Erythropoietin-Induced Changes in Bone and Bone Marrow in Mouse Models of Diet-Induced Obesity
Abstract
:1. Introduction
2. Results and Discussion
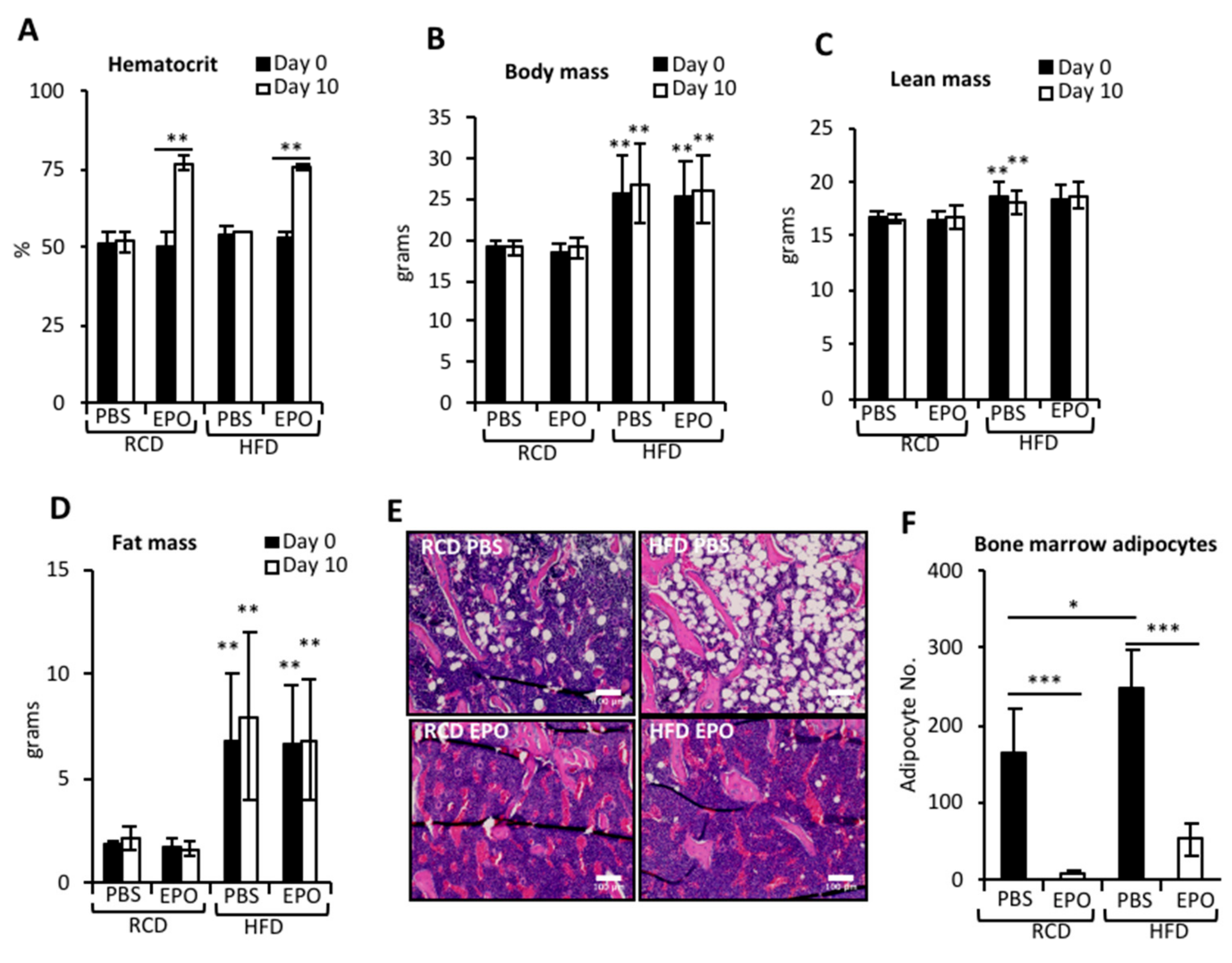
2.1. EPO Administration Reduces BMAT in Mice Fed Regular Chow or High-Fat Diet
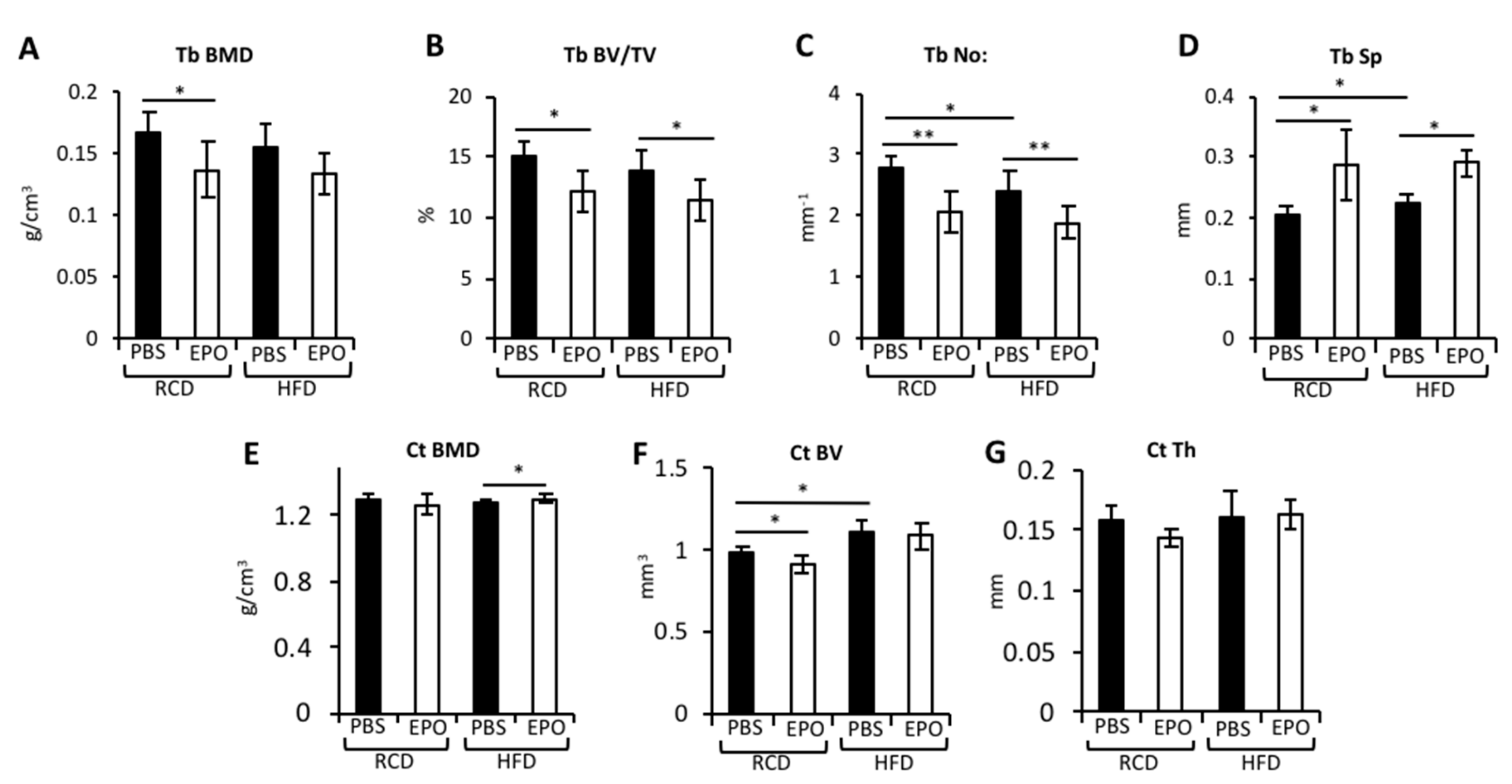
2.2. EPO Administration Reduces Trabecular Bone but Maintains Cortical Bone in HFD-Fed Mice
2.3. HFD Blunts EPO-Mediated Reduction of Cortical Osteocytes and Periosteal Osteoblasts
2.4. EPO Administration Did not Affect Osteoclasts
2.5. EPO Administration Restores Bone Marrow Cell Populations in HFD-Fed Mice
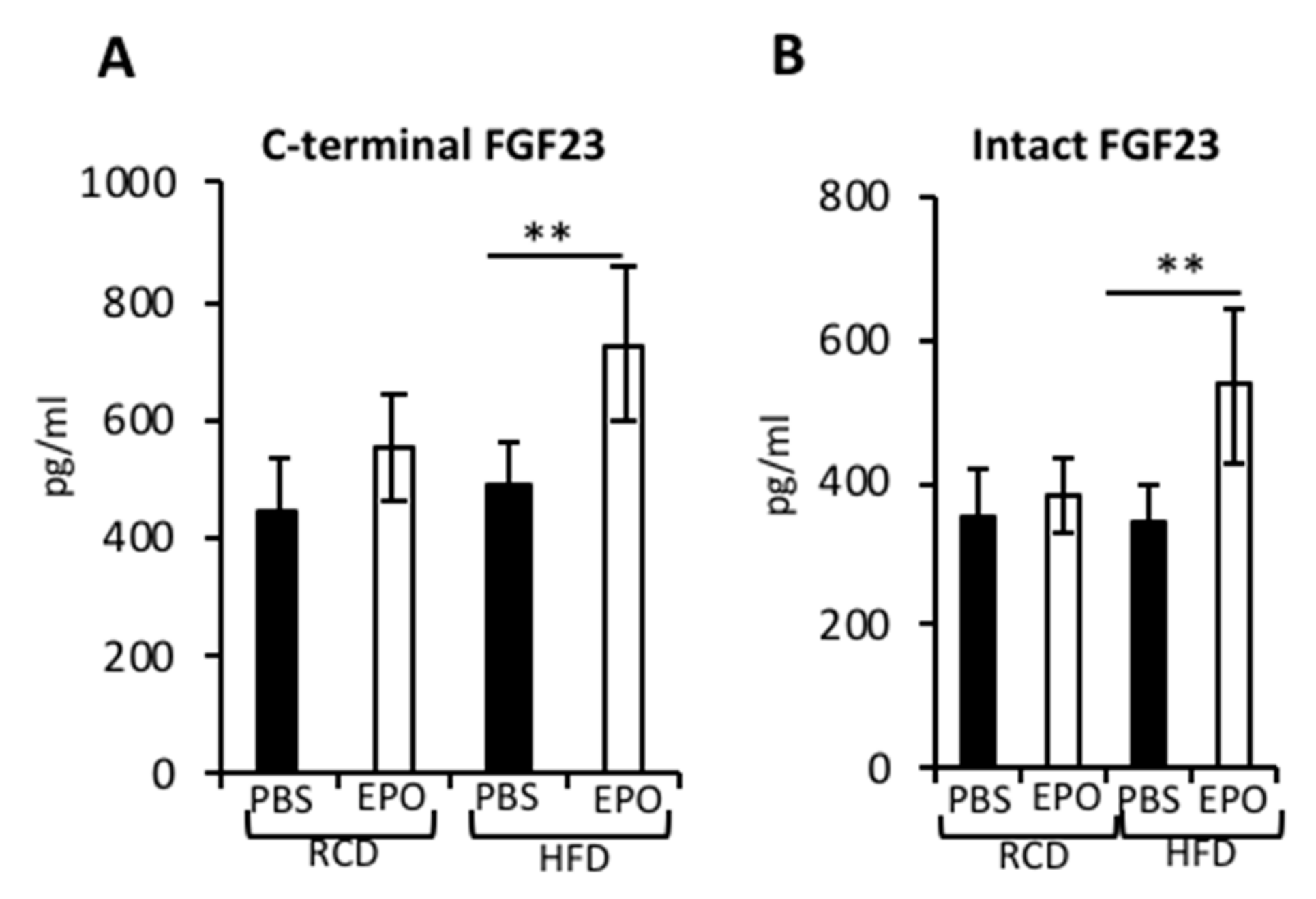
2.6. EPO Administration Increases Serum FGF23 Levels in HFD-Fed Mice
3. Discussion
4. Materials and Methods
4.1. Mice, High-Fat Diet Feeding and EPO Treatment
4.2. Micro-CT Analyses
4.3. Histology
4.4. FACS Analysis
4.5. ELISA
4.6. Statistical Analysis
Author Contributions
Funding
Conflicts of Interest
References
- Li, Y.; Meng, Y.; Yu, X. The Unique Metabolic Characteristics of Bone Marrow Adipose Tissue. Front. Endocrinol. 2019, 10, 69. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fazeli, P.K. Marrow fat and bone—New perspectives. J. Clin. Endocrinol. Metab. 2013, 98, 935–945. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suchacki, K.J.; Cawthorn, W.P.; Rosen, C.J. Bone marrow adipose tissue: Formation, function and regulation. Curr. Opin. Pharmacol. 2016, 28, 50–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bianco, P.; Robey, P.G. Skeletal stem cells. Development 2015, 142, 1023–1027. [Google Scholar] [CrossRef] [Green Version]
- Schwartz, A.V. Marrow fat and bone: Review of clinical findings. Front. Endocrinol. 2015, 6, 40. [Google Scholar] [CrossRef] [Green Version]
- Verma, S.; Rajaratnam, J.H.; Denton, J.; Hoyland, J.A.; Byers, R.J. Adipocytic proportion of bone marrow is inversely related to bone formation in osteoporosis. J. Clin. Pathol. 2002, 55, 693–698. [Google Scholar] [CrossRef]
- Ambrosi, T.H. Adipocyte Accumulation in the Bone Marrow during Obesity and Aging Impairs Stem Cell-Based Hematopoietic and Bone Regeneration. Cell Stem Cell 2017, 20, 771–784. [Google Scholar] [CrossRef] [Green Version]
- Devlin, M.J. Caloric restriction leads to high marrow adiposity and low bone mass in growing mice. J. Bone Miner. Res. 2010, 25, 2078–2088. [Google Scholar] [CrossRef]
- Costa, S.; Reagan, M.R. Therapeutic Irradiation: Consequences for Bone and Bone Marrow Adipose Tissue. Front. Endocrinol. 2019, 10, 587. [Google Scholar] [CrossRef] [Green Version]
- Albala, C. Obesity as a protective factor for postmenopausal osteoporosis. Int. J. Obes. Relat. Metab. Disord. 1996, 20, 1027–1032. [Google Scholar]
- Ishii, S. Pleiotropic effects of obesity on fracture risk: The Study of Women’s Health Across the Nation. J. Bone Miner. Res. 2014, 29, 2561–2570. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Compston, J.E. Obesity is not protective against fracture in postmenopausal women: GLOW. Am. J. Med. 2011, 124, 1043–1050. [Google Scholar] [CrossRef] [Green Version]
- Bredella, M.A. Determinants of bone microarchitecture and mechanical properties in obese men. J. Clin. Endocrinol. Metab. 2012, 97, 4115–4122. [Google Scholar] [CrossRef] [PubMed]
- Savvidis, C.; Tournis, S.; Dede, A.D. Obesity and bone metabolism. Hormones 2018, 17, 205–217. [Google Scholar] [CrossRef] [PubMed]
- Naveiras, O. Bone-marrow adipocytes as negative regulators of the haematopoietic microenvironment. Nature 2009, 460, 259–263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y. Erythropoietin action in stress response, tissue maintenance and metabolism. Int. J. Mol. Sci. 2014, 15, 10296–10333. [Google Scholar] [CrossRef] [Green Version]
- Holstein, J.H. Erythropoietin (EPO): EPO-receptor signaling improves early endochondral ossification and mechanical strength in fracture healing. Life Sci. 2007, 80, 893–900. [Google Scholar] [CrossRef]
- Mihmanli, A.; Dolanmaz, D.; Avunduk, M.C.; Erdemli, E. Effects of Recombinant Human Erythropoietin on Mandibular Distraction Osteogenesis. J. Oral Maxillofac. Surg. 2009, 67, 2337–2343. [Google Scholar] [CrossRef]
- Omlor, G.W. Increased bone formation in a rabbit long-bone defect model after single local and single systemic application of erythropoietin. Acta Orthop. 2016, 87, 425–431. [Google Scholar] [CrossRef] [Green Version]
- Rolfing, J.H. A single topical dose of erythropoietin applied on a collagen carrier enhances calvarial bone healing in pigs. Acta Orthop. 2014, 85, 201–209. [Google Scholar] [CrossRef]
- Singbrant, S. Erythropoietin couples erythropoiesis, B-lymphopoiesis, and bone homeostasis within the bone marrow microenvironment. Blood 2011, 117, 5631–5642. [Google Scholar] [CrossRef] [PubMed]
- Shiozawa, Y. Erythropoietin couples hematopoiesis with bone formation. PLoS ONE 2010, 5, e10853. [Google Scholar] [CrossRef] [PubMed]
- Hiram-Bab, S. Erythropoietin directly stimulates osteoclast precursors and induces bone loss. FASEB J. Off. Publ. Fed. Am. Soc. Exp. Biol. 2015, 29, 1890–1900. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Suresh, S.; de Castro, L.F.; Dey, S.; Robey, P.G.; Noguchi, C.T. Erythropoietin modulates bone marrow stromal cell differentiation. Bone Res. 2019, 7, 21. [Google Scholar] [CrossRef] [PubMed]
- Teng, R. Disrupted erythropoietin signalling promotes obesity and alters hypothalamus proopiomelanocortin production. Nat. Commun. 2011, 2, 520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L. PPARalpha and Sirt1 mediate erythropoietin action in increasing metabolic activity and browning of white adipocytes to protect against obesity and metabolic disorders. Diabetes 2013, 62, 4122–4131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Rogers, H.M.; Zhang, X.; Noguchi, C.T. Sex difference in mouse metabolic response to erythropoietin. FASEB J. 2017, 31, 2661–2673. [Google Scholar] [CrossRef] [Green Version]
- Alnaeeli, M. Erythropoietin signaling: A novel regulator of white adipose tissue inflammation during diet-induced obesity. Diabetes 2014, 63, 2415–2431. [Google Scholar] [CrossRef] [Green Version]
- Gautam, J. Micro-architectural changes in cancellous bone differ in female and male C57BL/6 mice with high-fat diet-induced low bone mineral density. Br. J. Nutr. 2014, 111, 1811–1821. [Google Scholar] [CrossRef] [Green Version]
- Bonewald, L.F. The amazing osteocyte. J. Bone Miner. Res. 2011, 26, 229–238. [Google Scholar] [CrossRef]
- Dwek, J.R. The periosteum: What is it, where is it, and what mimics it in its absence? Skelet. Radiol. 2010, 39, 319–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shu, L. High-fat diet causes bone loss in young mice by promoting osteoclastogenesis through alteration of the bone marrow environment. Calcif. Tissue Int. 2015, 96, 313–323. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hozumi, A. Bone marrow adipocytes support dexamethasone-induced osteoclast differentiation. Biochem. Biophys. Res. Commun. 2009, 382, 780–784. [Google Scholar] [CrossRef] [PubMed]
- Grover, A. Erythropoietin guides multipotent hematopoietic progenitor cells toward an erythroid fate. J. Exp. Med. 2014, 211, 181–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonewald, L.F.; Wacker, M.J. FGF23 production by osteocytes. Pediatr. Nephrol. 2013, 28, 563–568. [Google Scholar] [CrossRef] [PubMed]
- Clinkenbeard, E.L. Erythropoietin stimulates murine and human fibroblast growth factor-23, revealing novel roles for bone and bone marrow. Haematologica 2017, 102, e427–e430. [Google Scholar] [CrossRef]
- Daryadel, A. Erythropoietin stimulates fibroblast growth factor 23 (FGF23) in mice and men. Pflug. Arch. Eur. J. Physiol. 2018, 470, 1569–1582. [Google Scholar] [CrossRef]
- Coe, L.M. FGF-23 is a negative regulator of prenatal and postnatal erythropoiesis. J. Biol. Chem. 2014, 289, 9795–9810. [Google Scholar] [CrossRef] [Green Version]
- Jacobs, C.R. The mechanobiology of cancellous bone structural adaptation. J. Rehabil. Res. Dev. 2000, 37, 209–216. [Google Scholar]
- Styner, M. Bone marrow fat accumulation accelerated by high fat diet is suppressed by exercise. Bone 2014, 64, 39–46. [Google Scholar] [CrossRef] [Green Version]
- Metzger, C.E.; Narayanan, S.A. The Role of Osteocytes in Inflammatory Bone Loss. Front. Endocrinol. 2019, 10, 285. [Google Scholar] [CrossRef] [PubMed]
- van Bezooijen, R.L. Sclerostin is an osteocyte-expressed negative regulator of bone formation, but not a classical BMP antagonist. J. Exp. Med. 2004, 199, 805–814. [Google Scholar] [CrossRef] [PubMed]
- Nakashima, T. Evidence for osteocyte regulation of bone homeostasis through RANKL expression. Nat. Med. 2011, 17, 1231–1234. [Google Scholar] [CrossRef] [PubMed]
- Sato, M. Osteocytes regulate primary lymphoid organs and fat metabolism. Cell Metab. 2013, 18, 749–758. [Google Scholar] [CrossRef] [Green Version]
- Orwoll, E.S. Toward an expanded understanding of the role of the periosteum in skeletal health. J. Bone Miner. Res. 2003, 18, 949–954. [Google Scholar] [CrossRef]
- Yakar, S. Serum IGF-1 determines skeletal strength by regulating subperiosteal expansion and trait interactions. J. Bone Miner. Res. 2009, 24, 1481–1492. [Google Scholar] [CrossRef] [Green Version]
- Wu, C.L.; Diekman, B.O.; Jain, D.; Guilak, F. Diet-induced obesity alters the differentiation potential of stem cells isolated from bone marrow, adipose tissue and infrapatellar fat pad: The effects of free fatty acids. Int. J. Obes. 2013, 37, 1079–1087. [Google Scholar] [CrossRef] [Green Version]
- McGuire, T.R. Inflammation associated with obesity: Relationship with blood and bone marrow endothelial cells. Obesity 2011, 19, 2130–2136. [Google Scholar] [CrossRef]
- Maes, C. Osteoblast precursors, but not mature osteoblasts, move into developing and fractured bones along with invading blood vessels. Dev. Cell 2010, 19, 329–344. [Google Scholar] [CrossRef] [Green Version]
- Mirza, M.A. Circulating fibroblast growth factor-23 is associated with fat mass and dyslipidemia in two independent cohorts of elderly individuals. Arterioscler. Thromb. Vasc. Biol. 2011, 31, 219–227. [Google Scholar] [CrossRef] [Green Version]
- Glosse, P. A high-fat diet stimulates fibroblast growth factor 23 formation in mice through TNFalpha upregulation. Nutr. Diabetes 2018, 8, 36. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Emrich, I.E. Does a rise in plasma erythropoietin after high-altitude exposure affect FGF23 in healthy volunteers on a normal or low-phosphorus diet? Nutr. Metab. Cardiovasc. Dis. 2019, 29, 1361–1367. [Google Scholar] [CrossRef] [PubMed]
- Murali, S.K.; Andrukhova, O.; Clinkenbeard, E.L.; White, K.E.; Erben, R.G. Excessive Osteocytic Fgf23 Secretion Contributes to Pyrophosphate Accumulation and Mineralization Defect in Hyp Mice. PLoS Biol. 2016, 14, e1002427. [Google Scholar] [CrossRef] [PubMed] [Green Version]



© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Suresh, S.; Alvarez, J.C.; Dey, S.; Noguchi, C.T. Erythropoietin-Induced Changes in Bone and Bone Marrow in Mouse Models of Diet-Induced Obesity. Int. J. Mol. Sci. 2020, 21, 1657. https://doi.org/10.3390/ijms21051657
Suresh S, Alvarez JC, Dey S, Noguchi CT. Erythropoietin-Induced Changes in Bone and Bone Marrow in Mouse Models of Diet-Induced Obesity. International Journal of Molecular Sciences. 2020; 21(5):1657. https://doi.org/10.3390/ijms21051657
Chicago/Turabian StyleSuresh, Sukanya, Josue Caban Alvarez, Soumyadeep Dey, and Constance Tom Noguchi. 2020. "Erythropoietin-Induced Changes in Bone and Bone Marrow in Mouse Models of Diet-Induced Obesity" International Journal of Molecular Sciences 21, no. 5: 1657. https://doi.org/10.3390/ijms21051657




