Bone Marrow Aspirate Concentrate: Its Uses in Osteoarthritis
Abstract
:1. Introduction
2. Constitution
2.1. Cellular Contents in Bone Marrow Aspirate (BMA) and BMAC
2.2. Mesenchymal Stem Cells (MSCs)
2.3. Growth Factors and Cytokines
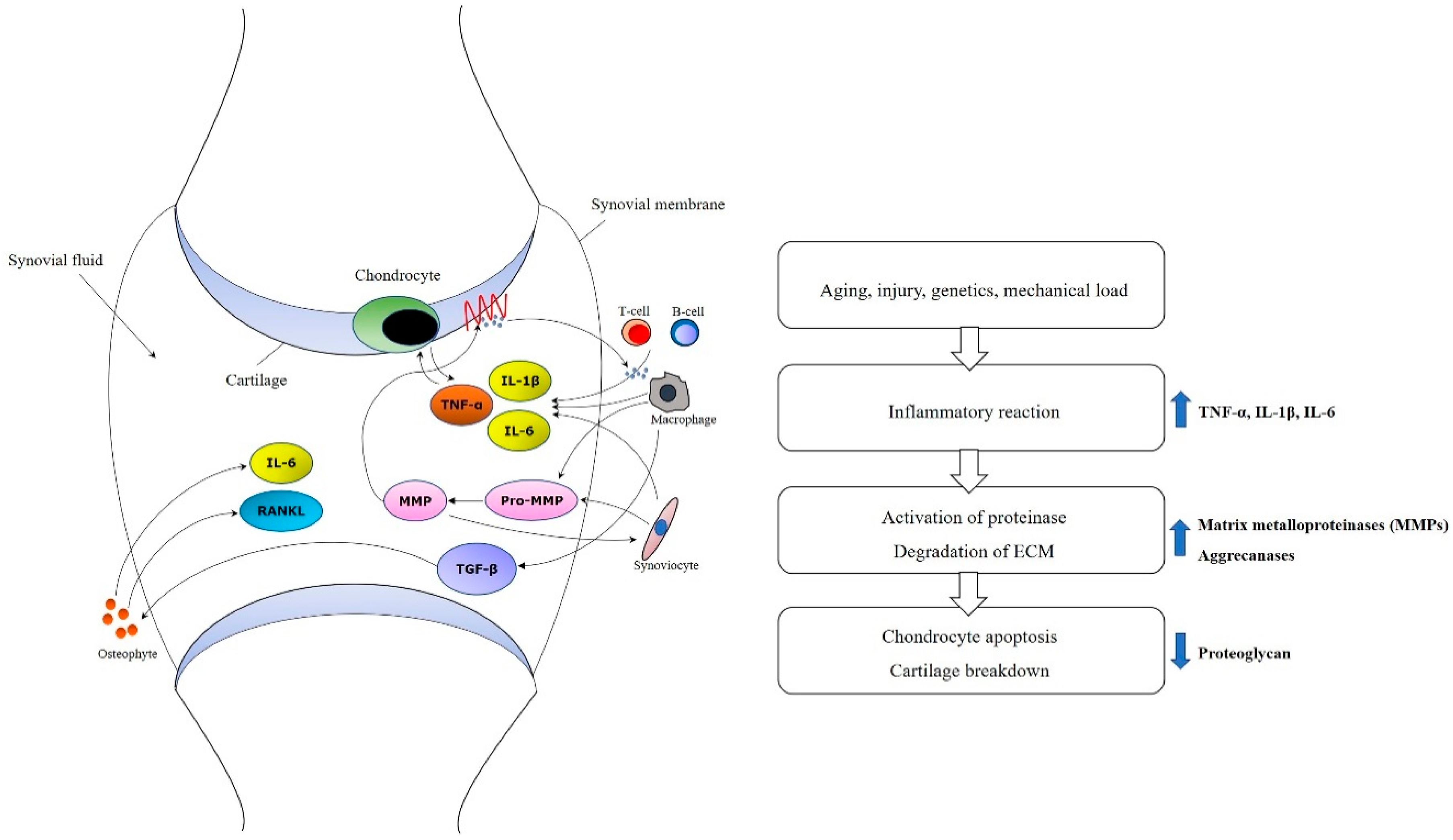
3. Pathophysiology and Issues of Osteoarthritis
4. Mechanisms of BMAC for Osteoarthritis
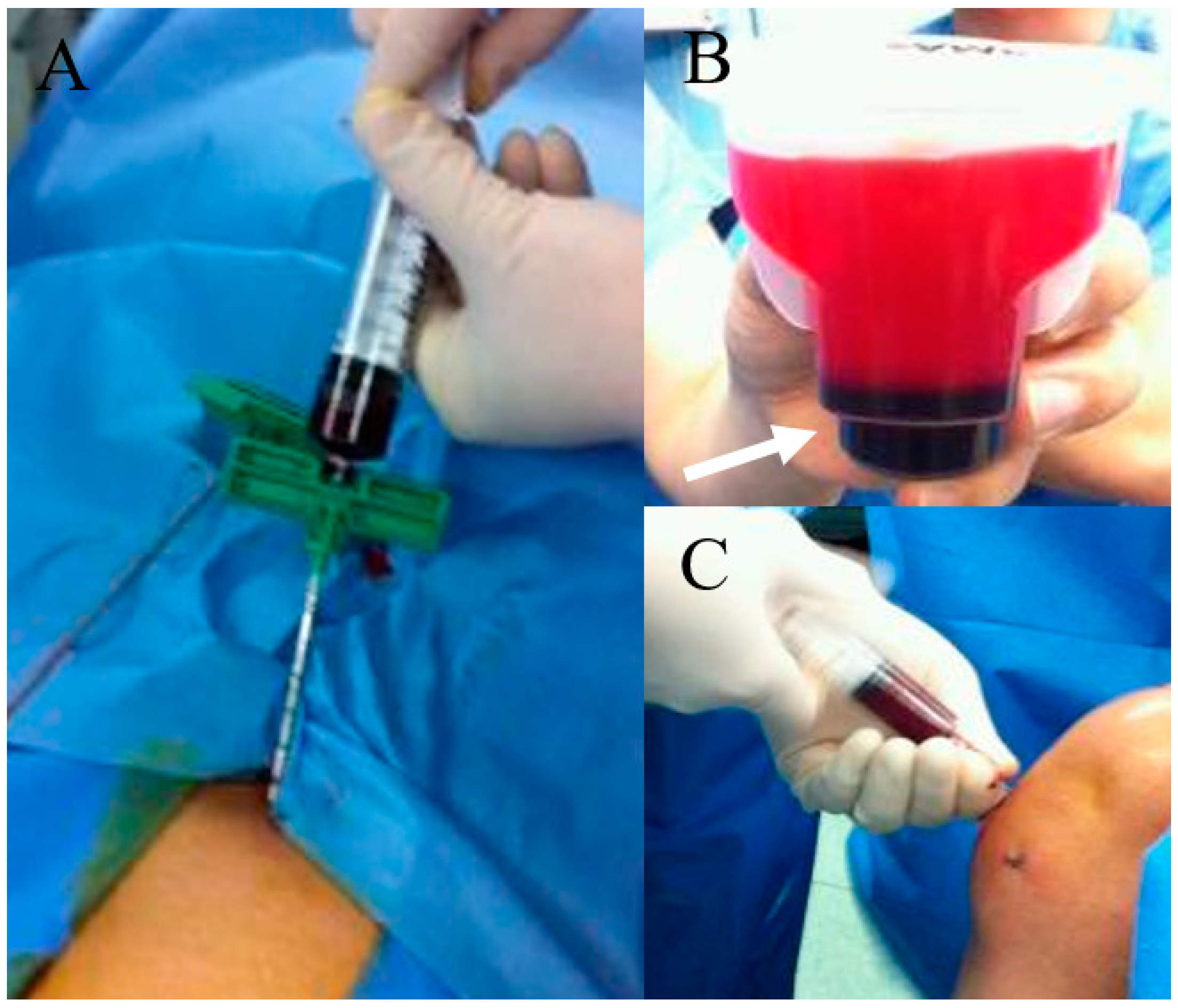
5. Issues on Harvest and Processing of BMAC
6. Application Modalities
7. Review of Clinical Studies with BMAC
8. Adverse Events
9. Advanced Technologies
9.1. Nanotechnologies
9.2. Other Smart Materials
10. Concluding Remarks and Perspectives
Author Contributions
Funding
Conflicts of Interest
References
- Mankin, H.J. The response of articular cartilage to mechanical injury. J. Bone Jt. Surg Am. 1982, 64, 460–466. [Google Scholar] [CrossRef]
- Goyal, D.; Keyhani, S.; Lee, E.H.; Hui, J.H.P. Evidence-based status of microfracture technique: A systematic review of level I and II studies. Arthroscopy 2013, 29, 1579–1588. [Google Scholar] [CrossRef] [PubMed]
- De Lange-Brokaar, B.J.; Ioan-Facsinay, A.; Van Osch, G.J.; Zuurmond, A.-M.; Schoones, J.; Toes, R.E.; Huizinga, T.W.; Kloppenburg, M. Synovial inflammation, immune cells and their cytokines in osteoarthritis: A review. Osteoarthr. Cartil. 2012, 20, 1484–1499. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, S.; Hawker, G.A.; Laporte, A.; Croxford, R.; Coyte, P.C. The economic burden of disabling hip and knee osteoarthritis (OA) from the perspective of individuals living with this condition. Rheumatol 2005, 44, 1531–1537. [Google Scholar] [CrossRef] [Green Version]
- Hawker, G.A.; Mian, S.; Bednis, K.; Stanaitis, I. Osteoarthritis year 2010 in review: Non-pharmacologic therapy. Osteoarthr. Cartil. 2011, 19, 366–374. [Google Scholar] [CrossRef] [Green Version]
- Chevalier, X.; Eymard, F.; Richette, P. Biologic agents in osteoarthritis: Hopes and disappointments. Nat. Rev. Rheumatol 2013, 9, 400–410. [Google Scholar] [CrossRef]
- Güler-Yüksel, M.; Allaart, C.F.; Watt, I.; Goekoop-Ruiterman, Y.P.M.; de Vries-Bouwstra, J.K.; van Schaardenburg, D.; van Krugten, M.V.; Dijkmans, B.A.C.; Huizinga, T.W.J.; Lems, W.F.; et al. Treatment with TNF-α inhibitor infliximab might reduce hand osteoarthritis in patients with rheumatoid arthritis. Osteoarthr. Cartil. 2010, 18, 1256–1262. [Google Scholar] [CrossRef] [Green Version]
- Mithoefer, K.; McAdams, T.; Williams, R.J.; Kreuz, P.C.; Mandelbaum, B.R. Clinical efficacy of the microfracture technique for articular cartilage repair in the knee: An evidence-based systematic analysis. Am. J. Sports Med. 2009, 37, 2053–2063. [Google Scholar] [CrossRef]
- Niemeyer, P.; Porichis, S.; Steinwachs, M.; Erggelet, C.; Kreuz, P.C.; Schmal, H.; Uhl, M.; Ghanem, N.; Südkamp, N.P.; Salzmann, G. Long-term outcomes after first-generation autologous chondrocyte implantation for cartilage defects of the knee. Am. J. Sports Med. 2014, 42, 150–157. [Google Scholar] [CrossRef]
- Gudas, R.; Gudaitė, A.; Mickevičius, T.; Masiulis, N.; Simonaitytė, R.; Čekanauskas, E.; Skurvydas, A. Comparison of osteochondral autologous transplantation, microfracture, or debridement techniques in articular cartilage lesions associated with anterior cruciate ligament injury: A prospective study with a 3-year follow-up. Arthroscopy 2013, 29, 89–97. [Google Scholar] [CrossRef]
- McCarrel, T.; Fortier, L. Temporal growth factor release from platelet-rich plasma, trehalose lyophilized platelets, and bone marrow aspirate and their effect on tendon and ligament gene expression. J. Orthop. Res. 2009, 27, 1033–1042. [Google Scholar] [CrossRef] [PubMed]
- Indrawattana, N.; Chen, G.; Tadokoro, M.; Shann, L.H.; Ohgushi, H.; Tateishi, T.; Tanaka, J.; Bunyaratvej, A. Growth factor combination for chondrogenic induction from human mesenchymal stem cell. Biochem. Biophys. Res. Commun. 2004, 320, 914–919. [Google Scholar] [CrossRef] [PubMed]
- Rubin, R.; Strayer, D.S.; Rubin, E. Rubin’s pathology: Clinicopathologic foundations of medicine; Lippincott Williams and Wilkins: Philadelphia, PA, USA, 2008. [Google Scholar]
- Lucas, D. The Bone Marrow Microenvironment for Hematopoietic Stem Cells. Adv. Exp. Med. Biol. 2017, 1041, 5–18. [Google Scholar] [PubMed]
- Buda, R.; Vannini, F.; Cavallo, M.; Grigolo, B.; Cenacchi, A.; Giannini, S. Osteochondral lesions of the knee: A new one-step repair technique with bone-marrow-derived cells. J. Bone Jt. Surg Am. 2010, 92 (Suppl. 2), 2–11. [Google Scholar] [CrossRef]
- Ipach, I.; Schäfer, R.; Lahrmann, J.; Kluba, T. Stiffness after knee arthrotomy: Evaluation of prevalence and results after manipulation under anaesthesia. Orthop. Traumatol. Surg. Res. 2011, 97, 292–296. [Google Scholar] [CrossRef] [Green Version]
- Huh, S.W.; Shetty, A.A.; Ahmed, S.; Lee, D.H.; Kim, S.J. Autologous bone-marrow mesenchymal cell induced chondrogenesis (MCIC). J. Clin. Orthop. Trauma 2016, 7, 153–156. [Google Scholar] [CrossRef] [Green Version]
- Chiang, H.; Hsieh, C.-H.; Lin, Y.-H.; Lin, S.; Tsai-Wu, J.-J.; Jiang, C.-C. Differences between chondrocytes and bone marrow-derived chondrogenic cells. Tissue Eng. Part. A 2011, 17, 2919–2929. [Google Scholar] [CrossRef]
- Filardo, G.; Madry, H.; Jelic, M.; Roffi, A.; Cucchiarini, M.; Kon, E. Mesenchymal stem cells for the treatment of cartilage lesions: From preclinical findings to clinical application in orthopaedics. Knee Surg. Sports Traumatol. Arthrosc. 2013, 21, 1717–1729. [Google Scholar] [CrossRef]
- Fortier, L.A.; Potter, H.G.; Rickey, E.J.; Schnabel, L.V.; Foo, L.F.; Chong, L.R.; Stokol, T.; Cheetham, J.; Nixon, A.J. Concentrated bone marrow aspirate improves full-thickness cartilage repair compared with microfracture in the equine model. J. Bone Jt. Surg. Am. 2010, 92, 1927–1937. [Google Scholar] [CrossRef] [Green Version]
- Johnson, R.G. Bone marrow concentrate with allograft equivalent to autograft in lumbar fusions. Spine 2014, 39, 695–700. [Google Scholar] [CrossRef]
- Koga, H.; Shimaya, M.; Muneta, T.; Nimura, A.; Morito, T.; Hayashi, M.; Suzuki, S.; Ju, Y.-J.; Mochizuki, T.; Sekiya, I. Local adherent technique for transplanting mesenchymal stem cells as a potential treatment of cartilage defect. Arthritis Res. 2008, 10, R84. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orozco, L.; Munar, A.; Soler, R.; Alberca, M.; Soler, F.; Huguet, M.; Sentís, J.; Sánchez, A.; García-Sancho, J. Treatment of knee osteoarthritis with autologous mesenchymal stem cells: A pilot study. Transplant 2013, 95, 1535–1541. [Google Scholar] [CrossRef] [PubMed]
- Cucchiarini, M.; Venkatesan, J.K.; Ekici, M.; Schmitt, G.; Madry, H. Human mesenchymal stem cells overexpressing therapeutic genes: From basic science to clinical applications for articular cartilage repair. Biomed. Mater. Eng. 2012, 22, 197–208. [Google Scholar] [CrossRef] [PubMed]
- Prockop, D.J. Marrow stromal cells as stem cells for nonhematopoietic tissues. Science 1997, 276, 71–74. [Google Scholar] [CrossRef] [Green Version]
- Lee, G.W.; Seo, M.-S.; Kang, K.-K.; Oh, S.-K. Epidural fat-derived mesenchymal stem cell: First report of epidural fat-derived mesenchymal stem cell. Asian Spine J. 2019, 13, 361. [Google Scholar] [CrossRef] [Green Version]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Johnstone, B.; Hering, T.M.; Caplan, A.I.; Goldberg, V.M.; Yoo, J.U. In vitrochondrogenesis of bone marrow-derived mesenchymal progenitor cells. Exp. Cell Res. 1998, 238, 265–272. [Google Scholar] [CrossRef]
- Fukumoto, T.; Sperling, J.; Sanyal, A.; Fitzsimmons, J.; Reinholz, G.; Conover, C.A.; O’Driscoll, S.W. Combined effects of insulin-like growth factor-1 and transforming growth factor-β1 on periosteal mesenchymal cells during chondrogenesis in vitro. Osteoarthr. Cartil. 2003, 11, 55–64. [Google Scholar] [CrossRef] [Green Version]
- Caplan, A.I.; Dennis, J.E. Mesenchymal stem cells as trophic mediators. J. Cell Biochem. 2006, 98, 1076–1084. [Google Scholar] [CrossRef]
- Cassano, J.M.; Kennedy, J.G.; Ross, K.A.; Fraser, E.J.; Goodale, M.B.; Fortier, L.A. Bone marrow concentrate and platelet-rich plasma differ in cell distribution and interleukin 1 receptor antagonist protein concentration. Knee Surg. Sports Traumatol. Arthrosc. 2018, 26, 333–342. [Google Scholar] [CrossRef]
- Gharibi, B.; Hughes, F.J. Effects of medium supplements on proliferation, differentiation potential, and in vitro expansion of mesenchymal stem cells. Stem Cells Transl. Med. 2012, 1, 771–782. [Google Scholar] [CrossRef] [PubMed]
- Tuli, R.; Tuli, S.; Nandi, S.; Huang, X.; Manner, P.A.; Hozack, W.J.; Danielson, K.G.; Hall, D.J.; Tuan, R.S. Transforming growth factor-β-mediated chondrogenesis of human mesenchymal progenitor cells involves N-cadherin and mitogen-activated protein kinase and Wnt signaling cross-talk. J. Biol. Chem. 2003, 278, 41227–41236. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ham, O.; Lee, C.Y.; Kim, R.; Lee, J.; Oh, S.; Lee, M.Y.; Kim, J.; Hwang, K.-C.; Maeng, L.-S.; Chang, W. Therapeutic potential of differentiated mesenchymal stem cells for treatment of osteoarthritis. Int. J. Mol. Sci. 2015, 16, 14961–14978. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jaime, P.; García-Guerrero, N.; Estella, R.; Pardo, J.; García-Álvarez, F.; Martinez-Lostao, L. CD56+/CD16− Natural Killer cells expressing the inflammatory protease granzyme A are enriched in synovial fluid from patients with osteoarthritis. Osteoarthr. Cartil. 2017, 25, 1708–1718. [Google Scholar] [CrossRef] [Green Version]
- Nigrovic, P.A.; Lee, D.M. Mast cells in inflammatory arthritis. Arthritis Res. 2004, 7, 1. [Google Scholar]
- Harrell, C.R.; Markovic, B.S.; Fellabaum, C.; Arsenijevic, A.; Volarevic, V. Mesenchymal stem cell-based therapy of osteoarthritis: Current knowledge and future perspectives. Biomed. Pharm. 2019, 109, 2318–2326. [Google Scholar] [CrossRef]
- Martin, J.A.; Buckwalter, J.A. The role of chondrocyte senescence in the pathogenesis of osteoarthritis and in limiting cartilage repair. J. Bone Jt. Surg Am. 2003, 85 (Suppl. 2), 106–110. [Google Scholar] [CrossRef]
- Roach, H.I.; Yamada, N.; Cheung, K.S.; Tilley, S.; Clarke, N.M.; Oreffo, R.O.; Kokubun, S.; Bronner, F. Association between the abnormal expression of matrix-degrading enzymes by human osteoarthritic chondrocytes and demethylation of specific CpG sites in the promoter regions. Arthritis Rheum. 2005, 52, 3110–3124. [Google Scholar] [CrossRef]
- Vincenti, M.P.; Brinckerhoff, C.E. Transcriptional regulation of collagenase (MMP-1, MMP-13) genes in arthritis: Integration of complex signaling pathways for the recruitment of gene-specific transcription factors. Arthritis Res. 2002, 4, 157. [Google Scholar] [CrossRef]
- Imam, M.A.; Holton, J.; Ernstbrunner, L.; Pepke, W.; Grubhofer, F.; Narvani, A.; Snow, M. A systematic review of the clinical applications and complications of bone marrow aspirate concentrate in management of bone defects and nonunions. Int. Orthop. 2017, 41, 2213–2220. [Google Scholar] [CrossRef]
- Jager, M.; Jelinek, E.M.; Wess, K.M.; Scharfstadt, A.; Jacobson, M.; Kevy, S.V.; Krauspe, R. Bone marrow concentrate: A novel strategy for bone defect treatment. Curr. Stem Cell Res. 2009, 4, 34–43. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, D.H.; Ryu, K.J.; Kim, J.W.; Kang, K.C.; Choi, Y.R. Bone marrow aspirate concentrate and platelet-rich plasma enhanced bone healing in distraction osteogenesis of the tibia. Clin. Orthop. Relat. Res. 2014, 472, 3789–3797. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potier, E.; Ferreira, E.; Dennler, S.; Mauviel, A.; Oudina, K.; Logeart-Avramoglou, D.; Petite, H. Desferrioxamine-driven upregulation of angiogenic factor expression by human bone marrow stromal cells. J. Tissue Eng. Regen. Med. 2008, 2, 272–278. [Google Scholar] [CrossRef] [PubMed]
- Huang, A.H.; Motlekar, N.A.; Stein, A.; Diamond, S.L.; Shore, E.M.; Mauck, R.L. High-throughput screening for modulators of mesenchymal stem cell chondrogenesis. Ann. Biomed. Eng. 2008, 36, 1909. [Google Scholar] [CrossRef]
- Uccelli, A.; Pistoia, V.; Moretta, L. Mesenchymal stem cells: A new strategy for immunosuppression? Trends Immunol. 2007, 28, 219–226. [Google Scholar] [CrossRef]
- Wang, Y.; Chen, X.; Cao, W.; Shi, Y. Plasticity of mesenchymal stem cells in immunomodulation: Pathological and therapeutic implications. Nat. Immunol. 2014, 15, 1009. [Google Scholar] [CrossRef]
- Wei, C.-C.; Lin, A.B.; Hung, S.-C. Mesenchymal stem cells in regenerative medicine for musculoskeletal diseases: Bench, bedside, and industry. Cell Transpl. 2014, 23, 505–512. [Google Scholar] [CrossRef]
- Fong, E.L.; Chan, C.K.; Goodman, S.B. Stem cell homing in musculoskeletal injury. Biomaterials 2011, 32, 395–409. [Google Scholar] [CrossRef] [Green Version]
- Acharya, C.; Adesida, A.; Zajac, P.; Mumme, M.; Riesle, J.; Martin, I.; Barbero, A. Enhanced chondrocyte proliferation and mesenchymal stromal cells chondrogenesis in coculture pellets mediate improved cartilage formation. J. Cell Physiol. 2012, 227, 88–97. [Google Scholar] [CrossRef]
- Pers, Y.-M.; Ruiz, M.; Noël, D.; Jorgensen, C. Mesenchymal stem cells for the management of inflammation in osteoarthritis: State of the art and perspectives. Osteoarthr. Cartil. 2015, 23, 2027–2035. [Google Scholar] [CrossRef] [Green Version]
- Oliver, K.; Awan, T.; Bayes, M. Single- Versus Multiple-Site Harvesting Techniques for Bone Marrow Concentrate: Evaluation of Aspirate Quality and Pain. Orthop J. Sports Med. 2017, 5, 2325967117724398. [Google Scholar] [CrossRef] [Green Version]
- Hernigou, P.; Homma, Y.; Flouzat Lachaniette, C.H.; Poignard, A.; Allain, J.; Chevallier, N.; Rouard, H. Benefits of small volume and small syringe for bone marrow aspirations of mesenchymal stem cells. Int. Orthop. 2013, 37, 2279–2287. [Google Scholar] [CrossRef] [PubMed]
- Yandow, S.M.; Van de Velde, S.K.; Siebert, J.; Perkins, S.L. The influence of aspiration volume on the number of osteoblastic progenitors obtained from bone marrow in children. J. Pediatr. Orthop. 2019, 39, 382–386. [Google Scholar] [CrossRef] [PubMed]
- Muschler, G.F.; Boehm, C.; Easley, K. Aspiration to obtain osteoblast progenitor cells from human bone marrow: The influence of aspiration volume. J. Bone Jt. Surg. Am. 1997, 79, 1699–1709. [Google Scholar] [CrossRef] [PubMed]
- Enea, D.; Cecconi, S.; Calcagno, S.; Busilacchi, A.; Manzotti, S.; Gigante, A. One-step cartilage repair in the knee: Collagen-covered microfracture and autologous bone marrow concentrate. A pilot study. Knee 2015, 22, 30–35. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gobbi, A.; Chaurasia, S.; Karnatzikos, G.; Nakamura, N. Matrix-induced autologous chondrocyte implantation versus multipotent stem cells for the treatment of large patellofemoral chondral lesions: A nonrandomized prospective trial. Cartilage 2015, 6, 82–97. [Google Scholar] [CrossRef] [Green Version]
- Gobbi, A.; Karnatzikos, G.; Sankineani, S.R. One-step surgery with multipotent stem cells for the treatment of large full-thickness chondral defects of the knee. Am. J. Sports Med. 2014, 42, 648–657. [Google Scholar] [CrossRef]
- Kim, J.-D.; Lee, G.W.; Jung, G.H.; Kim, C.K.; Kim, T.; Park, J.H.; Cha, S.S.; You, Y.-B. Clinical outcome of autologous bone marrow aspirates concentrate (BMAC) injection in degenerative arthritis of the knee. Eur. J. Orthop. Surg. Traumatol. 2014, 24, 1505–1511. [Google Scholar] [CrossRef]
- Hernigou, P.; Mathieu, G.; Poignard, A.; Manicom, O.; Beaujean, F.; Rouard, H. Percutaneous autologous bone-marrow grafting for nonunions. Surgical technique. J. Bone Jt. Surg. Am. 2006, 88, 322–327. [Google Scholar] [CrossRef] [Green Version]
- Gigante, A.; Cecconi, S.; Calcagno, S.; Busilacchi, A.; Enea, D. Arthroscopic knee cartilage repair with covered microfracture and bone marrow concentrate. Arthrosc. Tech. 2012, 1, e175–e180. [Google Scholar] [CrossRef]
- Skowroński, J.; Rutka, M. Osteochondral lesions of the knee reconstructed with mesenchymal stem cells-results. Ortop. Traumatol. Rehabil. 2013, 15, 195–204. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Davatchi, F.; Abdollahi, B.S.; Mohyeddin, M.; Shahram, F.; Nikbin, B. Mesenchymal stem cell therapy for knee osteoarthritis. Preliminary report of four patients. Int. J. Rheum. Dis. 2011, 14, 211–215. [Google Scholar] [CrossRef] [PubMed]
- Varma, H.; Dadarya, B.; Vidyarthi, A. The new avenues in the management of osteo-arthritis of knee-stem cells. J. Indian Med. Assoc. 2010, 108, 583–585. [Google Scholar] [PubMed]
- Zhao, D.; Cui, D.; Wang, B.; Tian, F.; Guo, L.; Yang, L.; Liu, B.; Yu, X. Treatment of early stage osteonecrosis of the femoral head with autologous implantation of bone marrow-derived and cultured mesenchymal stem cells. Bone 2012, 50, 325–330. [Google Scholar] [CrossRef]
- Hernigou, P.; Trousselier, M.; Roubineau, F.; Bouthors, C.; Chevallier, N.; Rouard, H.; Flouzat-Lachaniette, C.-H. Stem cell therapy for the treatment of hip osteonecrosis: A 30-year review of progress. Clin. Orthop. Surg. 2016, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Kouroupis, D.; Ahari, A.F.; Correa, D.; Shammaa, R. Intralesional Injection of Bone Marrow Aspirate Concentrate for the Treatment of Osteonecrosis of the Knee Secondary to Systemic Lupus Erythematosus: A Case Report. Front. Bioeng. Biotechnol. 2020, 8, 202. [Google Scholar] [CrossRef] [Green Version]
- Gobbi, A.; Whyte, G.P. Long-term Clinical Outcomes of One-Stage Cartilage Repair in the Knee With Hyaluronic Acid–Based Scaffold Embedded With Mesenchymal Stem Cells Sourced From Bone Marrow Aspirate Concentrate. Am. J. Sports Med. 2019, 47, 1621–1628. [Google Scholar] [CrossRef]
- Centeno, C.; Pitts, J.; Al-Sayegh, H.; Freeman, M. Efficacy of autologous bone marrow concentrate for knee osteoarthritis with and without adipose graft. Biomed. Res. Int. 2014, 2014, 370621. [Google Scholar] [CrossRef] [Green Version]
- Hauser, R.A.; Orlofsky, A. Regenerative injection therapy with whole bone marrow aspirate for degenerative joint disease: A case series. Clin. Med. Insights Arthritis Musculoskelet Disord. 2013, 6, 65–72. [Google Scholar] [CrossRef]
- Chahla, J.; Dean, C.S.; Moatshe, G.; Pascual-Garrido, C.; Serra Cruz, R.; LaPrade, R.F. Concentrated Bone Marrow Aspirate for the Treatment of Chondral Injuries and Osteoarthritis of the Knee: A Systematic Review of Outcomes. Orthop J. Sports Med. 2016, 4, 2325967115625481. [Google Scholar] [CrossRef] [Green Version]
- Shapiro, S.A.; Kazmerchak, S.E.; Heckman, M.G.; Zubair, A.C.; O’Connor, M.I. A Prospective, Single-Blind, Placebo-Controlled Trial of Bone Marrow Aspirate Concentrate for Knee Osteoarthritis. Am. J. Sports Med. 2017, 45, 82–90. [Google Scholar] [CrossRef] [PubMed]
- Arora, P.; Sindhu, A.; Dilbaghi, N.; Chaudhury, A.; Rajakumar, G.; Rahuman, A.A. Nano-regenerative medicine towards clinical outcome of stem cell and tissue engineering in humans. J. Cell Mol. Med. 2012, 16, 1991–2000. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maleki Dizaj, S.; Lotfipour, F.; Barzegar-Jalali, M.; Zarrintan, M.-H.; Adibkia, K. Ciprofloxacin HCl-loaded calcium carbonate nanoparticles: Preparation, solid state characterization, and evaluation of antimicrobial effect against Staphylococcus aureus. Artif. Cells Nanomed. Biotechnol. 2017, 45, 535–543. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chiu, L.; Waldman, S. Nanomaterials for Cartilage Tissue Engineering; IAPC Publishing: Zagreb, Croatia, 2016. [Google Scholar]
- Yang, Y.; Leong, K.W. Nanoscale surfacing for regenerative medicine. Wires Nanomed. Nanobiotechnol. 2010, 2, 478–495. [Google Scholar] [CrossRef] [PubMed]
- Gillogly, S.D.; Wheeler, K.S. Autologous chondrocyte implantation with collagen membrane. Sports Med. Arthrosc. Rev. 2015, 23, 118–124. [Google Scholar] [CrossRef] [PubMed]
- Pfeifer, C.G.; Berner, A.; Koch, M.; Krutsch, W.; Kujat, R.; Angele, P.; Nerlich, M.; Zellner, J. Higher ratios of hyaluronic acid enhance chondrogenic differentiation of human MSCs in a hyaluronic acid–gelatin composite scaffold. Materials 2016, 9, 381. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alves da Silva, M.L.; Martins, A.; Costa-Pinto, A.; Correlo, V.; Sol, P.; Bhattacharya, M.; Faria, S.; Reis, R.; Neves, N. Chondrogenic differentiation of human bone marrow mesenchymal stem cells in chitosan-based scaffolds using a flow-perfusion bioreactor. J. Tissue Eng. Regen Med. 2011, 5, 722–732. [Google Scholar] [CrossRef]
- Celik, C.; Mogal, V.T.; Hui, J.H.P.; Loh, X.J.; Toh, W.S. Injectable Hydrogels for Cartilage Regeneration. In Hydrogels; Springer: Berlin, Germany, 2018; pp. 315–337. [Google Scholar]
- Duarte Campos, D.F.; Drescher, W.; Rath, B.; Tingart, M.; Fischer, H. Supporting biomaterials for articular cartilage repair. Cartilage 2012, 3, 205–221. [Google Scholar] [CrossRef] [Green Version]
- Walvoort, M.T.; van den Elst, H.; Plante, O.J.; Kröck, L.; Seeberger, P.H.; Overkleeft, H.S.; van der Marel, G.A.; Codée, J.D. Automated Solid-Phase Synthesis of β-Mannuronic Acid Alginates. Angew. Chem. Int. Ed. 2012, 51, 4393–4396. [Google Scholar] [CrossRef]
- Perán, M.; García, M.A.; López-Ruiz, E.; Bustamante, M.; Jiménez, G.; Madeddu, R.; Marchal, J.A. Functionalized nanostructures with application in regenerative medicine. Int. J. Mol. Sci. 2012, 13, 3847–3886. [Google Scholar] [CrossRef] [Green Version]
- Johnstone, B.; Alini, M.; Cucchiarini, M.; Dodge, G.R.; Eglin, D.; Guilak, F.; Madry, H.; Mata, A.; Mauck, R.L.; Semino, C.E. Tissue engineering for articular cartilage repair—The state of the art. Eur. Cell Mater. 2013, 25, e67. [Google Scholar] [CrossRef] [PubMed]
- Eftekhari, A.; Maleki Dizaj, S.; Sharifi, S.; Salatin, S.; Rahbar Saadat, Y.; Zununi Vahed, S.; Samiei, M.; Ardalan, M.; Rameshrad, M.; Ahmadian, E. The Use of Nanomaterials in Tissue Engineering for Cartilage Regeneration; Current Approaches and Future Perspectives. Int. J. Mol. Sci. 2020, 21, 536. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, U.; Wiesmann, H.P. Bone and cartilage engineering; Springer Science & Business Media: Berlin, Germany, 2006. [Google Scholar]
- Carletti, E.; Motta, A.; Migliaresi, C. Scaffolds for tissue engineering and 3D cell culture. In Methods in Molecular Biology: 3D Cell Culture; Humana Press: Totowa, NJ, USA, 2011; pp. 17–39. [Google Scholar]
- Chung, S.; King, M.W. Design concepts and strategies for tissue engineering scaffolds. Biotechnol. Appl. Biochem. 2011, 58, 423–438. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, E.; Swain, R.J.; Evans, N.D.; Boonrungsiman, S.; Jell, G.; Ball, M.D.; Shean, T.A.; Oyen, M.L.; Porter, A.; Stevens, M.M. Comparative materials differences revealed in engineered bone as a function of cell-specific differentiation. Nat. Mater. 2009, 8, 763–770. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, J.; Rao, M.P.; MacDonald, N.C.; Khang, D.; Webster, T.J. Improved endothelial cell adhesion and proliferation on patterned titanium surfaces with rationally designed, micrometer to nanometer features. Acta Biomater. 2008, 4, 192–201. [Google Scholar] [CrossRef]
- Drury, J.L.; Mooney, D.J. Hydrogels for tissue engineering: Scaffold design variables and applications. Biomaterials 2003, 24, 4337–4351. [Google Scholar] [CrossRef]
- Belkas, J.S.; Munro, C.A.; Shoichet, M.S.; Midha, R. Peripheral nerve regeneration through a synthetic hydrogel nerve tube. Restor. Neurol. Neurosci. 2005, 23, 19–29. [Google Scholar]
- Erickson, I.E.; Huang, A.H.; Chung, C.; Li, R.T.; Burdick, J.A.; Mauck, R.L. Differential maturation and structure–function relationships in mesenchymal stem cell-and chondrocyte-seeded hydrogels. Tissue Eng. Part. A 2009, 15, 1041–1052. [Google Scholar] [CrossRef] [Green Version]
- Erickson, I.E.; Kestle, S.R.; Zellars, K.H.; Farrell, M.J.; Kim, M.; Burdick, J.A.; Mauck, R.L. High mesenchymal stem cell seeding densities in hyaluronic acid hydrogels produce engineered cartilage with native tissue properties. Acta Biomater. 2012, 8, 3027–3034. [Google Scholar] [CrossRef] [Green Version]
- Park, J.Y.; Gao, G.; Jang, J.; Cho, D.-W. 3D printed structures for delivery of biomolecules and cells: Tissue repair and regeneration. J. Mater. Chem. 2016, 4, 7521–7539. [Google Scholar] [CrossRef]
- Mortisen, D.; Peroglio, M.; Alini, M.; Eglin, D. Tailoring thermoreversible hyaluronan hydrogels by “click” chemistry and RAFT polymerization for cell and drug therapy. Biomacromolecules 2010, 11, 1261–1272. [Google Scholar] [CrossRef] [PubMed]
- Ávila, H.M.; Schwarz, S.; Rotter, N.; Gatenholm, P. 3D bioprinting of human chondrocyte-laden nanocellulose hydrogels for patient-specific auricular cartilage regeneration. Bioprinting 2016, 1, 22–35. [Google Scholar] [CrossRef]
- Giannitelli, S.M.; Accoto, D.; Trombetta, M.; Rainer, A. Current trends in the design of scaffolds for computer-aided tissue engineering. Acta Biomater. 2014, 10, 580–594. [Google Scholar] [CrossRef] [PubMed]
| Characteristics | Positive Markers | Negative Markers |
|---|---|---|
| Plastic adherent in vitro | CD44 | |
| Ability to form colony forming fibroblast | CD73 | CD34 |
| Ability to differentiate into mesodermal lineages (osteoblasts, adipocytes, chondroblasts, and tenocytes) | CD90 CD105 | CD45 CD133 |
| Promotion of hematopoiesis | CD166 | |
| Self-renewal potential | HLA-ABC |
| Authors | Publications/Year | Study Design/No. of Patients | Mean Age (Year) | Mean Follow-Up Period (Months) | Defect/Location | Treatment | Additional Factors | Harvest Volume/Kit Used | Outcomes | Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| Buda et al. [15] | J Bone Joint Surg Am/2010 | Case series/20 | N/A (15‒50) | 24.0 | ICRS grade III–IV lesion/MFC and LFC | BMAC + HA membrane | Platelet gel (platelet rich fibrin) | 60 mL/SmartPrep System | Significant clinical improvement; Subchondral bone & cartilage regeneration on MRI/histology | N/A |
| Gigante et al. [61] | Arthrosc Tech/2012 | A case report | 37.0 | 24.0 | 3.0 cm2 sized ICRS grade IV lesion/MFC | BMAC + fibrin glue | Microfx. | 60 mL/MarrowStim Concentration Kit | Asymptomatic; MRI at 12 months showed good defect filling with normal signal | N/A |
| Skowroński et al. [62] | Orthop Traumatol Rehabil/2013 | Retrospective comparative study/46 | 26.0 | 60.0 | >4cm2 width & >6mm deep/MFC | BMAC (21) vs. Peripheral blood MSCs (25) | Autologous spongy bone graft, collagen membrane | 27 mL/MarrowStim Concentration Kit | Clinical improvement in both groups; Peripheral blood MSCs group had superior results; Confirmed cartilage integration on MRI | N/A |
| Gobbi et al. [58] | Am J Sports Med/2014 | Case series/25 | 46.5 | 41.3 | Mean 8.3 cm2 sized ICRS grade IV lesion/MFC or patellar or trochlea | BMAC | Collagen membrane + fibrin glue | 60 mL/SmartPrep2 System | Significant clinical improvement; Good stability of implant and complete filling in 80% on MRI; Hyaline-like cartilage | N/A |
| Gobbi et al. [57] | Cartilage/2015 | Prospective comparative study/37 | M-ACI (43.1) vs. BMAC (45.4) | ≥36.0 | Mean size 7.1 cm2 (M-ACI) vs. 5.5 cm2 (BMAC) ICRS grade IV lesion/patella or trochlea | M-ACI (19) vs. BMAC (18) | HA scaffold + fibrin glue | 60 mL/SmartPrep2 System | Significant clinical improvement in both groups; no significant difference between the groups; Complete filling on MRI 76.0% (M-ACI) vs. 81.0% (BMAC); Hyaline-like features | N/A |
| Gobbi et al. [68] | Am J Sports Med/2019 | Case series/23 | 48.5 | 96.0 | Mean 6.5 cm2 sized ICRS grade IV lesion /MFC or patellar or trochlea | BMAC + HA-based scaffold | HTO; TTO; ACLR; LR | 60 mL/SmartPrep2 System | Good to excellent long-term clinical outcomes in full-thickness cartilage injury of the knee joint | N/A |
| Enea et al. [56] | Knee/2015 | Case series/9 | 43.0 | 29.0 | Mean size 2.6 cm2 with chondral defect Outerbridge type III- IV/MFC or LFC | BMAC + fibrin glue | Collagen membrane; Microfx.or partial menicectomy or synovectomy | 60 mL/MarrowStim Concentration Kit | Significant clinical improvement; Almost normal arthroscopic appearance of repaired cartilage; Regeneration potential to hyaline-like cartilage | N/A |
| Authors | Publications/Year | Study Design/No. of Patients | Mean Age (Year) | Mean Follow-Up Period (Months) | OA Grades | Treatment | Additional Factors | Harvest Volume/Kit Used | Outcomes | Complications |
|---|---|---|---|---|---|---|---|---|---|---|
| Hauser et al. [70] | Clin Med Insights Arthritis Musculoskelet Disord/2013 | Case series/7 (hip, knee, ankle OA) | 64.0 | 7.1 | N/A | Whole bone marrow injection | Dextrose prolotherapy | Not concentrated | Substantial gain in pain relief & functionality | N/A |
| Centeno et al. [69] | Biomed Res Int/2014 | Retrospective comparative study/840 | 54.3 vs. 59.9 | 10.4 vs. 10.7 | K–L grade 1,2,3,4 | BMAC alone (616) vs. BMAC + adipose graft (224) | PRP | Manual aspiration in a sterile ISO-7 class clean room and in ISO-5 class laminar flow cabinets | Encouraging clinical outcomes with a low rate of AEs; Better results in K–L 2 than K–L 3-4 (2.2 times); Adipose graft did not provide additional benefit | AEs rates 6.0% (BMAC alone) vs. 8,9% (BMAC + adipose graft) |
| Shapiro et al. [72] | Am J Sports Med/2017 | Prospective RCT/25 (bilateral knee OA) | 60.0 | 6.0 | K–L grade 1,2,3 | BMAC vs. Saline | PRP | 52 mL/Automated centrifuge (Magellan Autologous Platelet Separator System) | Pain relief did not differ significantly between both knees | N/A |
| Kim JD et al. [59] | Eur J Orthop Surg Traumatol/2014 | Case series/75 | 60.7 | 8.7 | K–L grade 1,2,3,4 | BMAC | Arthroscopic debridement; Microfx.; HTO | 120 mL/SmartPrep2 System | Significant clinical improvement; Better results in K–L 1-3 than K–L 4 | Swelling: 92.0% Pain: 41.3% |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kim, G.B.; Seo, M.-S.; Park, W.T.; Lee, G.W. Bone Marrow Aspirate Concentrate: Its Uses in Osteoarthritis. Int. J. Mol. Sci. 2020, 21, 3224. https://doi.org/10.3390/ijms21093224
Kim GB, Seo M-S, Park WT, Lee GW. Bone Marrow Aspirate Concentrate: Its Uses in Osteoarthritis. International Journal of Molecular Sciences. 2020; 21(9):3224. https://doi.org/10.3390/ijms21093224
Chicago/Turabian StyleKim, Gi Beom, Min-Soo Seo, Wook Tae Park, and Gun Woo Lee. 2020. "Bone Marrow Aspirate Concentrate: Its Uses in Osteoarthritis" International Journal of Molecular Sciences 21, no. 9: 3224. https://doi.org/10.3390/ijms21093224
APA StyleKim, G. B., Seo, M.-S., Park, W. T., & Lee, G. W. (2020). Bone Marrow Aspirate Concentrate: Its Uses in Osteoarthritis. International Journal of Molecular Sciences, 21(9), 3224. https://doi.org/10.3390/ijms21093224







