The Swing of Lipids at Peroxisomes and Endolysosomes in T Cell Activation
Abstract
1. Introduction
2. Endolysosomal System and Multivesicular Bodies
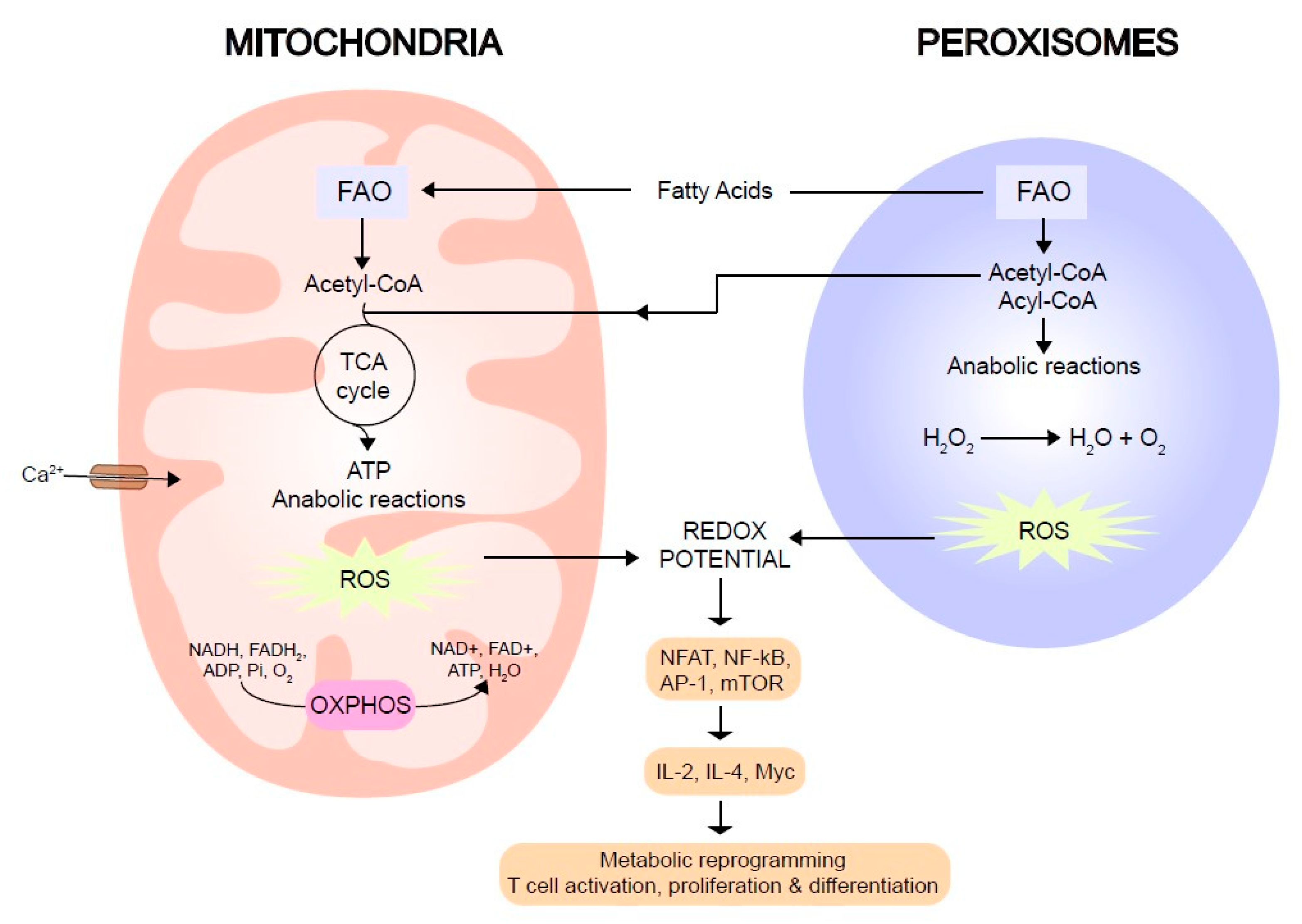
3. Mitochondria and Peroxisomes: Modulators of the Immune Synapse
4. Reactive Oxygen Species as Modulators of T Cell Activation
5. Lipids as a Source of Energy in Metabolic Reprogramming after T Cell Activation
6. Lipid second Messengers at the Immune Synapse (IS)
7. Bioactive Sphingolipids and T Cell Activation
8. Concluding Remarks
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
References
- Monks, C.R.; Freiberg, B.A.; Kupfer, H.; Sciaky, N.; Kupfer, A. Three-dimensional segregation of supramolecular activation clusters in T cells. Nature 1998, 395, 82–86. [Google Scholar] [CrossRef] [PubMed]
- Fooksman, D.R.; Vardhana, S.; Vasiliver-Shamis, G.; Liese, J.; Blair, D.A.; Waite, J.; Sacristan, C.; Victoria, G.D.; Zanin-Zhorov, A.; Dustin, M.L. Functional anatomy of T cell activation and synapse formation. Annu. Rev. Immunol. 2010, 28, 79–105. [Google Scholar] [CrossRef] [PubMed]
- Dieckmann, N.M.; Frazer, G.L.; Asano, Y.; Stinchcombe, J.C.; Griffiths, G.M. The cytotoxic T lymphocyte immune synapse at a glance. J. Cell Sci. 2016, 129, 2881–2886. [Google Scholar] [CrossRef] [PubMed]
- Wülfing, C.; Davis, M.M. A receptor/cytoskeletal movement triggered by costimulation during T cell activation. Science 1998, 282, 2266–2269. [Google Scholar] [CrossRef]
- Grakoui, A.; Bromley, S.K.; Sumen, C.; Davis, M.M.; Shaw, A.S.; Allen, P.M.; Dustin, M.L. The immunological synapse: A molecular machine controlling T cell activation. Science 1999, 285, 221–227. [Google Scholar] [CrossRef]
- Gomez, T.S.; Billadeau, D.D. T cell activation and the cytoskeleton: You can’t have one without the other. Adv. Immunol. 2008, 97, 1–64. [Google Scholar] [CrossRef]
- Ilani, T.; Vasiliver-Shamis, G.; Vardhana, S.; Bretscher, A.; Dustin, M.L. T cell antigen receptor signaling and immunological synapse stability require myosin IIA. Nat. Immunol. 2009, 10, 531–539. [Google Scholar] [CrossRef]
- Martin-Cofreces, N.B.; Baixauli, F.; Sanchez-Madrid, F. Immune synapse: Conductor of orchestrated organelle movement. Trends Cell Biol. 2014, 24, 61–72. [Google Scholar] [CrossRef]
- Vicente-Manzanares, M.; Sánchez-Madrid, F. Role of the cytoskeleton during leukocyte responses. Nat. Rev. Immunol. 2004, 4, 110–122. [Google Scholar] [CrossRef]
- Kupfer, A.; Swain, S.L.; Janeway, C.A.; Singer, S.J. The specific direct interaction of helper T cells and antigen-presenting B cells. Proc. Natl. Acad. Sci. USA 1986, 83, 6080–6083. [Google Scholar] [CrossRef]
- Varma, R.; Campi, G.; Yokosuka, T.; Saito, T.; Dustin, M.L. T cell receptor-proximal signals are sustained in peripheral microclusters and terminated in the central supramolecular activation cluster. Immunity 2006, 25, 117–127. [Google Scholar] [CrossRef]
- Martin-Cofreces, N.B.; Robles-Valero, J.; Cabrero, J.R.; Mittelbrunn, M.; Gordon-Alonso, M.; Sung, C.H.; Alarcon, B.; Vazquez, J.; Sanchez-Madrid, F. MTOC translocation modulates IS formation and controls sustained T cell signaling. J. Cell Biol. 2008, 182, 951–962. [Google Scholar] [CrossRef] [PubMed]
- Onnis, A.; Finetti, F.; Baldari, C.T. Vesicular Trafficking to the Immune Synapse: How to Assemble Receptor-Tailored Pathways from a Basic Building Set. Front. Immunol. 2016, 7, 50. [Google Scholar] [CrossRef] [PubMed]
- Quintana, A.; Schwindling, C.; Wenning, A.S.; Becherer, U.; Rettig, J.; Schwarz, E.C.; Hoth, M. T cell activation requires mitochondrial translocation to the immunological synapse. Proc. Natl. Acad. Sci. USA 2007, 104, 14418–14423. [Google Scholar] [CrossRef] [PubMed]
- Bromley, S.K.; Burack, W.R.; Johnson, K.G.; Somersalo, K.; Sims, T.N.; Sumen, C.; Davis, M.M.; Shaw, A.S.; Allen, P.M.; Dustin, M.L. The immunological synapse. Annu. Rev. Immunol. 2001, 19, 375–396. [Google Scholar] [CrossRef]
- Martin-Cofreces, N.B.; Vicente-Manzanares, M.; Sanchez-Madrid, F. Adhesive Interactions Delineate the Topography of the Immune Synapse. Front. Cell Dev. Biol. 2018, 6, 149. [Google Scholar] [CrossRef]
- Martin-Cofreces, N.B.; Sanchez-Madrid, F. Sailing to and Docking at the Immune Synapse: Role of Tubulin Dynamics and Molecular Motors. Front. Immunol. 2018, 9, 1174. [Google Scholar] [CrossRef]
- Purbhoo, M.A.; Liu, H.; Oddos, S.; Owen, D.M.; Neil, M.A.; Pageon, S.V.; French, P.M.; Rudd, C.E.; Davis, D.M. Dynamics of subsynaptic vesicles and surface microclusters at the immunological synapse. Sci. Signal. 2010, 3, ra36. [Google Scholar] [CrossRef]
- Martin-Cofreces, N.B.; Baixauli, F.; Lopez, M.J.; Gil, D.; Monjas, A.; Alarcon, B.; Sanchez-Madrid, F. End-binding protein 1 controls signal propagation from the T cell receptor. EMBO J. 2012, 31, 4140–4152. [Google Scholar] [CrossRef]
- Soares, H.; Henriques, R.; Sachse, M.; Ventimiglia, L.; Alonso, M.A.; Zimmer, C.; Thoulouze, M.I.; Alcover, A. Regulated vesicle fusion generates signaling nanoterritories that control T cell activation at the immunological synapse. J. Exp. Med. 2013, 210, 2415–2433. [Google Scholar] [CrossRef]
- Kienzle, C.; von Blume, J. Secretory cargo sorting at the trans-Golgi network. Trends Cell Biol. 2014, 24, 584–593. [Google Scholar] [CrossRef]
- Goldenring, J.R. Recycling endosomes. Curr. Opin. Cell Biol. 2015, 35, 117–122. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Sanchez-Madrid, F. Intercellular communication: Diverse structures for exchange of genetic information. Nat. Rev. Mol. Cell Biol. 2012, 13, 328–335. [Google Scholar] [CrossRef] [PubMed]
- Villarroya-Beltri, C.; Baixauli, F.; Gutierrez-Vazquez, C.; Sanchez-Madrid, F.; Mittelbrunn, M. Sorting it out: Regulation of exosome loading. Semin. Cancer Biol. 2014, 28, 3–13. [Google Scholar] [CrossRef] [PubMed]
- Torralba, D.; Baixauli, F.; Villarroya-Beltri, C.; Fernández-Delgado, I.; Latorre-Pellicer, A.; Acín-Pérez, R.; Martín-Cófreces, N.B.; Jaso-Tamame, Á.L.; Iborra, S.; Jorge, I.; et al. Priming of dendritic cells by DNA-containing extracellular vesicles from activated T cells through antigen-driven contacts. Nat. Commun. 2018. [Google Scholar] [CrossRef] [PubMed]
- Yáñez-Mó, M.; Barreiro, O.; Gordon-Alonso, M.; Sala-Valdés, M.; Sánchez-Madrid, F. Tetraspanin-enriched microdomains: A functional unit in cell plasma membranes. Trends Cell Biol. 2009, 19, 434–446. [Google Scholar] [CrossRef] [PubMed]
- Wildenberg, M.E.; Vos, A.C.; Wolfkamp, S.C.; Duijvestein, M.; Verhaar, A.P.; Te Velde, A.A.; van der Brink, G.R.; Hommes, D.W. Autophagy attenuates the adaptive immune response by destabilizing the immunologic synapse. Gastroenterology 2012, 142, 1493–1503. [Google Scholar] [CrossRef]
- Finetti, F.; Cassioli, C.; Cianfanelli, V.; Onnis, A.; Paccagnini, E.; Kabanova, A.; Baldari, C.T. The intraflagellar transport protein IFT20 controls lysosome biogenesis by regulating the post-Golgi transport of acid hydrolases. Cell Death Differ. 2020, 27, 310–328. [Google Scholar] [CrossRef]
- Finetti, F.; Capitani, N.; Baldari, C.T. Emerging Roles of the Intraflagellar Transport System in the Orchestration of Cellular Degradation Pathways. Front. Cell Dev. Biol. 2019, 7, 292. [Google Scholar] [CrossRef]
- Parkinson-Lawrence, E.J.; Shandala, T.; Prodoehl, M.; Plew, R.; Borlace, G.N.; Brooks, D.A. Lysosomal storage disease: Revealing lysosomal function and physiology. Physiol. (Bethesda) 2010, 25, 102–115. [Google Scholar] [CrossRef]
- Huber, L.A.; Teis, D. Lysosomal signaling in control of degradation pathways. Curr. Opin. Cell Biol. 2016, 39, 8–14. [Google Scholar] [CrossRef] [PubMed]
- Calabia-Linares, C.; Robles-Valero, J.; de la Fuente, H.; Perez-Martinez, M.; Martin-Cofreces, N.; Alfonso-Perez, M.; Gutierrez-Vazquez, C.; Mittelbrunn, M.; Ibiza, S.; Veiga, E.; et al. Endosomal clathrin drives actin accumulation at the immunological synapse. J. Cell Sci. 2011, 124, 820–830. [Google Scholar] [CrossRef] [PubMed]
- Pullan, L.; Mullapudi, S.; Huang, Z.; Baldwin, P.R.; Chin, C.; Sun, W.; Tsujimoto, S.; Kolodziej, S.J.; Stoops, J.K.; Lee, J.C.; et al. The endosome-associated protein Hrs is hexameric and controls cargo sorting as a master molecule. Structure 2006, 14, 661–671. [Google Scholar] [CrossRef] [PubMed]
- Bissig, C.; Gruenberg, J. Lipid sorting and multivesicular endosome biogenesis. Cold Spring Harb. Perspect. Biol. 2013, 5, a016816. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Boussac, M.; Veron, P.; Ricciardi-Castagnoli, P.; Raposo, G.; Garin, J.; Amigorena, S. Proteomic analysis of dendritic cell-derived exosomes: A secreted subcellular compartment distinct from apoptotic vesicles. J. Immunol. 2001, 166, 7309–7318. [Google Scholar] [CrossRef] [PubMed]
- Escola, J.M.; Kleijmeer, M.J.; Stoorvogel, W.; Griffith, J.M.; Yoshie, O.; Geuze, H.J. Selective enrichment of tetraspan proteins on the internal vesicles of multivesicular endosomes and on exosomes secreted by human B-lymphocytes. J. Biol. Chem. 1998, 273, 20121–20127. [Google Scholar] [CrossRef]
- Kalra, H.; Simpson, R.J.; Ji, H.; Aikawa, E.; Altevogt, P.; Askenase, P.; Bond, V.C.; Borras, F.E.; Breakefield, X.; Budnik, V.; et al. Vesiclepedia: A compendium for extracellular vesicles with continuous community annotation. PLoS Biol. 2012, 10, e1001450. [Google Scholar] [CrossRef]
- Skotland, T.; Sandvig, K.; Llorente, A. Lipids in exosomes: Current knowledge and the way forward. Prog. Lipid Res. 2017, 66, 30–41. [Google Scholar] [CrossRef]
- Mittelbrunn, M.; Gutierrez-Vazquez, C.; Villarroya-Beltri, C.; Gonzalez, S.; Sanchez-Cabo, F.; Gonzalez, M.A.; Bernard, A.; Sanchez-Madrid, F. Unidirectional transfer of microRNA-loaded exosomes from T cells to antigen-presenting cells. Nat. Commun. 2011, 2, 282. [Google Scholar] [CrossRef]
- Bobrie, A.; Colombo, M.; Raposo, G.; Thery, C. Exosome secretion: Molecular mechanisms and roles in immune responses. Traffic 2011, 12, 1659–1668. [Google Scholar] [CrossRef]
- Stoorvogel, W.; Kleijmeer, M.J.; Geuze, H.J.; Raposo, G. The biogenesis and functions of exosomes. Traffic 2002, 3, 321–330. [Google Scholar] [CrossRef] [PubMed]
- Thery, C.; Zitvogel, L.; Amigorena, S. Exosomes: Composition, biogenesis and function. Nat. Rev. Immunol. 2002, 2, 569–579. [Google Scholar] [CrossRef] [PubMed]
- Gould, S.J.; Booth, A.M.; Hildreth, J.E. The Trojan exosome hypothesis. Proc. Natl. Acad. Sci. USA 2003, 100, 10592–10597. [Google Scholar] [CrossRef]
- Bache, K.G.; Brech, A.; Mehlum, A.; Stenmark, H. Hrs regulates multivesicular body formation via ESCRT recruitment to endosomes. J. Cell Biol. 2003, 162, 435–442. [Google Scholar] [CrossRef] [PubMed]
- Hurley, J.H. ESCRT complexes and the biogenesis of multivesicular bodies. Curr. Opin. Cell Biol. 2008, 20, 4–11. [Google Scholar] [CrossRef]
- Muralidharan-Chari, V.; Clancy, J.; Plou, C.; Romao, M.; Chavrier, P.; Raposo, G.; D’Souza-Schorey, C. ARF6-regulated shedding of tumor cell-derived plasma membrane microvesicles. Curr. Biol. 2009, 19, 1875–1885. [Google Scholar] [CrossRef] [PubMed]
- Colombo, M.; Molita, C.; van Niel, G.; Kowal, J.; Vigneron, J.; Benaroch, P.; Manel, N.; Molita, L.F.; Thery, C.; Raposo, G. Analysis of ESCRT functions in exosome biogenesis, composition and secretion highlights the heterogeneity of extracellular vesicles. J. Cell Sci. 2013, 126, 5553–5565. [Google Scholar] [CrossRef]
- Joshi, R.P.; Koretzky, G.A. Diacylglycerol kinases: Regulated controllers of T cell activation, function, and development. Int. J. Mol. Sci. 2013, 14, 6649–6673. [Google Scholar] [CrossRef]
- Alonso, R.; Mazzeo, C.; Rodriguez, M.C. Diacylglycerol kinase alpha regulates the formation and polarisation of mature multivesicular bodies involved in the secretion of Fas ligand-containing exosomes in T lymphocytes. Cell Death Differ. 2011, 18, 1161–1173. [Google Scholar] [CrossRef]
- Bantug, R.; Galluzzi, L.; Kroemer, G.; Hess, C. The spectrum of T cell metabolism in health and disease. Nat. Rev. Immunol. 2018, 18, 19–34. [Google Scholar] [CrossRef]
- Desdín-Micó, G.; Soto-Heredero, G.; Mittelbrunn, M. Mitochondrial activity in T cells. Mitochondrion 2018, 41, 51–57. [Google Scholar] [CrossRef] [PubMed]
- Baixauli, F.; Martin-Cofreces, N.B.; Morlino, G.; Carrasco, Y.R.; Calabia-Linares, C.; Veiga, E.; Serrador, J.M.; Sanchez -Madrid, F. The mitochondrial fission factor dynamin-related protein 1 modulates T-cell receptor signalling at the immune synapse. EMBO J. 2011, 30, 1238–1250. [Google Scholar] [CrossRef] [PubMed]
- Quintana, A.; Kummerow, C.; Junker, C.; Becherer, U.; Hoth, M. Morphological changes of T cells following formation of the immunological synapse modulate intracellular calcium signals. Cell Calcium 2009, 45, 109–122. [Google Scholar] [CrossRef] [PubMed]
- Wang, R.; Dillon, C.P.; Shi, L.Z.; Milasta, S.; Carter, R.; Finkelstein, D.; McCormick, L.L.; Flitzgerald, P.; Chi, H.; Munger, J.; et al. The transcription factor Myc controls metabolic reprogramming upon T lymphocyte activation. Immunity 2011, 35, 871–882. [Google Scholar] [CrossRef] [PubMed]
- Kaminski, M.M.; Sauer, S.W.; Klemke, C.D.; Suss, D.; Okun, J.G.; Krammer, P.H.; Gulow, K. Mitochondrial reactive oxygen species control T cell activation by regulating IL-2 and IL-4 expression: Mechanism of ciprofloxacin-mediated immunosuppression. J. Immunol. 2010, 184, 4827–4841. [Google Scholar] [CrossRef]
- Sena, L.A.; Li, S.; Jairaman, A.; Prakriya, M.; Ezponda, T.; Hildeman, D.A.; Wang, C.R.; Schumacker, P.T.; Licht, J.D.; Perlman, H.; et al. Mitochondria are required for antigen-specific T cell activation through reactive oxygen species signaling. Immunity 2013, 38, 225–236. [Google Scholar] [CrossRef]
- Fransen, M.; Lismont, C.; Walton, P. The Peroxisome-Mitochondria Connection: How and Why? Int. J. Mol. Sci. 2017, 18, 1126. [Google Scholar] [CrossRef]
- Lazarow, P.B. Peroxisome biogenesis: Advances and conundrums. Curr. Opin. Cell Biol. 2003, 15, 489–497. [Google Scholar] [CrossRef]
- Tabak, F.; Braakman, I.; van der Zand, A. Peroxisome formation and maintenance are dependent on the endoplasmic reticulum. Annu. Rev. Biochem. 2013, 82, 723–744. [Google Scholar] [CrossRef]
- Sakai, Y.; Oku, M.; van der Klei, I.J.; Kiel, J.A. Pexophagy: Autophagic degradation of peroxisomes. Biochim. Biophys. Acta 2006, 1763, 1767–1775. [Google Scholar] [CrossRef]
- Robinson, A.; Waddington, K.E.; Pineda-Torra, I.; Jury, E.C. Transcriptional Regulation of T-Cell Lipid Metabolism: Implications for Plasma Membrane Lipid Rafts and T-Cell Function. Front. Immunol. 2017, 8, 1636. [Google Scholar] [CrossRef] [PubMed]
- Mothe-Satney, I.; Murdaca, J.; Sibille, B.; Rousseau, A.S.; Squillance, R.; Le Menn, G.; Rekima, A.; Larbret, F.; Pele, J.; Verhasselt, V.; et al. A role for Peroxisome Proliferator-Activated Receptor Beta in T cell development. Sci. Rep. 2016, 6, 34317. [Google Scholar] [CrossRef] [PubMed]
- Lismont, C.; Nordgren, M.; van Veldhoven, P.P.; Fransen, M. Redox interplay between mitochondria and peroxisomes. Front. Cell Dev. Biol. 2015, 3, 35. [Google Scholar] [CrossRef] [PubMed]
- Wanders, R.J.; Waterham, H.R.; Ferdinandusse, S. Metabolic Interplay between Peroxisomes and Other Subcellular Organelles Including Mitochondria and the Endoplasmic Reticulum. Front. Cell Dev. Biol. 2015, 3, 83. [Google Scholar] [CrossRef] [PubMed]
- Ganguli, G.; Mukherjee, U.; Sonawane, A. Peroxisomes and Oxidative Stress: Their Implications in the Modulation of Cellular Immunity During Mycobacterial Infection. Front. Microbiol. 2019, 10, 1121. [Google Scholar] [CrossRef]
- Panieri, E.; Santoro, M.M. ROS homeostasis and metabolism: A dangerous liason in cancer cells. Cell Death Dis. 2016, 7, e2253. [Google Scholar] [CrossRef]
- Brenner, D.; Mak, T.W. Mitochondrial cell death effectors. Curr. Opin. Cell Biol. 2009, 21, 871–877. [Google Scholar] [CrossRef]
- Huybrechts, S.J.; van Veldhoven, P.P.; Brees, C.; Mannaerts, G.P.; Los, G.V.; Fransen, M. Peroxisome dynamics in cultured mammalian cells. Traffic 2009, 10, 1722–1733. [Google Scholar] [CrossRef]
- Schmielau, J.; Finn, O.J. Activated granulocytes and granulocyte-derived hydrogen peroxide are the underlying mechanism of suppression of t-cell function in advanced cancer patients. Cancer Res. 2001, 61, 4756–4760. [Google Scholar]
- Schmielau, J.; Nalesnik, M.A.; Finn, O.J. Suppressed T-cell receptor zeta chain expression and cytokine production in pancreatic cancer patients. Clin. Cancer Res. 2001, 7 (Suppl. 3), 933s–939s. [Google Scholar]
- Klemke, M.; Samstag, Y. Molecular mechanisms mediating oxidative stress-induced T-cell suppression in cancer. Adv. Enzym. Regul. 2009, 49, 107–112. [Google Scholar] [CrossRef] [PubMed]
- Franchina, D.G.; Dostert, C.; Brenner, D. Reactive Oxygen Species: Involvement in T Cell Signaling and Metabolism. Trends Immunol. 2018, 39, 489–502. [Google Scholar] [CrossRef] [PubMed]
- Belikov, A.V.; Schraven, B.; Simeoni, L. T cells and reactive oxygen species. J. Biomed. Sci. 2015, 22, 85. [Google Scholar] [CrossRef]
- Sena, A.; Chandel, N.S. Physiological roles of mitochondrial reactive oxygen species. Mol. Cell 2012, 48, 158–167. [Google Scholar] [CrossRef] [PubMed]
- Brand, K.A.; Hermfisse, U. Aerobic glycolysis by proliferating cells: A protective strategy against reactive oxygen species. FASEB J. 1997, 11, 388–395. [Google Scholar] [CrossRef] [PubMed]
- Van der Windt, J.; Pearce, E.L. Metabolic switching and fuel choice during T-cell differentiation and memory development. Immunol. Rev. 2012, 249, 27–42. [Google Scholar] [CrossRef]
- Carr, E.L.; Kelman, A.; Wu, G.S.; Gopaul, R.; Senkevitch, E.; Aghvanyan, A.; Turay, A.M.; Frauwirth, K.A. Glutamine uptake and metabolism are coordinately regulated by ERK/MAPK during T lymphocyte activation. J. Immunol. 2010, 185, 1037–1044. [Google Scholar] [CrossRef]
- Jacobs, S.R.; Herman, C.E.; Maciver, N.J.; Wofford, J.A.; Wieman, H.L.; Hammen, J.J.; Rathmell, J.C. Glucose uptake is limiting in T cell activation and requires CD28-mediated Akt-dependent and independent pathways. J. Immunol. 2008, 180, 4476–4486. [Google Scholar] [CrossRef]
- Chang, C.H.; Curtis, J.D.; Maggi, L.B., Jr.; Faubert, B.; Villarino, A.V.; O’Sullivan, D.; Huang, S.C.; van der Windt, G.J.; Blagih, J.; Qiu, J.; et al. Posttranscriptional control of T cell effector function by aerobic glycolysis. Cell 2013, 153, 1239–1251. [Google Scholar] [CrossRef]
- Düvel, K.; Yecies, J.L.; Menon, S.; Raman, P.; Lipovsky, A.I.; Souza, A.L.; Triantafellow, E.; Ma, Q.; Gorski, R.; Cleaver, S.; et al. Activation of a metabolic gene regulatory network downstream of mTOR complex 1. Mol. Cell 2010, 39, 171–183. [Google Scholar] [CrossRef]
- Frauwirth, K.A.; Riley, J.L.; Harris, M.H.; Parry, R.V.; Rathmell, J.C.; Elstrom, R.L.; June, C.H.; Thompson, C.B. The CD28 signaling pathway regulates glucose metabolism. Immunity 2002, 16, 769–777. [Google Scholar] [CrossRef]
- Jackson, S.H.; Devadas, S.; Kwon, J.; Pinto, L.A.; Williams, M.S. T cells express a phagocyte-type NADPH oxidase that is activated after T cell receptor stimulation. Nat. Immunol. 2004, 5, 818–827. [Google Scholar] [CrossRef]
- Ron-Harel, N.; Santos, D.; Ghergurovich, J.M.; Sage, P.T.; Reddy, A.; Lovitch, S.B.; Dephoure, N.; Satterstrom, F.K.; Sheffer, M.; Spinelli, J.B.; et al. Mitochondrial Biogenesis and Proteome Remodeling Promote One-Carbon Metabolism for T Cell Activation. Cell Metab. 2016, 24, 104–117. [Google Scholar] [CrossRef]
- Youngblood, B.; Hale, J.S.; Kissick, H.T.; Ahn, E.; Xu, X.; Wieland, A.; Araki, K.; West, E.E.; Ghoneim, H.E.; Fan, Y.; et al. Effector CD8 T cells dedifferentiate into long-lived memory cells. Nature 2017, 552, 404–409. [Google Scholar] [CrossRef]
- Gray, S.M.; Kaech, S.M.; Staron, M.M. The interface between transcriptional and epigenetic control of effector and memory CD8⁺ T-cell differentiation. Immunol. Rev. 2014, 261, 157–168. [Google Scholar] [CrossRef]
- Sukumar, M.; Liu, J.; Mehta, G.U.; Patel, S.J.; Roychoudhuri, R.; Crompton, J.G.; Klebanoff, C.A.; Ji, Y.; Li, P.; Yu, Z.; et al. Mitochondrial Membrane Potential Identifies Cells with Enhanced Stemness for Cellular Therapy. Cell Metab. 2016, 23, 63–76. [Google Scholar] [CrossRef]
- Lochner, M.; Berod, L.; Sparwasser, T. Fatty acid metabolism in the regulation of T cell function. Trends Immunol. 2015, 36, 81–91. [Google Scholar] [CrossRef]
- Yang, K.; Shrestha, S.; Zeng, H.; Karmaus, P.W.; Neale, G.; Vogel, P.; Guertin, D.A.; Lamb, R.F.; Chi, H. T cell exit from quiescence and differentiation into Th2 cells depend on Raptor-mTORC1-mediated metabolic reprogramming. Immunity 2013, 39, 1043–1056. [Google Scholar] [CrossRef]
- McGarry, D.; Brown, N.F. The mitochondrial carnitine palmitoyltransferase system. From concept to molecular analysis. Eur. J. Biochem. 1997, 244, 1–14. [Google Scholar] [CrossRef]
- Longo, N.; Frigeni, M.; Pasquali, M. Carnitine transport and fatty acid oxidation. Biochim. Biophys. Acta 2016, 1863, 2422–2435. [Google Scholar] [CrossRef]
- Sayre, N.L.; Lechleiter, J.D. Fatty acid metabolism and thyroid hormones. Curr. Trends Endocinol. 2012, 6, 65–76. [Google Scholar]
- Houten, S.M.; Wanders, R.J. A general introduction to the biochemistry of mitochondrial fatty acid β-oxidation. J. Inherit. Metab. Dis. 2010, 33, 469–477. [Google Scholar] [CrossRef]
- Cluxton, D.; Petrasca, A.; Moran, B.; Fletcher, J.M. Differential Regulation of Human Treg and Th17 Cells by Fatty Acid Synthesis and Glycolysis. Front. Immunol. 2019, 10, 115. [Google Scholar] [CrossRef]
- Pan, Y.; Tian, T.; Park, C.O.; Lofftus, S.Y.; Mei, S.; Liu, X.; Luo, C.; O’Malley, J.T.; Gehad, A.; Teague, J.E.; et al. Survival of tissue-resident memory T cells requires exogenous lipid uptake and metabolism. Nature 2017, 543, 252–256. [Google Scholar] [CrossRef]
- Singleton, L.; Roybal, K.T.; Sun, Y.; Fu, G.; Gascoigne, N.R.; van Oers, N.S.; Wulfing, C. Spatiotemporal patterning during T cell activation is highly diverse. Sci. Signal. 2009, 2, ra15. [Google Scholar] [CrossRef]
- Zaru, R.; Berrie, C.P.; Iurisci, C.; Corda, D.; Valitutti, S. CD28 co-stimulates TCR/CD3-induced phosphoinositide turnover in human T lymphocytes. Eur. J. Immunol. 2001, 31, 2438–2447. [Google Scholar] [CrossRef]
- Sun, Y.; Dandekar, R.D.; Mao, Y.S.; Yin, H.L.; Wulfing, C. Phosphatidylinositol (4,5) bisphosphate controls T cell activation by regulating T cell rigidity and organization. PLoS ONE 2011, 6, e27227. [Google Scholar] [CrossRef]
- Zhang, L.; Mao, Y.S.; Janmey, P.A.; Yin, H.L. Phosphatidylinositol 4, 5 bisphosphate and the actin cytoskeleton. Subcell Biochem. 2012, 59, 177–215. [Google Scholar] [CrossRef]
- Rocha-Perugini, V.; Gordon-Alonso, M.; Sanchez-Madrid, F. PIP2: Choreographer of actin-adaptor proteins in the HIV-1 dance. Trends Microbiol. 2014, 22, 379–388. [Google Scholar] [CrossRef][Green Version]
- Andreu, Z.; Yáñez-Mó, M. Tetraspanins in extracellular vesicle formation and function. Front. Immunol. 2014, 5, 442. [Google Scholar] [CrossRef]
- Quann, E.J.; Merino, E.; Furuta, T.; Huse, M. Localized diacylglycerol drives the polarization of the microtubule-organizing center in T cells. Nat. Immunol. 2009, 10, 627–635. [Google Scholar] [CrossRef]
- Nakamura, Y.; Fukami, K. Regulation and physiological functions of mammalian phospholipase C. J. Biochem. 2017, 161, 315–321. [Google Scholar] [CrossRef]
- Ladygina, N.; Martin, B.R.; Altman, A. Dynamic palmitoylation and the role of DHHC proteins in T cell activation and anergy. Adv. Immunol. 2011, 109, 1–44. [Google Scholar] [CrossRef]
- Almeida, L.; Lochner, M.; Berod, L.; Sparwasser, T. Metabolic pathways in T cell activation and lineage differentiation. Semin. Immunol. 2016, 28, 514–524. [Google Scholar] [CrossRef]
- Udenwobele, D.I.; Su, R.C.; Good, S.V.; Ball, T.B.; Shrivastav, S.V.; Shrivastav, A. Myristoylation: An Important Protein Modification in the Immune Response. Front. Immunol. 2017, 8, 751. [Google Scholar] [CrossRef]
- Pereira-Leal, J.B.; Hume, A.N.; Seabra, M.C. Prenylation of Rab GTPases: Molecular mechanisms and involvement in genetic disease. FEBS Lett. 2001, 498, 197–200. [Google Scholar] [CrossRef]
- Phan, A.T.; Goldrath, A.W.; Glass, C.K. Metabolic and Epigenetic Coordination of T Cell and Macrophage Immunity. Immunity 2017, 46, 714–729. [Google Scholar] [CrossRef]
- Valapour, M.; Guo, J.; Schoroeder, J.T.; Keen, J.; Cianferoni, A.; Casolaro, V.; Georas, S.N. Histone deacetylation inhibits IL4 gene expression in T cells. J. Allergy Clin. Immunol. 2002, 109, 238–245. [Google Scholar] [CrossRef]
- Warren, J.L.; MacIver, N.J. Regulation of Adaptive Immune Cells by Sirtuins. Front. Endocrinol. 2019, 10, 466. [Google Scholar] [CrossRef]
- Harayama, T.; Riezman, H. Understanding the diversity of membrane lipid composition. Nat. Rev. Mol. Cell Biol. 2018, 19, 281–296. [Google Scholar] [CrossRef]
- Bartke, N.; Hannun, Y.A. Bioactive sphingolipids: Metabolism and function. J. Lipid Res. 2009, 50, S91–S96. [Google Scholar] [CrossRef]
- Avota, E.; de Lira, M.N.; Schneider-Schaulies, S. Sphingomyelin Breakdown in T Cells: Role of Membrane Compartmentalization in T Cell Signaling and Interference by a Pathogen. Front. Cell Dev. Biol. 2019, 7, 152. [Google Scholar] [CrossRef]
- Castro, B.M.; Prieto, M.; Silva, L.C. Ceramide: A simple sphingolipid with unique biophysical properties. Prog. Lipid Res. 2014, 54, 53–67. [Google Scholar] [CrossRef]
- Börtlein, C.; Draeger, A.; Schoenauer, R.; Kuhlemann, A.; Sauer, M.; Schneider-Schaulies, S.; Avota, E. The Neutral Sphingomyelinase 2 Is Required to Polarize and Sustain T Cell Receptor Signaling. Front. Immunol. 2018, 9, 815. [Google Scholar] [CrossRef]
- Trajkovic, K.; Hsu, C.; Chiantia, S.; Rajendran, L.; Wenzel, D.; Wieland, F.; Schwille, P.; Brugger, B.; Simons, M. Ceramide triggers budding of exosome vesicles into multivesicular endosomes. Science 2008, 319, 1244–1247. [Google Scholar] [CrossRef]
- Lamana, A.; Martin, P.; de la Fuente, H.; Martinez-Muñoz, L.; Cruz-Adalia, A.; Ramirez-Huesca, M.; Escribano, C.; Gollmer, K.; Mellado, M.; Stein, J.V.; et al. CD69 modulates sphingosine-1-phosphate-induced migration of skin dendritic cells. J. Investig. Dermatol. 2011, 131, 1503–1512. [Google Scholar] [CrossRef]
- Shiow, L.R.; Rosen, D.B.; Brdickova, N.; Xu, Y.; An, J.; Lanier, L.L.; Cyster, J.G.; Matloubian, M. CD69 acts downstream of interferon-alpha/beta to inhibit S1P1 and lymphocyte egress from lymphoid organs. Nature 2006, 440, 540–544. [Google Scholar] [CrossRef]
- Aoki, M.; Aoki, H.; Ramanathan, R.; Hait, N.C.; Takabe, K. Sphingosine-1-Phosphate Signaling in Immune Cells and Inflammation: Roles and Therapeutic Potential. Mediat. Inflamm. 2016, 2016, 8606878. [Google Scholar] [CrossRef]
- Chakraborty, P.; Vaena, S.G.; Thyagarajan, K.; Chaetterjee, S.; Al-Khami, A.; Selvam, S.P.; Nguyen, H.; Kang, I.; Wyatt, M.W.; Baliga, U.; et al. Pro-Survival Lipid Sphingosine-1-Phosphate Metabolically Programs T Cells to Limit Anti-tumor Activity. Cell Rep. 2019, 28, 1879–1893.e7. [Google Scholar] [CrossRef]
- Chi, H. Sphingosine-1-phosphate and immune regulation: Trafficking and beyond. Trends Pharm. Sci. 2011, 32, 16–24. [Google Scholar] [CrossRef]
- Karuppuchamy, T.; Tyler, C.J.; Lundborg, L.R.; Perez-Jeldres, T.; Kimball, A.K.; Clambey, E.T.; Jedlicka, P.; Rivera-Nieves, J. Sphingosine-1-Phosphate Lyase Inhibition Alters the S1P Gradient and Ameliorates Crohn’s-Like Ileitis by Suppressing Thymocyte Maturation. Inflamm. Bowel Dis. 2020, 26, 216–228. [Google Scholar] [CrossRef]
- Maceyka, M.; Harikumar, K.B.; Milstien, S.; Spiegel, S. Sphingosine-1-phosphate signaling and its role in disease. Trends Cell Biol. 2012, 22, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Colina, C.; Flores, A.; Castillo, C.; Garrido Mdel, R.; Israel, A.; DiPolo, R.; Benaim, G. Ceramide-1-P induces Ca2+ mobilization in Jurkat T-cells by elevation of Ins(1,4,5)-P3 and activation of a store-operated calcium channel. Biochem. Biophys. Res. Commun. 2005, 336, 54–60. [Google Scholar] [CrossRef] [PubMed]
- Stahelin, R.V.; Subramanian, P.; Vora, M.; Cho, W.; Chalfant, C.E. Ceramide-1-phosphate binds group IVA cytosolic phospholipase a2 via a novel site in the C2 domain. J. Biol. Chem. 2007, 282, 20467–20474. [Google Scholar] [CrossRef] [PubMed]
- Subramanian, P.; Stahelin, R.V.; Szulc, Z.; Bielawska, A.; Cho, W.; Chalfant, C.E. Ceramide 1-phosphate acts as a positive allosteric activator of group IVA cytosolic phospholipase A2 alpha and enhances the interaction of the enzyme with phosphatidylcholine. J. Biol. Chem. 2005, 280, 17601–17607. [Google Scholar] [CrossRef] [PubMed]
- Pettus, B.J.; Bielawska, A.; Subramanian, P.; Wijesinghe, D.S.; Maceyka, M.; Leslie, C.C.; Evans, J.H.; Freiberg, J.; Roddy, P.; Hannun, Y.A.; et al. Ceramide 1-phosphate is a direct activator of cytosolic phospholipase A2. J. Biol. Chem. 2004, 279, 11320–11326. [Google Scholar] [CrossRef] [PubMed]

© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dosil, S.G.; Rojas-Gomez, A.; Sánchez-Madrid, F.; Martín-Cófreces, N.B. The Swing of Lipids at Peroxisomes and Endolysosomes in T Cell Activation. Int. J. Mol. Sci. 2020, 21, 2859. https://doi.org/10.3390/ijms21082859
Dosil SG, Rojas-Gomez A, Sánchez-Madrid F, Martín-Cófreces NB. The Swing of Lipids at Peroxisomes and Endolysosomes in T Cell Activation. International Journal of Molecular Sciences. 2020; 21(8):2859. https://doi.org/10.3390/ijms21082859
Chicago/Turabian StyleDosil, Sara G., Amelia Rojas-Gomez, Francisco Sánchez-Madrid, and Noa B. Martín-Cófreces. 2020. "The Swing of Lipids at Peroxisomes and Endolysosomes in T Cell Activation" International Journal of Molecular Sciences 21, no. 8: 2859. https://doi.org/10.3390/ijms21082859
APA StyleDosil, S. G., Rojas-Gomez, A., Sánchez-Madrid, F., & Martín-Cófreces, N. B. (2020). The Swing of Lipids at Peroxisomes and Endolysosomes in T Cell Activation. International Journal of Molecular Sciences, 21(8), 2859. https://doi.org/10.3390/ijms21082859




