Soybean (Glycine max) Is Able to Absorb, Metabolize and Accumulate Fenbendazole in All Organs Including Beans
Abstract
:1. Introduction
2. Results and Discussion
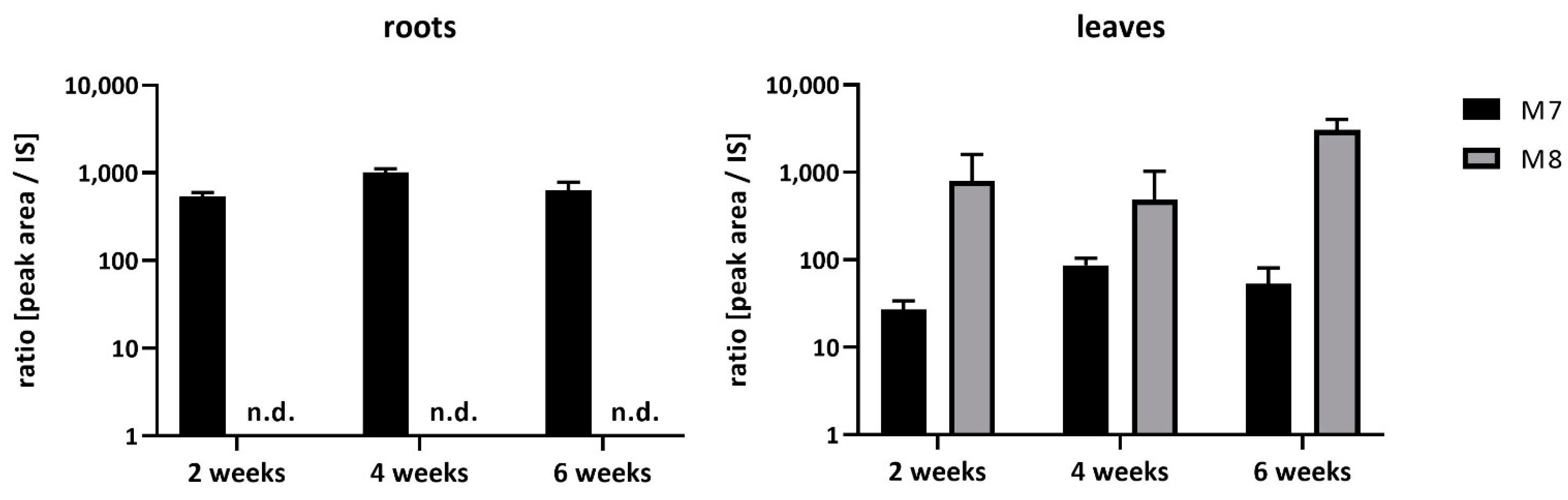
2.1. Uptake and Metabolism of FBZ in Soybeans
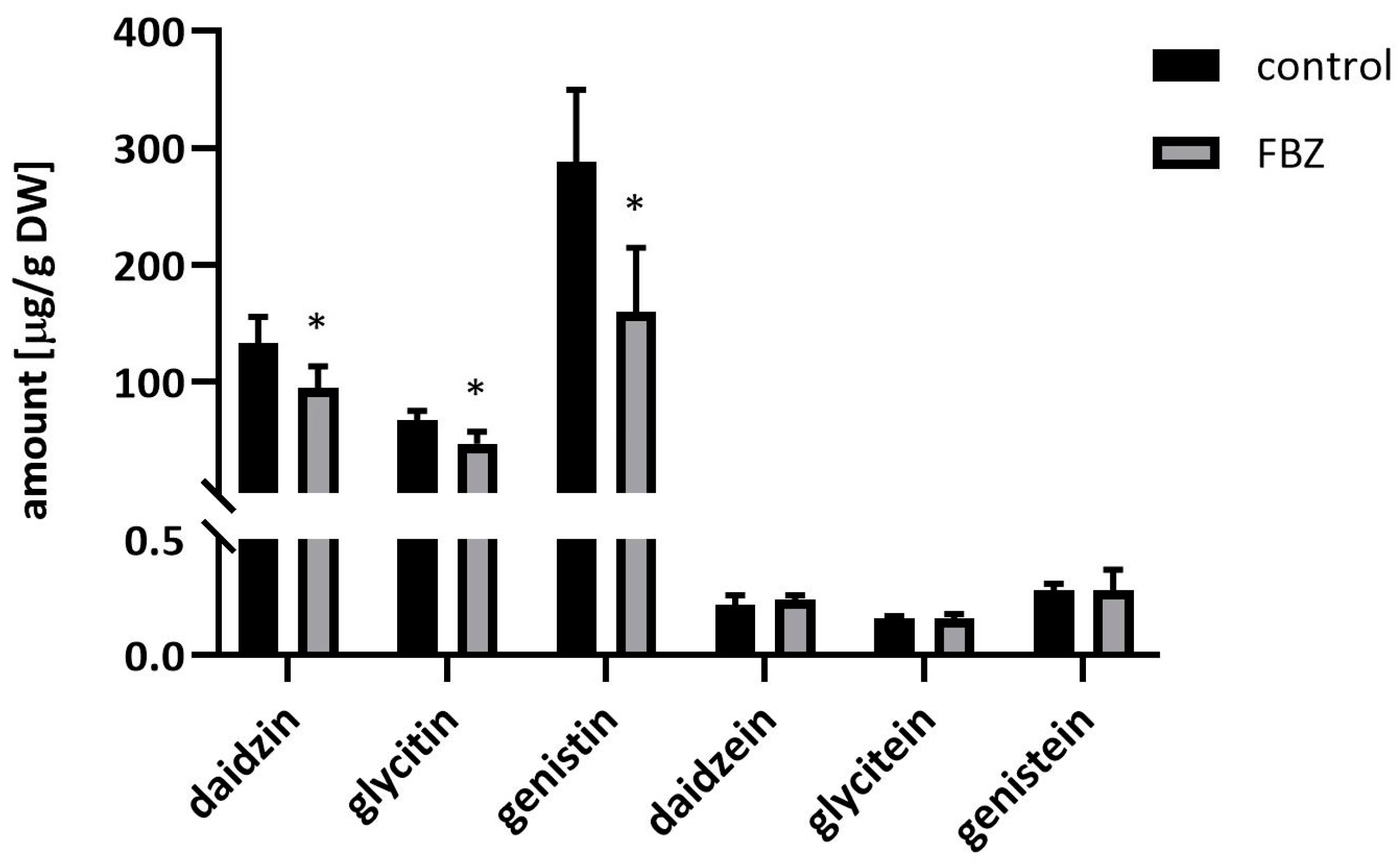
2.2. The Effect of FBZ on Isoflavones Content in Soybeans
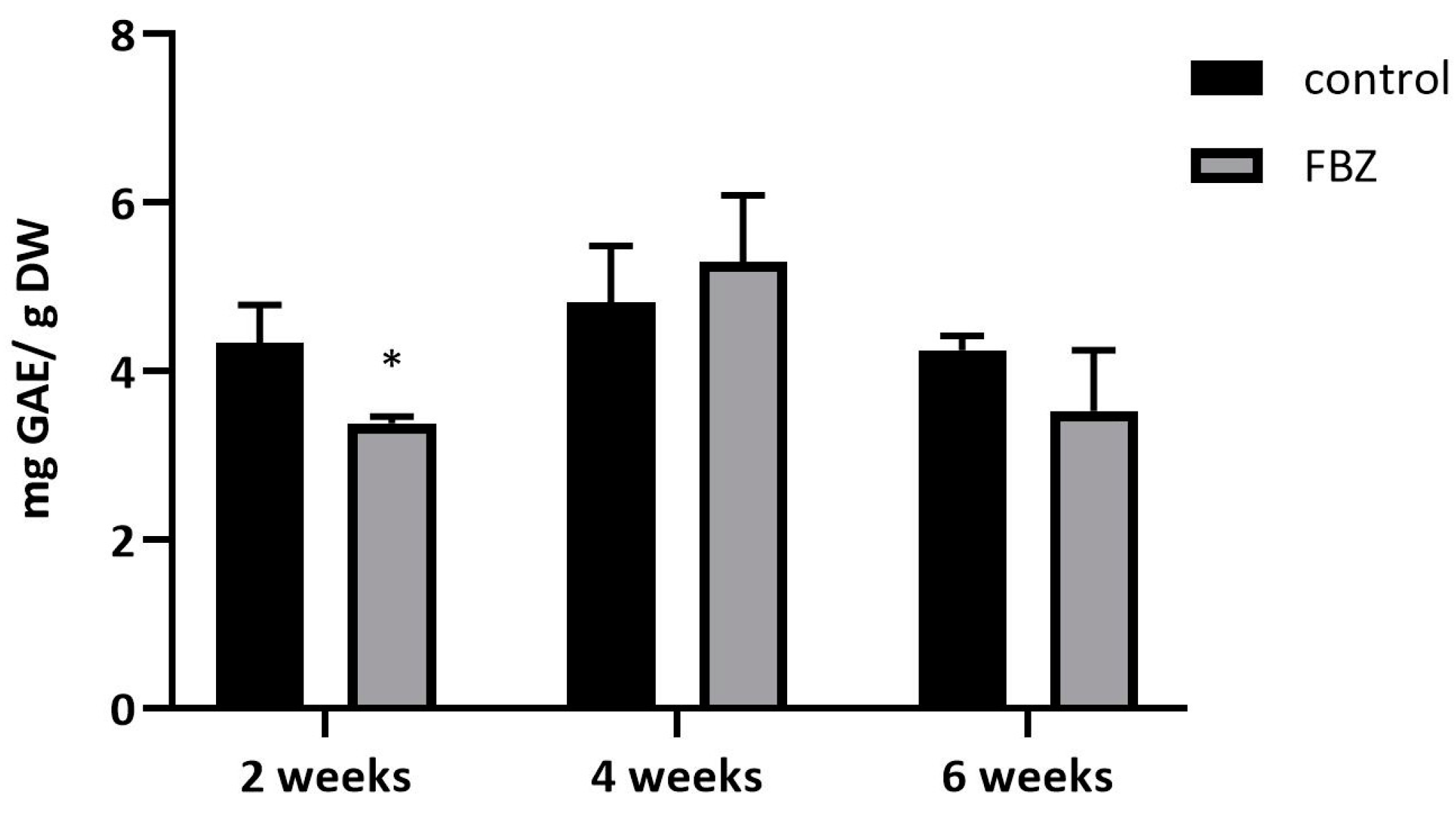
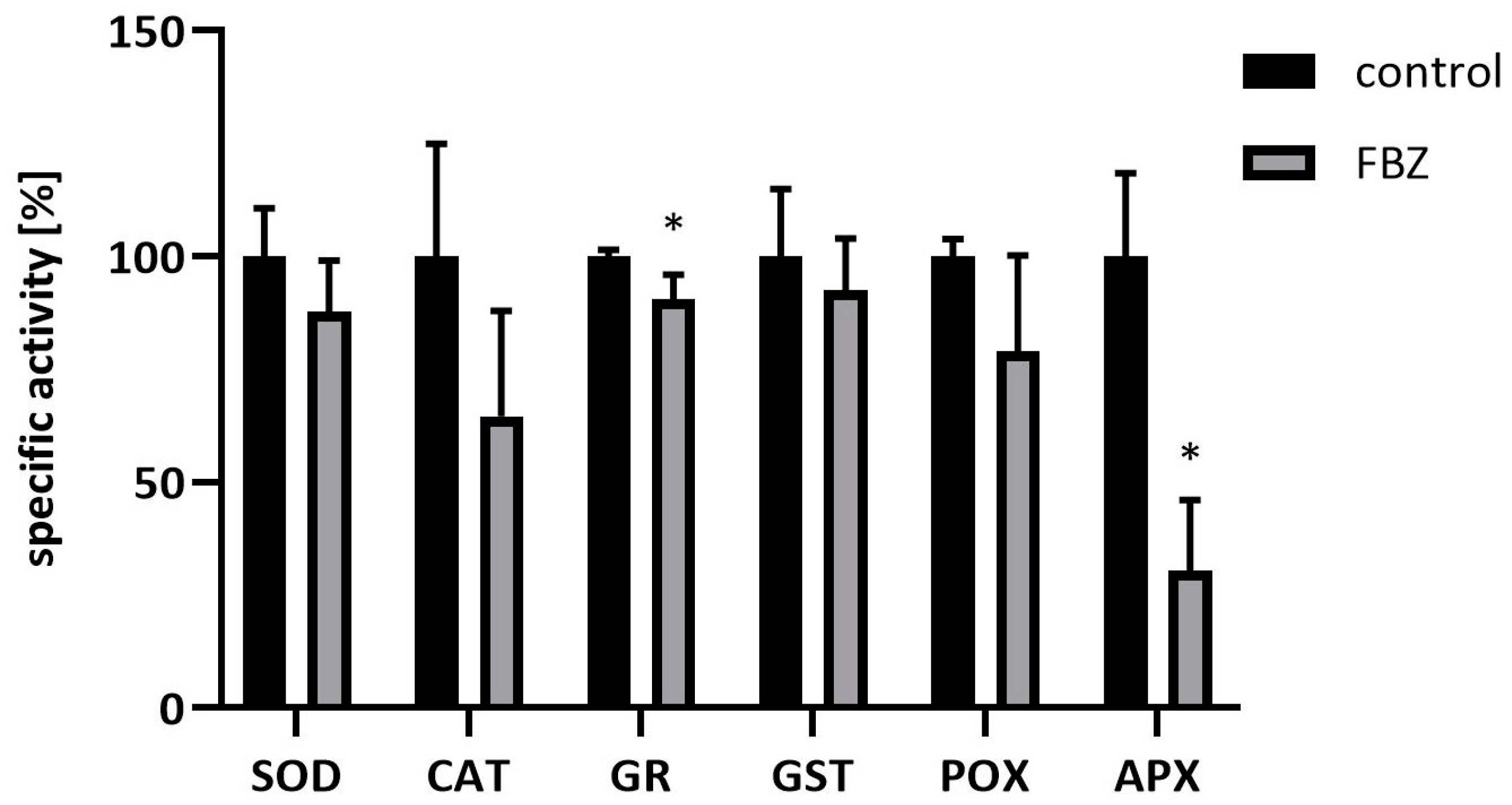
2.3. The Effect of FBZ on Antioxidant Enzyme Activities in Soybeans
3. Materials and Methods
3.1. In Vitro Cultivation of Soybean in Seedlings
3.2. Cultivation of Soybean Plants in a Greenhouse
3.3. HPLC Analysis of Isoflavones
3.4. UHPLC-MS/MS Analysis of FBZ and Metabolites
3.5. Total Phenol Content Assay
3.6. Antioxidant Enzymes Activity Assay
3.7. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- De Oliveira, H.C.; Joffe, L.S.; Simon, K.S.; Castelli, R.F.; Reis, F.C.G.; Bryan, A.M.; Borges, B.S.; Medeiros, L.C.S.; Bocca, A.L.; Del Poeta, M.; et al. Fenbendazole Controls In Vitro Growth, Virulence Potential, and Animal Infection in the Cryptococcus Model. Antimicrob. Agents Chemother. 2020, 64, e00286-20. [Google Scholar] [CrossRef]
- Dogra, N.; Kumar, A.; Mukhopadhyay, T. Fenbendazole acts as a moderate microtubule destabilizing agent and causes cancer cell death by modulating multiple cellular pathways. Sci. Rep. 2018, 8, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Gonzalez, A.E.; Codd, E.E.; Horton, J.; Garcia, H.H.; Gilman, R.H. Oxfendazole: A promising agent for the treatment and control of helminth infections in humans. Expert Rev. Anti-Infect. Ther. 2019, 17, 51–56. [Google Scholar] [CrossRef]
- Matthews, J.B. Anthelmintic resistance in equine nematodes. Int. J. Parasitol. Drugs Drug Resist. 2014, 4, 310–315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Boxall, A.B.A.; Kolpin, D.W.; Halling-Sørensen, B.; Tolls, J. Peer Reviewed: Are Veterinary Medicines Causing Environmental Risks? Environ. Sci. Technol. 2003, 37, 286A–294A. [Google Scholar] [CrossRef] [Green Version]
- Goodenough, A.E.; Webb, J.C.; Yardley, J. Environmentally-realistic concentrations of anthelmintic drugs affect survival and motility in the cosmopolitan earthworm Lumbricus terrestris (Linnaeus, 1758). Appl. Soil Ecol. 2019, 137, 87–95. [Google Scholar] [CrossRef]
- Bisognin, R.P.; Wolff, D.B.; Carissimi, E.; Prestes, O.D.; Zanella, R. Occurrence and fate of pharmaceuticals in effluent and sludge from a wastewater treatment plant in Brazil. Environ. Technol. 2021, 42, 2292–2303. [Google Scholar] [CrossRef] [PubMed]
- Kreuzig, R.; Blümlein, K.; Höltge, S. Fate of the Benzimidazole Antiparasitics Flubendazole and Fenbendazole in Manure and Manured Soils. CLEAN Soil Air Water 2007, 35, 488–494. [Google Scholar] [CrossRef]
- Bundschuh, M.; Hahn, T.; Ehrlich, B.; Höltge, S.; Kreuzig, R.; Schulz, R. Acute Toxicity and Environmental Risks of Five Veterinary Pharmaceuticals for Aquatic Macroinvertebrates. Bull. Environ. Contam. Toxicol. 2015, 96, 139–143. [Google Scholar] [CrossRef]
- Park, K.; Bang, H.W.; Park, J.; Kwak, I.-S. Ecotoxicological multilevel-evaluation of the effects of fenbendazole exposure to Chironomus riparius larvae. Chemosphere 2009, 77, 359–367. [Google Scholar] [CrossRef] [PubMed]
- Stuchlíková, L.R.; Skálová, L.; Szotáková, B.; Syslová, E.; Vokřál, I.; Vaněk, T.; Podlipná, R. Biotransformation of flubendazole and fenbendazole and their effects in the ribwort plantain (Plantago lanceolata). Ecotoxicol. Environ. Saf. 2018, 147, 681–687. [Google Scholar] [CrossRef] [PubMed]
- Syslová, E.; Landa, P.; Stuchlíková, L.R.; Matoušková, P.; Skálová, L.; Szotáková, B.; Navrátilová, M.; Vaněk, T.; Podlipná, R. Metabolism of the anthelmintic drug fenbendazole in Arabidopsis thaliana and its effect on transcriptome and proteome. Chemosphere 2019, 218, 662–669. [Google Scholar] [CrossRef] [PubMed]
- Horvat, A.; Babić, S.; Pavlović, D.; Ašperger, D.; Pelko, S.; Kaštelan-Macan, M.; Petrović, M.; Mance, A. Analysis, occurrence and fate of anthelmintics and their transformation products in the environment. TrAC Trends Anal. Chem. 2012, 31, 61–84. [Google Scholar] [CrossRef]
- Jaeger, L.H.; Carvalho-Costa, F.A. Status of benzimidazole resistance in intestinal nematode populations of livestock in Brazil: A systematic review. BMC Vet. Res. 2017, 13, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ismail, M.; William, S.; A Day, T.; Botros, S.; Tao, L.F.; Bennett, J.L.; Farghally, A.; Metwally, A. Resistance to praziquantel: Direct evidence from Schistosoma mansoni isolated from Egyptian villagers. Am. J. Trop. Med. Hyg. 1999, 60, 932–935. [Google Scholar] [CrossRef] [PubMed]
- Geerts, S.; Gryseels, B. Drug Resistance in Human Helminths: Current Situation and Lessons from Livestock. Clin. Microbiol. Rev. 2000, 13, 207–222. [Google Scholar] [CrossRef]
- Kellerová, P.; Stuchlíková, L.R.; Matoušková, P.; Štěrbová, K.; Lamka, J.; Navrátilová, M.; Vokřál, I.; Szotáková, B.; Skálová, L. Sub-lethal doses of albendazole induce drug metabolizing enzymes and increase albendazole deactivation in Haemonchus contortus adults. Vet. Res. 2020, 51, 1–17. [Google Scholar] [CrossRef] [PubMed]
- Alaswad, A.A.; Song, B.; Oehrle, N.W.; Wiebold, W.J.; Mawhinney, T.P.; Krishnan, H.B. Development of soybean experimental lines with enhanced protein and sulfur amino acid content. Plant Sci. 2021, 308, 110912. [Google Scholar] [CrossRef] [PubMed]
- Bártíková, H.; Podlipná, R.; Skálová, L. Veterinary drugs in the environment and their toxicity to plants. Chemosphere 2016, 144, 2290–2301. [Google Scholar] [CrossRef]
- Stuchlíková, L.; Jirásko, R.; Skálová, L.; Pavlík, F.; Szotáková, B.; Holčapek, M.; Vaněk, T.; Podlipná, R. Metabolic pathways of benzimidazole anthelmintics in harebell (Campanula rotundifolia). Chemosphere 2016, 157, 10–17. [Google Scholar] [CrossRef] [PubMed]
- Syslová, E.; Landa, P.; Navrátilová, M.; Stuchlíková, L.R.; Matoušková, P.; Skálová, L.; Szotáková, B.; Vaněk, T.; Podlipná, R. Ivermectin biotransformation and impact on transcriptome in Arabidopsis thaliana. Chemosphere 2019, 234, 528–535. [Google Scholar] [CrossRef]
- Gutierrez-Gonzalez, J.J.; Guttikonda, S.K.; Tran, L.-S.P.; Aldrich, D.L.; Zhong, R.; Yu, O.; Nguyen, H.T.; Sleper, D.A. Differential Expression of Isoflavone Biosynthetic Genes in Soybean During Water Deficits. Plant Cell Physiol. 2010, 51, 936–948. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lozovaya, V.V.; Lygin, A.V.; Ulanov, A.V.; Nelson, R.L.; Daydé, J.; Widholm, J.M. Effect of Temperature and Soil Moisture Status during Seed Development on Soybean Seed Isoflavone Concentration and Composition. Crop. Sci. 2005, 45, 1934–1940. [Google Scholar] [CrossRef] [Green Version]
- Riaz, L.; Mahmood, T.; Coyne, M.S.; Khalid, A.; Rashid, A.; Hayat, M.T.; Gulzar, A.; Amjad, M. Physiological and antioxidant response of wheat (Triticum aestivum) seedlings to fluoroquinolone antibiotics. Chemosphere 2017, 177, 250–257. [Google Scholar] [CrossRef]
- An, G.; Murry, D.J.; Gajurel, K.; Bach, T.; Deye, G.; Stebounova, L.V.; Codd, E.E.; Horton, J.; Gonzalez, A.E.; Garcia, H.H.; et al. Pharmacokinetics, Safety, and Tolerability of Oxfendazole in Healthy Volunteers: A Randomized, Placebo-Controlled First-in-Human Single-Dose Escalation Study. Antimicrob. Agents Chemother. 2019, 63. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bach, T.; Bae, S.; D’Cunha, R.; Winokur, P.; An, G. Development and validation of a simple, fast, and sensitive LC/MS/MS method for the quantification of oxfendazole in human plasma and its application to clinical pharmacokinetic study. J. Pharm. Biomed. Anal. 2019, 171, 111–117. [Google Scholar] [CrossRef] [PubMed]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bio Assays with Tobacco Tissue Cultures. Physiol. Plant. 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Lee, Y.-W.; Kim, J.-D.; Zheng, J.; Row, K.H. Comparisons of isoflavones from Korean and Chinese soybean and processed products. Biochem. Eng. J. 2007, 36, 49–53. [Google Scholar] [CrossRef]
- Liu, J.; Hu, B.; Liu, W.; Qin, W.; Wu, H.; Zhang, J.; Yang, C.; Deng, J.; Shu, K.; Du, J.; et al. Metabolomic tool to identify soybean [Glycine max (L.) Merrill] germplasms with a high level of shade tolerance at the seedling stage. Sci. Rep. 2017, 7, 42478. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Szymczak, G.; Wójciak-Kosior, M.; Sowa, I.; Zapała, K.; Strzemski, M.; Kocjan, R. Evaluation of isoflavone content and antioxidant activity of selected soy taxa. J. Food Compos. Anal. 2017, 57, 40–48. [Google Scholar] [CrossRef]
- Singleton, V.L.; Orthofer, R.; Lamuela-Raventós, R.M. Analysis of total phenols and other oxidation substrates and antioxidants by means of Folin-Ciocalteu reagent. In Methods in Enzymology; Academic Press: London, UK, 1998; Volume 299, pp. 152–178. [Google Scholar]
- Bradford, M.M. A rapid and sensitive method for the quantitation of microgram quantities of protein utilizing the principle of protein-dye binding. Anal. Biochem. 1976, 72, 248–254. [Google Scholar] [CrossRef]
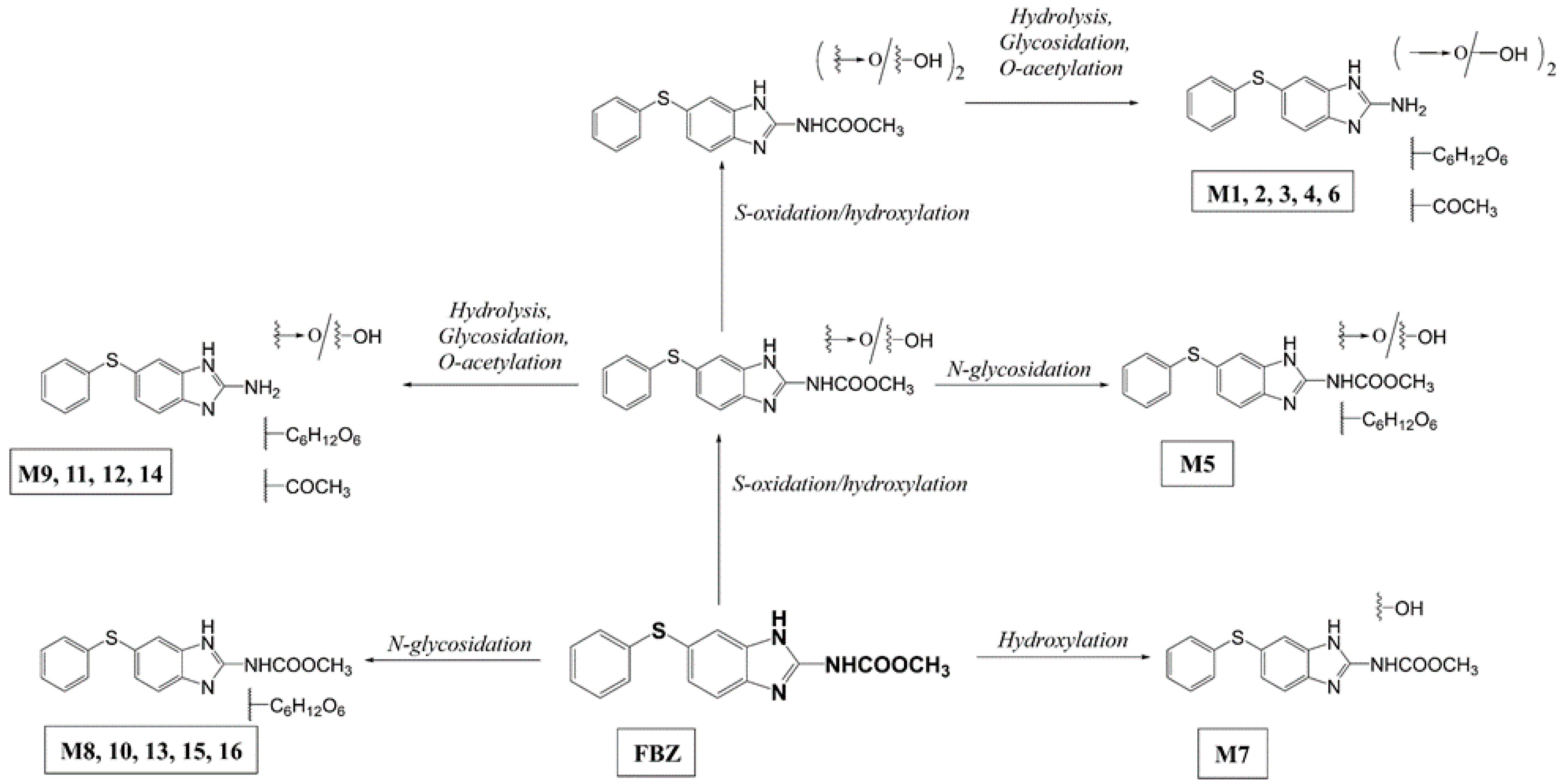

| Metabolite Designation | Roots | Leaves |
|---|---|---|
| M1 | 41 ± 8 | - |
| M2 | 8 ± 1 | - |
| M3 | 9 ± 3 | - |
| M4 | 109 ± 22 | |
| M5 | 185 ± 40 | - |
| M6 | 2 ± 0.02 | - |
| M7 | 20,845 ± 4374 | - |
| M8 | - | 6559 ± 2171 |
| M9 | 232 ± 71 | - |
| M10 | 10 ± 6 | 1047 ± 50 |
| M11 | 14 ± 3 | - |
| M12 | 19 ± 4 | - |
| M13 | 69 ± 17 | - |
| M14 | 200 ± 42 | - |
| M15 | 83 ± 36 | - |
| M16 | 1767 ± 648 | - |
| FBZ | 68,320 ± 14,998 | 141 ± 71 |
| M7 | M8 | M16 | FBZ | |
|---|---|---|---|---|
| roots | ||||
| 2 weeks | 539 ± 39 | 22 ± 4 | 1165 ± 6 | |
| 4 weeks | 1013 ± 67 | 16 ± 13 | 1830 ± 67 | |
| 6 weeks | 636 ± 101 | 49 ± 36 | 2911 ± 579 | |
| leaves | ||||
| 2 weeks | 27 ± 5 | 800 ± 566 | 15 ± 6 | |
| 4 weeks | 87 ± 12 | 483 ± 387 | 28 ± 11 | |
| 6 weeks | 53 ± 20 | 3057 ± 690 | 60 ± 23 | |
| pods | ||||
| 2 weeks | 0.104 ± 0.006 | 1.34 ± 0.27 | 0.471 ± 0.053 | |
| 4 weeks | 0.320 ± 0.075 | 3.87 ± 0.21 | 0.212 ± 0.031 | |
| 6 weeks | 0.581 ± 0.023 | 1.06 ± 0.36 | 0.477 ± 0.217 | |
| beans | ||||
| 2 weeks | 0.304 ± 0.007 | |||
| 4 weeks | 0.551 ± 0.408 | 0.486 ± 0.104 | ||
| 6 weeks | 0.552 ± 0.418 | 1.686 ± 1.319 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Podlipná, R.; Navrátilová, M.; Raisová Stuchlíková, L.; Moťková, K.; Langhansová, L.; Skálová, L.; Szotáková, B. Soybean (Glycine max) Is Able to Absorb, Metabolize and Accumulate Fenbendazole in All Organs Including Beans. Int. J. Mol. Sci. 2021, 22, 6647. https://doi.org/10.3390/ijms22136647
Podlipná R, Navrátilová M, Raisová Stuchlíková L, Moťková K, Langhansová L, Skálová L, Szotáková B. Soybean (Glycine max) Is Able to Absorb, Metabolize and Accumulate Fenbendazole in All Organs Including Beans. International Journal of Molecular Sciences. 2021; 22(13):6647. https://doi.org/10.3390/ijms22136647
Chicago/Turabian StylePodlipná, Radka, Martina Navrátilová, Lucie Raisová Stuchlíková, Kateřina Moťková, Lenka Langhansová, Lenka Skálová, and Barbora Szotáková. 2021. "Soybean (Glycine max) Is Able to Absorb, Metabolize and Accumulate Fenbendazole in All Organs Including Beans" International Journal of Molecular Sciences 22, no. 13: 6647. https://doi.org/10.3390/ijms22136647
APA StylePodlipná, R., Navrátilová, M., Raisová Stuchlíková, L., Moťková, K., Langhansová, L., Skálová, L., & Szotáková, B. (2021). Soybean (Glycine max) Is Able to Absorb, Metabolize and Accumulate Fenbendazole in All Organs Including Beans. International Journal of Molecular Sciences, 22(13), 6647. https://doi.org/10.3390/ijms22136647








