Chemical Defoliant Promotes Leaf Abscission by Altering ROS Metabolism and Photosynthetic Efficiency in Gossypium hirsutum
Abstract
:1. Introduction
2. Results
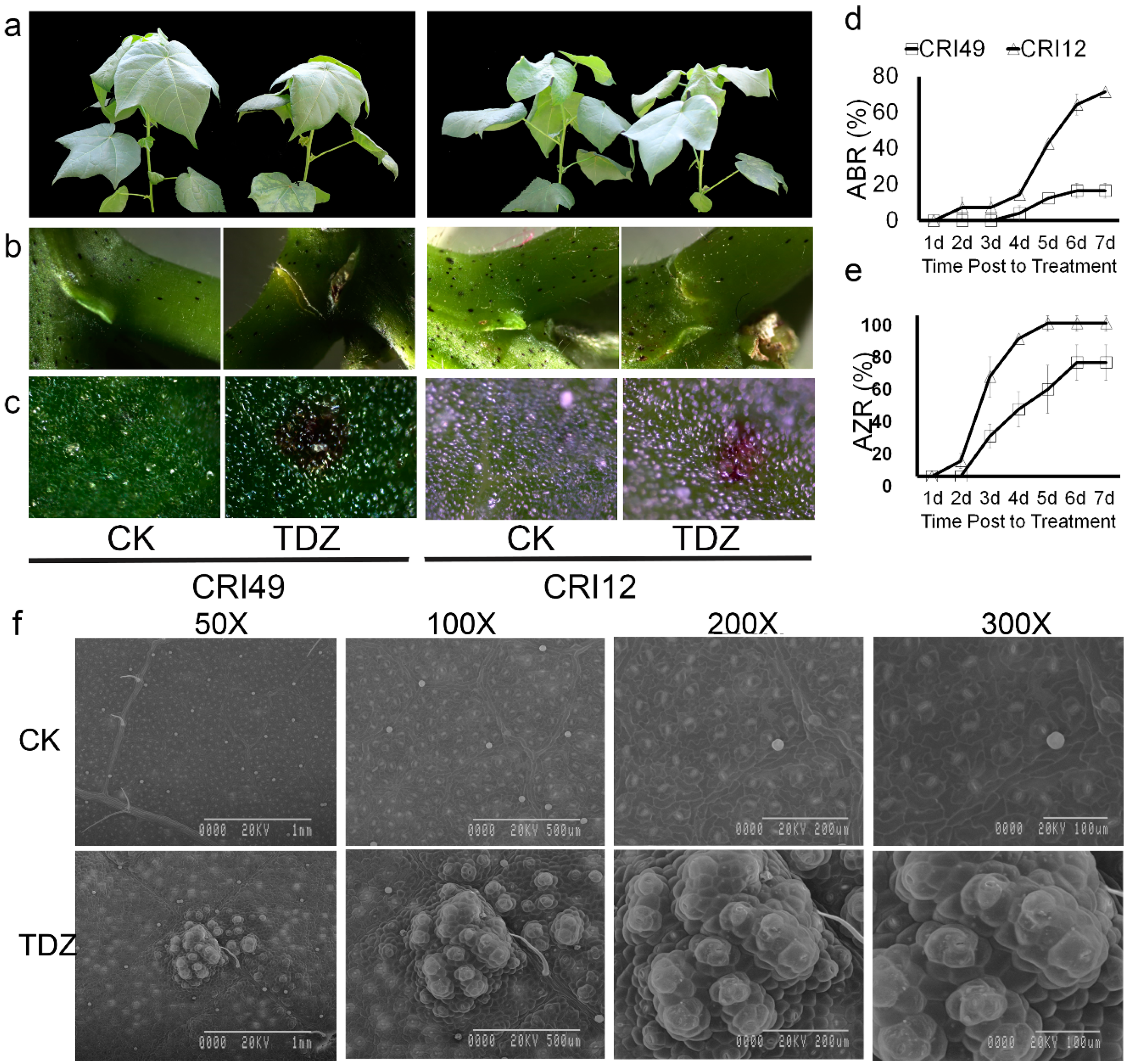
2.1. Morphology and Anatomical Features of Cotton Seedlings during Leaf Shedding
2.2. ROS Homeostasis during Abscission
2.3. Cotton Photosynthetic System during Abscission
2.4. Carbohydrate Contents in Leaf during Abscission
2.5. ROS Metabolism, Carbohydrate Metabolism and Photosynthesis Process Involved in Early Response under TDZ Treatment
2.6. Transcriptome Analysis
3. Discussion
3.1. Phenotypic Characteristic Damage under TDZ Treatment
3.2. ROS Homeostasis in Leaf Abscission in Respond to TDZ
3.3. Photosynthesis and Carbohydrate Metabolism in Leaf Abscission Induced by TDZ
3.4. The Potential Crosstalk among Phenotypic Characteristic, ROS, Photosynthesis, and Carbohydrate Metabolism in Leaf Abscission Induced by TDZ
4. Materials and Methods
4.1. Materials and Experimental Design
4.2. Abscission Rate and Formation Rate of Abscission Zone
4.3. Scanning Electron Microscopy of Leaf
4.4. Enzymes Activities, O2−, MDA, and H2O2 Content Assays in the Leaf
4.5. Photosynthetic Parameters, Carbohydrates and Soluble Protein Content
4.6. Measurement of Anthocyanin and Chlorophyll Contents
4.7. RNA Extraction, cDNA Library Construction, and Sequencing
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| TDZ | Thidiazuron |
| Pn | Net photosynthesis |
| Tr | Transpiration rate |
| Gs | Stomatal conductance |
| Ci | Intercellular carbon dioxide concentration |
| AZ | Abscission zone |
| H2O2 | Hydrogen peroxide |
| ROS | Reactive oxygen species |
| AZR | Abscission zone formation rate |
| ABR | Abscission rate |
| O2− | Superoxide anion free radical |
| MDA | Malondialdehyde |
| CAT | Catalase |
| POD | Peroxidase |
| SOD | Superoxide dismutase |
| Chla | Chlorophyll a |
| Chlb | Chlorophyll b |
| Cxc | Carotenoid |
| T Chl | Total chlorophyll |
| SS | Soluble sugar |
| FAA | Free amino acid |
References
- Patharkar, O.R.; Walker, J.C. Advances in abscission signaling. J. Exp. Bot. 2018, 69, 733–740. [Google Scholar] [CrossRef] [PubMed]
- Olsson, V.; Butenko, M.A. Abscission in plants. Curr. Biol. 2018, 28, R338–R339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patharkar, O.R.; Gassmann, W.; Walker, J.C. Leaf shedding as an anti-bacterial defense in Arabidopsis cauline leaves. PLoS Genet. 2017, 13, e1007132. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Taylor, J.E.; Whitelaw, C.A. Signals in abscission. New Phytol. 2001, 151, 323–339. [Google Scholar] [CrossRef]
- Jackson, M.B.; Osborne, D.J. Ethylene, the natural regulator of leaf abscission. Nature 1970, 225, 1019–1022. [Google Scholar] [CrossRef]
- Basu, M.M.; Gonzalez-Carranza, Z.H.; Azam-Ali, S.; Tang, S.Y.; Shahid, A.A.; Roberts, J.A. The manipulation of auxin in the abscission zone cells of Arabidopsis flowers reveals that indoleacetic acid signaling is a prerequisite for organ shedding. Plant Physiol. 2013, 162, 96–106. [Google Scholar] [CrossRef] [Green Version]
- Celton, J.M.; Dheilly, E.; Guillou, M.C.; Simonneau, F.; Juchaux, M.; Costes, E.; Laurens, F.; Renou, J.P. Additional amphivasal bundles in pedicel pith exacerbate central fruit dominance and induce self-thinning of lateral fruitlets in Apple(1[C][W]). Plant Physiol. 2014, 164, 1930–1951. [Google Scholar] [CrossRef] [Green Version]
- Jin, X.; Zimmermann, J.; Polle, A.; Fischer, U. Auxin is a long-range signal that acts independently of ethylene signaling on leaf abscission in Populus. Front. Plant Sci. 2015, 6, 634. [Google Scholar] [CrossRef] [Green Version]
- Xu, J.; Chen, L.; Sun, H.; Wusiman, N.; Sun, W.N.; Li, B.Q.; Gao, Y.; Kong, J.; Zhang, D.W.; Zhang, X.L.; et al. Crosstalk between cytokinin and ethylene signaling pathways regulates leaf abscission in cotton in response to chemical defoliants. J. Exp. Bot. 2019, 70, 1525–1538. [Google Scholar] [CrossRef] [Green Version]
- Roberts, J.A.; Elliott, K.A.; Gonzalez-Carranza, Z.H. Abscission, dehiscence, and other cell separation processes. Annu. Rev. Plant Biol. 2002, 53, 131–158. [Google Scholar] [CrossRef]
- Kim, J.; Sundaresan, S.; Philosoph-Hadas, S.; Yang, R.H.; Meir, S.; Tucker, M.L. Examination of the abscission-associated transcriptomes for soybean, tomato, and Arabidopsis highlights the conserved biosynthesis of an extensible extracellular matrix and boundary layer. Front. Plant Sci. 2015, 6, 1109. [Google Scholar] [CrossRef] [Green Version]
- Rhee, S.Y.; Osborne, E.; Poindexter, P.D.; Somerville, C.R. Microspore separation in the quartet 3 mutants of Arabidopsis is impaired by a defect in a developmentally regulated polygalacturonase required for pollen mother cell wall degradation. Plant Physiol. 2003, 133, 1170–1180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, M.; Munemura, I.; Tomita, R.; Kobayashi, K. Reactive oxygen species in leaf abscission signaling. Plant Signal. Behav. 2008, 3, 1014–1015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sakamoto, M.; Munemura, I.; Tomita, R.; Kobayashi, K. Involvement of hydrogen peroxide in leaf abscission signaling, revealed by analysis with an in vitro abscission system in Capsicum plants. Plant J. 2008, 56, 13–27. [Google Scholar] [CrossRef]
- Jordan, W.R.; Morgan, P.W.; Davenport, T.L. Water stress enhances ethylene-mediated leaf abscission in Cotton. Plant Physiol. 1972, 50, 756–758. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agusti, J.; Gimeno, J.; Merelo, P.; Serrano, R.; Cercos, M.; Conesa, A.; Talon, M.; Tadeo, F.R. Early gene expression events in the laminar abscission zone of abscission-promoted citrus leaves after a cycle of water stress/rehydration: Involvement of CitbHLH1. J. Exp. Bot. 2012, 63, 6079–6091. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Michaeli, R.; Philosoph-Hadas, S.; Riov, J.; Shahak, Y.; Ratner, K.; Meir, S. Chilling-induced leaf abscission of Ixora coccinea plants. III. Enhancement by high light via increased oxidative processes. Physiol. Plantarum. 2001, 113, 338–345. [Google Scholar] [CrossRef]
- Xie, X.L.; He, Z.Q.; Chen, N.F.; Tang, Z.Z.; Wang, Q.; Cai, Y. The roles of environmental factors in regulation of oxidative stress in plant. Biomed. Res. Int. 2019, 2019, 9732325. [Google Scholar] [CrossRef]
- Liao, W.B.; Wang, G.; Li, Y.Y.; Wang, B.; Zhang, P.; Peng, M. Reactive oxygen species regulate leaf pulvinus abscission zone cell separation in response to water-deficit stress in cassava. Sci. Rep.-UK 2016, 6, 21542. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Goldental-Cohen, S.; Burstein, C.; Biton, I.; Ben Sasson, S.; Sadeh, A.; Many, Y.; Doron-Faigenboim, A.; Zemach, H.; Mugira, Y.; Schneider, D.; et al. Ethephon induced oxidative stress in the olive leaf abscission zone enables development of a selective abscission compound. BMC Plant Biol. 2017, 17, 87. [Google Scholar] [CrossRef]
- Yang, Z.Q.; Zhong, X.M.; Fan, Y.; Wang, H.C.; Li, J.G.; Huang, X.M. Burst of reactive oxygen species in pedicel-mediated fruit abscission after carbohydrate supply was cut off in longan (Dimocarpus longan). Front. Plant Sci. 2015, 6, 360. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iglesias, D.J.; Tadeo, F.R.; Primo-Millo, E.; Talon, M. Carbohydrate and ethylene levels related to fruitlet drop through abscission zone A in citrus. Trees-Struct. Funct. 2006, 20, 348–355. [Google Scholar] [CrossRef]
- Domingos, S.; Fino, J.; Cardoso, V.; Sanchez, C.; Ramalho, J.C.; Larcher, R.; Paulo, O.S.; Oliveira, C.M.; Goulao, L.F. Shared and divergent pathways for flower abscission are triggered by gibberellic acid and carbon starvation in seedless Vitis vinifera L. BMC Plant Biol. 2016, 16, 38. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, T.; Hu, Y.; Jiang, W.; Fang, L.; Guan, X.; Chen, J.; Zhang, J.; Saski, C.A.; Scheffler, B.E.; Stelly, D.M.; et al. Sequencing of allotetraploid cotton (Gossypium hirsutum L. acc. TM-1) provides a resource for fiber improvement. Nat. Biotechnol. 2015, 33, 531–537. [Google Scholar] [CrossRef] [Green Version]
- Du, M.W.; Li, Y.; Tian, X.L.; Duan, L.S.; Zhang, M.C.; Tan, W.M.; Xu, D.Y.; Li, Z.H. The phytotoxin coronatine induces abscission-related gene expression and boll ripening during defoliation of cotton. PLoS ONE 2014, 9, e97652. [Google Scholar] [CrossRef] [Green Version]
- Wang, H.M.; Gao, K.; Fang, S.; Zhou, Z.G. Cotton yield and defoliation efficiency in response to nitrogen and harvest aids. Agron. J. 2019, 111, 250–256. [Google Scholar] [CrossRef]
- Du, M.W.; Ren, X.M.; Tian, X.L.; Duan, L.S.; Zhang, M.C.; Tan, W.M.; Li, Z.H. Evaluation of harvest aid chemicals for the cotton-winter wheat double cropping system. J. Integr. Agric. 2013, 12, 273–282. [Google Scholar] [CrossRef]
- Nisler, J.; Kopecny, D.; Koncitikova, R.; Zatloukal, M.; Bazgier, V.; Berka, K.; Zalabak, D.; Briozzo, P.; Strnad, M.; Spichal, L. Novel thidiazuron-derived inhibitors of cytokinin oxidase/dehydrogenase. Plant Mol. Biol. 2016, 92, 235–248. [Google Scholar] [CrossRef]
- Grossmann, K. Induction of leaf abscission in cotton is a common effect of urea- and adenine-type cytokinins. Plant Physiol. 1991, 95, 234–237. [Google Scholar] [CrossRef] [Green Version]
- Suttle, J.C. Involvement of ethylene in the action of the cotton defoliant thidiazuron. Plant Physiol. 1985, 78, 272–276. [Google Scholar] [CrossRef]
- Botton, A.; Eccher, G.; Forcato, C.; Ferrarini, A.; Begheldo, M.; Zermiani, M.; Moscatello, S.; Battistelli, A.; Velasco, R.; Ruperti, B.; et al. Signaling pathways mediating the induction of apple fruitlet abscission. Plant Physiol. 2011, 155, 185–208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patharkar, O.R.; Walker, J.C. Core mechanisms regulating developmentally timed and environmentally triggered abscission. Plant Physiol. 2016, 172, 510–520. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, J.; Song, C.P.; Wang, B.; Zhou, J.; Kangasjarvi, J.; Zhu, J.K.; Gong, Z. Reactive oxygen species signaling and stomatal movement in plant responses to drought stress and pathogen attack. J. Integr. Plant Biol. 2018, 60, 805–826. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agurla, S.; Gahir, S.; Munemasa, S.; Murata, Y.; Raghavendra, A.S. Mechanism of stomatal closure in plants exposed to drought and cold stress. Adv. Exp. Med. Biol. 2018, 1081, 215–232. [Google Scholar] [PubMed]
- Wang, Z.; Wang, F.; Hong, Y.; Huang, J.; Shi, H.; Zhu, J.K. Two chloroplast proteins suppress drought resistance by affecting ros production in guard cells. Plant Physiol. 2016, 172, 2491–2503. [Google Scholar] [CrossRef]
- Qiu, Z.; Zhu, L.; He, L.; Chen, D.; Zeng, D.; Chen, G.; Hu, J.; Zhang, G.; Ren, D.; Dong, G.; et al. DNA damage and reactive oxygen species cause cell death in the rice local lesions 1 mutant under high light and high temperature. New Phytol. 2019, 222, 349–365. [Google Scholar] [CrossRef]
- Mishra, A.; Khare, S.; Trivedi, P.K.; Nath, P. Effect of ethylene, 1-MCP, ABA and IAA on break strength, cellulase and polygalacturonase activities during cotton leaf abscission. S. Afr. J. Bot. 2008, 74, 282–287. [Google Scholar] [CrossRef] [Green Version]
- Xu, Y.; Magwanga, R.O.; Yang, X.; Jin, D.; Cai, X.; Hou, Y.; Wei, Y.; Zhou, Z.; Wang, K.; Liu, F. Genetic regulatory networks for salt-alkali stress in Gossypium hirsutum with differing morphological characteristics. BMC Genom. 2020, 21, 15. [Google Scholar] [CrossRef] [Green Version]
- Singh, R.; Singh, S.; Parihar, P.; Mishra, R.K.; Tripathi, D.K.; Singh, V.P.; Chauhan, D.K.; Prasad, S.M. Reactive oxygen species (ROS): Beneficial companions of plants’ developmental processes. Front. Plant Sci. 2016, 7, 1299. [Google Scholar] [CrossRef] [Green Version]
- Liang, D. A Salutary role of reactive oxygen species in intercellular tunnel-mediated communication. Front. Cell Dev. Biol. 2018, 6, 2. [Google Scholar] [CrossRef] [Green Version]
- Dauphinee, A.N.; Fletcher, J.I.; Denbigh, G.L.; Lacroix, C.R.; Gunawardena, A.H. Remodelling of lace plant leaves: Antioxidants and ROS are key regulators of programmed cell death. Planta 2017, 246, 133–147. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, S.; Oshita, S.; Makino, Y.; Wang, Q.H.; Kawagoe, Y.; Uchida, T. Oxidative capacity of nanobubbles and its effect on seed germination. ACS Sustain. Chem. Eng. 2016, 4, 1347–1353. [Google Scholar] [CrossRef]
- Li, T.; Shi, D.; Wu, Q.; Zhang, Z.; Qu, H.; Jiang, Y. Sodium para-aminosalicylate delays pericarp browning of litchi fruit by inhibiting ROS-mediated senescence during postharvest storage. Food Chem. 2019, 278, 552–559. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Magwanga, R.O.; Cai, X.; Zhou, Z.; Wang, X.; Wang, Y.; Zhang, Z.; Jin, D.; Guo, X.; Wei, Y.; et al. Deep transcriptome analysis reveals reactive oxygen species (ROS) network evolution, response to abiotic stress, and regulation of fiber development in cotton. Int. J. Mol. Sci. 2019, 20, 1863. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sha, S.; Cheng, M.; Hu, K.; Zhang, W.; Yang, Y.; Xu, Q. Toxic effects of Pb on Spirodela polyrhiza (L.): Subcellular distribution, chemical forms, morphological and physiological disorders. Ecotoxicol. Environ. Saf. 2019, 181, 146–154. [Google Scholar] [CrossRef]
- Zhao, D.Q.; Li, T.T.; Hao, Z.J.; Cheng, M.L.; Tao, J. Exogenous trehalose confers high temperature stress tolerance to herbaceous peony by enhancing antioxidant systems, activating photosynthesis, and protecting cell structure. Cell Stress Chaperones 2019, 24, 247–257. [Google Scholar] [CrossRef]
- You, J.; Chan, Z. ROS regulation during abiotic stress responses in crop plants. Front. Plant Sci. 2015, 6, 1092. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kaur, N.; Dhawan, M.; Sharma, I.; Pati, P.K. Interdependency of reactive oxygen species generating and scavenging system in salt sensitive and salt tolerant cultivars of rice. BMC Plant Biol. 2016, 16, 131. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, Z.H.; Mahmood, K.; Rothstein, S.J. ROS induces anthocyanin production via late biosynthetic genes and anthocyanin deficiency confers the hypersensitivity to ROS-generating stresses in Arabidopsis. Plant Cell Physiol. 2017, 58, 1364–1377. [Google Scholar] [CrossRef]
- Moustaka, J.; Panteris, E.; Adamakis, I.D.S.; Tanou, G.; Giannakoula, A.; Eleftheriou, E.P.; Moustakas, M. High anthocyanin accumulation in poinsettia leaves is accompanied by thylakoid membrane unstacking, acting as a photoprotective mechanism, to prevent ROS formation. Environ. Exp. Bot. 2018, 154, 44–55. [Google Scholar] [CrossRef]
- Xu, Z.H.; Rothstein, S.J. ROS-Induced anthocyanin production provides feedback protection by scavenging ROS and maintaining photosynthetic capacity in Arabidopsis. Plant Signal. Behav. 2018, 13, 1364–1377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Asada, K. Production and action of active oxygen species in photosynthetic tissues. In Causes of Photooxidative Stress and Amelioration of Defense Systems in Plants; Foyer, C., Mullineaux, P., Eds.; CRC Press Inc.: Boca Raton, FL, USA, 1994; pp. 77–104. [Google Scholar]
- Yoon, H.I.; Zhang, W.; Son, J.E. Optimal duration of drought stress near harvest for promoting bioactive compounds and antioxidant capacity in kale with or without UV-B radiation in plant factories. Plants 2020, 9, 295. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, J.; Zhao, X.; Chory, J. The Arabidopsis transcriptome responds specifically and dynamically to high light stress. Cell Rep. 2019, 29, 4186–4199. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kräutler, B.; Matile, P. Solving the riddle of chlorophyll breakdown. Acc. Chem. Res. 1999, 32, 35–43. [Google Scholar] [CrossRef]
- Lin, M.; Pang, C.Y.; Fan, S.L.; Song, M.Z.; Wei, H.L.; Yu, S.X. Global analysis of the Gossypium hirsutum L. transcriptome during leaf senescence by RNA-seq. BMC Plant Biol. 2015, 15, 43. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.; Rozhon, W.; Poppenberger, B. The role of hormones in the aging of plants—A mini-review. Gerontology 2014, 60, 49–55. [Google Scholar] [CrossRef]
- Lichtenthaler, H. Chlorophylls and carotenoids: Pigments of photosynthesis. Methods Enzymol. 1987, 148, 350–352. [Google Scholar]
- Green, B.R.; Pichersky, E.; Kloppstech, K. Chlorophyll a/b-binding proteins: An extended family. Trends Biochem. Sci. 1991, 16, 181–186. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, R.; Tanaka, A. Chlorophyll cycle regulates the construction and destruction of the light-harvesting complexes. Biochim. Biophys. Acta 2011, 1807, 968–976. [Google Scholar] [CrossRef] [Green Version]
- Meguro, M.; Ito, H.; Takabayashi, A.; Tanaka, R.; Tanaka, A. Identification of the 7-hydroxymethyl chlorophyll a reductase of the chlorophyll cycle in Arabidopsis. Plant Cell 2011, 23, 3442–3453. [Google Scholar] [CrossRef] [Green Version]
- Hofius, D.; Börnke, F.A.J. Chapter 13—Photosynthesis, carbohydrate metabolism and source–sink relations. In Potato Biology and Biotechnology; Vreugdenhil, D., Bradshaw, J., Gebhardt, C., Govers, F., Mackerron, D.K.L., Taylor, M.A., Ross, H.A., Eds.; Elsevier Science B.V.: Amsterdam, The Netherlands, 2007; pp. 257–285. [Google Scholar]
- Osipenkova, O.V.; Odintsova, M.S.; Iurina, N.P. The influence of light, hormonal, and carbohydrate signal systems on ELIP genes expression in gun-mutants Arabidopsis thaliana. Prikl. Biokhimiia i Mikrobiol. 2010, 46, 363–371. [Google Scholar] [CrossRef]
- Giannopolitis, C.N.; Ries, S.K. Superoxide dismutases: I. Occurrence in higher plants. Plant Physiol. 1977, 59, 309–314. [Google Scholar] [CrossRef] [PubMed]
- Aebi, H. Catalase in vitro. Methods Enzymol. 1984, 105, 121–126. [Google Scholar] [PubMed]
- Maehly, A.C.; Chance, B. The assay of catalases and peroxidases. Methods Biochem. Anal. 1954, 1, 357–424. [Google Scholar] [PubMed]
- Wang, L.R.; Hu, W.; Zahoor, R.; Yang, X.N.; Wang, Y.H.; Zhou, Z.G.; Meng, Y.L. Cool temperature caused by late planting affects seed vigor via altering kernel biomass and antioxidant metabolism in cotton (Gossypium hirsutum L.). Field Crop. Res. 2019, 236, 145–154. [Google Scholar] [CrossRef]
- Velikova, V.; Yordanov, I.; Edreva, A. Oxidative stress and some antioxidant systems in acid rain-treated bean plants—Protective role of exogenous polyamines. Plant Sci. 2000, 151, 59–66. [Google Scholar] [CrossRef]
- Hu, W.; Dai, Z.; Yang, J.S.; Snider, J.L.; Wang, S.S.; Meng, Y.L.; Wang, Y.H.; Chen, B.L.; Zhao, W.Q.; Zhou, Z.G. Cultivar sensitivity of cotton seed yield to potassium availability is associated with differences in carbohydrate metabolism in the developing embryo. Field Crop. Res. 2017, 214, 301–309. [Google Scholar] [CrossRef]
- Hu, W.; Yang, J.S.; Meng, Y.L.; Wang, Y.H.; Chen, B.L.; Zhao, W.Q.; Oosterhuis, D.M.; Zhou, Z.G. Potassium application affects carbohydrate metabolism in the leaf subtending the cotton (Gossypium hirsutum L.) boll and its relationship with boll biomass. Field Crop. Res. 2015, 179, 120–131. [Google Scholar] [CrossRef]
- Zahoor, R.; Dong, H.R.; Abid, M.; Zhao, W.Q.; Wang, Y.H.; Zhou, Z.G. Potassium fertilizer improves drought stress alleviation potential in cotton by enhancing photosynthesis and carbohydrate metabolism. Environ. Exp. Bot. 2017, 137, 73–83. [Google Scholar] [CrossRef]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
- Jeong, S.W.; Das, P.K.; Jeoung, S.C.; Song, J.Y.; Lee, H.K.; Kim, Y.K.; Kim, W.J.; Park, Y.I.; Yoo, S.D.; Choi, S.B.; et al. Ethylene suppression of sugar-induced anthocyanin pigmentation in Arabidopsis. Plant Physiol. 2010, 154, 1514–1531. [Google Scholar] [CrossRef] [PubMed] [Green Version]





© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Jin, D.; Wang, X.; Xu, Y.; Gui, H.; Zhang, H.; Dong, Q.; Sikder, R.K.; Yang, G.; Song, M. Chemical Defoliant Promotes Leaf Abscission by Altering ROS Metabolism and Photosynthetic Efficiency in Gossypium hirsutum. Int. J. Mol. Sci. 2020, 21, 2738. https://doi.org/10.3390/ijms21082738
Jin D, Wang X, Xu Y, Gui H, Zhang H, Dong Q, Sikder RK, Yang G, Song M. Chemical Defoliant Promotes Leaf Abscission by Altering ROS Metabolism and Photosynthetic Efficiency in Gossypium hirsutum. International Journal of Molecular Sciences. 2020; 21(8):2738. https://doi.org/10.3390/ijms21082738
Chicago/Turabian StyleJin, Dingsha, Xiangru Wang, Yanchao Xu, Huiping Gui, Hengheng Zhang, Qiang Dong, Ripon Kumar Sikder, Guozheng Yang, and Meizhen Song. 2020. "Chemical Defoliant Promotes Leaf Abscission by Altering ROS Metabolism and Photosynthetic Efficiency in Gossypium hirsutum" International Journal of Molecular Sciences 21, no. 8: 2738. https://doi.org/10.3390/ijms21082738
APA StyleJin, D., Wang, X., Xu, Y., Gui, H., Zhang, H., Dong, Q., Sikder, R. K., Yang, G., & Song, M. (2020). Chemical Defoliant Promotes Leaf Abscission by Altering ROS Metabolism and Photosynthetic Efficiency in Gossypium hirsutum. International Journal of Molecular Sciences, 21(8), 2738. https://doi.org/10.3390/ijms21082738




