Can Human Oral Mucosa Stem Cells Differentiate to Corneal Epithelia?
Abstract
:1. Introduction
2. Results
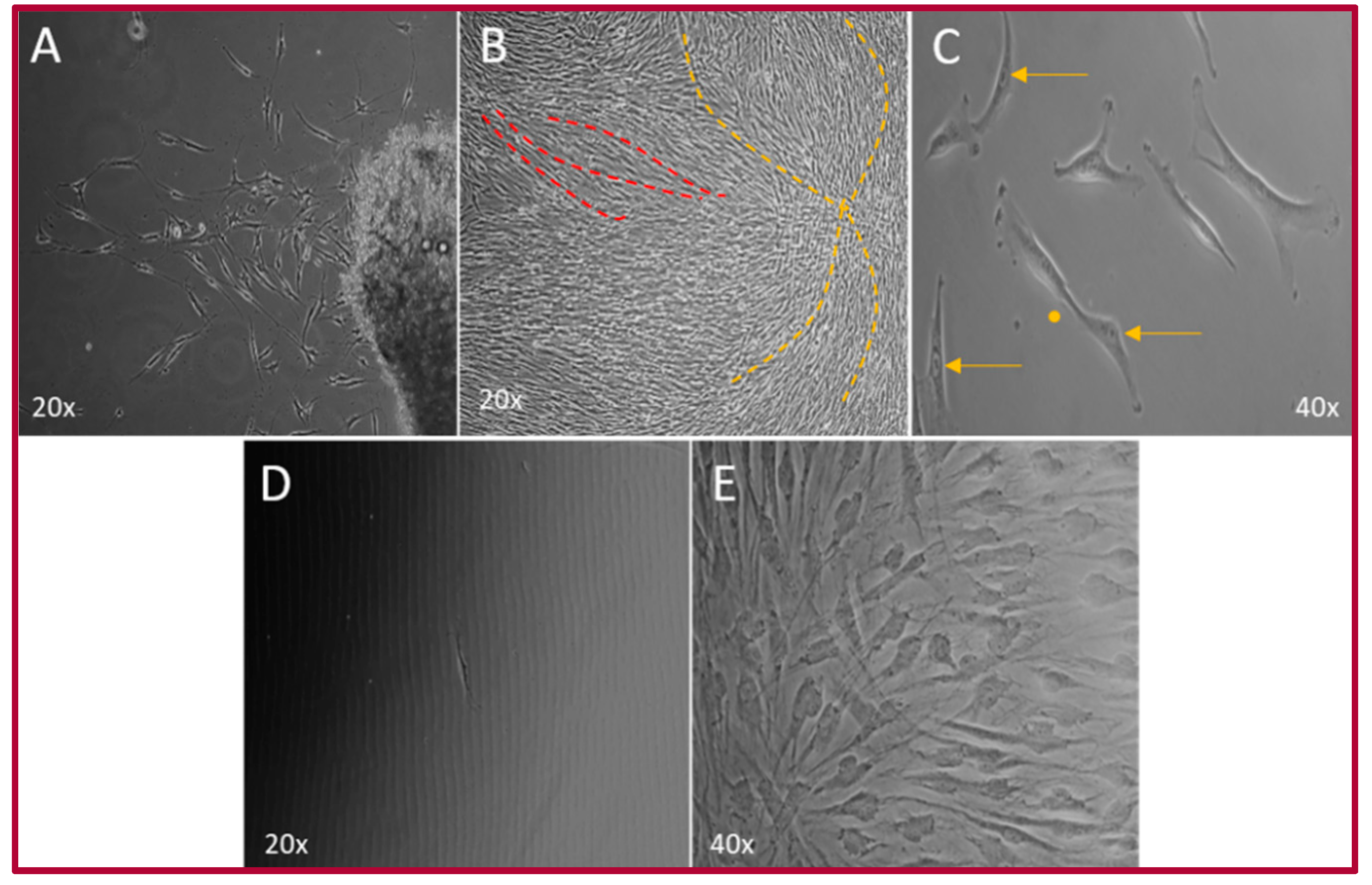
2.1. Isolation of the hOMSCs
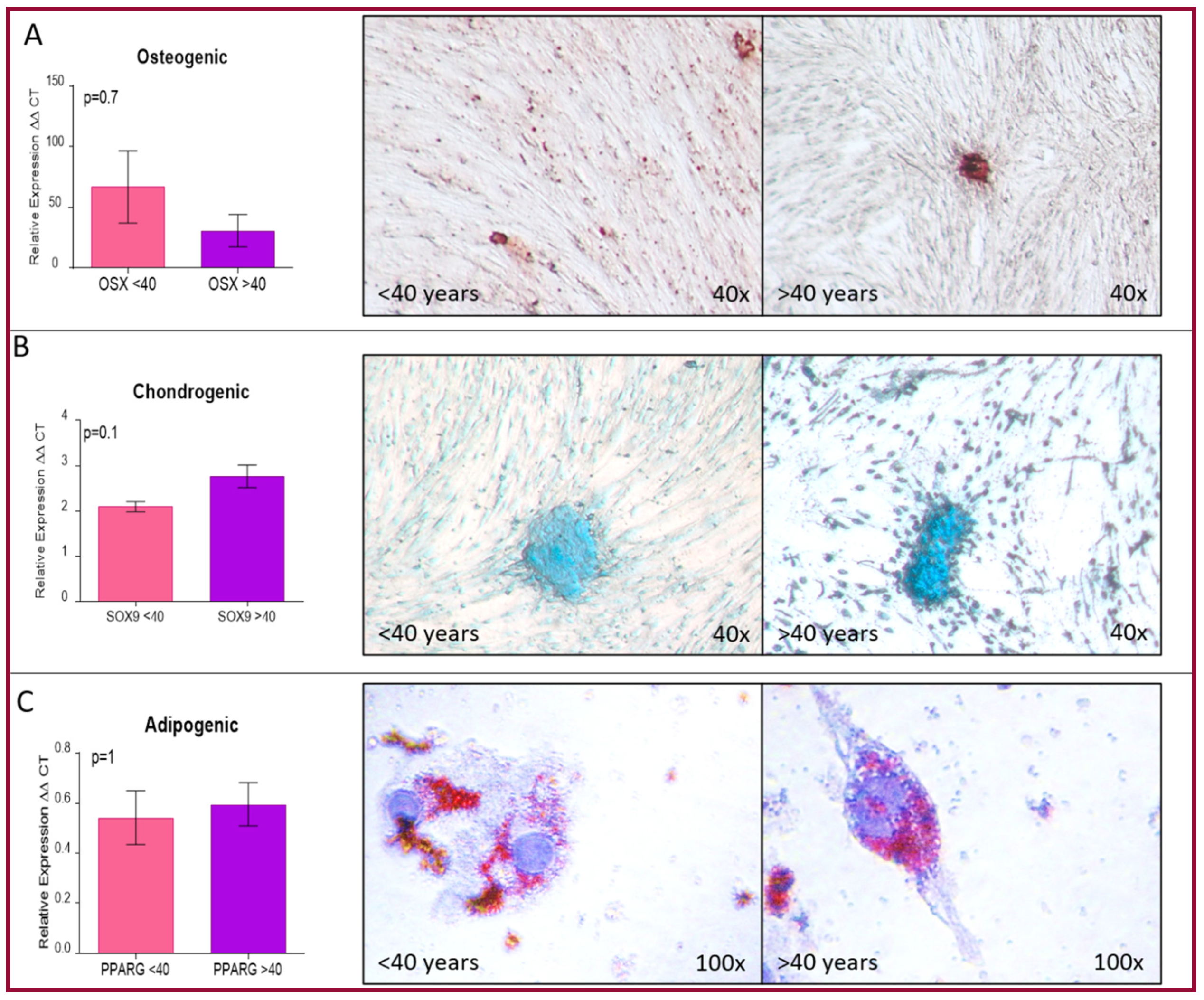
2.2. Cellular Characterization of hOMSCs
2.3. Epithelial Differentiation
3. Discussion
4. Materials and Methods
4.1. Culture Media
Transport Media
4.2. hOMSCs Isolation
4.3. Limbal Epithelial Cells
4.4. Cellular Characterization
4.4.1. Cell Number and Viability
4.4.2. Cell Proliferation
4.4.3. Mesenchymal Differentiation
4.4.4. RT-qPCR
4.4.5. Immunofluorescence
4.4.6. Flow Cytometry
4.5. Epithelial Differentiation
4.5.1. Epithelial Differentiation
4.5.2. Immunofluorescence was Performed as Previously Described
4.5.3. Histology and Immunofluorescence
4.6. Microscopic Analysis
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| MSC | Mesenchymal stem cells |
| NC | Neural crest |
| hOMSCs | Human oral mucosa stem cells |
| LESC | Limbal epithelial stem cells |
| LESCD | Limbal epithelial stem cells deficiency |
| RT-qPCR | Reverse transcriptase–real time polymerase chain reaction |
| LEC | Limbal epithelial cells |
| SHEM | Supplemental hormonal epithelium medium |
| DMEM/F12 | Dulbecco’s Modified Eagle medium/Ham 12 medium |
| ESC | Embryonic stem cells |
| MEM | Minimum essential medium |
| FBS | Fetal bovine serum |
| hEGF | Human epidermal growth factor |
| DMSO | Dymethylsulfoxide |
| ITS | Insulin- transferrin-selenium |
| PBS | Phosphate buffered saline |
| EDTA | Ethylenediaminetetraacetic acid |
| PFA | Paraformaldehyde |
| PBST | PBS + 0.1% Tween 20 |
| BSA | Bovine serum albumin |
| PC | Polycarbonate |
References
- Lakshmipathy, U.; Verfaillie, C. Stem cell plasticity. Blood Rev. 2005, 19, 29–38. [Google Scholar] [CrossRef]
- Dominici, M.; Le Blanc, K.; Mueller, I.; Slaper-Cortenbach, I.; Marini, F.; Krause, D.; Deans, R.; Keating, A.; Prockop, D.; Horwitz, E. Minimal criteria for defining multipotent mesenchymal stromal cells. The International Society for Cellular Therapy position statement. Cytotherapy 2006, 8, 315–317. [Google Scholar] [CrossRef]
- Nombela-Arrieta, C.; Ritz, J.; Silberstein, L.E. The elusive nature and function of mesenchymal stem cells. Nat. Rev. Mol. Cell Biol. 2011, 12, 126–131. [Google Scholar] [CrossRef] [Green Version]
- Dupin, E.; Coelho-Aguiar, J.M. Isolation and differentiation properties of neural crest stem cells. Cytometry A 2013, 83, 38–47. [Google Scholar] [CrossRef]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry—Part I: Stem cell sources. J. Prosthodont. Res. 2012, 56, 151–165. [Google Scholar] [CrossRef] [Green Version]
- Egusa, H.; Sonoyama, W.; Nishimura, M.; Atsuta, I.; Akiyama, K. Stem cells in dentistry—Part II: Clinical applications. J. Prosthodont. Res. 2012, 56, 229–248. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Abu Eid, R.; Sawair, F.; Landini, G.; Saku, T. Age and the architecture of oral mucosa. Age 2012, 34, 651–658. [Google Scholar] [CrossRef] [PubMed]
- Marynka-Kalmani, K.; Treves, S.; Yafee, M.; Rachima, H.; Gafni, Y.; Cohen, M.A.; Pitaru, S. The lamina propria of adult human oral mucosa harbors a novel stem cell population. Stem Cells 2010, 28, 984–995. [Google Scholar] [CrossRef] [PubMed]
- Ganz, J.; Arie, I.; Ben-Zur, T.; Dadon-Nachum, M.; Pour, S.; Araidy, S.; Pitaru, S.; Offen, D. Astrocyte-like cells derived from human oral mucosa stem cells provide neuroprotection in vitro and in vivo. Stem Cells Transl. Med. 2014, 3, 375–386. [Google Scholar] [CrossRef]
- Ganz, J.; Arie, I.; Buch, S.; Zur, T.B.; Barhum, Y.; Pour, S.; Araidy, S.; Pitaru, S.; Offen, D. Dopaminergic-like neurons derived from oral mucosa stem cells by developmental cues improve symptoms in the hemi-parkinsonian rat model. PLoS ONE 2014, 9, e100445. [Google Scholar] [CrossRef] [Green Version]
- Gafni, Y.; Rachima, H.; Marynks-Kalmani, K.; Blatt, A.; Vered, Z.; Pitaru, S. A new in vivo/in vitro model for assessing the capacity of human derived oral mucosa stem cells to colonize the infarcted myocardium. Stem Cell Stud. 2011, 1, e6. [Google Scholar] [CrossRef]
- Sonia, L.L. Células Troncales Derivadas de la Lámina Propia de la Mucosa Bucal: Su Potencial en la Regeneración Ósea In Vivo. Master Thesis, Universidad Nacional Autónoma de México, Mexico City, Mexico, June 2017. Available online: https://tesiunam.dgb.unam.mx/F?func=find-b-0&local_base=TES01 (accessed on 28 May 2021).
- Treves-Manusevitz, S.; Hoz, L.; Rachima, H.; Montoya, G.; Tzur, E.; Vardimon, A.; Narayanan, A.S.; Amar, S.; Arzate, H.; Pitaru, S. Stem cells of the lamina propria of human oral mucosa and gingiva develop into mineralized tissues in vivo. J. Clin. Periodontol. 2013, 40, 73–81. [Google Scholar] [CrossRef] [PubMed]
- Romero-Mendez, J. Boletín Estadístico-Informativo del Centro Nacional de Trasplantes. BEI Cenatra 2019, IV, 55. [Google Scholar]
- Forrester, J.V.; Dick, A.D.; McMenamin, P.G.; Roberts, F.; Pearlman, E. The Eye, 4th ed.; Elsevier: Amsterdam, The Netherlands, 2016. [Google Scholar]
- Cui, D.; Naftel, J.P.; Daley, W.P.; Lynch, J.C.; Haines, D.E.; Yang, G.; Fratkin, J.D. Atlas of Histology with Functional and Clinical Correlations, 1st ed.; Lippincot, Williams & Wilkins: Baltimore, MD, USA, 2011; p. 456. [Google Scholar]
- Navas, A.; Magaña-Guerrero, F.S.; Domínguez-López, A.; Chávez-García, C.; Partido, G.; Graue-Hernández, E.O.; Sánchez-García, F.J.; Garfias, Y. Anti-Inflammatory and Anti-Fibrotic Effects of Human Amniotic Membrane Mesenchymal Stem Cells and Their Potential in Corneal Repair. Stem Cells Transl. Med. 2018, 7, 906–917. [Google Scholar] [CrossRef]
- Nishida, K. Tissue engineering of the cornea. Cornea 2003, 22, S28–S34. [Google Scholar] [CrossRef] [PubMed]
- Tsai, R.J.; Li, L.M.; Chen, J.K. Reconstruction of damaged corneas by transplantation of autologous limbal epithelial cells. N. Engl. J. Med. 2000, 343, 86–93. [Google Scholar] [CrossRef]
- Nishida, K.; Yamato, M.; Hayashida, Y.; Watanabe, K.; Yamamoto, K.; Adachi, E.; Nagai, S.; Kikuchi, A.; Maeda, N.; Watanabe, H.; et al. Corneal reconstruction with tissue-engineered cell sheets composed of autologous oral mucosal epithelium. N. Engl. J. Med. 2004, 351, 1187–1196. [Google Scholar] [CrossRef] [Green Version]
- Koizumi, N.; Inatomi, T.; Suzuki, T.; Sotozono, C.; Kinoshita, S. Cultivated corneal epithelial stem cell transplantation in ocular surface disorders. Ophthalmology 2001, 108, 1569–1574. [Google Scholar] [CrossRef]
- Zhang, L.; Coulson-Thomas, V.J.; Ferreira, T.G.; Kao, W.W. Mesenchymal stem cells for treating ocular surface diseases. BMC Ophthalmol. 2015, 15 (Suppl. 1), 155. [Google Scholar] [CrossRef] [Green Version]
- Harkin, D.G.; Foyn, L.; Bray, L.J.; Sutherland, A.J.; Li, F.J.; Cronin, B.G. Concise reviews: Can mesenchymal stromal cells differentiate into corneal cells? A systematic review of published data. Stem Cells 2015, 33, 785–791. [Google Scholar] [CrossRef] [Green Version]
- Serna-Ojeda, J.C.; García-Mejía, M.; Graue-Hernández, E.O.; Navas, A.; Garfias, Y. Short-Term Results Analysis in the Allogenic Transplantation of Limbal Stem Cells Expanded on Amniotic Membrane in Patients with Bilateral Limbal Stem Cell Deficiency. J. Ocul. Pharmacol. Ther. 2020, 36, 238–246. [Google Scholar] [CrossRef]
- Jirsova, K.; Jones, G.L. Amniotic membrane in ophthalmology: Properties, preparation, storage and indications for grafting—A review. Cell Tissue Bank 2017, 18, 193–204. [Google Scholar] [CrossRef]
- Kaltschmidt, B.; Kaltschmidt, C.; Widera, D. Adult craniofacial stem cells: Sources and relation to the neural crest. Stem Cell Rev. Rep. 2012, 8, 658–671. [Google Scholar] [CrossRef] [PubMed]
- Weidgang, C.E.; Seufferlein, T.; Kleger, A.; Mueller, M. Pluripotency Factors on Their Lineage Move. Stem Cells Int. 2016, 2016, 6838253. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, L.; Michowski, W.; Kolodziejczyk, A.; Sicinski, P. The cell cycle in stem cell proliferation, pluripotency and differentiation. Nat. Cell Biol. 2019, 21, 1060–1067. [Google Scholar] [CrossRef] [PubMed]
- Li, M.; Izpisua Belmonte, J.C. Deconstructing the pluripotency gene regulatory network. Nat. Cell Biol. 2018, 20, 382–392. [Google Scholar] [CrossRef]
- Thomson, M.; Liu, S.J.; Zou, L.N.; Smith, Z.; Meissner, A.; Ramanathan, S. Pluripotency factors in embryonic stem cells regulate differentiation into germ layers. Cell 2011, 145, 875–889. [Google Scholar] [CrossRef] [Green Version]
- Heavner, W.; Pevny, L. Eye development and retinogenesis. Cold Spring Harb. Perspect. Biol. 2012, 4, a008391. [Google Scholar] [CrossRef] [Green Version]
- Utheim, T.P. Concise review: Transplantation of cultured oral mucosal epithelial cells for treating limbal stem cell deficiency-current status and future perspectives. Stem Cells 2015, 33, 1685–1695. [Google Scholar] [CrossRef]
- Utheim, T.P.; Utheim, Ø.; Khan, Q.E.; Sehic, A. Culture of Oral Mucosal Epithelial Cells for the Purpose of Treating Limbal Stem Cell Deficiency. J. Funct. Biomater. 2016, 7, 5. [Google Scholar] [CrossRef] [Green Version]
- Nowell, C.S.; Odermatt, P.D.; Azzolin, L.; Hohnel, S.; Wagner, E.F.; Fantner, G.E.; Lutolf, M.P.; Barrandon, Y.; Piccolo, S.; Radtke, F. Chronic inflammation imposes aberrant cell fate in regenerating epithelia through mechanotransduction. Nat. Cell Biol. 2016, 18, 168–180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alberts, B.; Johnson, A.; Lewis, J.; Morgan, D.; Raff, M.; Roberts, K.; Walter, P. Molecular Biology of the Cell, 6th ed.; Garland Science: New York, NY, USA, 2015; p. 1465. [Google Scholar]
- Brightbill, F. Corneal Surgery: Theory, Technique and Tissue, 4th ed.; Mosby: Maryland Heights, MO, USA, 2009; p. 877. [Google Scholar]
- Masterton, S.; Ahearne, M. Mechanobiology of the corneal epithelium. Exp. Eye Res. 2018, 177, 122–129. [Google Scholar] [CrossRef] [PubMed]
- Dziasko, M.A.; Daniels, J.T. Anatomical Features and Cell-Cell Interactions in the Human Limbal Epithelial Stem Cell Niche. Ocul. Surf. 2016, 14, 322–330. [Google Scholar] [CrossRef] [PubMed]
- Ma, J.; Wang, Y.; Wei, P.; Jhanji, V. Biomechanics and structure of the cornea: Implications and association with corneal disorders. Surv. Ophthalmol. 2018, 63, 851–861. [Google Scholar] [CrossRef] [PubMed]
- Di Iorio, E.; Barbaro, V.; Ruzza, A.; Ponzin, D.; Pellegrini, G.; De Luca, M. Isoforms of DeltaNp63 and the migration of ocular limbal epithelial cells. Stem Cells 2005, 23, 63–73. [Google Scholar]
- Ksander, B.R.; Kolovou, P.E.; Wilson, B.J.; Saab, K.R.; Guo, Q.; Ma, J.; McGuire, S.P.; Gregory, M.S.; Vincent, W.J.; Perez, V.L.; et al. ABCB5 is a limbal stem cell gene required for corneal development and repair. Nature 2014, 511, 353–357. [Google Scholar] [CrossRef] [Green Version]
- Wells, W.A. Is transdifferentiation in trouble? J. Cell Biol. 2002, 157, 15–18. [Google Scholar] [CrossRef]
- Wagner, B.K. Grand challenge commentary: Chemical transdifferentiation and regenerative medicine. Nat. Chem. Biol. 2010, 6, 877–879. [Google Scholar] [CrossRef]
- Hybiak, J.; Jankowska, K.; Machaj, F.; Rosik, J.; Broniarek, I.; Żyluk, A.; Hilderman, G.C.; Małecki, A.; Łos, M.J.; Urasińska, E. Reprogramming and transdifferentiation—Two key processes for regenerative medicine. Eur. J. Pharmacol. 2020, 882, 173202. [Google Scholar] [CrossRef]
- Kim, H.Y.; Jackson, T.R.; Davidson, L.A. On the role of mechanics in driving mesenchymal-to-epithelial transitions. Semin. Cell Dev. Biol. 2017, 67, 113–122. [Google Scholar] [CrossRef]
- Gouveia, R.M.; Vajda, F.; Wibowo, J.A.; Figueiredo, F.; Connon, C.J. YAP, ΔNp63, and β-Catenin Signaling Pathways are Involved in the Modulation of Corneal Epithelial Stem Cell Phenotype Induced by Substrate Stiffness. Cells 2019, 8, 347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gouveia, R.M.; Lepert, G.; Gupta, S.; Mohan, R.R.; Paterson, C.; Connon, C.J. Assessment of corneal substrate biomechanics and its effect on epithelial stem cell maintenance and differentiation. Nat. Commun. 2019, 10, 1496. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, H.J.; Nam, S.M.; Choi, S.K.; Seo, K.Y.; Kim, H.O.; Chung, S.H. Comparative study of substrate free and amniotic membrane scaffolds for cultivation of limbal epithelial sheet. Sci. Rep. 2018, 8, 14628. [Google Scholar] [CrossRef]
- Monteiro, B.G.; Loureiro, R.R.; Cristovam, P.C.; Covre, J.L.; Gomes, J.P.; Kerkis, I. Amniotic membrane as a biological scaffold for dental pulp stem cell transplantation in ocular surface reconstruction. Arq. Bras. Oftalmol. 2019, 82, 32–37. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Holan, V.; Trosan, P.; Cejka, C.; Javorkova, E.; Zajicova, A.; Hermankova, B.; Chudickova, M.; Cejkova, J. A Comparative Study of the Therapeutic Potential of Mesenchymal Stem Cells and Limbal Epithelial Stem Cells for Ocular Surface Reconstruction. Stem Cells Transl. Med. 2015, 4, 1052–1063. [Google Scholar] [CrossRef]
- Chávez-García, C.; Jiménez-Corona, A.; Graue-Hernández, E.O.; Zaga-Clavellina, V.; García-Mejía, M.; Jiménez-Martínez, M.C.; Garfias, Y. Ophthalmic indications of amniotic membrane transplantation in Mexico: An eight years Amniotic Membrane Bank experience. Cell Tissue Bank 2016, 17, 261–268. [Google Scholar] [CrossRef]
- Zen 2, 2.0.0.0; Carl Zeiss Microscopy GmbH: Jena, Germany, 2011.
- Motulsky, D.H. Graphpad, 8th ed.; GraphPad Software: San Diego, CA, USA, 2021. [Google Scholar]





| Gene | Sequence 5′-3′ | Size (nt) | Tm (°C) | |
|---|---|---|---|---|
| SOX9 | F | GTA ATC CGG GTG GTC CTT CT | 20 | 58.8 |
| R | GAC GCT GGG CAA GCT CT | 17 | 59.68 | |
| OSX | F | GCC AGA AGC TGT GAA ACC TC | 20 | 59.12 |
| R | GCT GCA AGC TCT CCA TAA CC | 20 | 58.98 | |
| PPAR-γ | F | GAG AGA TCC ACG GAG CTG AT | 20 | 58.67 |
| R | AGG CCA TTT TGT CAA ACG AG | 20 | 56.91 | |
| GAPDH | F | CAACGGATTTGGTCGTATTGG | 21 | 59.4 |
| R | GCAACAATATCCACTTTACCAAGAGTTAA | 29 | 59.5 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
López, S.; Hoz, L.; Tenorio, E.P.; Buentello, B.; Magaña, F.S.; Wintergerst, A.; Navas, A.; Garfias, Y.; Arzate, H. Can Human Oral Mucosa Stem Cells Differentiate to Corneal Epithelia? Int. J. Mol. Sci. 2021, 22, 5976. https://doi.org/10.3390/ijms22115976
López S, Hoz L, Tenorio EP, Buentello B, Magaña FS, Wintergerst A, Navas A, Garfias Y, Arzate H. Can Human Oral Mucosa Stem Cells Differentiate to Corneal Epithelia? International Journal of Molecular Sciences. 2021; 22(11):5976. https://doi.org/10.3390/ijms22115976
Chicago/Turabian StyleLópez, Sonia, Lía Hoz, Eda Patricia Tenorio, Beatriz Buentello, Fátima Sofía Magaña, Ana Wintergerst, Alejandro Navas, Yonathan Garfias, and Higinio Arzate. 2021. "Can Human Oral Mucosa Stem Cells Differentiate to Corneal Epithelia?" International Journal of Molecular Sciences 22, no. 11: 5976. https://doi.org/10.3390/ijms22115976
APA StyleLópez, S., Hoz, L., Tenorio, E. P., Buentello, B., Magaña, F. S., Wintergerst, A., Navas, A., Garfias, Y., & Arzate, H. (2021). Can Human Oral Mucosa Stem Cells Differentiate to Corneal Epithelia? International Journal of Molecular Sciences, 22(11), 5976. https://doi.org/10.3390/ijms22115976






