Abstract
Patients with pancreatic ductal adenocarcinoma (PDAC) normally have a poor long-term prognosis. However, some rare cases of long-term survivors have been reported. The tumor microenvironment, consisting of cellular and stromal components, possibly plays an important role and might influence prognosis. In this context, the role of tumor-infiltrating B-cells and its impact on the survival in patients with PDAC remains controversial. We therefore aimed to assess the prognostic value of CD20-positive B-cells and CD20-positive B-cell aggregates as well as CD138, IgM, Pax5, and Ki67 on the survival of patients with PDAC using immunohistochemistry of FFPE pancreatectomy tissue sections from patients that underwent primary surgery for pT3- and R0-pancreatic adenocarcinoma between 1995 and 2016. Patients with PDAC were matched and grouped in 16 long-term-survivors (LTS, median overall survival (OS): 96 months [range: 61–177 months]) and 16 short-term-survivors (STS, median OS: 16 months [range: 7–32 months]). CD20-positive B-cells and B-cell aggregates in the tumor infiltration zone were significantly upregulated in the LTS-group compared to the STS-group (p = 0.0499 respectively p = 0.0432). Regarding the entire patient cohort (n = 32) CD20 positive B-cell aggregates in the tumor infiltration zone were an independent prognostic marker for overall survival in multivariate analysis (HR 9.2, CI 1.6–51.4, p = 0.012). These results underline the importance of tumor-associated B-cells for prognosis of patients with PDAC. The detailed role of B cells in the pathomechanism of PDAC should be further investigated for predicting outcome, identifying appropriate treatment regimens, and developing novel therapeutic options.
1. Introduction
Pancreatic ductal adenocarcinoma (PDAC) is associated with a poor prognosis, accounting for the seventh leading cause of cancer-related mortality worldwide [1]. In 2018, PDAC was diagnosed in about 458,000 people worldwide and more than 432,000 died of this disease in the same year [2]. The 5- and 10-year survival rates are low, ranging in Germany from 4% to 17% and 2% to 12%, respectively [3]. Although progress has been made in multimodal treatment approaches, the mortality rate of PDAC is still increasing throughout the years. Surgery is considered the only potential curative treatment for PDAC but is reserved for the minority of patients with non-metastatic and locally resectable tumors. Most patients with PDAC remain asymptomatic until the disease develops to an advanced inoperable stage, leading to its disappointing prognosis [4].
The determination of prognostic factors in patients with PDAC is essential for predicting the outcome and for identifying appropriate treatment strategies. Known clinical prognostic parameters include age, tumor stage, lymph node status, grading, perineural invasion, and for resected patients’ resection status, adjuvant chemotherapy and hospital volume [5,6,7].
Moreover, it has long been recognized that the tumor microenvironment and an involvement of the immune system play a distinctive role in the biological behavior of cancer [8]. PDACs are characterized by an immunosuppressive microenvironment due to the dysfunction of the immune system, which is a result of the involvement of multiple types of immune cells, including cancer-associated fibroblasts, regulatory T cells, myeloid-derived suppressor cells, tumor-associated macrophages, and tumor-infiltrating lymphocytes [9]. Until now, most studies have largely focused on the T cell compartment. The function of tumor-infiltrating B-cells, however, and its impact on survival in patients with PDAC, remains unclear [10,11].
The aim of the present study was to examine the prognostic role of B-cells and B-cell aggregates on the survival of patients with PDAC using CD20—a transmembrane phosphoprotein that is expressed on B-lymphocytes in different stages of development [12]. Additionally, the following B-cell associated markers were investigated: CD138—a transmembrane receptor, which participates in cell proliferation, cell migration, and cell-matrix interactions and is also known as syndecan-1 [13]; IgM—an immunoglobulin that occurs on the surface of B-cells as well as freely circulating in the blood [14]; Pax 5—a nuclear transcription factor, which is required for B cell development [15]; and Ki67—a nuclear protein expressed by cells in the proliferative phase [16].
2. Materials and Methods
2.1. Patients
We analyzed patients with previously untreated PDAC, who underwent primary surgery at the university hospital Erlangen, Germany, during the period between 1995 and 2016. Patients included in this analysis had to meet following additional criteria: Complete macroscopic and microscopic surgical resection (R0), pT3-category in histopathological examination, no in-hospital-mortality, and survival of a minimum of one month.
Patients’ clinical and pathological data were obtained from the Erlangen Cancer Registry of the Department of Surgery. The detailed documentation allowed a classification of pathology and staging of all patients according to the eighth edition of the tumor–node–metastasis (TNM) classification system [17].
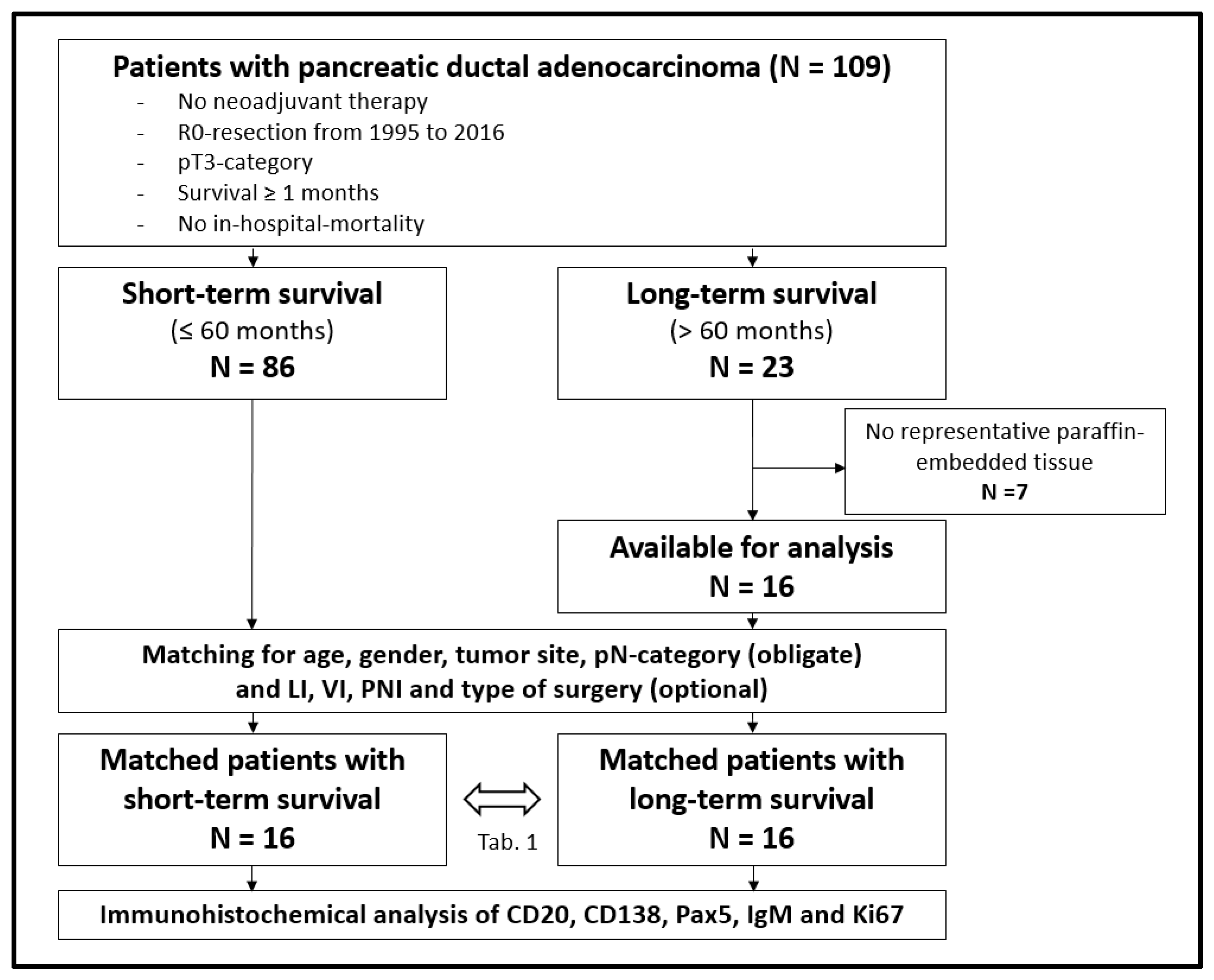
A total of 109 patients met the inclusion criteria. Eighty-six of the 109 patients had a survival of no longer than 60 months (short-term survival (STS)), while 23 patients showed a survival over 60 months (long-term survival (LTS)). After exclusion of seven patients with no representative formalin-fixed paraffin-embedded surgical samples in the LTS-groups, 16 LTS-patients were matched with the STS-group. Obligate matching criteria were age (difference of up to 9 years), gender, tumor site, and histopathological pN-category. In addition, matching of lymphatic invasion, vascular invasion, perineural invasion, grading, and type of surgery was carried out as far as possible. There were at least 16 matched patients in each group for immunohistochemical analysis (Figure 1).
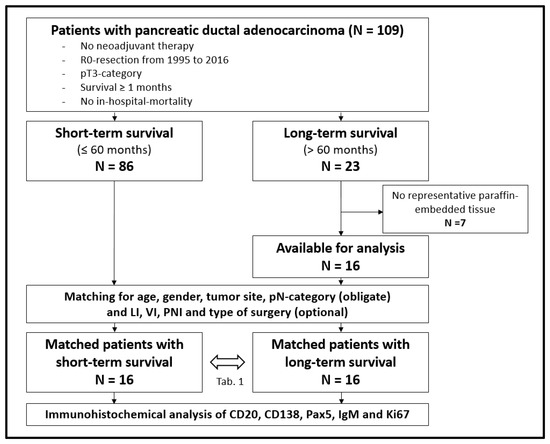
Figure 1.
Flow chart for patient inclusion; LI = lymphatic invasion, VI = vascular invasion, PNI = perineural invasion.
2.2. Immunohistochemical Staining and Scoring
Formalin-fixed paraffin-embedded tissue blocks of the 32 patients were anonymously reviewed by an experienced pathologist and the most representative block of each patient with the most inflammation in the tumor infiltration zone was selected for immunohistochemical staining.
Sections (1 µm) of the paraffin-embedded tissue blocks were cut with Leica microtom and mounted on slides. Slides were stained with hematoxylin and immunohistochemically with the primary antibodies CD20 (Dako, Ely, UK), CD138 (Zytomed Systems, Berlin, Germany), IgM (Dako, Ely, UK), Pax5 (BD Biosciences, Barking, UK), and Ki67 (Dako, Ely, UK) using the Benchmark Ultra system (Roche, Mannheim, Germany). Subsequently, slides were stained with the chromogen DAB (Roche, Mannheim, Germany) followed by a hemtoxylin and bluing reagent counterstain (Roche, Mannheim, Germany). Staining of a positive and a negative tissue control was done.
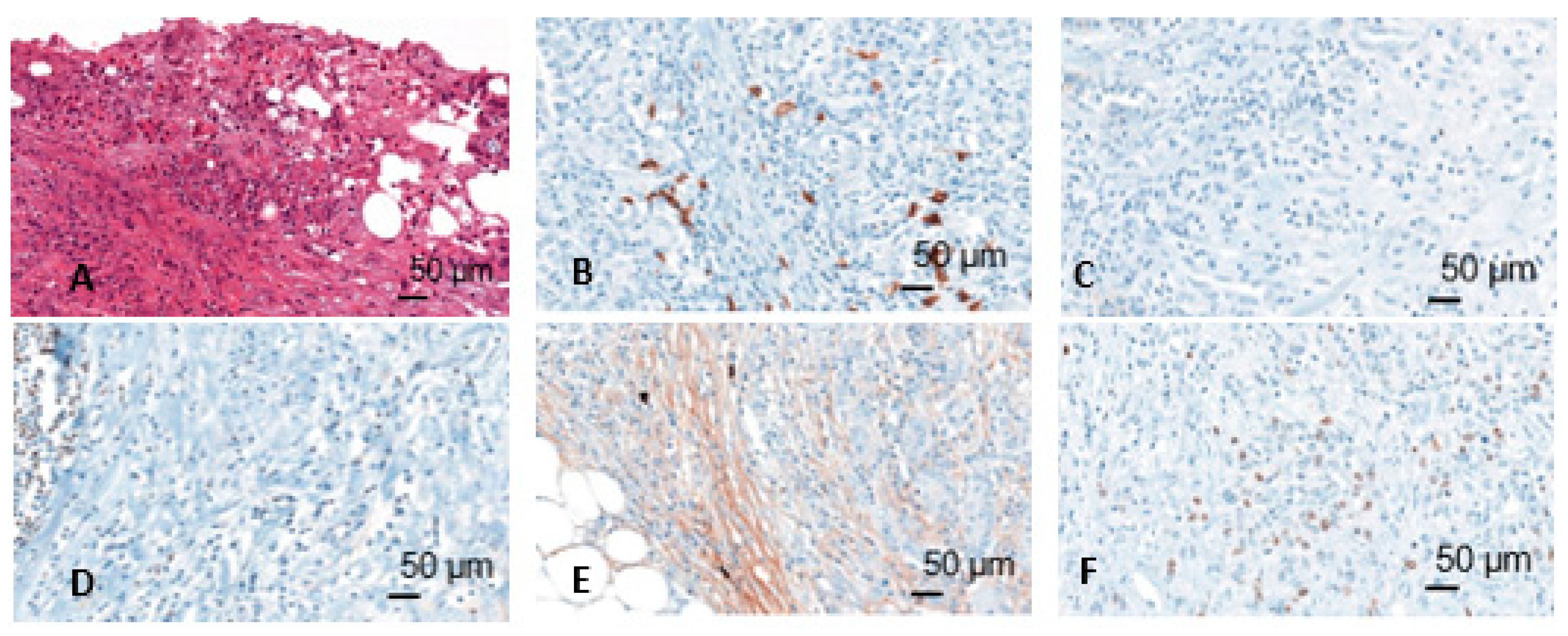
Immunohistochemically stained slides were scanned by Panoramic 250 Flash II scanner (3D Histech, Budapest, Hungary) at 40× magnification. The scans were blindly analyzed using CaseViewer software. First, the tumor infiltration zone was determined on the basis of the HE staining. After evaluation of the positive and negative tissue controls, the assessment of expression of CD20, CD138, Pax5, IgM, and Ki67 was performed (Figure 2). For CD20, CD138, Pax5, and IgM 10 counting areas were defined at 10× magnification and the positive cells in these areas were counted at 20× magnification. For Ki67, 500 cells were counted, and a quotient of positive and negative cells was formed. Cells were assessed in two compartments: Tumor and tumor infiltration zone. Moreover, CD20 positive lymphocytes aggregates in the tumor infiltration zone were measured.
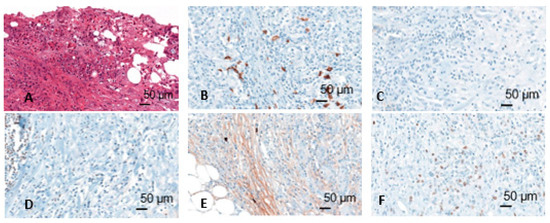
Figure 2.
Different immunohistochemical stainings (HE (A), CD20 (B), CD138 (C), Pax5 (D), IgM (E), Mib (F)); figures from the dissertation of KM.
2.3. Statistical Analysis
Data analysis was performed with SPSS software (SPSS Inc., Chicago, IL, USA). Comparisons of metric and ordinal data were calculated with the Student t-test or Mann Whitney U test. The Chi-square test was used for categorical data. Statistical significance was set at p < 0.05. Overall survival was calculated from the date of surgery to the day of death or last follow-up. The Kaplan–Meier method was used to plot the survival curves and Log-Rang test to compare influencing factors in univariate analysis. For correlation analysis Spearman coefficient was determined meaning 0.0 to ≤0.2 no correlation, >0.2 to ≤0.5 moderate correlation, and >0.5 high correlation. The results of the immunohistochemical markers (CD20, CD20-positive aggregates in the tumor compartment, CD20-positive aggregates in the tumor infiltration zone, CD138, IgM, Pax5, and Mib) were assessed for outliers using GraphPad QuickCalcs. Three outliers for CD20 and Pax5 and one outlier for IgM and CD138 were excluded from analysis due to insufficient staining. Dichotomized labelling (low vs. high expression) was based on the median value of immune marker expression. Multivariate analysis was performed using Cox regression model with backward elimination, including a log-likelihood adjustment. Backward elimination was applied with inclusion of all immunhistochemical parameters from the starting model. Variables were included if the p-values were less than 0.05 and were removed if the p-values were greater than 0.10.
3. Results
3.1. Clinicopathological Characteristics
The study cohort includes 32 patients (mean age 64.7 years [range 47–78], 69% female) with mainly in the head localized PDACs grouped into 16 short- (STS) and 16 long-term-survivors (LTS). Next to the obligate matching parameters type of surgery, grading, lymphatic, vascular and perineural invasion, as well as frequency of adjuvant chemotherapy, CA19-9 and CEA did not differ between the two groups. Adjuvant chemotherapy was performed in 34% of patients using gemcitabine alone in 45% of adjuvant treated patients and a gemcitabine-based combination therapy in 45% of adjuvant-treated patients (Table 1).

Table 1.
Clinicopathological characteristics of matched short-term survival patients (STS, n = 16) and long-term survival patients (LTS, n = 16); data presented as n (%); * obligate matching; + 3× Gemcitabine, 2× Gemcitabine + 5 FU, 1× Gemcitabine + Cisplatin + Vinorelbin + Paclitaxel, 1× unknown; # 2× Gemcitabine, 1× Gemcitabine + 5 FU, 1× Gemcitabine + Erlotinib.
3.2. Immune Marker and Lymphoid Aggregates
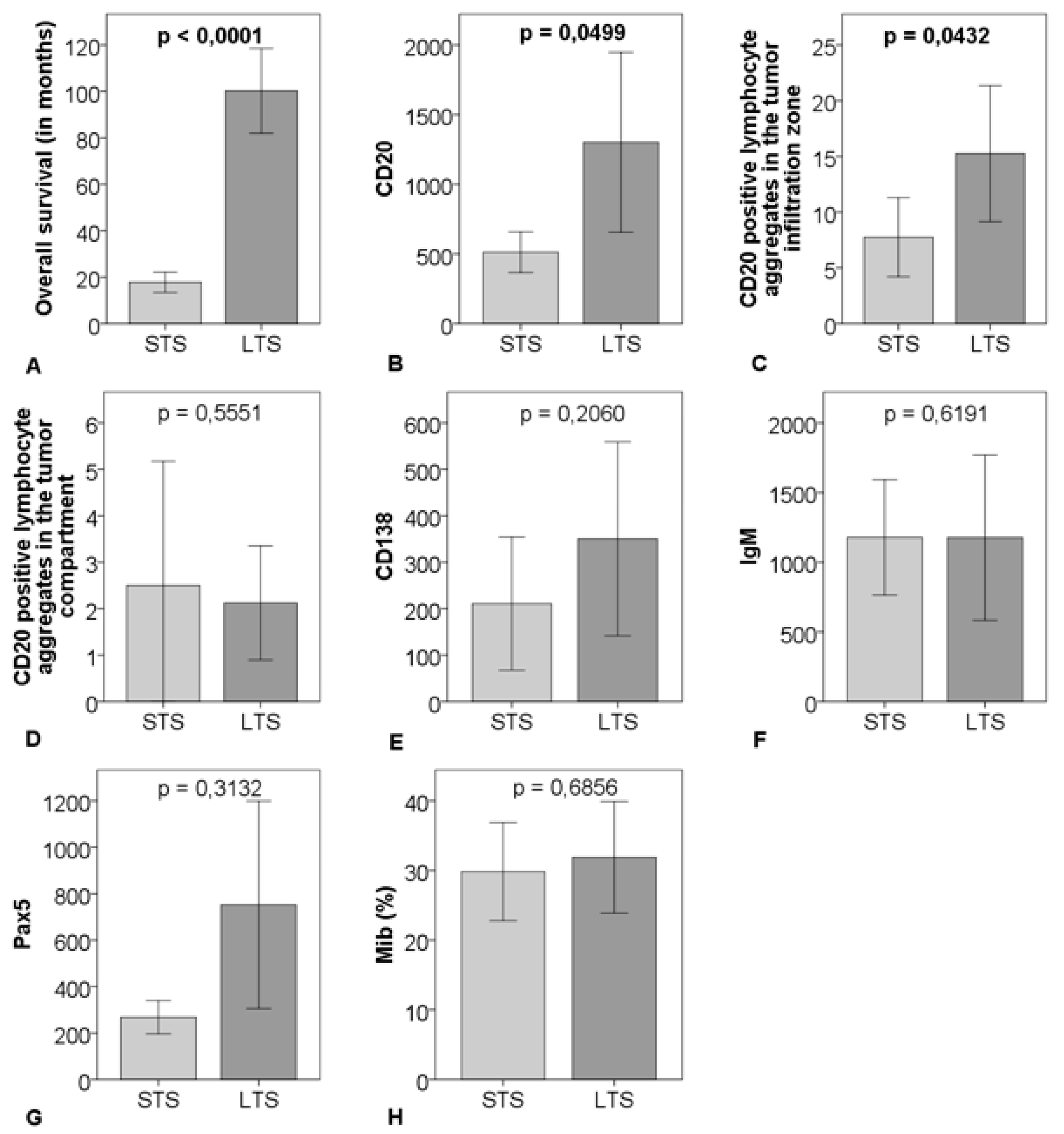
CD20-positve B-cells and CD20-positive B-cell aggregates in the tumor infiltration zone were significantly upregulated in the LTS-group compared to the STS-group (p = 0.0499 respectively p = 0.0432). There were no significant differences regarding CD20-positive B-cell aggregates in the tumor compartment, Pax5, CD138, Mib, and IgM comparing the two groups (Figure 3 and Figure 4).
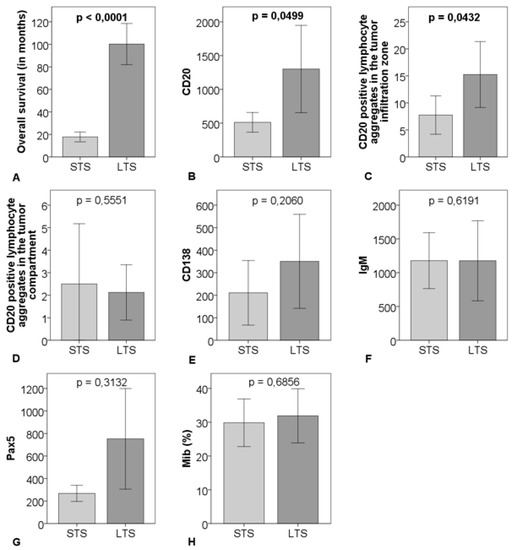
Figure 3.
Comparison of survival (A), cell count of CD20 (B), CD20-positive B-cell aggregates in the tumor infiltration zone (C), and in the tumor compartment (D), CD138 (E), IgM (F), Pax5 (G), percentage of Mib (H) between 16 matched short-term survivors (STS) and 16 matched long-term survivors (LTS); outliner excluded > CD20: n = 29, CD138: n = 31, IgM: n = 31, Pax5: n = 29; the graphs show mean and 95% CI including p-value.
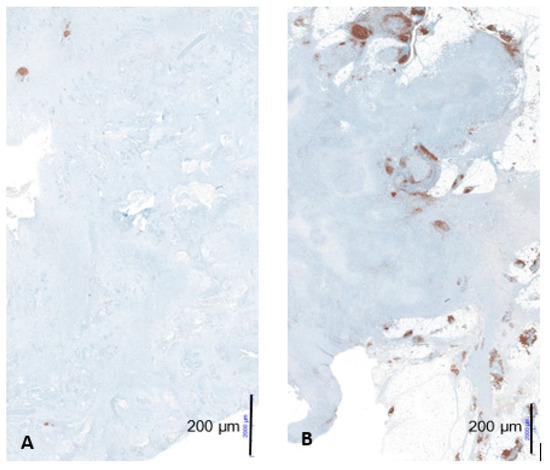
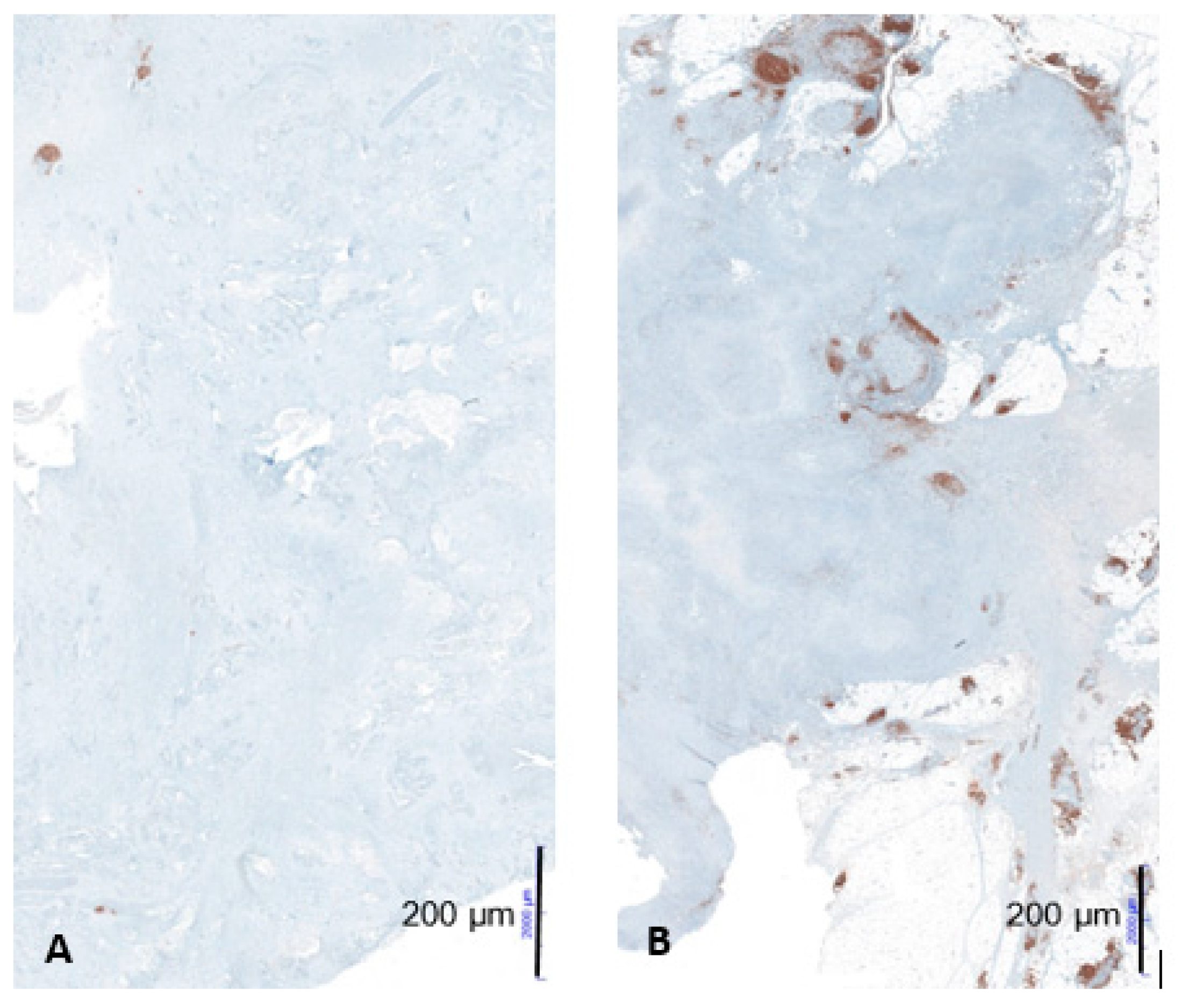
Figure 4.
Example of low CD20-positive B-cell aggregates in a patient with short-term survival (A) and high CD20-positive B-cell aggregates in a patient with long-term survival (B) at 10× magnification; figures from the dissertation of KM.
In the LTS-group, CD20 showed a high correlation with CD20-positive B-cell aggregates in the tumor infiltration zone, Pax 5, CD138, Mib, and IgM (spearman rho: 0.6124, 0.9029, 0.5857, 0.6357, and 0.5956, respectively). In the STS-group, CD20 correlated highly with CD20-positive B-cell aggregates in the tumor infiltration zone, Pax 5, and CD138 (spearmen rho: 0.5709, 0.8542, and 0.5209, respectively). Moderate and no correlation existed between CD20 and Mib and IgM, respectively, in the STS-group (spearman rho: 0.4813 and 0.1298, respectively).
3.3. Correlation of Immune Marker Expression with Clinicopathological Characteristics
Regarding the impact of clinicopathological characteristics on the expression of the investigated markers (CD20, CD20-positive B-cell aggregates in the tumor compartment and in the tumor infiltration zone, CD138, IgM, Pax5, and Mib), there was a significant association between high CD20-expression and an age ≥65 years (p = 0.025). High IgM-expression was more often found in the pancreatic head and therefore associated with a higher rate of pancreatic head resections (p = 0.018 and p = 0.024, respectively) (Table 2).

Table 2.
Impact of clinicopathological characteristics on CD20, CD20-aggregates, CD138, IgM, Pax5, and Mib expression; data presented as n (%); * outliner excluded > CD20: n = 29, CD138: n =31, IgM: n =31, Pax5: n =29; bold p-values are significant (<0.05); ** data incomplete.
3.4. Multivariate Analysis
Regarding the entire patient cohort (n = 32), high expression of CD20-positive B-cell aggregates in the tumor infiltration zone was an independent prognostic marker for overall survival in multivariate analysis (HR 9.2, CI 1.6–51.4, p = 0.012). The other investigated immune markers showed no significant impact on overall survival in multivariate analysis (Table 3).

Table 3.
Multivariate analysis of the impact of different immune markers on overall survival in the entire patient cohort (n = 32); * outliner excluded > CD20: n = 29, CD138: n = 31, IgM: n = 31, Pax5: n = 29; bold values are significant (p < 0.05).
4. Discussion
The tumor microenvironment is known to be an important parameter influencing the biological behavior of carcinomas and therefore prognosis of cancer patients. Several studies revealed especially tumor-associated lymphocytes (TALs) to be associated with good prognosis in pancreatic cancer as well as in different solid tumors [8,18,19,20,21,22] but focused largely on the T cell compartment. However, the role of B-cells and its impact on survival in patients with PDAC remains controversial [10,11].
CD20 is a 33–37 kDa transmembrane phosphoprotein, which is expressed on B-lymphocyte precursors and mature B-lymphocytes. CD20 positive B-cell infiltration has been associated with improved patient survival as well as increased immunotherapy response in various cancers [23,24]. Until now, there were only two studies investigating the association of CD20-positive B-cells with survival in patients with pancreatic carcinomas. Tewari et al. examined 81 patients with pancreatic ductal carcinomas and showed that high CD20-positive lymphocyte levels were associated with an improved survival [10]. In contrast, an investigation of pancreatectomy tissue sections of 141 primary resected pancreatic ductal adenocarcinomas by Diana et al. revealed no correlation of CD20 and prognostic outcome [11].
Our study is the first analysis comparing matched groups. In our cohort, CD20-positive B-cells and CD20-positive B-cell aggregates in the tumor infiltration zone were significantly upregulated in the LTS-group compared to the STS-group indicating a positive association of B-cells with prognosis. Moreover CD20-positive B-cell aggregates in the tumor infiltration zone were proved to be an independent prognostic marker for overall survival in multivariate analysis (HR 9.2, CI 1.6–51.4, p = 0.012).
However, the specific role of B-cells in the pathophysiological mechanism of PDAC remains unclear. The investigated B-cell associated markers (CD138, PAX 5, MiB, IgM) showed no specific pattern in our study and were therefore unable to indicate a potential pathway.
B-cells are known to constitute effector cells and to promote tumor-specific activation of cytotoxic T-cells via antigen presentation [25]: B-cells can internalize antigen that binds to their B-cell receptor and present it to helper T-cells. Unlike T-cells, B-cells can recognize soluble antigen for which their B-cell receptor is specific. Whereas antigen-presenting cells such as dendritic cells and macrophages ingest and present antigens nonspecifically, a B-cell generally presents only an antigen that it specifically recognizes. Naive helper T cells are then activated by binding to a foreign peptide bound to class II MHC proteins on the surface of a dendritic cell. Once activated, the effector helper T cell can then activate a B-cell that specifically displays the same complex of foreign peptide and class II MHC protein on its surface. The display of antigen on the B-cell surface reflects the selectivity with which it takes up foreign proteins from the extracellular fluid. These foreign proteins are selected by the antigen receptors on the surface of the B-cell and are ingested by receptor-mediated endocytosis. They are then degraded and recycled to the cell surface in the form of peptides bound to class II MHC proteins. Thus, the helper T-cell activates those B-cells with receptors that specifically recognize the antigen that initially activated the T-cell, although the T- and B-cells usually recognize distinct antigenic determinants on the antigen. In secondary antibody responses, memory B-cells themselves can act as antigen-presenting cells and activate helper T-cells, as well as being the subsequent targets of the effector helper T-cells. The mutually reinforcing actions of helper T-cells and B-cells lead to an immune response that is both intense and highly specific. Thus, it is reasonable to assume that interaction between various immune cells such as CD20-positive B-cells with T-cells represent an active immune response leading to less tumor immune evasion resulting in a better prognosis in patients with PDAC.
In contrast, there are preclinical studies reporting a protumorigenic role of B-cell subtypes [26,27,28]. Another interesting hypothesis explaining the B-cell-upregulation could be that some of the B-cells could even be malignant, as a recent study showed that in patient-derived xenograft mice models pancreatic cancer tumors can grow as a lymphocytic tumor containing CD20-expressing B-cells [29].
This suggests that the role of B-cells in pancreatic tumorgenesis seems to be more complex and not fully understood. However, the accumulation of B-cells into lymphoid aggregates close to T- and other cells seems to play a decisive prognostic role, as our investigation confirms, although the reasons for this are also not known [11,30].
In our cohort, age ≥65 years was significantly associated with a high CD20 expression. As advanced age is normally associated with a poorer prognosis, it is unclear to what extent age plays a role in CD20 expression. However, high CD20 expression as well as high expression of CD20-positive B-cell aggregates in the tumor infiltration zone were not associated with any other prognosis influencing clinicopathological characteristics like lymph node status (N) or grading (G) indicating a potentially underlying pathway. A possible interesting aspect could also be an impact of B-cells on the efficacy of adjuvant chemotherapy. Unfortunately, an investigation of this was not possible due to the small number of cases and the high heterogeneity of chemotherapeutics.
This study has some limitations. First, the small sample size limited the statistical power and the retrospective design of our study may have incurred some bias. Second the assessment and counting of immune markers is always somewhat arbitrary; therefore, a digital method might improve the reproducibility. Third, the examination of the exact pathomechanism was limited because, due to the retrospective design of the study, there were no blood samples or fresh tissue samples available for blood analysis or mass cytometric analysis, which could have provided even more findings regarding the pathomechanism of B-cells in pancreatic cancer.
5. Conclusions
This study is the first to report an association of CD20-positive B-cells with the long-term prognosis of patients with pancreatic ductal adenocarcinomas in an accurately matched cohort. As new promising immune therapies presently arise, the interaction and role of CD20-positive B-cells in the development of pancreatic adenocarcinoma should be further investigated for predicting outcome and develop novel therapy options.
Author Contributions
Conceptualization, K.M. and G.F.W.; writing—original draft preparation, M.B., S.M., S.K., and G.F.W.; writing—review and editing, all authors. All authors have read and agree to the published version of the manuscript.
Funding
This research received no external funding.
Acknowledgments
The present work was performed in partial fulfillment of the requirements for obtaining the degree “med” for K.M.
Conflicts of Interest
The authors declare no conflict of interest.
Abbreviations
| PDAC | Pancreatic ductal adenocarcinoma |
| LTS | Long-term survivors |
| STS | Short-term survivors |
| OS | Overall survival |
| HR | Hazard ratio |
| CI | Confidence interval |
| TNM | Tumor-node-metastasis |
| CA19-9 | Carbohydrate antigen 19-9 |
| CEA | Carcinoembryonic antigen |
| TALs | Tumor-associated lymphocytes |
| kDa | Kilodalton |
| MHC | Major histocompatibility complex |
| LI | Lymphatic invasion |
| VI | Vascular invasion |
| PI | Perineural invasion |
| FU | Fluoruracil |
References
- Bray, F.; Ferlay, J.; Soerjomataram, I.; Siegel, R.L.; Torre, L.A.; Jemal, A. Global cancer statistics 2018: GLOBOCAN estimates of incidence and mortality worldwide for 36 cancers in 185 countries. CA Cancer J. Clin. 2018, 68, 394–424. [Google Scholar] [CrossRef] [PubMed]
- Siegel, R.L.; Miller, K.D.; Jemal, A. Cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 7–30. [Google Scholar] [CrossRef] [PubMed]
- Kaatsch, P.; Spix, C.; Katalinic, A.; Hentschel, S.; Luttmann, S.; Waldeyer-Sauerland, M.; Waldmann, A.; Christ, M.; Folkerts, J.; Hansmann, J.; et al. Gesellschaft der Epidemiologischen Krebsregister in Deutschland e.V.: Krebs in Deutschland 2015/16, 12th ed.; Robert-Koch-Institut: Berlin, Germany, 2019; Available online: http://www.gekid.de/Doc/krebs_in_deutschland_2019.pdf (accessed on 20 February 2020).
- Kamisawa, T.; Wood, L.D.; Itoi, T.; Takaori, K. Pancreatic cancer. Lancet 2016, 388, 73–85. [Google Scholar] [CrossRef]
- Kardosh, A.; Lichtensztajn, D.Y.; Gubens, M.A.; Kunz, P.L.; Fisher, G.A.; Clarke, C.A. Long-Term Survivors of Pancreatic Cancer: A California Population-Based Study. Pancreas 2018, 47, 958–966. [Google Scholar] [CrossRef]
- Stark, A.P.; Sacks, G.D.; Rochefort, M.M.; Donahue, T.R.; Reber, H.A.; Tomlinson, J.S.; Dawson, D.W.; Eibl, G.; Hines, O.J. Long-term survival in patients with pancreatic ductal adenocarcinoma. Surgery 2016, 159, 1520–1527. [Google Scholar] [CrossRef]
- Krautz, C.; Nimptsch, U.; Weber, G.F.; Mansky, T.; Grützmann, R. Effect of Hospital Volume on In-hospital Morbidity and Mortality Following Pancreatic Surgery in Germany. Ann. Surg. 2018, 267, 411–417. [Google Scholar] [CrossRef]
- Ino, Y.; Yamazaki-Itoh, R.; Shimada, K.; Iwasaki, M.; Kosuge, T.; Kanai, Y.; Hiraoka, N. Immune cell infiltration as an indicator of the immune microenvironment of pancreatic cancer. Br. J. Cancer 2013, 108, 914–923. [Google Scholar] [CrossRef]
- Wormann, S.M.; Diakopoulos, K.N.; Lesina, M.; Algul, H. The immune network in pancreatic cancer development and progression. Oncogene 2014, 33, 2956–2967. [Google Scholar] [CrossRef]
- Tewari, N.; Zaitoun, A.M.; Arora, A.; Madhusudan, S.; Ilyas, M.; Lobo, D.N. The presence of tumour-associated lymphocytes confers a good prognosis in pancreatic ductal adenocarcinoma: An immunohistochemical study of tissue microarrays. BMC Cancer 2013, 13, 436. [Google Scholar] [CrossRef]
- Diana, A.; Wang, L.M.; D’Costa, Z.; Azad, A.; Silva, M.A.; Soonawalla, Z.; Allen, P.; Liu, S.; McKenna, W.G.; Muschel, R.J.; et al. Prognostic role and correlation of CA9, CD31, CD68 and CD20 with the desmoplastic stroma in pancreatic ductal adenocarcinoma. Oncotarget 2016, 7, 72819–72832. [Google Scholar] [CrossRef][Green Version]
- Golay, J.T.; Clark, E.A.; Beverley, P.C. The CD20 (Bp35) antigen is involved in activation of B cells from the G0 to the G1 phase of the cell cycle. J. Immunol. 1985, 135, 3795–3801. [Google Scholar] [PubMed]
- Juuti, A.; Nordling, S.; Lundin, J.; Louhimo, J.; Haglund, C. Syndecan-1 expression--a novel prognostic marker in pancreatic cancer. Oncology 2005, 68, 97–106. [Google Scholar] [CrossRef]
- Casali, P.; Schettino, E.W. Structure and function of natural antibodies. Curr. Top. Microbiol. Immunol. 1996, 210, 167–179. [Google Scholar] [PubMed]
- Kanteti, R.; Nallasura, V.; Loganathan, S.; Tretiakova, M.; Kroll, T.; Krishnaswamy, S.; Faoro, L.; Cagle, P.; Husain, A.N.; Vokes, E.E.; et al. PAX5 is expressed in small-cell lung cancer and positively regulates c-Met transcription. Lab. Investig. 2009, 89, 301–314. [Google Scholar] [CrossRef] [PubMed]
- Gerdes, J.; Schwab, U.; Lemke, H.; Stein, H. Production of a mouse monoclonal antibody reactive with a human nuclear antigen associated with cell proliferation. Int. J. Cancer 1983, 31, 13–20. [Google Scholar] [CrossRef] [PubMed]
- Brierley, J.D.; Gospodarowicz, M.K.; Wittekind, C. TNM Classification of Malignant Tumours, 8th ed.; Wiley-Blackwell: Oxford, UK, 2017. [Google Scholar]
- Droeser, R.; Zlobec, I.; Kilic, E.; Guth, U.; Heberer, M.; Spagnoli, G.; Oertli, D.; Tapia, C. Differential pattern and prognostic significance of CD4+, FOXP3+ and IL-17+ tumor infiltrating lymphocytes in ductal and lobular breast cancers. BMC Cancer 2012, 12, 134. [Google Scholar] [CrossRef] [PubMed]
- Ahmed, M.A.; Aleskandarany, M.A.; Rakha, E.A.; Moustafa, R.Z.; Benhasouna, A.; Nolan, C.; Green, A.R.; Ilyas, M.; Ellis, I.O. A CD44(−)/CD24(+) phenotype is a poor prognostic marker in early invasive breast cancer. Breast Cancer Res. Treat. 2011, 133, 979–995. [Google Scholar] [CrossRef]
- Ahmed, M.A.; Al-Attar, A.; Kim, J.; Watson, N.F.; Scholefield, J.H.; Durrant, L.G.; Ilyas, M. CD24 shows early upregulation and nuclear expression but is not a prognostic marker in colorectal cancer. J. Clin. Pathol. 2009, 62, 1117–1122. [Google Scholar] [CrossRef]
- Dieu-Nosjean, M.C.; Giraldo, N.A.; Kaplon, H.; Germain, C.; Fridman, W.H.; Sautès-Fridman, C. Tertiary lymphoid structures, drivers of the anti-tumor responses in human cancers. Immunol. Rev. 2016, 271, 260–275. [Google Scholar] [CrossRef]
- Hiraoka, N.; Ino, Y.; Yamazaki-Itoh, R.; Kanai, Y.; Kosuge, T.; Shimada, K. Intratumoral tertiary lymphoid organ is a favourable prognosticator in patients with pancreatic cancer. Br. J. Cancer 2015, 112, 1782–1790. [Google Scholar] [CrossRef]
- Petitprez, F.; de Reyniès, A.; Keung, E.Z.; Chen, T.W.; Sun, C.M.; Calderaro, J.; Jeng, Y.M.; Hsiao, L.P.; Lacroix, L.; Bougoüin, A.; et al. B cells are associated with survival and immunotherapy response in sarcoma. Nature 2020. [Google Scholar] [CrossRef]
- Helmink, B.A.; Reddy, S.M.; Gao, J.; Zhang, S.; Basar, R.; Thakur, R.; Yizhak, K.; Sade-Feldman, M.; Blando, J.; Han, G.; et al. B cells and tertiary lymphoid structures promote immunotherapy response. Nature 2020. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, A.J.; Coussens, L.M. B cells and their mediators as targets for therapy in solid tumors. Exp. Cell Res. 2013, 319, 1644–1649. [Google Scholar] [CrossRef] [PubMed]
- Gunderson, A.J.; Kaneda, M.M.; Tsujikawa, T.; Nguyen, A.V.; Affara, N.I.; Ruffell, B.; Gorjestani, S.; Liudahl, S.M.; Truitt, M.; Olson, P.; et al. Bruton Tyrosine Kinase-Dependent Immune Cell Cross-talk Drives Pancreas Cancer. Cancer Discov. 2016, 6, 270–285. [Google Scholar] [CrossRef] [PubMed]
- Lee, K.E.; Spata, M.; Bayne, L.J.; Buza, E.L.; Durham, A.C.; Allman, D.; Vonderheide, R.H.; Simon, M.C. Hif1a Deletion Reveals Pro-Neoplastic Function of B Cells in Pancreatic Neoplasia. Cancer Discov. 2016, 6, 256–269. [Google Scholar] [CrossRef] [PubMed]
- Pylayeva-Gupta, Y.; Das, S.; Handler, J.S.; Hajdu, C.H.; Coffre, M.; Koralov, S.B.; Bar-Sagi, D. IL35-Producing B Cells Promote the Development of Pancreatic Neoplasia. Cancer Discov. 2016, 6, 247–255. [Google Scholar] [CrossRef]
- Bondarenko, G.; Ugolkov, A.; Rohan, S.; Kulesza, P.; Dubrovskyi, O.; Gursel, D.; Mathews, J.; O’Halloran, T.V.; Wei, J.J.; Mazar, A.P. Patient-Derived Tumor Xenografts are Susceptible to Formation of Human Lymphocytic Tumors. Neoplasia 2015, 17, 735–741. [Google Scholar] [CrossRef]
- Castino, G.F.; Cortese, N.; Capretti, G.; Serio, S.; Di Caro, G.; Mineri, R.; Magrini, E.; Grizzi, F.; Cappello, P.; Novelli, F.; et al. Spatial distribution of B cells predicts prognosis in human pancreatic adenocarcinoma. Oncoimmunology 2016, 5, e1085147. [Google Scholar] [CrossRef]
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).