACCCATTCCTTCTTG |
| IL-1β | XM_006498795.4 | AGGCTCCGAGATGAACAACA | TTGTCGTTGCTTGGTTCTCC |
| IL-6 | NM_031168.2 | TAGTCCTTCCTACCCCAATTTCC | TTGGTCCTTAGCCACTCCTTC |
| TNF-α | NM_013693.3 | GCTGAGCTCAAACCCTGGTA | AGTACTTGGGCAGATTGACCT |
| iNOS | NM_010927.4 | CAACGTGAAGAAAACCCCTTGT | AACATTCTGTGCTGTCCCAGT |
| HO-1 | NM_010442.2 | CACGCATATACCCGCTACCT | CCAGAGTGTTCATTCGAGCA |
| β-actin | NM_007393.5 | GTGGGAATGGGTCAGAAGGA | CTTCTCCATGTCGTCCCAGT |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Ji, Y.; Yin, W.; Liang, Y.; Sun, L.; Yin, Y.; Zhang, W. Anti-Inflammatory and Anti-Oxidative Activity of Indole-3-Acetic Acid Involves Induction of HO-1 and Neutralization of Free Radicals in RAW264.7 Cells. Int. J. Mol. Sci. 2020, 21, 1579. https://doi.org/10.3390/ijms21051579
Ji Y, Yin W, Liang Y, Sun L, Yin Y, Zhang W. Anti-Inflammatory and Anti-Oxidative Activity of Indole-3-Acetic Acid Involves Induction of HO-1 and Neutralization of Free Radicals in RAW264.7 Cells. International Journal of Molecular Sciences. 2020; 21(5):1579. https://doi.org/10.3390/ijms21051579
Chicago/Turabian StyleJi, Yun, Wenzhen Yin, Yuan Liang, Lijun Sun, Yue Yin, and Weizhen Zhang. 2020. "Anti-Inflammatory and Anti-Oxidative Activity of Indole-3-Acetic Acid Involves Induction of HO-1 and Neutralization of Free Radicals in RAW264.7 Cells" International Journal of Molecular Sciences 21, no. 5: 1579. https://doi.org/10.3390/ijms21051579
APA StyleJi, Y., Yin, W., Liang, Y., Sun, L., Yin, Y., & Zhang, W. (2020). Anti-Inflammatory and Anti-Oxidative Activity of Indole-3-Acetic Acid Involves Induction of HO-1 and Neutralization of Free Radicals in RAW264.7 Cells. International Journal of Molecular Sciences, 21(5), 1579. https://doi.org/10.3390/ijms21051579