Chondrogenic Potential of Pellet Culture Compared to High-Density Culture on a Bacterial Cellulose Hydrogel
Abstract
:1. Introduction
2. Results
2.1. Histology and Visual Grading
2.2. Gene Expression
3. Discussion
4. Materials and Methods
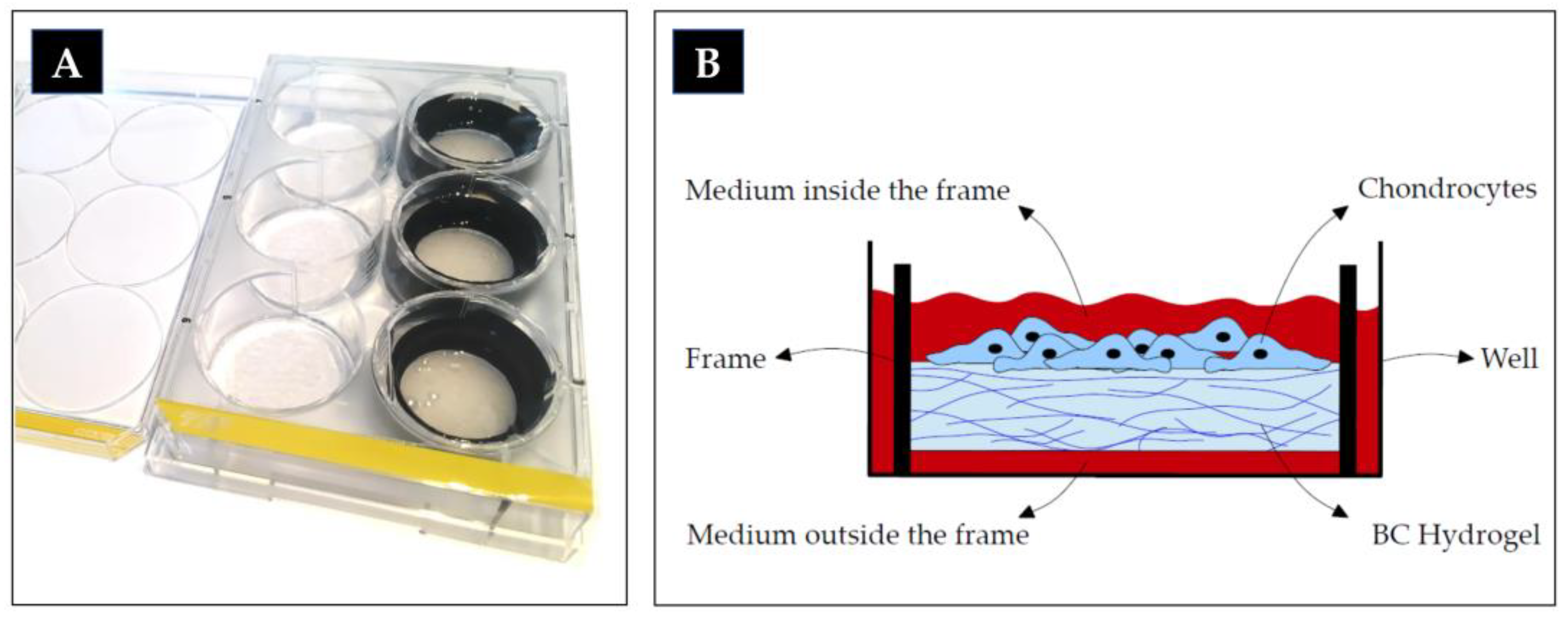
4.1. Bacterial Cellulose (BC) Hydrogel Matrix
4.2. Cell Culture
4.3. Histology
4.4. Visual Histologic Grading System
4.5. RNA Extraction
4.6. Quantitative Real-Time Polymerase Chain Reaction (q-PCR)
4.7. Statistical Analysis
5. Conclusions
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| 3D | three dimensional |
| ACAN | Cartilage-specific proteoglycan core protein |
| BC | bacterial cellulose |
| COL1A1 | Collagen type I alpha 1 chain |
| COL2A1 | Collagen type II alpha 1 chain |
| DMEM | Dulbecco’s modified eagle’s medium |
| ECM | extracellular matrix |
| FCS | fetal calf serum |
| GAPDH | Glycerinaldehyd-3-phosphat-dehydrogenase |
| HD | high density |
| LD | low density |
| MACT | matrix-associated chondrocyte transplantation |
| MMP13 | matrix metalloproteinase 13 |
| non-OA | non-osteoarthritic |
| OA | osteoarthritic |
| p | p-value, probability |
| PBS | Phosphate buffered saline |
| qPCR | Quantitative real-time polymerase chain reaction |
| SOX9 | SRY (sex determining region Y)-box 9 |
References
- Schulze-Tanzil, G.; de Souza, P.; Villegas Castrejon, H.; John, T.; Merker, H.J.; Scheid, A.; Shakibaei, M. Redifferentiation of dedifferentiated human chondrocytes in high-density cultures. Cell Tissue Res. 2002, 308, 371–379. [Google Scholar] [CrossRef]
- Caron, M.M.; Emans, P.J.; Coolsen, M.M.; Voss, L.; Surtel, D.A.; Cremers, A.; van Rhijn, L.W.; Welting, T.J. Redifferentiation of dedifferentiated human articular chondrocytes: Comparison of 2D and 3D cultures. Osteoarthr. Cartil. 2012, 20, 1170–1178. [Google Scholar] [CrossRef] [Green Version]
- Francioli, S.E.; Candrian, C.; Martin, K.; Heberer, M.; Martin, I.; Barbero, A. Effect of three-dimensional expansion and cell seeding density on the cartilage-forming capacity of human articular chondrocytes in type II collagen sponges. J. Biomed. Mater. Res. Part A 2010, 95, 924–931. [Google Scholar] [CrossRef]
- Yang, J.J.; Chen, Y.M.; Liu, J.F.; Kurokawa, T.; Gong, J.P. Spontaneous redifferentiation of dedifferentiated human articular chondrocytes on hydrogel surfaces. Tissue Eng. Part A 2010, 16, 2529–2540. [Google Scholar] [CrossRef] [Green Version]
- Redeker, J.I.; Schmitt, B.; Grigull, N.P.; Braun, C.; Buttner, A.; Jansson, V.; Mayer-Wagner, S. Effect of electromagnetic fields on human osteoarthritic and non-osteoarthritic chondrocytes. BMC Complement. Altern. Med. 2017, 17, 402. [Google Scholar] [CrossRef] [Green Version]
- Zhu, G.; Mayer-Wagner, S.; Schroder, C.; Woiczinski, M.; Blum, H.; Lavagi, I.; Krebs, S.; Redeker, J.I.; Holzer, A.; Jansson, V.; et al. Comparing effects of perfusion and hydrostatic pressure on gene profiles of human chondrocyte. J. Biotechnol. 2015, 210, 59–65. [Google Scholar] [CrossRef]
- Francioli, S.E.; Martin, I.; Sie, C.P.; Hagg, R.; Tommasini, R.; Candrian, C.; Heberer, M.; Barbero, A. Growth factors for clinical-scale expansion of human articular chondrocytes: Relevance for automated bioreactor systems. Tissue Eng. 2007, 13, 1227–1234. [Google Scholar] [CrossRef] [Green Version]
- Rai, M.F.; Rachakonda, P.S.; Manning, K.; Palissa, C.; Sittinger, M.; Ringe, J.; Schmidt, M.F. Molecular and phenotypic modulations of primary and immortalized canine chondrocytes in different culture systems. Res. Vet. Sci. 2009, 87, 399–407. [Google Scholar] [CrossRef] [PubMed]
- Stewart, M.C.; Saunders, K.M.; Burton-Wurster, N.; Macleod, J.N. Phenotypic stability of articular chondrocytes in vitro: The effects of culture models, bone morphogenetic protein 2, and serum supplementation. J. Bone Miner. Res. Off. J. Am. Soc. Bone Miner. Res. 2000, 15, 166–174. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; McCaffery, J.M.; Spencer, R.G.; Francomano, C.A. Hyaline cartilage engineered by chondrocytes in pellet culture: Histological, immunohistochemical and ultrastructural analysis in comparison with cartilage explants. J. Anat. 2004, 205, 229–237. [Google Scholar] [CrossRef] [PubMed]
- Sophia Fox, A.J.; Bedi, A.; Rodeo, S.A. The basic science of articular cartilage: Structure, composition, and function. Sports Health 2009, 1, 461–468. [Google Scholar] [CrossRef] [PubMed]
- Roman, M.; Haring, A.P.; Bertucio, T.J. The growing merits and dwindling limitations of bacterial cellulose-based tissue engineering scaffolds. Curr. Opin. Chem. Eng. 2019, 24, 98–106. [Google Scholar] [CrossRef]
- Barbero, A.; Grogan, S.P.; Mainil-Varlet, P.; Martin, I. Expansion on specific substrates regulates the phenotype and differentiation capacity of human articular chondrocytes. J. Cell. Biochem. 2006, 98, 1140–1149. [Google Scholar] [CrossRef] [PubMed]
- Yadav, V.; Paniliatis, B.J.; Shi, H.; Lee, K.; Cebe, P.; Kaplan, D.L. Novel in vivo-degradable cellulose-chitin copolymer from metabolically engineered Gluconacetobacter xylinus. Appl. Environ. Microbiol. 2010, 76, 6257–6265. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- An, S.J.; Lee, S.H.; Huh, J.B.; Jeong, S.I.; Park, J.S.; Gwon, H.J.; Kang, E.S.; Jeong, C.M.; Lim, Y.M. Preparation and Characterization of Resorbable Bacterial Cellulose Membranes Treated by Electron Beam Irradiation for Guided Bone Regeneration. Int. J. Mol. Sci. 2017, 18, 8426. [Google Scholar] [CrossRef] [PubMed]
- Feil, G.; Horres, R.; Schulte, J.; Mack, A.F.; Petzoldt, S.; Arnold, C.; Meng, C.; Jost, L.; Boxleitner, J.; Kiessling-Wolf, N.; et al. Bacterial cellulose shifts transcriptome and proteome of cultured endothelial cells towards native differentiation. Mol. Cell. Proteom. Mcp 2017, 16, 1563–1577. [Google Scholar] [CrossRef] [Green Version]
- Basu, P.; Saha, N.; Alexandrova, R.; Andonova-Lilova, B.; Georgieva, M.; Miloshev, G.; Saha, P. Biocompatibility and biological efficiency of inorganic calcium filled bacterial cellulose based hydrogel scaffolds for bone bioengineering. Int. J. Mol. Sci. 2018, 19, 3980. [Google Scholar] [CrossRef] [Green Version]
- Martinez Avila, H.; Feldmann, E.M.; Pleumeekers, M.M.; Nimeskern, L.; Kuo, W.; de Jong, W.C.; Schwarz, S.; Muller, R.; Hendriks, J.; Rotter, N.; et al. Novel bilayer bacterial nanocellulose scaffold supports neocartilage formation in vitro and in vivo. Biomaterials 2015, 44, 122–133. [Google Scholar] [CrossRef] [Green Version]
- Ahrem, H.; Pretzel, D.; Endres, M.; Conrad, D.; Courseau, J.; Muller, H.; Jaeger, R.; Kaps, C.; Klemm, D.O.; Kinne, R.W. Laser-structured bacterial nanocellulose hydrogels support ingrowth and differentiation of chondrocytes and show potential as cartilage implants. Acta Biomater. 2014, 10, 1341–1353. [Google Scholar] [CrossRef]
- Svensson, A.; Nicklasson, E.; Harrah, T.; Panilaitis, B.; Kaplan, D.L.; Brittberg, M.; Gatenholm, P. Bacterial cellulose as a potential scaffold for tissue engineering of cartilage. Biomaterials 2005, 26, 419–431. [Google Scholar] [CrossRef]
- Andersson, J.; Stenhamre, H.; Backdahl, H.; Gatenholm, P. Behavior of human chondrocytes in engineered porous bacterial cellulose scaffolds. J. Biomed. Mater. Res. Part A 2010, 94, 1124–1132. [Google Scholar] [CrossRef] [PubMed]
- Cigan, A.D.; Roach, B.L.; Nims, R.J.; Tan, A.R.; Albro, M.B.; Stoker, A.M.; Cook, J.L.; Vunjak-Novakovic, G.; Hung, C.T.; Ateshian, G.A. High seeding density of human chondrocytes in agarose produces tissue-engineered cartilage approaching native mechanical and biochemical properties. J. Biomech. 2016, 49, 1909–1917. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayer-Wagner, S.; Schiergens, T.S.; Sievers, B.; Redeker, J.I.; Schmitt, B.; Buettner, A.; Jansson, V.; Muller, P.E. Scaffold-free 3D cellulose acetate membrane-based cultures form large cartilaginous constructs. J. Tissue Eng. Regen. Med. 2011, 5, 151–155. [Google Scholar] [CrossRef] [PubMed]
- Naumann, A.; Dennis, J.E.; Aigner, J.; Coticchia, J.; Arnold, J.; Berghaus, A.; Kastenbauer, E.R.; Caplan, A.I. Tissue engineering of autologous cartilage grafts in three-dimensional in vitro macroaggregate culture system. Tissue Eng. 2004, 10, 1695–1706. [Google Scholar] [CrossRef]
- Parreno, J.; Bianchi, V.J.; Sermer, C.; Regmi, S.C.; Backstein, D.; Schmidt, T.A.; Kandel, R.A. Adherent agarose mold cultures: An in vitro platform for multi-factorial assessment of passaged chondrocyte redifferentiation. J. Orthop. Res. Off. Publ. Orthop. Res. Soc. 2018, 36, 2392–2405. [Google Scholar] [CrossRef] [Green Version]
- Foldager, C.B. Advances in autologous chondrocyte implantation and related techniques for cartilage repair. Dan. Med J. 2013, 60, B4600. [Google Scholar]
- Grogan, S.P.; Barbero, A.; Winkelmann, V.; Rieser, F.; Fitzsimmons, J.S.; O’Driscoll, S.; Martin, I.; Mainil-Varlet, P. Visual histological grading system for the evaluation of in vitro-generated neocartilage. Tissue Eng. 2006, 12, 2141–2149. [Google Scholar] [CrossRef] [Green Version]
- Albrecht, C.; Tichy, B.; Nurnberger, S.; Hosiner, S.; Zak, L.; Aldrian, S.; Marlovits, S. Gene expression and cell differentiation in matrix-associated chondrocyte transplantation grafts: A comparative study. Osteoarthr. Cartil. 2011, 19, 1219–1227. [Google Scholar] [CrossRef] [Green Version]
- Daley, W.P.; Peters, S.B.; Larsen, M. Extracellular matrix dynamics in development and regenerative medicine. J. Cell Sci. 2008, 121, 255–264. [Google Scholar] [CrossRef] [Green Version]
- Yadav, V.; Sun, L.; Panilaitis, B.; Kaplan, D.L. In vitro chondrogenesis with lysozyme susceptible bacterial cellulose as a scaffold. J. Tissue Eng. Regen. Med. 2015, 9, E276–E288. [Google Scholar] [CrossRef]
- Dehne, T.; Karlsson, C.; Ringe, J.; Sittinger, M.; Lindahl, A. Chondrogenic differentiation potential of osteoarthritic chondrocytes and their possible use in matrix-associated autologous chondrocyte transplantation. Arthritis Res. Ther. 2009, 11, R133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jeyakumar, V.; Halbwirth, F.; Niculescu-Morzsa, E.; Bauer, C.; Zwickl, H.; Kern, D.; Nehrer, S. Chondrogenic gene expression differences between chondrocytes from osteoarthritic and non-oa trauma joints in a 3D collagen type I hydrogel. Cartilage 2017, 8, 191–198. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neri, S.; Mariani, E.; Cattini, L.; Facchini, A. Long-term in vitro expansion of osteoarthritic human articular chondrocytes do not alter genetic stability: A microsatellite instability analysis. J. Cell. Physiol. 2011, 226, 2579–2585. [Google Scholar] [CrossRef] [PubMed]
- Dunda, S.E.; Ranker, M.; Pallua, N.; Machens, H.G.; Ravichandran, A.; Schantz, J.T. In vitro and in vivo biocompatibility of a novel, 3-dimensional cellulose matrix structure. Handchir. Mikrochir. Plast. Chir. Organ Dtsch. Arb. Handchir. Organ Dtsch. Arb. Mikrochir. Peripher. Nerven Gefasse 2015, 47, 378–383. [Google Scholar] [CrossRef] [Green Version]
- Vandesompele, J.; De Preter, K.; Pattyn, F.; Poppe, B.; Van Roy, N.; De Paepe, A.; Speleman, F. Accurate normalization of real-time quantitative RT-PCR data by geometric averaging of multiple internal control genes. Genome Biol. 2002, 3, Research0034. [Google Scholar] [CrossRef] [Green Version]
- Varas, L.; Ohlsson, L.B.; Honeth, G.; Olsson, A.; Bengtsson, T.; Wiberg, C.; Bockermann, R.; Jarnum, S.; Richter, J.; Pennington, D.; et al. Alpha10 integrin expression is up-regulated on fibroblast growth factor-2-treated mesenchymal stem cells with improved chondrogenic differentiation potential. Stem Cells Dev. 2007, 16, 965–978. [Google Scholar] [CrossRef]
- Pauly, S.; Klatte, F.; Strobel, C.; Schmidmaier, G.; Greiner, S.; Scheibel, M.; Wildemann, B. Characterization of tendon cell cultures of the human rotator cuff. Eur. Cells Mater. 2010, 20, 84–97. [Google Scholar] [CrossRef]
- RTPrimerDB. RTPrimerDB ID 309. Available online: http://www.rtprimerdb.org/assay_report.php?assay_id=309 (accessed on 14 August 2019).
- Hong, H.; Park, Y.K.; Choi, M.S.; Ryu, N.H.; Song, D.K.; Suh, S.I.; Nam, K.Y.; Park, G.Y.; Jang, B.C. Differential down-regulation of COX-2 and MMP-13 in human skin fibroblasts by glucosamine-hydrochloride. J. Dermatol. Sci. 2009, 56, 43–50. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2(-Delta Delta C(T)) Method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]

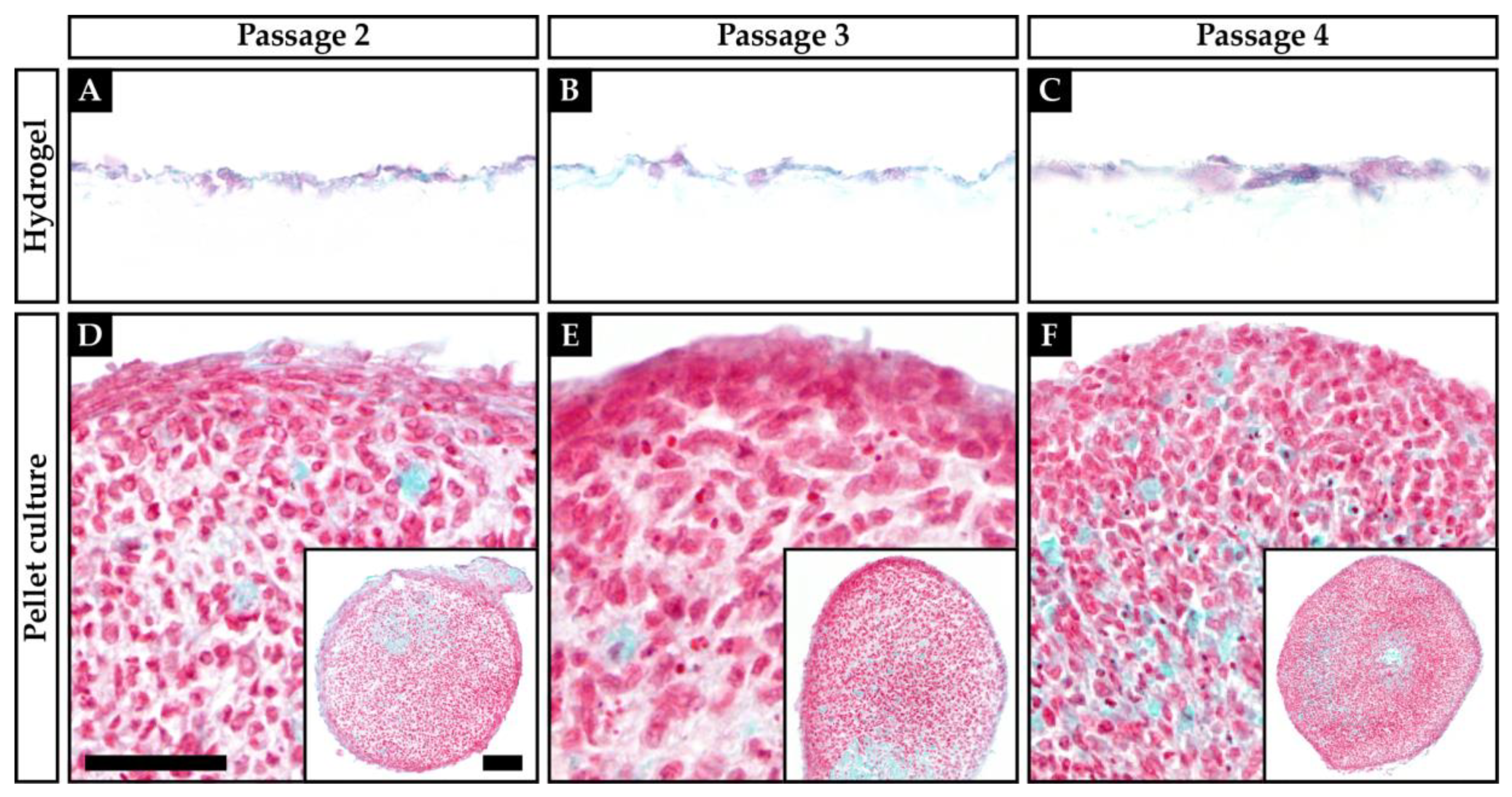
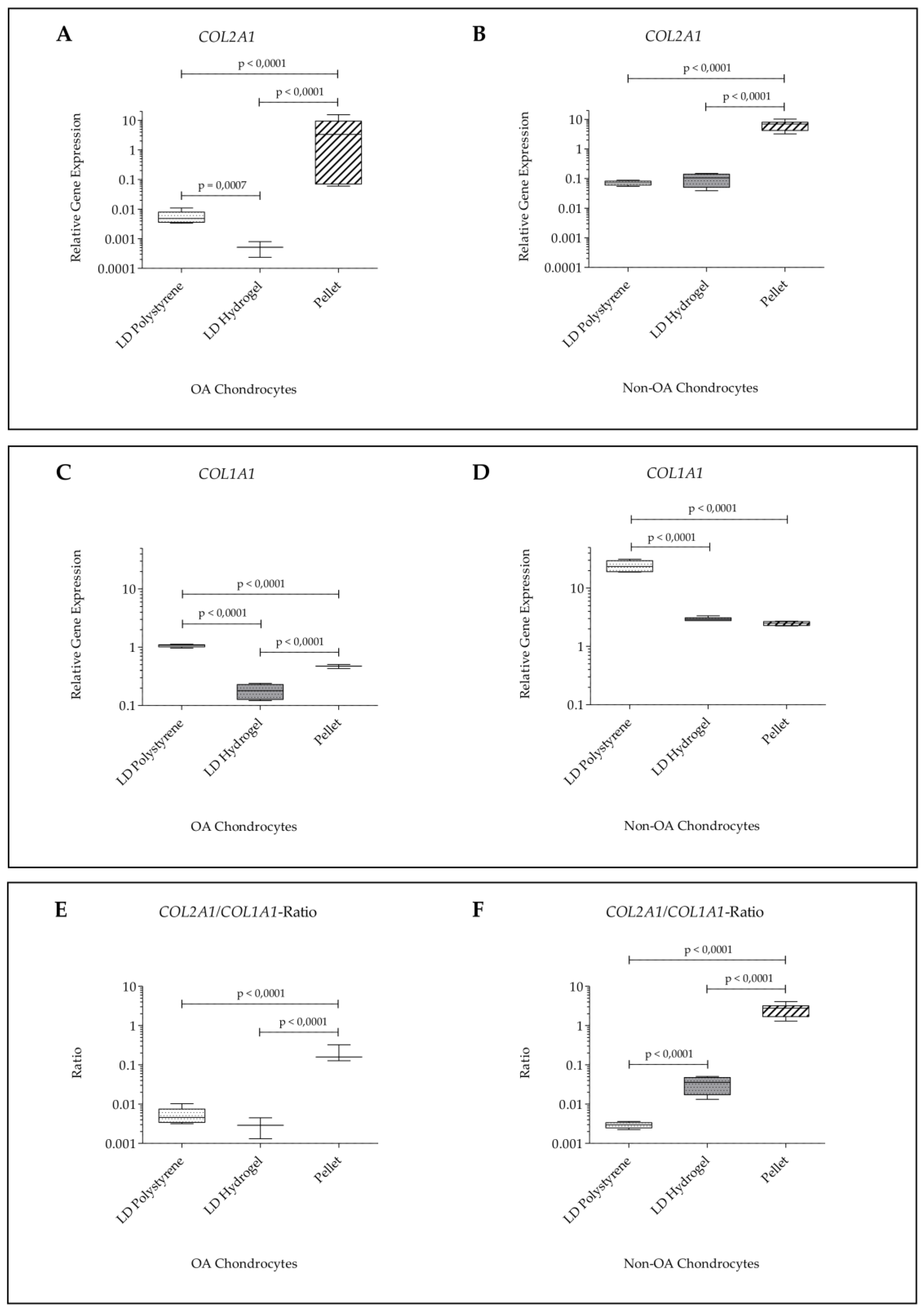

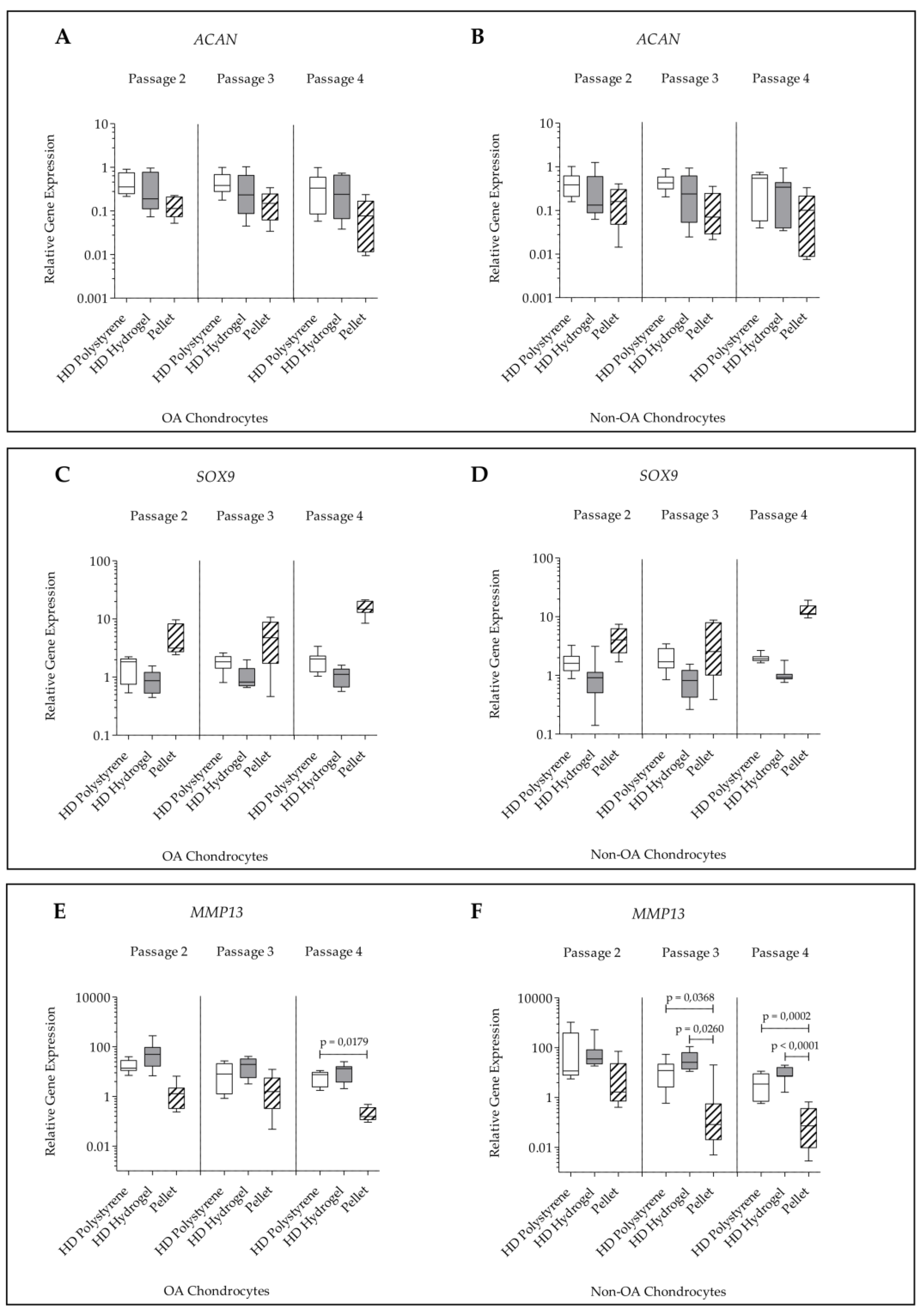
| Score | Culture | Passage | A | B | C | Sum |
|---|---|---|---|---|---|---|
| OA | Hydrogel | 2 | 1 | 2 | 2 | 5 |
| 3 | 1 | 2 | 2 | 5 | ||
| 4 | 1 | 2 | 2 | 5 | ||
| Pellet | 2 | 2 | 3 | 3 | 8 | |
| 3 | 2 | 2 | 3 | 7 | ||
| 4 | 2 | 3 | 3 | 8 | ||
| Non-OA | Hydrogel | 2 | 1 | 2 | 1 | 4 |
| 3 | 1 | 2 | 2 | 5 | ||
| 4 | 1 | 2 | 2 | 5 | ||
| Pellet | 2 | 2 | 3 | 3 | 8 | |
| 3 | 2 | 3 | 3 | 8 | ||
| 4 | 2 | 3 | 3 | 8 |
| Gene | Primer Sequence | Reference | |
|---|---|---|---|
| GAPDH | forward | TGCACCACCAACTGCTTAGC | [35] |
| reverse | GGCATGGACTGTGGTCATGAG | ||
| COL2A1 | forward | GTTATCGAGTACCGGTCACAGAAG | [36] |
| reverse | AGTACTTGGGTCCTTTGGGTTTG | ||
| ACAN | forward | CAGCACCAGCATCCCAGA | [36] |
| reverse | CAGCAGTTGATTCTGATTCACG | ||
| COL1A1 | forward | TGACCTCAAGATGTGCCACT | [37] |
| reverse | ACCAGACATGCCTCTTGTCC | ||
| SOX9 | forward | AGACCTTTGGGCTGCCTTAT | [38] |
| reverse | TAGCCTCCCTCACTCCAAGA | ||
| MMP13 | forward | GACTTCACGATGGCATTGCTG | [39] |
| reverse | GCATCAACCTGCTGAGGATGC | ||
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Grigull, N.P.; Redeker, J.I.; Schmitt, B.; Saller, M.M.; Schönitzer, V.; Mayer-Wagner, S. Chondrogenic Potential of Pellet Culture Compared to High-Density Culture on a Bacterial Cellulose Hydrogel. Int. J. Mol. Sci. 2020, 21, 2785. https://doi.org/10.3390/ijms21082785
Grigull NP, Redeker JI, Schmitt B, Saller MM, Schönitzer V, Mayer-Wagner S. Chondrogenic Potential of Pellet Culture Compared to High-Density Culture on a Bacterial Cellulose Hydrogel. International Journal of Molecular Sciences. 2020; 21(8):2785. https://doi.org/10.3390/ijms21082785
Chicago/Turabian StyleGrigull, Nele Pascale, Julia Isabelle Redeker, Bärbel Schmitt, Maximilian Michael Saller, Veronika Schönitzer, and Susanne Mayer-Wagner. 2020. "Chondrogenic Potential of Pellet Culture Compared to High-Density Culture on a Bacterial Cellulose Hydrogel" International Journal of Molecular Sciences 21, no. 8: 2785. https://doi.org/10.3390/ijms21082785
APA StyleGrigull, N. P., Redeker, J. I., Schmitt, B., Saller, M. M., Schönitzer, V., & Mayer-Wagner, S. (2020). Chondrogenic Potential of Pellet Culture Compared to High-Density Culture on a Bacterial Cellulose Hydrogel. International Journal of Molecular Sciences, 21(8), 2785. https://doi.org/10.3390/ijms21082785







