Secretome Analysis of Arabidopsis–Trichoderma atroviride Interaction Unveils New Roles for the Plant Glutamate:Glyoxylate Aminotransferase GGAT1 in Plant Growth Induced by the Fungus and Resistance against Botrytis cinerea
Abstract
:1. Introduction
2. Results
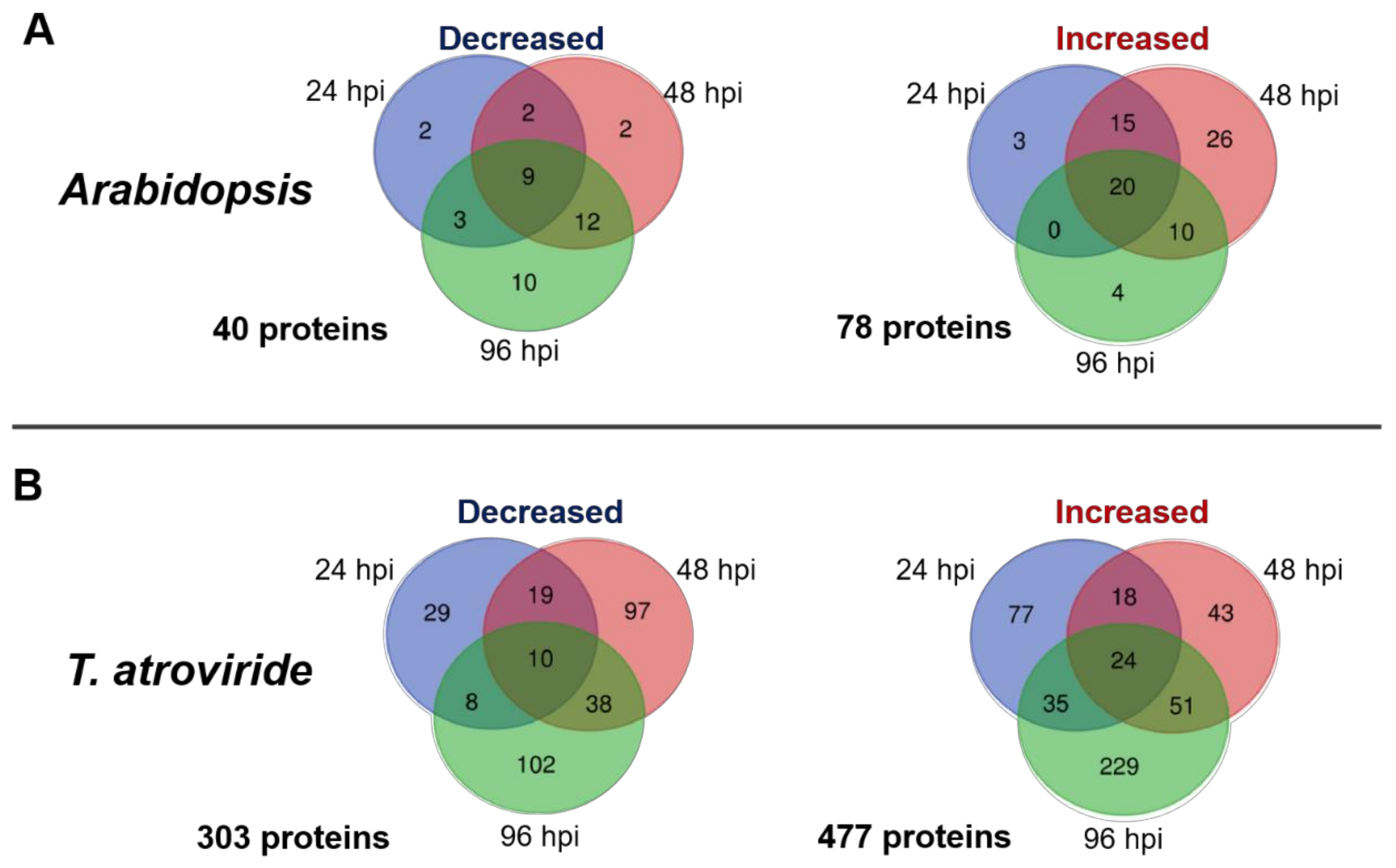
2.1. Time-Course Analysis of the Arabidopsis–T. atroviride Interaction Secretome
2.2. Arabidopsis and T. atroviride Proteins Are Conventionally and Unconventionally Secreted during their Symbiosis
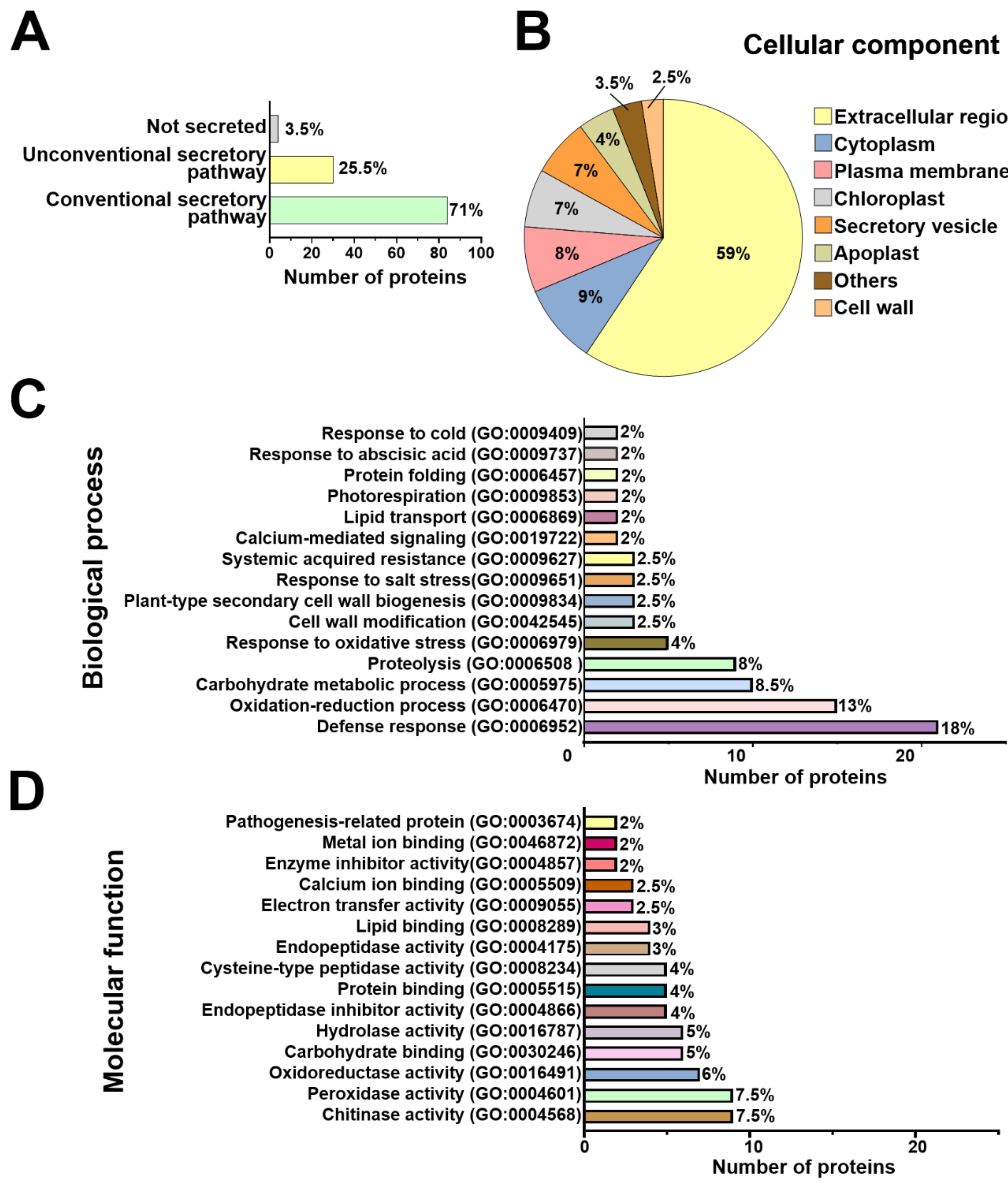
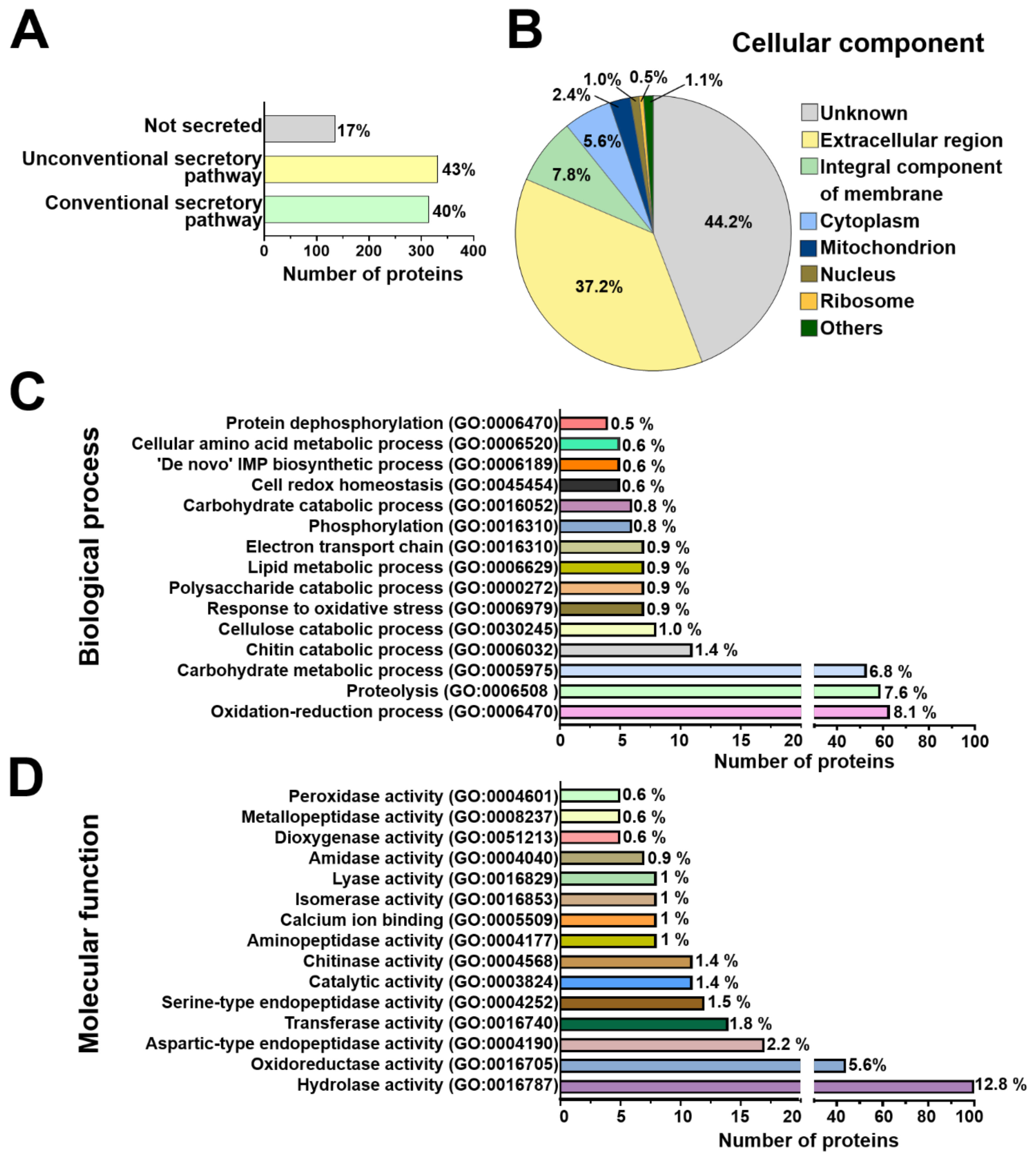
2.3. Functional Annotation of Arabidopsis and T. atroviride Secreted Proteins
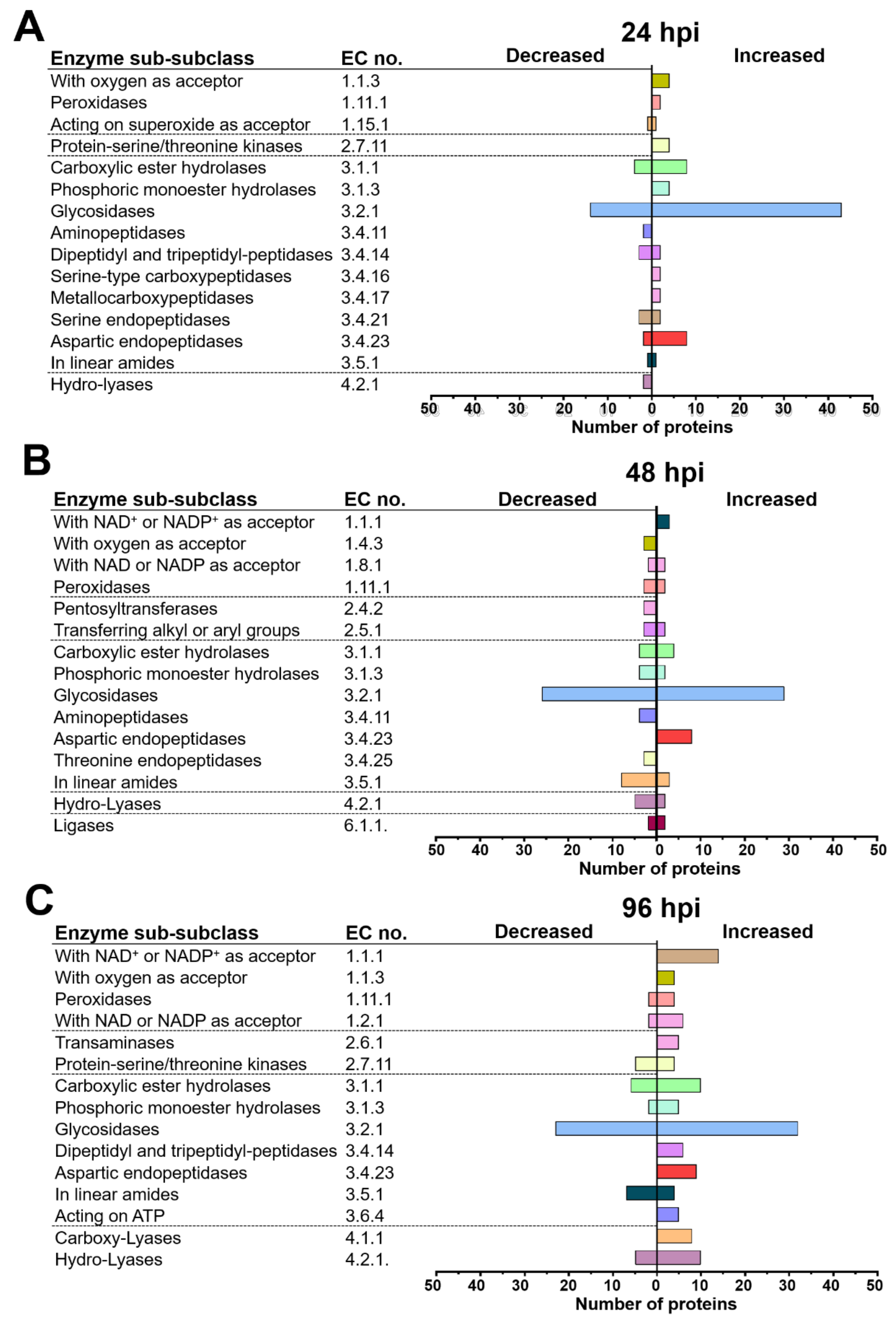
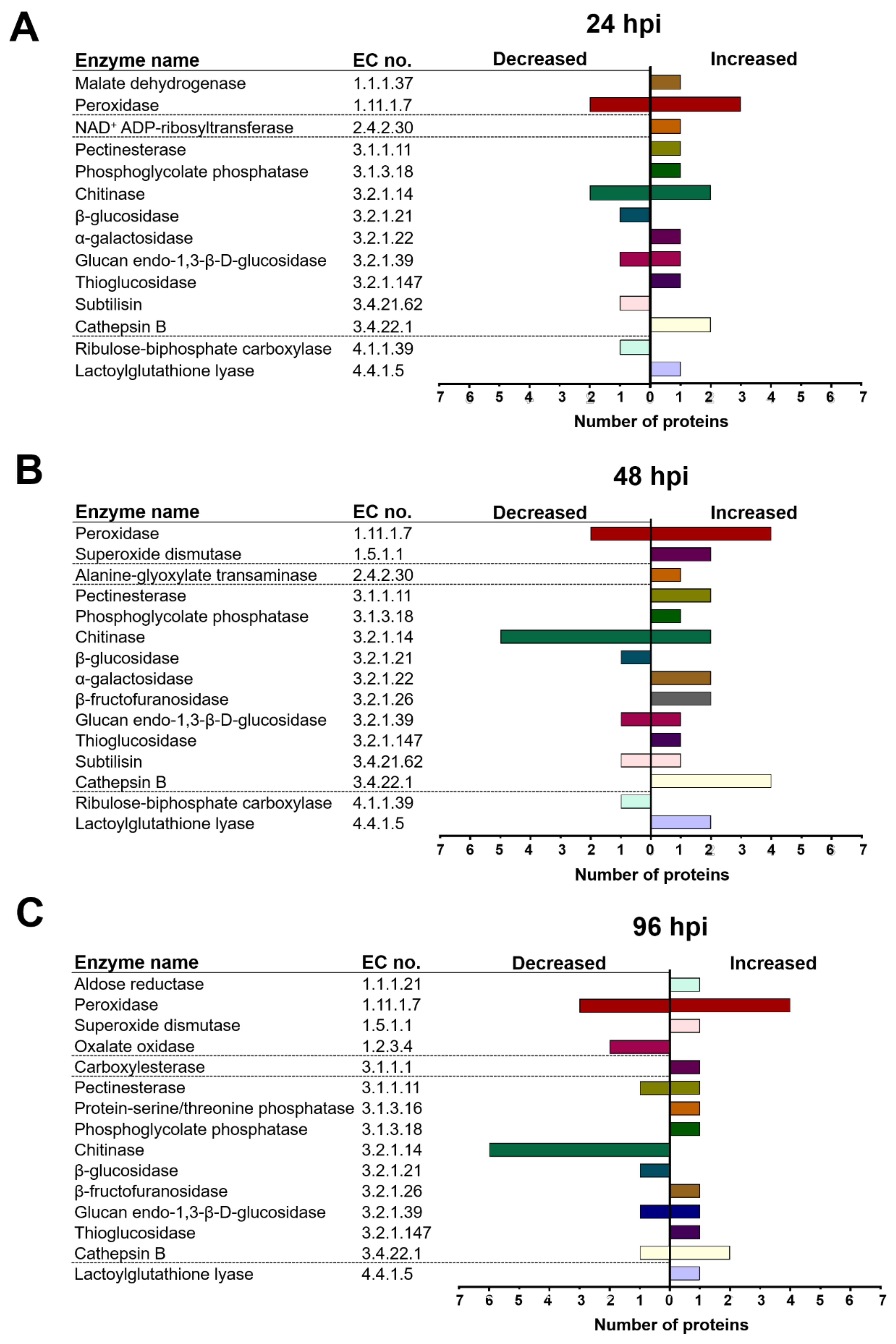
2.4. T. atroviride and Arabidopsis Secretomes Were Enriched with a Plethora of Enzymes
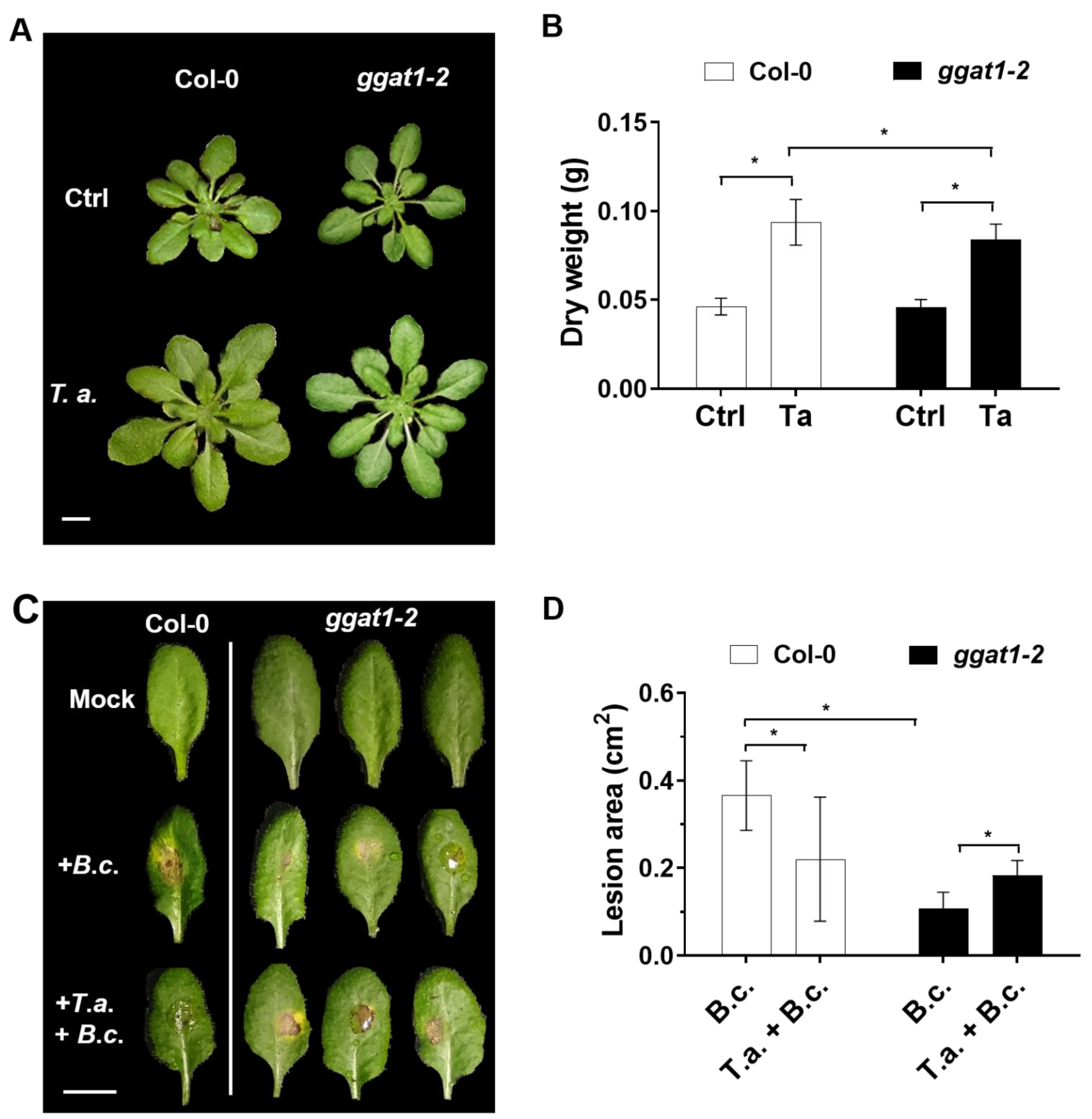
2.5. GGAT1 Plays a Minor Role in Plant Growth Stimulation by T. atroviride
2.6. GGAT1 Is Potentially Involved in the Negative Regulation of the Systemic Resistance against B. cinerea
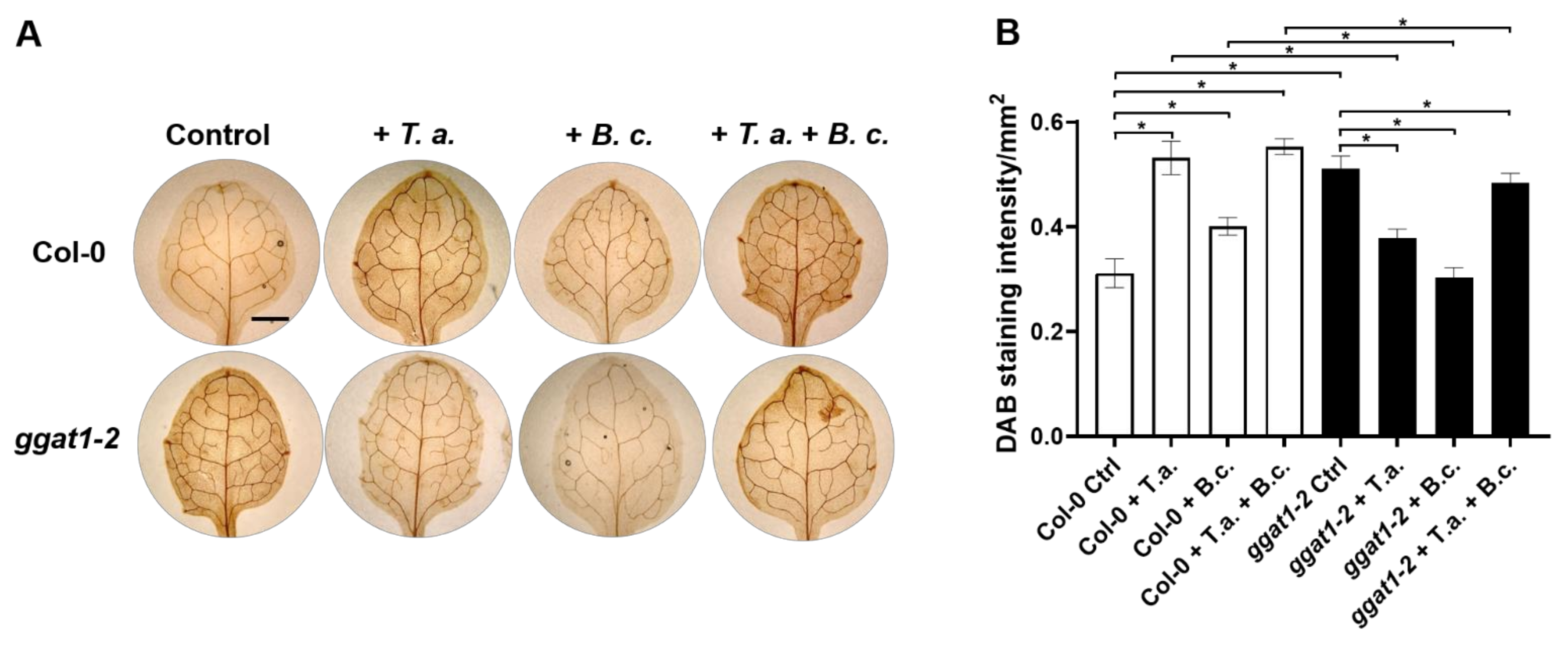
2.7. In Arabidopsis, GGAT1 Participates in the Resistance against B. cinerea, Tentatively through a Mechanism Involving H2O2 Production
3. Discussion
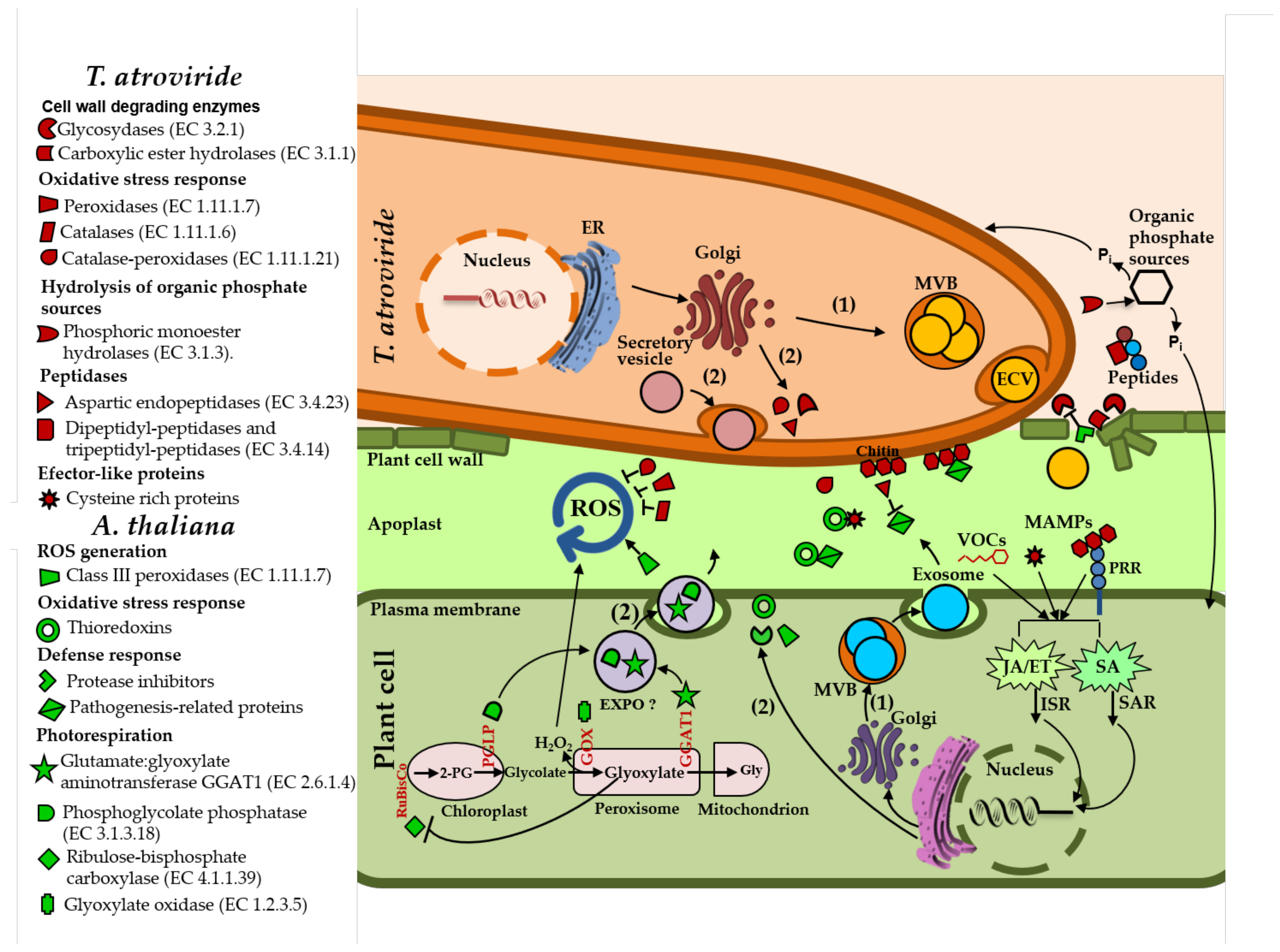
3.1. Arabidopsis and T. atroviride Secreted a Diverse Array of Proteins during Their Interaction
3.2. Arabidopsis and T. atroviride Secrete Proteins through Conventional and Non-Conventional Secretion Pathways
3.3. T. atroviride Secretes Enzymes Involved in Oxidative Stress Response, Cell Wall Degradation, Hydrolysis of Organic Phosphate Sources, and Peptidases during Its Interaction with Arabidopsis
3.4. Arabidopsis Responds to the Presence of T. atroviride by Secreting Enzymes Involved in ROS Generation, Oxidative Stress Response, Defense Response, and Photorespiration
3.5. GGAT1 Is Partially Required for Plant Growth Stimulation by T. atroviride
3.6. GGAT1 Negatively Regulates the Plant Systemic Resistance against B. cinerea Tentatively through a Mechanism that Involves Altered H2O2 Production
4. Materials and Methods
4.1. Plant and Fungal Growth Conditions for In Vitro Experiments
4.2. Secretome System for Arabidopsis and T. atroviride Interaction Analysis
4.3. Secretome Samples Concentration
4.4. Identification of Secreted Proteins by Gel-Free Shotgun Proteomics
4.5. Bioinformatic Analysis of Secreted Proteins
4.6. Plant-Growth Promotion and Plant–Pathogen Challenge Assays
4.7. Detection of Hydrogen Peroxide (H2O2) in Arabidopsis Leaves Using 3,3-diaminobenzidine (DAB)
4.8. Colonization Assay
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rehman Hakeem, K.; Akhtar Sayeed, M. Plant, Soil and Microbes; Springer: Berlin/Heidelberg, Germany, 2016; Volume 2, ISBN 9783319295725. [Google Scholar]
- Malinovsky, F.G.; Fangel, J.U.; Willats, W.G.T. The role of the cell wall in plant immunity. Front. Plant Sci. 2014, 5, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De-la-Pena, C.; Badri, D.V.; Loyola-Vargas, V.M. Plant root secretions and their interactions with neighbors. In Secretions and Exudates in Biological Systems; Springer: Berlin/Heidelberg, Germany, 2012; pp. 1–26. [Google Scholar] [CrossRef]
- Kubicek, C.P.; Starr, T.L.; Glass, N.L. Plant cell wall-degrading enzymes and their secretion in plant-pathogenic fungi. Annu. Rev. Phytopathol. 2014, 52, 427–451. [Google Scholar] [CrossRef] [PubMed]
- Druzhinina, I.S.; Chenthamara, K.; Zhang, J.; Atanasova, L.; Yang, D.; Miao, Y.; Rahimi, M.J.; Grujic, M.; Cai, F.; Pourmehdi, S.; et al. Massive lateral transfer of genes encoding plant cell wall-degrading enzymes to the mycoparasitic fungus Trichoderma from its plant-associated hosts. PLoS Genet. 2018, 14, 1–33. [Google Scholar] [CrossRef] [PubMed]
- Pieterse, M.J.; Zamioudis, C.; Berendsen, R.L.; Weller, D.M.; Van Wees, S.C.M.; Bakker, P.A.H.M. Induced Systemic Resistance by Beneficial Microbes. Annu. Rev. Phytopathol. 2014, 52, 347–375. [Google Scholar] [CrossRef] [Green Version]
- Ning, Y.; Liu, W.; Wang, G.L. Balancing Immunity and Yield in Crop Plants. Trends Plant Sci. 2017, 22, 1069–1079. [Google Scholar] [CrossRef]
- Bürger, M.; Chory, J. Stressed out about Hormones: How Plants Orchestrate Immunity. Cell Host Microbe 2019, 26, 163–172. [Google Scholar] [CrossRef]
- Burdett, H.; Bentham, A.R.; Williams, S.J.; Dodds, P.N.; Anderson, P.A.; Banfield, M.J.; Kobe, B. The Plant “Resistosome”: Structural Insights into Immune Signaling. Cell Host Microbe 2019, 26, 193–201. [Google Scholar] [CrossRef] [Green Version]
- Ramirez-prado, J.S.; Abulfaraj, A.A.; Rayapuram, N.; Benhamed, M. Plant Immunity: From Signaling to Epigenetic Control of Defense. Trends Plant Sci. 2018, 23, 833–844. [Google Scholar] [CrossRef]
- Tanveer, T.; Shaheen, K.; Parveen, S.; Kazi, A.G.; Ahmad, P. Plant secretomics: Identification, isolation, and biological significance under environmental stress. Plant Signal. Behav. 2014, 9, 37–41. [Google Scholar] [CrossRef] [Green Version]
- Davis, D.J.; Kang, B.-H.; Heringer, A.S.; Wilkop, T.E.; Drakakaki, G. Unconventional Protein Secretion in Plants; Pompa, A., De Marchis, F., Eds.; Humana Press: New York, NY, USA, 2016; Volume 1459, ISBN 9781493938025. [Google Scholar]
- Rabouille, C. Pathways of Unconventional Protein Secretion. Trends Cell Biol. 2017, 27, 230–240. [Google Scholar] [CrossRef]
- Plett, J.M.; Daguerre, Y.; Wittulsky, S.; Vayssières, A.; Deveau, A.; Melton, S.J. Effector MiSSP7 of the mutualistic fungus Laccaria bicolor stabilizes the Populus JAZ6 protein and represses jasmonic acid (JA) responsive genes. Proc. Natl. Acad. Sci. USA 2014, 111. [Google Scholar] [CrossRef] [Green Version]
- Rebolledo-Prudencio, O.G.; Dautt-Castro, M.; Estrada-Rivera, M.; del González-López, M.C.; Jijón-Moreno, S.; Casas-Flores, S. Trichoderma in the rhizosphere: An approach toward a long and successful symbiosis with plants. In New and Future Developments in Microbial Biotechnology and Bioengineering; Elsevier: Amsterdam, The Netherlands, 2020; pp. 3–38. ISBN 9780128194539. [Google Scholar]
- Kloppholz, S.; Kuhn, H.; Requena, N. Report A Secreted Fungal Effector of Glomus intraradices Promotes Symbiotic Biotrophy. Curr. Biol. 2011, 21, 1204–1209. [Google Scholar] [CrossRef] [Green Version]
- Jaroszuk-ściseł, J.; Tyśkiewicz, R.; Nowak, A.; Ozimek, E.; Majewska, M.; Hanaka, A.; Tyśkiewicz, K.; Pawlik, A.; Janusz, G. Phytohormones (Auxin, gibberellin) and ACC deaminase in vitro synthesized by the mycoparasitic Trichoderma DEMTKZ3A0 strain and changes in the level of auxin and plant resistance markers in wheat seedlings inoculated with this strain conidia. Int. J. Mol. Sci. 2019, 20, 4923. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bononi, L.; Chiaramonte, J.B.; Pansa, C.C.; Moitinho, M.A.; Melo, I.S. Phosphorus-solubilizing Trichoderma spp. from Amazon soils improve soybean plant growth. Sci. Rep. 2020, 10, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada-Rivera, M.; del Rocha-Medina, M.C.; Pérez-Robles, D.A.; Casas-Flores, S.; Rebolledo-Prudencio, O.G.; del González-López, M.C. Trichoderma histone deacetylase HDA-2 modulates multiple responses in Arabidopsis. Plant Physiol. 2019, 52. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Phoka, N.; Suwannarach, N.; Lumyong, S.; Ito, S.I.; Matsui, K.; Arikit, S.; Sunpapao, A. Role of volatiles from the endophytic fungus Trichoderma asperelloides psu-p1 in biocontrol potential and in promoting the plant growth of Arabidopsis thaliana. J. Fungi 2020, 6, 341. [Google Scholar] [CrossRef]
- Salas-Marina, M.A.; Isordia-Jasso, M.I.; Islas-Osuna, M.A.; Delgado-Sánchez, P.; Jiménez-Bremont, J.F.; Rodríguez-Kessler, M.; Rosales-Saavedra, M.T.; Herrera-Estrella, A.; Casas-Flores, S. The Epl1 and Sm1 proteins from Trichoderma atroviride and Trichoderma virens differentially modulate systemic disease resistance against different life style pathogens in Solanum lycopersicum. Front. Plant Sci. 2015, 6, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Aamir, M.; Kashyap, S.P.; Zehra, A.; Dubey, M.K.; Singh, V.K.; Ansari, W.A.; Upadhyay, R.S.; Singh, S. Trichoderma erinaceum Bio-Priming Modulates the WRKYs Defense Programming in Tomato Against the Fusarium oxysporum f. sp. lycopersici (Fol) Challenged Condition. Front. Plant Sci. 2019, 10. [Google Scholar] [CrossRef] [Green Version]
- Rebolledo-Prudencio, O.G. La Maquinaria de Silenciamiento Génico Mediada por sRNAs de Arabidopsis thaliana y su Papel en el Establecimiento de una Relación Benéfica con Trichoderma atroviride. Master’s Thesis, Instituto Potosino de Investigación Científica y Tecnológica (IPICYT), San Luis Potosí, Mexico, 2015. [Google Scholar]
- Der Wang, K.; Gorman, Z.; Huang, P.C.; Kenerley, C.M.; Kolomiets, M.V. Trichoderma virens colonization of maize roots triggers rapid accumulation of 12-oxophytodienoate and two ⍺-ketols in leaves as priming agents of induced systemic resistance. Plant Signal. Behav. 2020, 18–22. [Google Scholar] [CrossRef]
- Van Oosten, V.R.; Bodenhausen, N.; Reymond, P.; Van Pelt, J.A.; Van Loon, L.C.; Dicke, M.; Pieterse, C.M.J. Differential effectiveness of microbially induced resistance against herbivorous insects in Arabidopsis. Mol. Plant-Microbe Interact. 2008, 21, 919–930. [Google Scholar] [CrossRef] [Green Version]
- Nogueira-Lopez, G.; Greenwood, D.R.; Middleditch, M.; Winefield, C.; Eaton, C.; Steyaert, J.M. The Apoplastic Secretome of Trichoderma virens During Interaction with Maize Roots Shows an Inhibition of Plant Defence and Scavenging Oxidative Stress Secreted Proteins. Front. Plant Sci. 2018, 9, 1–23. [Google Scholar] [CrossRef]
- Lamdan, N.; Shalaby, S.; Ziv, T.; Kenerley, C.M.; Horwitz, B.A. Secretome of Trichoderma Interacting with Maize Roots: Role in Induced Systemic Resistance. Mol. Cell. Proteom. 2015, 14, 1054–1063. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rutter, B.D.; Innes, R.W. Extracellular vesicles isolated from the leaf apoplast carry stress-response proteins. Plant Physiol. 2017, 173, 728–741. [Google Scholar] [CrossRef] [Green Version]
- Cortázar, A.R.; Aransay, A.M.; Alfaro, M.; Oguiza, J.A.; Lavín, J.L. SECRETOOL: Integrated secretome analysis tool for fungi. Amino Acids 2014, 46, 471–473. [Google Scholar] [CrossRef]
- Bendtsen, J.D.; Jensen, L.J.; Blom, N.; Von Heijne, G.; Brunak, S. Feature-based prediction of non-classical and leaderless protein secretion. Protein Eng. Des. Sel. 2004, 17, 349–356. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Poschmann, G.; Waldera-Lupa, D.; Rafiee, N.; Kollmann, M.; Stühler, K. OutCyte: A novel tool for predicting unconventional protein secretion. Sci. Rep. 2019, 9, 1–9. [Google Scholar] [CrossRef] [PubMed]
- Conesa, A.; Götz, S.; García-Gómez, J.M.; Terol, J.; Talón, M.; Robles, M. Blast2GO: A universal tool for annotation, visualization and analysis in functional genomics research. Bioinformatics 2005, 21, 3674–3676. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schomburg, I.; Chang, A.; Ebeling, C.; Gremse, M.; Heldt, C.; Huhn, G.; Schomburg, D. BRENDA, the enzyme database: Updates and major new developments. Nucleic Acids Res. 2004, 32, 431–433. [Google Scholar] [CrossRef] [Green Version]
- Verslues, P.E.; Kim, Y.S.; Zhu, J.K. Altered ABA, proline and hydrogen peroxide in an Arabidopsis glutamate:glyoxylate aminotransferase mutant. Plant Mol. Biol. 2007, 64, 205–217. [Google Scholar] [CrossRef]
- Thürich, J.; Meichsner, D.; Furch, A.C.U.; Pfalz, J.; Krüger, T.; Kniemeyer, O.; Brakhage, A.; Oelmüller, R. Arabidopsis thaliana responds to colonisation of Piriformospora indica by secretion of symbiosis-specific proteins. PLoS ONE 2018, 13, e209658. [Google Scholar] [CrossRef] [Green Version]
- Morán-Diez, E.; Hermosa, R.; Ambrosino, P.; Cardoza, R.E.; Gutiérrez, S.; Lorito, M.; Monte, E. The ThPG1 Endopolygalacturonase Is Required for the Trichoderma harzianum—Plant Beneficial Interaction. Mol. Plant Microbe Interact. 2009, 22, 1021–1031. [Google Scholar] [CrossRef] [Green Version]
- Sun, X.; Su, X. Harnessing the knowledge of protein secretion for enhanced protein production in filamentous fungi. World J. Microbiol. Biotechnol. 2019, 35, 1–10. [Google Scholar] [CrossRef] [PubMed]
- Heard, W.; Sklenář, J.; Tomé, D.F.A.; Robatzek, S.; Jones, A.M.E. Identification of regulatory and cargo proteins of endosomal and secretory pathways in Arabidopsis thaliana by proteomic dissection. Mol. Cell. Proteom. 2015, 14, 1796–1813. [Google Scholar] [CrossRef] [Green Version]
- Miura, N.; Ueda, M. Evaluation of Unconventional Protein Secretion by Saccharomyces cerevisiae and other Fungi. Cells 2018, 7, 128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giraldo, M.C.; Dagdas, Y.F.; Gupta, Y.K.; Mentlak, T.A.; Yi, M.; Martinez-Rocha, A.L.; Saitoh, H.; Terauchi, R.; Talbot, N.J.; Valent, B. Two distinct secretion systems facilitate tissue invasion by the rice blast fungus Magnaporthe oryzae. Nat. Commun. 2013, 4, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Valkonen, M.; Kalkman, E.R.; Saloheimo, M.; Penttilä, M.; Read, N.D.; Duncan, R.R. Spatially segregated SNARE protein interactions in living fungal cells. J. Biol. Chem. 2007, 282, 22775–22785. [Google Scholar] [CrossRef] [Green Version]
- De Paula, R.G.; Antoniêto, A.C.C.; Nogueira, K.M.V.; Ribeiro, L.F.C.; Rocha, M.C.; Malavazi, I.; Almeida, F.; Silva, R.N. Extracellular vesicles carry cellulases in the industrial fungus Trichoderma reesei. Biotechnol. Biofuels 2019, 12, 1–14. [Google Scholar] [CrossRef]
- Smulevich, G.; Jakopitsch, C.; Droghetti, E.; Obinger, C. Probing the structure and bifunctionality of catalase-peroxidase (KatG). J. Inorg. Biochem. 2006, 100, 568–585. [Google Scholar] [CrossRef]
- Tanabe, S.; Ishii-Minami, N.; Saitoh, K.I.; Otake, Y.; Kaku, H.; Shibuya, N.; Nishizawa, Y.; Minami, E. The role of catalase-peroxidase secreted by Magnaporthe oryzae during early infection of rice cells. Mol. Plant-Microbe Interact. 2011, 24, 163–171. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Houfani, A.A.; Anders, N.; Spiess, A.C.; Baldrian, P.; Benallaoua, S. Insights from enzymatic degradation of cellulose and hemicellulose to fermentable sugars—A review. Biomass Bioenergy 2020, 134, 105481. [Google Scholar] [CrossRef]
- Singh, G.; Verma, A.K.; Kumar, V. Catalytic properties, functional attributes and industrial applications of β-glucosidases. 3 Biotech 2016, 6, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Zhu, N.; Yang, J.; Lin, Y.; Liu, J.; Wang, R.; Wang, F.; Yuan, H. A novel bifunctional acetyl xylan esterase/arabinofuranosidase from Penicillium chrysogenum P33 enhances enzymatic hydrolysis of lignocellulose. Microb. Cell Fact. 2017, 16, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Su, L.; Chen, J.; Wu, J. Cutinase: Characteristics, preparation, and application. Biotechnol. Adv. 2013, 31, 1754–1767. [Google Scholar] [CrossRef]
- Wang, X.X.; Hoffland, E.; Feng, G.; Kuyper, T.W. Phosphate uptake from phytate due to hyphae-mediated phytase activity by arbuscular mycorrhizal maize. Front. Plant Sci. 2017, 8, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Corrêa, T.L.R.; de Araújo, E.F. Fungal phytases: From genes to applications. Braz. J. Microbiol. 2020, 51, 1009–1020. [Google Scholar] [CrossRef] [PubMed]
- Saravanakumar, K.; Arasu, V.S.; Kathiresan, K. Effect of Trichoderma on soil phosphate solubilization and growth improvement of Avicennia marina. Aquat. Bot. 2013, 104, 101–105. [Google Scholar] [CrossRef]
- Mara, P.R.; Isabel, C.M.C.J.; dos Luiz, C.R.S.; Marcos, A.S.; Flvia, D.P.; Edson, L.S.; Fabiano, G.S. Phosphate solubilization and phytohormone production by endophytic and rhizosphere Trichoderma isolates of guanandi (Calophyllum brasiliense Cambess). Afr. J. Microbiol. Res. 2014, 8, 2616–2623. [Google Scholar] [CrossRef] [Green Version]
- Chagas, L.F.B.; De Castro, H.G.; Colonia, B.S.O.; De Carvalho Filho, M.R.; Miller, L.D.O.; Chagas, A.F.J. Efficiency of Trichoderma spp. as a growth promoter of cowpea (Vigna unguiculata) and analysis of phosphate solubilization and indole acetic acid synthesis. Rev. Bras. Bot. 2016, 39, 437–445. [Google Scholar] [CrossRef]
- Rawat, R.; Tewari, L. Effect of abiotic stress on phosphate solubilization by biocontrol fungus Trichoderma sp. Curr. Microbiol. 2011, 62, 1521–1526. [Google Scholar] [CrossRef]
- Zhao, L.; Liu, Q.; Zhang, Y.Q.; Cui, Q.Y.; Liang, Y.C. Effect of acid phosphatase produced by Trichoderma asperellum Q1 on growth of Arabidopsis under salt stress. J. Integr. Agric. 2017, 16, 1341–1346. [Google Scholar] [CrossRef] [Green Version]
- Tandon, A.; Fatima, T.; Anshu; Shukla, D.; Tripathi, P.; Srivastava, S.; Singh, P.C. Phosphate solubilization by Trichoderma koningiopsis (NBRI-PR5) under abiotic stress conditions. J. King Saud Univ. Sci. 2020, 32, 791–798. [Google Scholar] [CrossRef]
- Schenk, G.; Mitić, N.Š.; Hanson, G.R.; Comba, P. Purple acid phosphatase: A journey into the function and mechanism of a colorful enzyme. Coord. Chem. Rev. 2013, 257, 473–482. [Google Scholar] [CrossRef]
- De Oliveira Ornela, P.H.; Souza Guimarães, L.H. Purification and characterization of an alkalistable phytase produced by Rhizopus microsporus var. microsporus in submerged fermentation. Process Biochem. 2019, 81, 70–76. [Google Scholar] [CrossRef]
- Jashni, M.K.; Mehrabi, R.; Collemare, J.; Mesarich, C.H.; de Wit, P.J.G.M. The battle in the apoplast: Further insights into the roles of proteases and their inhibitors in plant–pathogen interactions. Front. Plant Sci. 2015, 6, 1–7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Han, L.B.; Li, Y.B.; Wang, F.X.; Wang, W.Y.; Liu, J.; Wu, J.H.; Zhong, N.Q.; Wu, S.J.; Jiao, G.L.; Wang, H.Y.; et al. The Cotton Apoplastic Protein CRR1 stabilizes chitinase 28 to facilitate defense against the fungal pathogen Verticillium dahliae. Plant Cell 2019, 31, 520–536. [Google Scholar] [CrossRef]
- Slavokhotova, A.A.; Naumann, T.A.; Price, N.P.J.; Rogozhin, E.A.; Andreev, Y.A.; Vassilevski, A.A.; Odintsova, T.I. Novel mode of action of plant defense peptides—Hevein-like antimicrobial peptides from wheat inhibit fungal metalloproteases. FEBS J. 2014, 281, 4754–4764. [Google Scholar] [CrossRef] [PubMed]
- Martino, E.; Morin, E.; Grelet, G.A.; Kuo, A.; Kohler, A.; Daghino, S.; Barry, K.W.; Cichocki, N.; Clum, A.; Dockter, R.B.; et al. Comparative genomics and transcriptomics depict ericoid mycorrhizal fungi as versatile saprotrophs and plant mutualists. New Phytol. 2018, 217, 1213–1229. [Google Scholar] [CrossRef] [Green Version]
- Nanda, A.K.; Andrio, E.; Marino, D.; Pauly, N.; Dunand, C. Reactive oxygen species during plant-microorganism early interactions. J. Integr. Plant Biol. 2010, 52, 195–204. [Google Scholar] [CrossRef]
- Daudi, A.; Cheng, Z.; O’Brien, J.A.; Mammarella, N.; Khan, S.; Ausubel, F.M.; Bolwell, G.P. The Apoplastic Oxidative Burst Peroxidase in Arabidopsis Is a Major Component of Pattern-Triggered Immunity. Plant 2012, 24, 275–287. [Google Scholar] [CrossRef] [Green Version]
- O’Brien, J.A.; Daudi, A.; Finch, P.; Butt, V.S.; Whitelegge, J.P.; Souda, P.; Ausubel, F.M.; Bolwell, G.P. A peroxidase-dependent apoplastic oxidative burst in cultured arabidopsis cells functions in MAMP-elicited defense. Plant Physiol. 2012, 158, 2013–2027. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kámán-Tóth, E.; Dankó, T.; Gullner, G.; Bozsó, Z.; Palkovics, L.; Pogány, M. Contribution of cell wall peroxidase- and NADPH oxidase-derived reactive oxygen species to Alternaria brassicicola-induced oxidative burst in Arabidopsis. Mol. Plant Pathol. 2019, 20, 485–499. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.B.; Han, L.B.; Wang, H.Y.; Zhang, J.; Sun, S.T.; Feng, D.Q.; Yang, C.L.; Sun, Y.D.; Zhong, N.Q.; Xia, G.X. The thioredoxin GbNRX1 plays a crucial role in homeostasis of apoplastic reactive oxygen species in response to Verticillium dahliae infection in cotton. Plant Physiol. 2016, 170, 2392–2406. [Google Scholar] [CrossRef] [Green Version]
- Zhang, C.J.; Zhao, B.C.; Ge, W.N.; Zhang, Y.F.; Song, Y.; Sun, D.Y.; Guo, Y. An apoplastic h-type thioredoxin is involved in the stress response through regulation of the apoplastic reactive oxygen species in rice. Plant Physiol. 2011, 157, 1884–1899. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.; Kim, S.M.; Lee, R.T. Thioredoxin and thioredoxin target proteins: From molecular mechanisms to functional significance. Antioxid. Redox Signal. 2013, 18, 1165–1207. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Patwari, P.; Higgins, L.J.; Chutkow, W.A.; Yoshioka, J.; Lee, R.T. The interaction of thioredoxin with Txnip: Evidence for formation of a mixed disulfide by disulfide exchange. J. Biol. Chem. 2006, 281, 21884–21891. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Brader, G.; Palva, E.T. Kunitz trypsin inhibitor: An antagonist of cell death triggered by phytopathogens and fumonisin B1 in Arabidopsis. Mol. Plant 2008, 1, 482–495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Renko, M.; Sabotič, J.; Turk, D. β-Trefoil inhibitors—From the work of Kunitz onward. Biol. Chem. 2012, 393, 1043–1054. [Google Scholar] [CrossRef]
- Laluk, K.; Mengiste, T. The Arabidopsis extracellular UNUSUAL SERINE PROTEASE INHIBITOR functions in resistance to necrotrophic fungi and insect herbivory. Plant J. 2011, 68, 480–494. [Google Scholar] [CrossRef]
- Jain, D.; Khurana, J.P. Role of pathogenesis-related (PR) proteins in plant defense mechanism. Mol. Asp. Plant Pathog. Interact. 2018, 265–281. [Google Scholar] [CrossRef]
- Bauwe, H. Photorespiration—Damage Repair Pathway of the Calvin-Benson Cycle. In Plant Mitochondria; Logan, D., Ed.; Wiley Online Library: Hoboken, NJ, USA, 2018; Volume 50, pp. 293–342. ISBN 9781119312994. [Google Scholar]
- Schwarte, S.; Bauwe, H. Identification of the photorespiratory 2-phosphoglycolate phosphatase, PGLP1, in Arabidopsis. Plant Physiol. 2007, 144, 1580–1586. [Google Scholar] [CrossRef] [Green Version]
- Igarashi, D.; Miwa, T.; Seki, M.; Kobayashi, M.; Kato, T.; Tabata, S.; Shinozaki, K.; Ohsumi, C. Identification of photorespiratory glutamate:glyoxylate aminotransferase (GGAT) gene in Arabidopsis. Plant J. 2003, 33, 975–987. [Google Scholar] [CrossRef]
- Lu, Y.; Li, Y.; Yang, Q.; Zhang, Z.; Chen, Y.; Zhang, S.; Peng, X.X. Suppression of glycolate oxidase causes glyoxylate accumulation that inhibits photosynthesis through deactivating Rubisco in rice. Physiol. Plant. 2014, 150, 463–476. [Google Scholar] [CrossRef] [PubMed]
- Fiorini, L.; Guglielminetti, L.; Mariotti, L.; Curadi, M.; Picciarelli, P.; Scartazza, A.; Sarrocco, S.; Vannacci, G. Trichoderma harzianum T6776 modulates a complex metabolic network to stimulate tomato cv. Micro-Tom growth. Plant Soil 2016, 400, 351–366. [Google Scholar] [CrossRef]
- Oljira, A.M.; Hussain, T.; Waghmode, T.R.; Zhao, H.; Sun, H.; Liu, X.; Wang, X.; Liu, B. Trichoderma enhances net photosynthesis, water use efficiency, and growth of wheat (Triticum aestivum L.) under salt stress. Microorganisms 2020, 8, 1565. [Google Scholar] [CrossRef] [PubMed]
- Segarra, G.; Casanova, E.; Bellido, D.; Odena, M.A.; Oliveira, E.; Trillas, I. Proteome, salicylic acid, and jasmonic acid changes in cucumber plants inoculated with Trichoderma asperellum strain T34. Proteomics 2007, 7, 3943–3952. [Google Scholar] [CrossRef] [PubMed]
- Shoresh, M.; Harman, G.E. The molecular basis of shoot responses of maize seedlings to Trichoderma harzianum T22 inoculation of the root: A proteomic approach. Plant Physiol. 2008, 147, 2147–2163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellero, Y.; Lamothe-Sibold, M.; Jossier, M.; Hodges, M. Arabidopsis thaliana ggt1 photorespiratory mutants maintain leaf carbon/nitrogen balance by reducing RuBisCO content and plant growth. Plant J. 2015, 83, 1005–1018. [Google Scholar] [CrossRef] [PubMed]
- Liepman, A.H.; Olsen, L.J. Alanine aminotransferase homologs catalyze the glutamate:Glyoxylate aminotransferase reaction in peroxisomes of Arabidopsis. Plant Physiol. 2003, 131, 215–227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dellero, Y.; Jossier, M.; Schmitz, J.; Maurino, V.G.; Hodges, M. Photorespiratory glycolate-glyoxylate metabolism. J. Exp. Bot. 2016, 67, 3041–3052. [Google Scholar] [CrossRef] [Green Version]
- Asselbergh, B.; Curvers, K.; França, S.C.; Audenaert, K.; Vuylsteke, M.; Van Breusegem, F.; Höfte, M. Resistance to Botrytis cinerea in sitiens, an abscisic acid-deficient tomato mutant, involves timely production of hydrogen peroxide and cell wall modifications in the epidermis. Plant Physiol. 2007, 144, 1863–1877. [Google Scholar] [CrossRef] [Green Version]
- Anderson, J.P.; Badruzsaufari, E.; Schenk, P.M.; Manners, J.M.; Desmond, O.J.; Ehlert, C.; Maclean, D.J.; Ebert, P.R.; Kazan, K. Antagonistic interaction between abscisic acid and jasmonate-ethylene signaling pathways modulates defense gene expression and disease resistance in Arabidopsis. Plant Cell 2004, 16, 3460–3479. [Google Scholar] [CrossRef] [Green Version]
- Contreras-Cornejo, H.A.; Macías-Rodríguez, L.; Beltrán-Peña, E.; Herrera-Estrella, A.; López-Bucio, J. Trichoderma-induced plant immunity likely involves both hormonal- and camalexindependent mechanisms in Arabidopsis thaliana and confers resistance against necrotrophic fungus Botrytis cinerea. Plant Signal. Behav. 2011, 6, 1554–1563. [Google Scholar] [CrossRef] [Green Version]
- Amselem, J.; Cuomo, C.A.; Van Kan, J.A.L.; Viaud, M.; Benito, E.P.; Couloux, A.; Coutinho, P.M.; De Vries, R.P.; Dyer, P.S.; Fillinger, S.; et al. Genomic Analysis of the Necrotrophic Fungal Pathogens Sclerotinia sclerotiorum and Botrytis cinerea. PLoS Genet. 2011, 7, e1002230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Estrada-Rivera, M.; Angel, M.; Dautt-Castro, M.; Arenas-Huertero, C.; Herrera-Estrella, A.; Casas-Flores, S. IPA-1 a Putative Chromatin Remodeler/Helicase-Related Protein of Trichoderma virens Plays Important Roles in Antibiosis Against Rhizoctonia solani and Induction of Arabidopsis Systemic Disease Resistance. Mol. Plant-Microbe Interact. 2020, 33, 808–824. [Google Scholar] [CrossRef] [PubMed]
- Bateman, A. UniProt: A worldwide hub of protein knowledge. Nucleic Acids Res. 2019, 47, D506–D515. [Google Scholar] [CrossRef] [Green Version]
- Altschul, S.F.; Gish, W.; Miller, W.; Myers, E.W.; Lipman, D.J. Basic local alignment search tool. J. Mol. Biol. 1990, 215, 403–410. [Google Scholar] [CrossRef]
- Potter, S.C.; Luciani, A.; Eddy, S.R.; Park, Y.; Lopez, R.; Finn, R.D. HMMER web server: 2018 update. Nucleic Acids Res. 2018, 46, W200–W204. [Google Scholar] [CrossRef] [Green Version]
- Almagro Armenteros, J.J.; Tsirigos, K.D.; Sønderby, C.K.; Petersen, T.N.; Winther, O.; Brunak, S.; von Heijne, G.; Nielsen, H. SignalP 5.0 improves signal peptide predictions using deep neural networks. Nat. Biotechnol. 2019, 37, 420–423. [Google Scholar] [CrossRef]
- Krogh, A.; Larsson, È.; Von Heijne, G.; Sonnhammer, E.L.L. Predicting Transmembrane Protein Topology with a Hidden Markov Model: Application to Complete Genomes. J. Mol. Biol. 2001, 305, 567–580. [Google Scholar] [CrossRef] [Green Version]
- Daudi, A.; O’Brien, J.A. Detection of Hydrogen Peroxide by DAB Staining in Arabidopsis leaves. Bio-Protocol 2012, 2, e263. [Google Scholar] [CrossRef] [Green Version]

| JGI Id * | Enzyme Name ** | EC No. ** | Confidence Score ** | Fold Change *** (Log2 Mean) | ||
|---|---|---|---|---|---|---|
| Response to oxidative stress | 24 h | 48 h | 96 h | |||
| 300451 | Peroxidase | EC 1.11.1.17 | 0.72 | 9.1 | 1.9 | 1.1 |
| 88379 | Catalase-peroxidase | EC 1.11.1.21 | 1.7 | 2.1 | −1.0 | 0.5 |
| 300992 | Thioredoxin-dependent peroxiredoxin | EC 1.11.1.24 | 1.57 | 0 | 3.4 | 6.9 |
| 297668 | Catalase | EC 1.11.1.6 | 2.5 | 0 | −4.9 | 10.8 |
| 94401 | Glutathione peroxidase | EC 1.11.1.9 | 0.76 | 0 | 3.3 | 0.2 |
| 215831 | Superoxide dismutase | EC 1.15.1.1 | 0.76 | 4.7 | 1.0 | 0.4 |
| 299895 | Cytochrome-c peroxidase | EC 1.11.1.5 | 0.94 | 0 | −2.9 | −2.9 |
| 155960 | Catalase-peroxidase | EC 1.11.1.21 | 1.7 | 3.7 | −6.5 | 0.2 |
| 298583 | Superoxide dismutase | EC 1.15.1.1 | 2.57 | 0 | −2.5 | −2.2 |
| Cell-wall degrading enzymes | ||||||
| 84753 | Acetylxylan esterase | EC 3.1.1.72 | 0.94 | 6.2 | 3.8 | 2.4 |
| 297844 | Cutinase | EC 3.1.1.74 | 0.72 | 5.9 | 5.9 | 4.0 |
| 296657 | Mannan endo-1,6-α-mannosidase | EC 3.2.1.101 | 1.62 | 5.6 | 2.9 | 4.0 |
| 44429 | Xyloglucan-specific endo-β-1,4-glucanase | EC 3.2.1.151 | 0.81 | 4.9 | 2.8 | 4.5 |
| 314392 | Cellulase | EC 3.2.1.4 | 0.94 | 4.4 | 7.2 | 10.1 |
| 221999 | Cellulase | EC 3.2.1.4 | 0.94 | 3.1 | 4.7 | 5.1 |
| 88310 | Endo-1,4-β-xylanase | EC 3.2.1.8 | 0.72 | 2.4 | 9.3 | 3.7 |
| 44894 | Cellulose 1,4-β-cellobiosidase | EC 3.2.1.91 | 1.89 | 4.2 | 5.4 | 5.0 |
| 88458 | Cellulose 1,4-β-cellobiosidase | EC 3.2.1.91 | 1.89 | 4.1 | 5.4 | 6.9 |
| 91075 | Glucan 1,3-β-glucosidase | EC 3.2.1.58 | 0.76 | 4.8 | 2.3 | 0 |
| 139054 | β-glucosidase | EC 3.2.1.21 | 0.94 | 4.7 | 5.6 | 10.4 |
| 42986 | β-glucosidase | EC 3.2.1.21 | 1.7 | −4.8 | −5.9 | −3.8 |
| 223991 | β-glucosidase | EC 3.2.1.21 | 0.94 | −6.5 | −2.2 | −2.9 |
| 302027 | β-glucosidase | EC 3.2.1.21 | 0.94 | −8.2 | −6.6 | −0.9 |
| 161158 | Xylan 1,4-β-xylosidase | EC 3.2.1.37 | 0.94 | −2.3 | −2.3 | −1.6 |
| 161159 | Xylan 1,4-β-xylosidase | EC 3.2.1.37 | 0.94 | −4.0 | −4.9 | −0.9 |
| Proteolysis | ||||||
| 90832 | Pepsin A | EC 3.4.23.1 | 0.76 | 6.4 | 3.4 | 0 |
| 142040 | Aspergillopepsin I | EC 3.4.23.18 | 1.81 | 2.4 | 3.9 | 0.2 |
| 298116 | Aspergillopepsin II | EC 3.4.23.19 | 0.76 | 3.9 | 3.4 | 0 |
| 137451 | Penicillopepsin | EC 3.4.23.20 | 0.81 | 2.6 | 6.1 | 9.2 |
| 131866 | Penicillopepsin | EC 3.4.23.20 | 0.86 | 1.5 | 3.8 | 5.2 |
| 33651 | Rhizopuspepsin | EC 3.4.23.21 | 0.76 | 0.6 | 3.1 | 6.2 |
| 176535 | Candidapepsin | EC 3.4.23.24 | 0.76 | 3.8 | 3.5 | 0 |
| 28954 | Candidapepsin | EC 3.4.23.24 | 0.76 | 2.5 | 0.1 | 13.0 |
| 292296 | Candidapepsin | EC 3.4.23.24 | 0.76 | 2.2 | 0.4 | 7.5 |
| 34007 | Candidapepsin | EC 3.4.23.24 | 0.76 | 1.6 | 0.4 | 9.2 |
| 220221 | Tripeptidyl-peptidase II | EC 3.4.14.10 | 0.94 | −5.4 | −4.4 | 0 |
| 36337 | Deuterolysin | EC 3.4.24.39 | 1.7 | 0 | −1.4 | −8.4 |
| 40863 | Aspergillopepsin I | EC 3.4.23.18 | 0.81 | −2.1 | −2.7 | 0 |
| 54917 | Dipeptidyl-peptidase II | EC 3.4.14.2 | 0.72 | −3.0 | −0.1 | 0 |
| 321810 | C5a peptidase | EC 3.4.21.110 | 0.76 | −3.5 | 0 | −10.5 |
| Dephosphorylation | ||||||
| 215617 | 3-phytase | EC 3.1.3.8 | 0.76 | 4.7 | 0 | 0.8 |
| 44629 | Acid phosphatase | EC 3.1.3.2 | 1.7 | 2.0 | −1.2 | 0.6 |
| 298464 | Inositol-phosphate phosphatase | EC 3.1.3.25 | 1.62 | 0 | 4.3 | 7.1 |
| 298832 | 5′-nucleotidase | EC 3.1.3.5 | 0.94 | −1.4 | 0.9 | 4.3 |
| 89336 | Protein-serine/threonine phosphatase | EC 3.1.3.16 | 1.7 | 3.0 | 0.9 | 3.9 |
| 147790 | Phosphoglycolate phosphatase | EC 3.1.3.18 | 0.81 | 0 | 0 | 4.5 |
| Locus_Tag * | Gene Symbol * | Enzyme Name ** | EC. No. ** | Confidence Score ** | Fold Change *** (Log2 Mean) | ||
|---|---|---|---|---|---|---|---|
| Response to oxidative stress | 24 h | 48 h | 96 h | ||||
| At3g49120 | PER34 | Peroxidase | EC 1.11.1.17 | 2.70 | 6.0 | 6.8 | 3.1 |
| At2g38380 | PER22 | Peroxidase | EC 1.11.1.17 | 2.70 | 2.2 | 8.2 | 7.6 |
| At1g05260 | PER3 | Peroxidase | EC 1.11.1.17 | 2.70 | 2.0 | 8.2 | 4.4 |
| At4g11290 | PER39 | Peroxidase | EC 1.11.1.17 | 2.70 | 0 | 2.6 | 0 |
| At3g32980 | PER32 | Peroxidase | EC 1.11.1.17 | 2.70 | 0 | −1.8 | 3.8 |
| At3g11630 | BAS1A | Thioredoxin-dependent peroxiredoxin | EC 1.11.1.24 | 3.70 | 1.9 | 3.0 | 0 |
| At2g28190 | SODC | Superoxide dismutase | EC 1.15.1.1 | 3.70 | 0 | 2.7 | 0 |
| At4g25100 | FDSD1 | Superoxide dismutase | EC 1.15.1.1 | 2.65 | 0 | 4.3 | 3.7 |
| At5g05340 | PER52 | Peroxidase | EC 1.11.1.17 | 2.70 | −1.6 | −2.8 | −2.8 |
| At5g64120 | PER71 | Peroxidase | EC 1.11.1.17 | 2.70 | −2.3 | −4.5 | −7.0 |
| At5g64100 | PER69 | Peroxidase | EC 1.11.1.17 | 2.70 | −3.0 | −1.6 | −6.7 |
| Defense response | |||||||
| At4g01610 | CATB3 | Cathepsin B | EC 3.4.22.1 | 1.74 | 0 | 3.9 | 0 |
| At3g19390 | RD21C | Cathepsin B | EC 3.4.22.1 | 1.82 | 0 | 6.6 | 3.4 |
| At5g60360 | ALP | Cathepsin B | EC 3.4.22.1 | 0.94 | 3.6 | 8.7 | 6.2 |
| At5g43060 | RD21B | Cathepsin B | EC 3.4.22.1 | 1.82 | 5.8 | 0.1 | 1.6 |
| At1g47128 | RD21A | Cathepsin B | EC 3.4.22.1 | 1.82 | 0.2 | 5.6 | −2.1 |
| At1g03220 | F15K9.17 | Chitinase | EC 3.2.1.14 | 0.81 | 0 | 2.5 | 1.9 |
| At4g16260 | At4g16260 | Glucan endo-1,3-β-d-glucosidase | EC 3.2.1.39 | 1.89 | 4.8 | 8.4 | 2.6 |
| At4g19810 | CHIC | Chitinase | EC 3.2.1.14 | 2.70 | 0 | −2.4 | −2.1 |
| At2g43620 | CHI62 | Chitinase | EC 3.2.1.14 | 2.70 | 0 | −2.8 | −1.9 |
| At2g43610 | CHI61 | Chitinase | EC 3.2.1.14 | 2.70 | −1.4 | −3.3 | −6.4 |
| At2g43570 | CHI | Chitinase | EC 3.2.1.14 | 2.70 | −2.1 | −4.9 | −9.2 |
| At3g54420 | CH5 | Chitinase | EC 3.2.1.14 | 2.70 | −2.7 | −5.8 | −7.8 |
| At1g75040 | PR5 | Glucan endo-1,3-β-d-glucosidase | EC 3.2.1.39 | 0.86 | −5.5 | −6.8 | −7.9 |
| Photorespiration | |||||||
| At5g36790 | PGP1B | Phosphoglycolate phosphatase | EC 3.1.3.18 | 2.70 | 3.9 | 6.3 | 4.4 |
| At1g23310 | GGAT1 | Glutamate:glyoxylate aminotransferase 1 | EC 2.6.1.4 | 2.70 | 0 | 3.5 | 0 |
| AtCg00490 | RBCL | Ribulose-bisphosphate carboxylase | EC 4.1.1.39 | 3.70 | −3.5 | −2.4 | 0 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
González-López, M.d.C.; Jijón-Moreno, S.; Dautt-Castro, M.; Ovando-Vázquez, C.; Ziv, T.; Horwitz, B.A.; Casas-Flores, S. Secretome Analysis of Arabidopsis–Trichoderma atroviride Interaction Unveils New Roles for the Plant Glutamate:Glyoxylate Aminotransferase GGAT1 in Plant Growth Induced by the Fungus and Resistance against Botrytis cinerea. Int. J. Mol. Sci. 2021, 22, 6804. https://doi.org/10.3390/ijms22136804
González-López MdC, Jijón-Moreno S, Dautt-Castro M, Ovando-Vázquez C, Ziv T, Horwitz BA, Casas-Flores S. Secretome Analysis of Arabidopsis–Trichoderma atroviride Interaction Unveils New Roles for the Plant Glutamate:Glyoxylate Aminotransferase GGAT1 in Plant Growth Induced by the Fungus and Resistance against Botrytis cinerea. International Journal of Molecular Sciences. 2021; 22(13):6804. https://doi.org/10.3390/ijms22136804
Chicago/Turabian StyleGonzález-López, María del Carmen, Saúl Jijón-Moreno, Mitzuko Dautt-Castro, Cesaré Ovando-Vázquez, Tamar Ziv, Benjamin A. Horwitz, and Sergio Casas-Flores. 2021. "Secretome Analysis of Arabidopsis–Trichoderma atroviride Interaction Unveils New Roles for the Plant Glutamate:Glyoxylate Aminotransferase GGAT1 in Plant Growth Induced by the Fungus and Resistance against Botrytis cinerea" International Journal of Molecular Sciences 22, no. 13: 6804. https://doi.org/10.3390/ijms22136804
APA StyleGonzález-López, M. d. C., Jijón-Moreno, S., Dautt-Castro, M., Ovando-Vázquez, C., Ziv, T., Horwitz, B. A., & Casas-Flores, S. (2021). Secretome Analysis of Arabidopsis–Trichoderma atroviride Interaction Unveils New Roles for the Plant Glutamate:Glyoxylate Aminotransferase GGAT1 in Plant Growth Induced by the Fungus and Resistance against Botrytis cinerea. International Journal of Molecular Sciences, 22(13), 6804. https://doi.org/10.3390/ijms22136804






