Rapid Hormetic Responses of Photosystem II Photochemistry of Clary Sage to Cadmium Exposure
Abstract
:1. Introduction
2. Results
2.1. Cadmium Accumulation and Elemental Concentrations
2.2. Chlorophyll a and Chlorophyll b Content after Cadmium Exposure
2.3. The Efficiency of Photosystem II after Cadmium Exposure
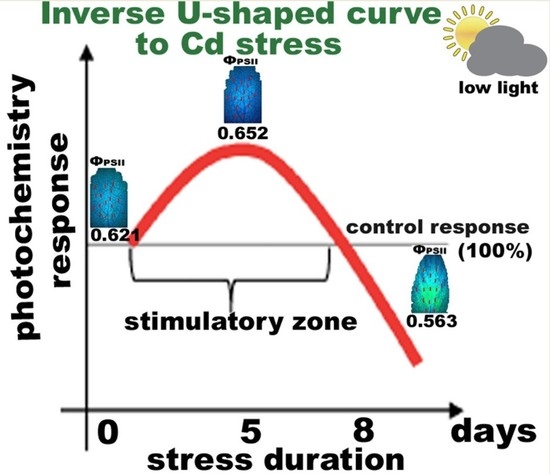
2.4. Changes in the Quantum Yields and the Fraction of Open Photosystem II Reaction Centers after Cadmium Exposure under Low Light
2.5. Changes in Non-Photochemical Fluorescence Quenching and Electron Transport Rate after Cadmium Exposure under Low Light
2.6. Changes in Excess Excitation Energy under Low and High Light after Cadmium Exposure
2.7. Changes in the Quantum Yields under High Light after Cadmium Exposure
2.8. Changes in Non-Photochemical Fluorescence Quenching, Electron Transport Rate, and the Fraction of Open Photosystem II Reaction Centers under High Light after Cadmium Exposure
2.9. Chlorophyll a Fluorescence Images under Low and High Light
2.10. Lipid Peroxidation and Hydrogen Peroxide (H2O2) after Cadmium Exposure
2.11. Chloroplast Ultrastructure after Cadmium Exposure
3. Discussion
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Cadmium Treatment
4.3. Determination of Elemental Concentration by Inductively Coupled Plasma Mass Spectrometry (ICP-MS)
4.4. Measurements of Chlorophyll a and Chlorophyll b Content
4.5. Chlorophyll Fluorescence Imaging Analysis
4.6. Determination of Oxidative Damage
4.7. Leaf Ultrastructure Observations by Transmission Electron Microscopy
4.8. Statistical Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
Appendix A.1. ICP-MS Operational Conditions
Appendix A.2. Calibration Solutions
Appendix A.3. ICP-MS Instrumental Settings
Appendix A.4. Data Validation
Appendix A.5. Validation Parameters
References
- Küpper, H.; Parameswaran, A.; Leitenmaier, B.; Trtílek, M.; Šerlík, I. Cadmium induced inhibition of photosynthesis and long-term acclimation to cadmium stress in the hyperaccumulator Thlaspi caerulescens. New Phytol. 2007, 175, 655–674. [Google Scholar]
- Sharma, R.K.; Agrawal, M.; Marshall, F. Heavy metal contamination of soil and vegetables in suburban areas of Varanasi, India. Ecotoxicol. Environ. Saf. 2007, 66, 258–266. [Google Scholar] [CrossRef] [PubMed]
- Clemens, S.; Ma, J.F. Toxic heavy metal and metalloid accumulation in crop plants and foods. Ann. Rev. Plant Biol. 2016, 67, 489–512. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bayçu, G.; Moustaka, J.; Gevrek-Kürüm, N.; Moustakas, M. Chlorophyll fluorescence imaging analysis for elucidating the mechanism of photosystem II acclimation to cadmium exposure in the hyperaccumulating plant Noccaea caerulescens. Materials 2018, 11, 2580. [Google Scholar] [CrossRef] [Green Version]
- Dobrikova, A.G.; Apostolova, E.L. Damage and protection of the photosynthetic apparatus under cadmium stress. In Cadmium Toxicity and Tolerance in Plants: From Physiology to Remediation, 1st ed.; Hasanuzzaman, M., Prasad, M.N.V., Fujita, M., Eds.; Academic Press: Cambridge, MA, USA, 2019; pp. 275–298. [Google Scholar]
- Moustakas, M.; Hanć, A.; Dobrikova, A.; Sperdouli, I.; Adamakis, I.D.S.; Apostolova, E. Spatial heterogeneity of cadmium effects on Salvia sclarea leaves revealed by chlorophyll fluorescence imaging analysis and laser ablation inductively coupled plasma mass spectrometry. Materials 2019, 12, 2953. [Google Scholar] [CrossRef] [Green Version]
- Bayçu, G.; Gevrek-Kürüm, N.; Moustaka, J.; Csatári, I.; Rognes, S.E.; Moustakas, M. Cadmium-zinc accumulation and photosystem II responses of Noccaea caerulescens to Cd and Zn exposure. Environ. Sci. Pollut. Res. 2017, 24, 2840–2850. [Google Scholar] [CrossRef]
- Carvalho, M.E.A.; Castro, P.R.C.; Azevedo, R.A. Hormesis in plants under Cd exposure: From toxic to beneficial element? J. Hazard. Mater. 2020, 384, 121434. [Google Scholar] [CrossRef]
- Muszynska, E.; Hanus-Fajerska, E.; Ciarkowska, K. Studies on lead and cadmium toxicity in Dianthus carthusianorum calamine ecotype cultivated in vitro. Plant Biol. 2018, 20, 474–482. [Google Scholar] [CrossRef]
- Carvalho, M.E.A.; Piotto, F.A.; Franco, M.R.; Rossi, M.L.; Martinelli, A.P.; Cuypers, A.; Azevedo, R.A. Relationship between Mg, B and Mn status and tomato tolerance against Cd toxicity. J. Environ. Manag. 2019, 240, 84–92. [Google Scholar] [CrossRef]
- Kato, F.H.; Carvalho, M.E.A.; Gaziola, S.A.; Piotto, F.A.; Azevedo, R.A. Lysine metabolism and amino acid profile in maize grains from plants subjected to cadmium exposure. Sci. Agric. 2020, 77, e20180095. [Google Scholar] [CrossRef]
- Assunção, A.G.L.; Bookum, W.M.; Nelissen, H.J.M.; Vooijs, R.; Schat, H.; Ernst, W.H.O. Differential metal-specific tolerance and accumulation patterns among Thlaspi caerulescens populations originating from different soil types. New Phytol. 2003, 159, 411–419. [Google Scholar] [CrossRef]
- Sperdouli, I.; Moustakas, M. Spatio-temporal heterogeneity in Arabidopsis thaliana leaves under drought stress. Plant Biol. 2012, 14, 118–128. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Moustakas, M. Leaf developmental stage modulates metabolite accumulation and photosynthesis contributing to acclimation of Arabidopsis thaliana to water deficit. J. Plant Res. 2014, 127, 481–489. [Google Scholar] [CrossRef] [PubMed]
- Sperdouli, I.; Moustakas, M. A better energy allocation of absorbed light in photosystem II and less photooxidative damage contribute to acclimation of Arabidopsis thaliana young leaves to water deficit. J. Plant Physiol. 2014, 171, 587–593. [Google Scholar] [CrossRef] [PubMed]
- Agathokleous, E. Environmental hormesis, a fundamental non-monotonic biological phenomenon with implications in ecotoxicology and environmental safety. Ecotoxicol. Environ. Saf. 2018, 148, 1042–1053. [Google Scholar] [CrossRef] [Green Version]
- Christou, A.; Michael, C.; Fatta-Kassinos, D.; Fotopoulos, V. Can the pharmaceutically active compounds released in agroecosystems be considered as emerging plant stressors? Environ. Int. 2018, 114, 360–364. [Google Scholar] [CrossRef]
- Muszynska, E.; Labudda, M. Dual role of metallic trace elements in stress biology-from negative to beneficial impact on plants. Int. J. Mol. Sci. 2019, 20, 3117. [Google Scholar] [CrossRef] [Green Version]
- Agathokleous, E.; Feng, Z.; Peñuelas, J. Chlorophyll hormesis: Are chlorophylls major components of stress biology in higher plants? Sci. Total Environ. 2020, 726, 138637. [Google Scholar] [CrossRef]
- Mittler, R. ROS are good. Trends Plant Sci. 2017, 22, 11–19. [Google Scholar] [CrossRef] [Green Version]
- Czarnocka, W.; Karpiński, S. Friend or foe? Reactive oxygen species production, scavenging and signaling in plant response to environmental stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Harayama, H. On the non-monotonic, hermetic photoprotective response of plants to stress. Dose-Response 2019, 17, 1–3. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bellini, E.; De Tullio, M.C. Ascorbic acid and ozone: Novel perspectives to explain an elusive relationship. Plants 2019, 8, 122. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rozhko, T.V.; Nogovitsyna, E.I.; Badun, G.A.; Lukyanchuk, A.N.; Kudryasheva, N.S. Reactive Oxygen Species and low-dose effects of tritium on bacterial cells. J. Environ. Radioact. 2019, 208–209, 106035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Adamakis, I.D.S.; Sperdouli, I.; Eleftheriou, E.P.; Moustakas, M. Hydrogen peroxide production by the spot-like mode action of bisphenol A. Front. Plant Sci. 2020, 11, 1196. [Google Scholar] [CrossRef] [PubMed]
- Malea, P.; Charitonidou, K.; Sperdouli, I.; Mylona, Z.; Moustakas, M. Zinc uptake, photosynthetic efficiency and oxidative stress in the seagrass Cymodocea nodosa exposed to ZnO nanoparticles. Materials 2019, 12, 2101. [Google Scholar] [CrossRef] [Green Version]
- Sperdouli, I.; Moustaka, J.; Antonoglou, O.; Adamakis, I.-D.S.; Dendrinou-Samara, C.; Moustakas, M. Leaf age-dependent effects of foliar-sprayed CuZn nanoparticles on photosynthetic efficiency and ROS generation in Arabidopsis thaliana. Materials 2019, 12, 2498. [Google Scholar] [CrossRef] [Green Version]
- Roach, T.; Na, C.S.; Stöggl, W.; Krieger-Liszkay, A. The non-photochemical quenching protein LHCSR3 prevents oxygen-dependent photoinhibition in Chlamydomonas reinhardtii. J. Exp. Bot. 2020, 71, 2650–2660. [Google Scholar] [CrossRef]
- Moustakas, M.; Bayçu, G.; Sperdouli, I.; Eroğlu, H.; Eleftheriou, E.P. Arbuscular mycorrhizal symbiosis enhances photosynthesis in the medicinal herb Salvia fruticosa by improving photosystem II photochemistry. Plants 2020, 9, 962. [Google Scholar] [CrossRef]
- Nishiyama, Y.; Yamamoto, H.; Allakhverdiev, S.I.; Inaba, M.; Yokota, A.; Murata, N. Oxidative stress inhibits the repair of photodamage to the photosynthetic machinery. EMBO J. 2001, 20, 5587–5594. [Google Scholar] [CrossRef]
- Murata, N.; Takahashi, S.; Nishiyama, Y.; Allakhverdiev, S.I. Photoinhibition of photosystem II under environmental stress. Biochim. Biophys. Acta 2007, 1767, 414–421. [Google Scholar] [CrossRef] [Green Version]
- Kale, R.; Hebert, A.E.; Frankel, L.K.; Sallans, L.; Bricker, T.M.; Pospíšil, P. Amino acid oxidation of the D1 and D2 proteins by oxygen radicals during photoinhibition of Photosystem II. Proc. Natl. Acad. Sci. USA 2017, 114, 2988–2993. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szopiński, M.; Sitko, K.; Gieroń, Z.; Rusinowski, S.; Corso, M.; Hermans, C.; Verbruggen, N.; Małkowski, E. Toxic Effects of Cd and Zn on the photosynthetic apparatus of the Arabidopsis halleri and Arabidopsis arenosa pseudo-metallophytes. Front. Plant Sci. 2019, 10, 748. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: A compelling platform for sophisticated plant science. Trends Plant Sci. 2019, 24, 318–327. [Google Scholar] [CrossRef] [PubMed]
- Shahid, M.; Niazi, N.K.; Rinklebe, J.; Bundschuh, J.; Dumat, C.; Pinelli, E. Trace elements-induced phytohormesis: A critical review and mechanistic interpretation. Crit. Rev. Environ. Sci. Technol. 2020, 50, 1984–2015. [Google Scholar] [CrossRef]
- Małkowski, E.; Sitko, K.; Szopiński, M.; Gieroń, Z.; Pogrzeba, M.; Kalaji, H.M.; Zieléznik-Rusinowska, P. Hormesis in plants: The role of oxidative stress, auxins and photosynthesis in corn treated with Cd or Pb. Int. J. Mol. Sci. 2020, 21, 2099. [Google Scholar] [CrossRef] [Green Version]
- Kudryasheva, N.S.; Rozhko, T.V. Effect of low-dose ionizing radiation on luminous marine bacteria: Radiation hormesis and toxicity. J. Environ. Radioact. 2015, 142, 68–77. [Google Scholar] [CrossRef]
- Agathokleous, E.; Kitao, M.; Calabrese, E.J. Hormesis: Highly generalizable and beyond laboratory. Trends Plant Sci. 2020, 25, 1076–1086. [Google Scholar] [CrossRef]
- Calabrese, E.J. Hormetic mechanisms. Crit. Rev. Toxicol. 2013, 43, 580–606. [Google Scholar] [CrossRef]
- Agathokleous, E.; Calabrese, E.J. Hormesis: The dose response for the 21st Century: The future has arrived. Toxicology 2019, 425, 152249. [Google Scholar] [CrossRef]
- Agathokleous, E.; Feng, Z.; Iavicoli, I.; Calabrese, E.J. The two faces of nanomaterials: A quantification of hormesis in algae and plants. Environ. Int. 2019, 131, 105044. [Google Scholar] [CrossRef]
- Calabrese, E.J. Evidence that hormesis represents an ‘‘overcompensation’’ response to a disruption in homeostasis. Ecotoxicol. Environ. Saf. 1999, 42, 135–137. [Google Scholar] [CrossRef] [PubMed]
- Kuźma, L.; Kalemba, D.; Rózalski, M.; Rózalska, B.; Wieckowska-Szakiel, M.; Krajewska, U.; Wysokińska, H. Chemical composition and biological activities of essential oil from Salvia sclarea plants regenerated in vitro. Molecules 2009, 14, 1438–1447. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zheljazkov, V.D.; Nielsen, N.E. Growing clary sage (Salvia sclarea L.) in heavy metal-polluted areas. Acta Hortic. 1996, 426, 309–328. [Google Scholar] [CrossRef]
- Khan, N.A.; Singh, S.; Anjum, N.A.; Nazar, R. Cadmium effects on carbonic anhydrase, photosynthesis, dry mass and antioxidative enzymes in wheat (Triticum aestivum) under low and sufficient zinc. J. Plant Interact. 2008, 3, 31–37. [Google Scholar] [CrossRef]
- Vassilev, A.; Perez-Sanz, A.; Semane, B.; Carleer, R.; Vangronsveld, J. Cadmium accumulation and tolerance of two Salix genotypes hydroponically grown in presence of cadmium. J. Plant Nutr. 2005, 28, 2159–2177. [Google Scholar] [CrossRef]
- Ismail, S.; Khan, F.; Iqbal, M.Z. Phytoremediation: Assessing tolerance of tree species against heavy metal (Pb and Cd) toxicity. Pak. J. Bot. 2013, 45, 2181–2186. [Google Scholar]
- Dixit, V.; Pandey, V.; Shyam, R. Differential oxidative responses to cadmium in roots and leaves of pea (Pisum sativum). J. Exp. Bot. 2001, 52, 1101–1109. [Google Scholar] [CrossRef] [Green Version]
- Gratão, P.L.; Monteiro, C.C.; Tezotto, T.; Carvalho, R.F.; Alves, L.R.; Peters, L.P.; Azevedo, R.A. Cadmium stress antioxidant responses and root-to-shoot communication in grafted tomato plants. BioMetals 2015, 28, 803–816. [Google Scholar] [CrossRef]
- Mizushima, M.Y.B.; Ferreira, B.G.; França, M.G.C.; Almeida, A.A.F.; Cortez, P.A.; Silva, J.V.S.; Jesus, R.M.; Prasad, M.N.V.; Mangabeira, P.A.O. Ultrastructural and metabolic disorders induced by short-term cadmium exposure in Avicennia schaueriana plants and its excretion through leaf salt glands. Plant Biol. 2019, 21, 844–853. [Google Scholar] [CrossRef]
- Caldelas, C.; Weiss, D.J. Zinc homeostasis and isotopic fractionation in plants: A review. Plant Soil 2017, 411, 17–46. [Google Scholar] [CrossRef] [Green Version]
- Moustakas, M.; Bayçu, G.; Gevrek-Kürüm, N.; Moustaka, J.; Csatári, I.; Rognes, S.E. Spatiotemporal heterogeneity of photosystem II function during acclimation to zinc exposure and mineral nutrition changes in the hyperaccumulator Noccaea caerulescens. Environ. Sci. Pollut. Res. 2019, 26, 6613–6624. [Google Scholar] [CrossRef] [PubMed]
- White, P.J.; Brown, P.H. Plant nutrition for sustainable development and global health. Ann. Bot. Lond. 2010, 105, 1073–1080. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leitenmaier, B.; Küpper, H. Cadmium uptake and sequestration kinetics in individual leaf cell protoplasts of the Cd/Zn hyperaccumulator Thlaspi caerulescens. Plant Cell Environ. 2011, 34, 208–219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maestri, E.; Marmiroli, M.; Visioli, G.; Marmiroli, N. Metal tolerance and hyperaccumulation: Costs and trade-offs between traits and environment. Environ. Exp. Bot. 2010, 68, 1–13. [Google Scholar] [CrossRef]
- Wójcik, M.; Dresler, S.; Plak, A.; Tukiendorf, A. Naturally evolved enhanced Cd tolerance of Dianthus carthusianorum L. is not related to accumulation of thiol peptides and organic acids. Environ. Sci. Pollut. Res. 2015, 22, 7906–7917. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.S.; Dietz, K.J.; Mimura, T. Vacuolar compartmentalization as indispensable component of heavy metal detoxification in plants. Plant Cell Environ. 2016, 39, 1112–1126. [Google Scholar] [CrossRef]
- Verbruggen, N.; Hermans, C.; Schat, H. Mechanisms to cope with arsenic or cadmium excess in plants. Curr. Opin. Plant Biol. 2009, 12, 364–372. [Google Scholar] [CrossRef]
- He, H.; Wang, X.; Wu, M.; Guo, L.; Fan, C.; Peng, Q. Cadmium and lead affect the status of mineral nutrients in alfalfa grown on a calcareous soil. Soil Sci. Plant Nutr. 2020, 66, 506–514. [Google Scholar] [CrossRef]
- Perfus-Barbeoch, L.; Leonhardt, N.; Vavaddeur, A.; Forestier, C. Heavy metal toxicity: Cadmium permeates through calcium channels and disturbs the plant water status. Plant J. 2002, 32, 539–548. [Google Scholar] [CrossRef]
- Gallego, S.M.; Pena, L.B.; Barcia, R.A.; Azpilicueta, C.E.; Iannone, M.F.; Rosales, E.P.; Zawoznik, M.S.; Groppa, M.D.; Benavides, M.P. Unravelling cadmium toxicity and tolerance in plants: Insight into regulatory mechanisms. Environ. Exp. Bot. 2012, 83, 33–46. [Google Scholar] [CrossRef]
- Suzuki, N. Alleviation by calcium of cadmium-induced root growth inhibition in Arabidopsis seedlings. Plant Biotechnol. J. 2005, 22, 19–25. [Google Scholar] [CrossRef] [Green Version]
- Li, S.; Yu, J.; Zhu, M.; Zhao, F.; Luan, S. Cadmium impairs ion homeostasis by altering K+ and Ca2+ channel activities in rice root hair cells. Plant Cell Environ. 2012, 35, 1998–2013. [Google Scholar] [CrossRef] [PubMed]
- Chou, T.S.; Chao, Y.Y.; Huang, W.D.; Hong, C.Y.; Kao, C.H. Effect of magnesium deficiency on antioxidant status and cadmium toxicity in rice seedlings. J. Plant Physiol. 2011, 168, 1021–1030. [Google Scholar] [CrossRef] [PubMed]
- Hermans, C.; Chen, J.; Coppens, F.; Inzé, D.; Verbruggen, N. Low magnesium status in plants enhances tolerance to cadmium exposure. New Phytol. 2011, 192, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Kudo, H.; Kudo, K.; Uemura, M.; Kawai, S. Magnesium inhibits cadmium translocation from roots to shoots, rather than the uptake from roots, in barley. Botany 2015, 93, 345–351. [Google Scholar] [CrossRef]
- Borišev, M.; Pajevic, S.; Nikolic, N.; Orlovic, S.; Župunski, M.; Pilipovic, A.; Kebert, M. Magnesium and iron deficiencies alter Cd accumulation in Salix viminalis L. Int. J. Phytoremediat. 2016, 18, 164–170. [Google Scholar] [CrossRef]
- Carvalho, M.E.A.; Castro, P.R.C.; Kozak, M.; Azevedo, R.A. The sweet side of misbalanced nutrients in cadmium-stressed plants. Ann. Appl. Biol. 2020, 176, 275–284. [Google Scholar] [CrossRef]
- Das, P.; Samantaray, S.; Rout, G.R. Studies on cadmium toxicity in plants: A review. Environ. Pollut. 1997, 98, 29–36. [Google Scholar] [CrossRef]
- Clemens, S. Toxic metal accumulation, responses to exposure and mechanisms of tolerance in plants. Biochimie 2006, 88, 1707–1719. [Google Scholar] [CrossRef]
- Yoshihara, T.; Hodoshima, H.; Miyano, Y.; Shoji, K.; Shimada, H.; Goto, F. Cadmium inducible Fe deficiency responses observed from macro and molecular views in tobacco plants. Plant Cell Rep. 2006, 25, 365–373. [Google Scholar] [CrossRef]
- Xu, S.S.; Lin, S.Z.; Lai, Z.X. Cadmium impairs iron homeostasis in Arabidopsis thaliana by increasing the polysaccharide contents and the iron-binding capacity of root cell walls. Plant Soil 2015, 392, 71–85. [Google Scholar] [CrossRef]
- Sasaki, A.; Yamaji, N.; Yokosho, K.; Ma, J.F. Nramp5 is a major transporter responsible for manganese and cadmium uptake in rice. Plant Cell 2012, 24, 2155–2167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, F.J.; Wang, P. Arsenic and cadmium accumulation in rice and mitigation strategies. Plant Soil 2020, 446, 1–21. [Google Scholar] [CrossRef]
- Guo, Q.; Meng, L.; Zhang, Y.N.; Mao, P.C.; Tian, X.X.; Li, S.S.; Zhang, L. Antioxidative systems, metal ion homeostasis and cadmium distribution in Iris lactea exposed to cadmium stress. Ecotoxicol. Environ. Saf. 2017, 139, 50–55. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.E.; Feng, Y.; He, Z.L.; Stoffella, P.J. Molecular mechanisms of heavy metal hyperaccumulation and phytoremediation. J. Trace Elem. Med. Biol. 2005, 18, 339–353. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.F.; Xia, H.P.; Li, Z.A.; Zhuang, P.; Cao, B. Potential of four forage grasses in remediation of Cd and Zn contaminated soils. Bioresour. Technol. 2010, 101, 2063–2066. [Google Scholar] [CrossRef]
- Yoon, J.; Cao, X.; Zhou, Q.; Ma, L.Q. Accumulation of Pb, Cu, and Zn in native plants growing on a contaminated Florida site. Sci. Total Environ. 2006, 368, 456–464. [Google Scholar] [CrossRef]
- Lokhande, V.H.; Srivastava, S.; Patade, V.Y.; Dwivedi, S.; Tripathi, R.D.; Nikam, T.D.; Suprasanna, P. Investigation of arsenic accumulation and tolerance potential of Sesuvium portulacastrum (L.) L. Chemosphere 2011, 82, 529–534. [Google Scholar] [CrossRef]
- Feng, J.; Lin, Y.; Yang, Y.; Shen, Q.; Huang, J.; Wang, S.; Zhu, X.; Li, Z. Tolerance and bioaccumulation of Cd and Cu in Sesuvium portulacastrum. Ecotoxicol. Environ. Saf. 2018, 147, 306–312. [Google Scholar] [CrossRef]
- Müller, P.; Li, X.P.; Niyogi, K.K. Non-photochemical quenching. A response to excess light energy. Plant Physiol. 2001, 125, 1558–1566. [Google Scholar] [CrossRef] [Green Version]
- Gawroński, P.; Witoń, D.; Vashutina, K.; Bederska, M.; Betliński, B.; Rusaczonek, A.; Karpiński, S. Mitogen-activated protein kinase 4 is a salicylic acid-independent regulator of growth but not of photosynthesis in Arabidopsis. Mol. Plant 2014, 7, 1151–1166. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moustaka, J.; Tanou, G.; Giannakoula, A.; Panteris, E.; Eleftheriou, E.P.; Moustakas, M. Anthocyanin accumulation in poinsettia leaves and its functional role in photo-oxidative stress. Environ. Exp. Bot. 2020, 175, 104065. [Google Scholar] [CrossRef]
- Li, X.; Wakao, S.; Fischer, B.B.; Niyogi, K.K. Sensing and responding to excess light. Annu. Rev. Plant Biol. 2009, 60, 239–260. [Google Scholar] [CrossRef] [PubMed]
- Fu, X.; Liu, C.; Li, Y.; Liao, S.; Cheng, H.; Tu, Y.; Zhu, X.; Chen, K.; He, Y.; Wang, G. The coordination of OsbZIP72 and OsMYBS2 with reverse roles regulates the transcription of OsPsbS1 in rice. New Phytol. 2020. [Google Scholar] [CrossRef]
- Külheim, C.; Ågren, J.; Jansson, S. Rapid regulation of light harvesting and plant fitness in the field. Science 2002, 297, 91–93. [Google Scholar] [CrossRef] [PubMed]
- Li, X.; Müller-Moulé, P.; Gilmore, A.M.; Niyogi, K.K. PsbS-dependent enhancement of feedback de-excitation protects photosystem II from photoinhibition. Proc. Natl. Acad. Sci. USA 2002, 99, 15222–15227. [Google Scholar] [CrossRef] [Green Version]
- Moustaka, J.; Moustakas, M. Photoprotective mechanism of the non-target organism Arabidopsis thaliana to paraquat exposure. Pest. Biochem. Physiol. 2014, 111, 1–6. [Google Scholar] [CrossRef]
- Moustaka, J.; Tanou, G.; Adamakis, I.D.; Eleftheriou, E.P.; Moustakas, M. Leaf age dependent photoprotective and antioxidative mechanisms to paraquat-induced oxidative stress in Arabidopsis thaliana. Int. J. Mol. Sci. 2015, 16, 13989–14006. [Google Scholar] [CrossRef] [Green Version]
- Ruban, A.V. Nonphotochemical chlorophyll fluorescence quenching: Mechanism and effectiveness in protecting plants from photodamage. Plant Physiol. 2016, 170, 1903–1916. [Google Scholar] [CrossRef] [Green Version]
- Kalaji, M.H.; Carpentier, R.; Allakhverdiev, S.I.; Bosa, K. Fluorescence parameters as an early indicator of light stress in barley. J. Photochem. Photobiol. B 2012, 112, 1–6. [Google Scholar] [CrossRef]
- Moustaka, J.; Ouzounidou, G.; Bayçu, G.; Moustakas, M. Aluminum resistance in wheat involves maintenance of leaf Ca2+ and Mg2+ content, decreased lipid peroxidation and Al accumulation, and low photosystem II excitation pressure. BioMetals 2016, 29, 611–623. [Google Scholar] [CrossRef] [PubMed]
- Moustaka, J.; Ouzounidou, G.; Sperdouli, I.; Moustakas, M. Photosystem II is more sensitive than photosystem I to Al3+ induced phytotoxicity. Materials 2018, 11, 1772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Takahashi, S.; Badger, M.R. Photoprotection in plants: A new light on photosystem II damage. Trends Plant Sci. 2011, 16, 53–60. [Google Scholar] [CrossRef] [PubMed]
- Dietz, K.J.; Pfannschmidt, T. Novel regulators in photosynthetic redox control of plant metabolism and gene expression. Plant Physiol. 2011, 155, 1477–1485. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kendig, E.L.; Le, H.H.; Belcher, S.M. Defining hormesis: Evaluation of a complex concentration response phenomenon. Int. J. Toxicol. 2010, 29, 235–246. [Google Scholar] [CrossRef]
- Szopiński, M.; Sitko, K.; Rusinowski, S.; Zieleźnik-Rusinowska, P.; Corso, M.; Rostański, A.; Rojek-Jelonek, M.; Verbruggen, N.; Małkowski, E. Different strategies of Cd tolerance and accumulation in Arabidopsis halleri and Arabidopsis arenosa. Plant Cell Environ. 2020, 43, 3002–3019. [Google Scholar] [CrossRef]
- Tang, L.; Yao, A.; Yuan, M.; Tang, Y.; Liu, J.; Liu, X.; Qiu, R. Transcriptional up-regulation of genes involved in photosynthesis of the Zn/Cd hyperaccumulator Sedum alfredii in response to zinc and cadmium. Chemosphere 2016, 164, 190–200. [Google Scholar] [CrossRef]
- Lombi, E.; Zhao, F.J.; Dunham, S.J.; McGrath, S.P. Cadmium accumulation in populations of Thlaspi caerulescens and Thlaspi goesingense. New Phytol. 2000, 145, 11–20. [Google Scholar] [CrossRef] [Green Version]
- Faller, P.; Kienzler, K.; Krieger-Liszkay, A. Mechanism of Cd2+ toxicity: Cd2+ inhibits photoactivation of photosystem II by competitive binding to the essential Ca2+ site. Biochim. Biophys. Acta 2005, 1706, 158–164. [Google Scholar] [CrossRef] [Green Version]
- Ekmekçi, Y.; Tanyolaç, D.; Ayhan, B. Effects of cadmium on antioxidant enzyme and photosynthetic activities in leaves of two maize cultivars. J. Plant Physiol. 2008, 65, 600–611. [Google Scholar] [CrossRef]
- Parmar, P.; Kumari, N.; Sharma, V. Structural and functional alterations in photosynthetic apparatus of plants under cadmium stress. Bot. Stud. 2013, 54, 45. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dobrikova, A.G.; Yotsova, E.K.; Börner, A.; Landjeva, S.P.; Apostolova, E.L. The wheat mutant DELLA-encoding gene (Rht-B1c) afects plant photosynthetic responses to cadmium stress. Plant Physiol. Biochem. 2017, 114, 10–18. [Google Scholar] [CrossRef]
- Yotsova, E.K.; Dobrikova, A.G.; Stefanov, M.; Misheva, S.; Bardácová, M.; Matusíková, I.; Zideková, L.; Blehová, A.; Apostolova, E. Effects of cadmium on two wheat cultivars depending on different nitrogen supply. Plant Physiol. Biochem. 2020, 155, 789–799. [Google Scholar] [CrossRef]
- Hanć, A.; Małecka, A.; Kutrowska, A.; Bagniewska-Zadworna, A.; Tomaszewska, B.; Barałkiewicz, D. Direct analysis of elemental biodistribution in pea seedlings by LA-ICP-MS, EDX and confocal microscopy: Imaging and quantification. Microchem. J. 2016, 128, 305–311. [Google Scholar] [CrossRef]
- Małecka, A.; Konkolewska, A.; Hanć, A.; Barałkiewicz, B.; Ciszewska, L.; Ratajczak, E.; Staszak, A.M.; Kmita, H.; Jarmuszkiewicz, W. Insight into the phytoremediation capability of Brassica juncea (v. Malopolska): Metal accumulation and antioxidant enzyme activity. Int. J. Mol. Sci. 2019, 20, 4355–4372. [Google Scholar]
- Adamakis, I.D.S.; Malea, P.; Sperdouli, I.; Panteris, E.; Kokkinidi, D.; Moustakas, M. Evaluation of the spatiotemporal effects of bisphenol A on the leaves of the seagrass Cymodocea nodosa. J. Hazard. Mater. 2021, 404, 124001. [Google Scholar] [CrossRef]
- Arena, C.; Figlioli, F.; Sorrentino, M.C.; Izzo, L.G.; Capozzi, F.; Giordano, S.; Spagnuolo, V. Ultrastructural, protein and photosynthetic alterations induced by Pb and Cd in Cynara cardunculus L. and its potential for phytoremediation. Ecotoxicol. Environ. Saf. 2017, 145, 83–89. [Google Scholar] [CrossRef]
- Sorrentino, M.C.; Capozzi, F.; Amitrano, C.; Giordano, S.; Arena, C.; Spagnuolo, V. Performance of three cardoon cultivars in an industrial heavy metal contaminated soil: Effects on morphology, cytology and photosynthesis. J. Hazard. Mater. 2018, 351, 131–137. [Google Scholar] [CrossRef]
- Delhaize, E.; Schachtman, D.; Kochian, L.; Ryan, P.R. Mineral Nutrient Acquisition, Transport, and Utilization. In Biochemistry and Molecular Biology of Plants, 2nd ed.; Buchanan, B.B., Gruissem, W., Russell, L.J., Eds.; John Wiley & Sons, Ltd.: Hoboken, NJ, USA, 2015; pp. 1101–1131. [Google Scholar]
- Souza, V.L.; de Almeida, A.A.; Lima, S.G.; de M Cascardo, J.C.; da C Silva, D.; Mangabeira, P.A.; Gomes, F.P. Morphophysiological responses and programmed cell death induced by cadmium in Genipa americana L. (Rubiaceae). BioMetals 2011, 24, 59–71. [Google Scholar] [CrossRef]
- Barcelo, J.; Vazquez, M.D.; Poschenrieder, C.H. Structural and ultrastructural disorders in cadmium-treated bush bean plants (Phaseolus vulgaris L.). New Phytol. 1988, 108, 37–49. [Google Scholar] [CrossRef]
- Ouzounidou, G.; Moustakas, M.; Eleftheriou, E.P. Physiological and ultrastructural effects of cadmium on wheat (Triticum aestivum L.) leaves. Arch. Environ. Contam. Toxicol. 1997, 32, 154–160. [Google Scholar] [CrossRef] [PubMed]
- Daud, M.K.; Variath, M.T.; Ali, S.; Najeeb, U.; Jamil, M.; Hayat, Y.; Dawoo, M.; Khan, M.I.; Zaffar, M.; Cheema, S.A.; et al. Cadmium-induced ultramorphological and physiological changes in leaves of two transgenic cotton cultivars and their wild relative. J. Hazard. Mater. 2009, 168, 614–625. [Google Scholar] [CrossRef] [PubMed]
- Najeeb, U.; Jilani, G.; Ali, S.; Sarwar, M.; Xu, L.; Zhou, W. Insights into cadmium induced physiological and ultra-structural disorders in Juncus effusus L. and its remediation through exogenous citric acid. J. Hazard. Mater. 2011, 186, 565–574. [Google Scholar] [CrossRef] [PubMed]
- Pietrini, F.; Iannelli, M.A.; Pasqualini, S.; Massacci, A. Interaction of cadmium with glutathione and photosynthesis in developing leaves and chloroplasts of Phragmites australis (Cav.) Trin. ex Steudel. Plant Physiol. 2003, 133, 829–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gratão, P.L.; Monteiro, C.C.; Rossi, M.L.; Martinelli, A.P.; Peres, L.E.P.; Medici, L.O.; Lea, P.J.; Azevedo, R.A. Differential ultrastructural changes in tomato hormonal mutants exposed to cadmium. Environ. Exp. Bot. 2009, 67, 387–394. [Google Scholar] [CrossRef]
- Vazquez, M.D.; Poschenrieder, C.; Barcelo, J. Pulvinus structure and leaf abscission in cadmium-treated bean plants (Phaseolus vulgaris). Can. J. Bot. 1989, 67, 2756–2764. [Google Scholar] [CrossRef]
- Savvides, A.; Ali, S.; Tester, M.; Fotopoulos, V. Chemical priming of plants against multiple abiotic stresses: Mission possible? Trends Plant Sci. 2016, 21, 329–340. [Google Scholar] [CrossRef] [Green Version]
- Tanou, G.; Fotopoulos, V.; Molassiotis, A. Priming against environmental challenges and proteomics in plants: Update and agricultural perspectives. Front. Plant Sci. 2012, 3, 216. [Google Scholar] [CrossRef] [Green Version]
- Calabrese, E.J. Preconditioning is hormesis part II: How the conditioning dose mediates protection: Dose optimization within temporal and mechanistic frameworks. Pharmacol. Res. 2016, 110, 265–275. [Google Scholar] [CrossRef]
- Lambrev, P.H.; Miloslavina, Y.; Jahns, P.; Holzwarth, A.R. On the relationship between non-photochemical quenching and photoprotection of photosystem II. Biochim. Biophys. Acta 2012, 1817, 760–769. [Google Scholar] [CrossRef] [Green Version]
- Moustakas, M.; Malea, P.; Zafeirakoglou, A.; Sperdouli, I. Photochemical changes and oxidative damage in the aquatic macrophyte Cymodocea nodosa exposed to paraquat-induced oxidative stress. Pest. Biochem. Physiol. 2016, 126, 28–34. [Google Scholar] [CrossRef] [PubMed]
- Moustakas, M.; Malea, P.; Haritonidou, K.; Sperdouli, I. Copper bioaccumulation, photosystem II functioning and oxidative stress in the seagrass Cymodocea nodosa exposed to copper oxide nanoparticles. Environ. Sci. Pollut. Res. 2017, 24, 16007–16018. [Google Scholar] [CrossRef]
- Antonoglou, O.; Moustaka, J.; Adamakis, I.D.; Sperdouli, I.; Pantazaki, A.; Moustakas, M.; Dendrinou-Samara, C. Nanobrass CuZn nanoparticles as foliar spray non phytotoxic fungicides. ACS Appl. Mater. Interfaces 2018, 10, 4450–4461. [Google Scholar] [CrossRef]
- Dobrikova, A.; Apostolova, E.; Hanć, A.; Yotsova, E.; Borisova, P.; Sperdouli, I.; Adamakis, I.D.S.; Moustakas, M. Cadmium toxicity on Salvia sclarea: An integrative response of elemental uptake, oxidative stress markers, leaf structure and photosynthesis. Ecotoxicol. Environ. Saf. 2021. [Google Scholar] [CrossRef]
- Borowiak, K.; Budka, A.; Lisiak-Zielińska, M.; Hanć, A.; Zbierska, J.; Barałkiewicz, D.; Kayzer, D.; Gaj, R.; Szymczak-Graczyk, A.; Kanclerz, J. Accumulation of airborne toxic elements and photosynthetic performance of Lolium multiflorum L. leaves. Processes 2020, 8, 1013. [Google Scholar] [CrossRef]
- Lichtenthaler, H.K. Chlorophylls and carotenoids pigments of photosynthetic membranes. Methods Enzymol. 1987, 148, 350–382. [Google Scholar]
- Moustaka, J.; Panteris, E.; Adamakis, I.D.S.; Tanou, G.; Giannakoula, A.; Eleftheriou, E.P.; Moustakas, M. High anthocyanin accumulation in poinsettia leaves is accompanied by thylakoid membrane unstacking, acting as a photoprotective mechanism, to prevent ROS formation. Environ. Exp. Bot. 2018, 154, 44–55. [Google Scholar] [CrossRef]
- Hodges, D.M.; DeLong, J.M.; Forney, C.F.; Prange, R.K. Improving the thiobarbituric acid–reactive–substances assay for estimating lipid peroxidation in plant tissues containing anthocyanin and other interfering compounds. Planta 1999, 207, 604–611. [Google Scholar] [CrossRef]
- Hossain, M.A.; Hasanuzzaman, M.; Fujita, M. Up-regulation of antioxidant and glyoxalase systems by exogenous glycinebetaine and proline in mung bean confer tolerance to cadmium stress. Physiol. Mol. Biol. Plants 2010, 26, 259–272. [Google Scholar] [CrossRef] [Green Version]
- Daudi, A.; O’Brien, J.A. Detection of hydrogen peroxide by DAB staining in Arabidopsis leaves. BioProtocol. 2012, 2, e263. [Google Scholar] [CrossRef] [Green Version]














| Parameter | Definition | Calculation |
|---|---|---|
| Fv/Fm | Maximum efficiency of PSII photochemistry | Calculated as (Fm − Fo)/Fm |
| Fv/Fo | Efficiency of the water-splitting complex on the donor side of PSII | Calculated as (Fm − Fo)/Fo |
| Fv′/Fm′ | The efficiency of open PSII reaction centers | Calculated as (Fm′ − Fo′)/Fm′ |
| ΦPSII | The effective quantum yield of PSII photochemistry | Calculated as (Fm′ − Fs)/Fm′ |
| qp | The photochemical quenching, that is the redox state of the plastoquinone pool, is a measure of the number of open PSII reaction centers | Calculated as (Fm′ − Fs)/(Fm′ − Fo′) |
| NPQ | The non-photochemical quenching that reflects heat dissipation of excitation energy | Calculated as (Fm − Fm′)/Fm′ |
| ETR | The relative PSII electron transport rate | Calculated as ΦPSII × PAR × c × abs, where PAR is the photosynthetically active radiation c is 0.5, and abs is the total light absorption of the leaf taken as 0.84 |
| ΦNPQ | The quantum yield of regulated non-photochemical energy loss in PSII, that is heat dissipation for photoprotection | Calculated as Fs/Fm′ − Fs/Fm |
| ΦNO | The quantum yield of non-regulated energy loss in PSII | Calculated as Fs/Fm |
| EXC | Excess excitation energy | Calculated as (Fv/Fm − ΦPSII)/(Fv/Fm) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2020 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Adamakis, I.-D.S.; Sperdouli, I.; Hanć, A.; Dobrikova, A.; Apostolova, E.; Moustakas, M. Rapid Hormetic Responses of Photosystem II Photochemistry of Clary Sage to Cadmium Exposure. Int. J. Mol. Sci. 2021, 22, 41. https://doi.org/10.3390/ijms22010041
Adamakis I-DS, Sperdouli I, Hanć A, Dobrikova A, Apostolova E, Moustakas M. Rapid Hormetic Responses of Photosystem II Photochemistry of Clary Sage to Cadmium Exposure. International Journal of Molecular Sciences. 2021; 22(1):41. https://doi.org/10.3390/ijms22010041
Chicago/Turabian StyleAdamakis, Ioannis-Dimosthenis S., Ilektra Sperdouli, Anetta Hanć, Anelia Dobrikova, Emilia Apostolova, and Michael Moustakas. 2021. "Rapid Hormetic Responses of Photosystem II Photochemistry of Clary Sage to Cadmium Exposure" International Journal of Molecular Sciences 22, no. 1: 41. https://doi.org/10.3390/ijms22010041
APA StyleAdamakis, I.-D. S., Sperdouli, I., Hanć, A., Dobrikova, A., Apostolova, E., & Moustakas, M. (2021). Rapid Hormetic Responses of Photosystem II Photochemistry of Clary Sage to Cadmium Exposure. International Journal of Molecular Sciences, 22(1), 41. https://doi.org/10.3390/ijms22010041