Bisphenol a Interferes with Uterine Artery Features and Impairs Rat Feto-Placental Growth
Abstract
:1. Introduction
2. Results
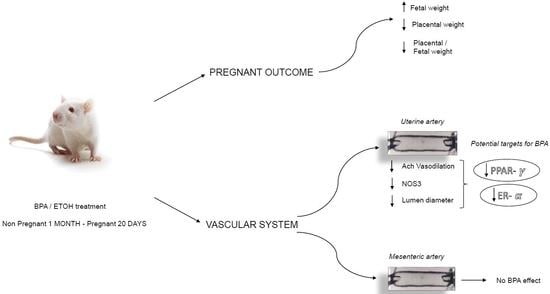
2.1. BPA Impact on Fetal and Placental Outcomes
2.2. BPA Impact on Maternal Uterine Arteries
3. Discussion and Conclusions
4. Material and Methods
4.1. Animals and Treatments
4.2. Reproductive Performance
4.3. Isolated Vessel Preparation
4.4. Reactivity Study
4.5. RNA Isolation and Gene Expression
4.6. Chemicals and Solutions
4.7. Statistical Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- European Commission Joint Research Centre Institute for Health and Consumer Protection. European Union Risk Assessment Report 2008. Human Health Addendum of April 2008. 4,4′-isopropylidenediphenol (Bisphenol A). Part 2 Human Health; Publications Office of the European Union: Luxembourg, 2010.
- Vandenberg, L.N.; Hauser, R.; Marcus, M.; Olea, N.; Welshons, W.V. Human exposure to bisphenol A (BPA). Reprod. Toxicol. 2007, 24, 139–177. [Google Scholar] [CrossRef]
- Goodson, A.; Robin, H.; Summerfield, W.; Cooper, I. Migration of bisphenol A from can coating effects of damage, storage conditions and heating. Food Addit. Contam. 2004, 21, 1015–1026. [Google Scholar] [CrossRef]
- Goodson, A.; Summerfield, W.; Cooper, I. Survey of bisphenol A and bisphenol F in canned foods. Food Addit. Contam. 2002, 19, 796–802. [Google Scholar] [CrossRef]
- Thomson, B.M.; Grounds, P.R. Bisphenol A in canned foods in New Zealand: An exposure assessment. Food Addit. Contam. 2005, 22, 65–72. [Google Scholar] [CrossRef] [Green Version]
- Brede, C.; Fjeldal, I.; Herikstad, H. Increasedd migration levels of bisphenol A from polycarbonate baby bottles after dishwashing, boiling and brushing. Food Addit. Contam. 2003, 20, 684–689. [Google Scholar] [CrossRef] [PubMed]
- Geens, T.; Goeyens, L.; Covaci, A. Are potential sources for human exposure to bisphenol-A overlooked? Int. J. Hyg. Environ. Health 2011, 214, 339–347. [Google Scholar] [CrossRef]
- Calafat, A.M.; Kuklenyik, Z.; Reidy, J.A.; Caudill, S.P.; Ekong, J.; Needham, L.L. Urinary concentrations of bisphenol A and 4-nonylphenol in a human reference population. Environ. Health Perspect. 2000, 113, 391–395. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Calafat, A.M.; Ye, X.; Wong, L.Y.; Reidy, J.A.; Needham, L.L. Exposure of the U.S. population to bisphenol A and 4-ter-tiaryoctylphenol:2003–2004. Environ. Health Perspect. 2008, 116, 39–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lakind, J.S.; Naiman, D.Q. Bisphenol A (BPA) daily in- takes in the United States: Estimates from the 2003–2004 NHANES urinary BPA data. J. Expo. Sci. Environ. Epidemiol. 2008, 18, 608–615. [Google Scholar] [CrossRef] [Green Version]
- Padmanabhan, V.; Siefert, K.; Ransom, S.; Johnson, T.; Pinkerton, J.; Anderson, L.; Tao, L.; Kannan, K. Maternal bisphenol-A levels at delivery: A looming problem? J. Perinatol. 2008, 28, 258–263. [Google Scholar] [CrossRef] [Green Version]
- Schönfelder, G.; Wittfoht, W.; Hopp, H.; Talsness, C.E.; Paul, M.; Chahoud, I. Parent bisphenol A accumulation in the human maternal–fetal–placental unit. Environ. Health Perspect. 2002, 110, 703–707. [Google Scholar] [CrossRef]
- Edlow, A.G.; Chein, M.; Smith, N.A.; Lu, C.; McElrath, T.F. Bisphenol A exposure: Concentration of conjugated and unconjugated bisphenol A in amniotic fluid in the second and third trimesters. Reprod. Toxicol. 2012, 34, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Jangwoo, L.; Kyungho, C.; Jeongim, P.; Hyo-Bang, M.; Gyuyeon, C.; Jeong, J.L.; Eunsook, S.; Hai-joong, K.; So-Hee, E.; Gun-Ha, K.; et al. Bisphenol A distribution in serum, urine, placenta, breast milk, and umbilical cord serum in a birth panel of mother-neonate pairs. Sci. Total Environ. 2018, 626, 1494–1501. [Google Scholar]
- Jagne, J.; White, D.; Jefferson, F. Endocrine- Disrupting Chemicals: Adverse Effects of Bisphenol A and Parabens to Women’s Health. Water Air Soil Pollut. 2016, 227, 182. [Google Scholar] [CrossRef]
- Mørck, T.J.; Sorda, G.; Bechi, N.; Rasmussen, B.S.; Nielsen, J.B.; Ietta, F.; Rytting, E.; Mathiesen, L.; Paulesu, L.; Knudsen, L. Placental transport and in vitro effects of Bisphenol A. Reprod. Toxicol. 2010, 30, 131–137. [Google Scholar] [CrossRef]
- Narciso, L.; Ietta, F.; Romagnoli, R.; Paulesu, L.; Mantovani, A.; Tait, S. Effects of Bisphenol A on endogenous retroviral envelopes expression and trophoblast fusion in BeWo cells. Reprod. Toxicol. 2019, 89, 35–44. [Google Scholar] [CrossRef] [PubMed]
- Spagnoletti, A.; Paulesu, L.; Mannelli, C.; Ermini, L.; Romagnoli, R.; Cintorino, M.; Ietta, F. Low concentrations of Bisphenol A and para-Nonylphenol affect extravillous pathway of human trophoblast cells. Mol. Cell Endocrinol. 2015, 5, 56–64. [Google Scholar] [CrossRef]
- Benincasa, L.; Mandalà, M.; Paulesu, L.; Barberio, L.; Ietta, F. Prenatal Nutrition Containing Bisphenol A Affects Placenta Glucose Transfer: Evidence in Rats and Human Trophoblast. Nutrients 2020, 12, 1375. [Google Scholar] [CrossRef]
- Aghajanova, L.; Giudice, L.C. Effect of bisphenol A on human endometrial stromal fibroblasts in vitro. Reprod. Biomed. Online 2011, 22, 249–256. [Google Scholar] [CrossRef] [Green Version]
- Mannelli, C.; Szóstek, A.; Lukasik, C.; Carotenuto, C.; Ietta, F.; Romagnoli, R.; Ferretti, C.; Paulesu, L.; Wolczynski, S.; Skarzynski, D. Bisphenol A modulates receptivity and secretory function of human decidual cells: An in vitro study. Reproduction 2015, 150, 115–125. [Google Scholar] [CrossRef] [Green Version]
- Forte, M.; Mita, L.; Cobellis, L.; Merafina, V.; Specchio, R.; Rossi, S.; Mita, D.G.; Mosca, L.; Castaldi, M.A.; De Falco, M.; et al. Triclosan and bisphenol a affect decidualization of human endometrial stromal cells. Mol. Cell Endocrinol. 2016, 15, 74–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Burstyn, I.; Martin, W.J.; Beesoon, S.; Bamforth, F.; Li, Q.; Yasui, Y.; Cherry, M.N. Maternal Exposure to Bisphenol-A and Fetal Growth Restriction: A Case-Referent Study. Int. J. Environ. Res. Public Health 2013, 10, 7001–7014. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Leclerc, F.; Dubois, M.-F.; Aris, A. Maternal, placental and fetal exposure to bisphenol A in women with and without preeclampsia. Hypertens Pregnancy 2014, 33, 341–348. [Google Scholar] [CrossRef] [PubMed]
- Müller, J.E.; Meyer, N.; Santamaria, C.G.; Schumacher, A.; Luque, E.H.; Zenclussen, M.L.; Rodriguez, H.A.; Zenclussen, A.C. Bisphenol A exposure during early pregnancy impairs uterine spiral artery remodeling and provokes intrauterine growth restriction in mice. Sci. Rep. 2018, 8, 1–10. [Google Scholar] [CrossRef]
- Lunell, N.O.; Nylund, L.E.; Lewander, R.; Sarby, B. Uteroplacental blood flow in pre-eclampsia measurements with indium-113m and a computer-linked camera. Clin. Exp. Hypertens B 1982, 1, 105–117. [Google Scholar] [CrossRef]
- USEPA. IRIS (Integrated Risk Information System); US Environmental Protection Agency: Washington, DC, USA, 2012. Available online: http://www.epa.gov/iris/index.html (accessed on 1 June 2021).
- Yingying, Z.; Shiyu, T.; Cong, Y.; Yan, L.; Zaizhao, W. Non-monotonic dose-response effect of bisphenol A on rare minnow Gobiocypris rarus ovarian development. Chemosphere 2016, 144, 304–311. [Google Scholar]
- Cheng, Y.; Cui, Y.; Chen, H.; Xie, W. Thyroid disruption effects of environmental level perfluorooctane sulfonates (PFOS) in Xenopus laevis. Ecotoxicology 2011, 20, 2069–2078. [Google Scholar] [CrossRef] [PubMed]
- Tachibana, T.; Wakimoto, Y.; Nakamuta, N.; Phichitraslip, T.; Wakitani, S.; Kusakabe, K.; Hondo, E.; Kiso, Y. Effects of Bisphenol A (BPA) on Placentation and Survival of the Neonates in Mice. J. Reprod. Dev. 2007, 53, 509–514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Benachour, N.; Aris, A. Toxic effects of low doses of Bisphenol-A on human placental cells. Toxicol. Appl. Pharmacol. 2009, 241, 322–328. [Google Scholar] [CrossRef]
- Naeye, R.L. Do placental weights have clinical significance? Hum. Pathol. 1987, 18, 387–391. [Google Scholar] [CrossRef]
- Molteni, R.A.; Stys, S.J.; Battaglia, F.C. Relationship of fetal and placental weight in human beings: Fetal/placental weight ratios at various gestational ages and birth weight distributions. J. Reprod. Med. 1978, 21, 327–334. [Google Scholar]
- Robertson, C.M.; Svenson, L.W.; Kyle, J.M. Birth weight by gestational age for Alberta liveborn infants, 1985 through 1998. J. Obstet. Gynaecol. Can. 2002, 24, 138–148. [Google Scholar] [CrossRef]
- Fox, G.E.; Van, W.R.; Resau, J.H.; Sun, C.J. The effect of immersion formaldehyde fixation on human placental weight. Arch. Pathol. Lab. Med. 1991, 115, 726–728. [Google Scholar]
- Eriksson, J.; Forsen, T.; Tuomilehto, J.; Osmond, C.; Barker, D. Foetal and childhood growth and hypertension in adult life. Hypertension 2000, 36, 790–794. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Palmer, S.K.; Zamudio, S.; Con, C.; Parker, S.; Stamm, E.; Moore, L.G. Quantitative estimation of human uterine artery blood flow and pelvic blood flow redistribution in pregnancy. Obstet. Gynecol. 1992, 80, 1000–1006. [Google Scholar]
- Osol, G.; Ko, L.N.; Mandalà, M. Plasticity of the Maternal Vasculature During Pregnancy. Annu. Rev. Physiol. 2019, 81, 89–111. [Google Scholar] [CrossRef] [PubMed]
- Krause, B.J.; Garrasco-Wong, I.; Caniuguir, A.; Carvajal, J.; Farías, M.; Casanello, P. Endothelial eNOS/arginase imbalance contributes to vascular dysfunction in IUGR umbilical and placental vessels. Placenta 2013, 34, 20–28. [Google Scholar] [CrossRef]
- Johal, T.; Lees, C.C.; Everett, T.R.; Wilkinson, I.B. The nitric oxide pathway and possible therapeutic options in pre.eclampsia. Br. J. Clin. Pharmacol. 2014, 78, 244–257. [Google Scholar] [CrossRef] [Green Version]
- Kim, J.Y.; Jeong, H.G. Down-regulation of inducible nitric oxide synthase and tumor necrosis factor-alpha expression by bisphenol A via nuclear factor-kappaB inactivation in macrophages. Cancer Lett. 2003, 196, 69–76. [Google Scholar]
- Ni, Y.; Meyer, M.; Osol, G. Gestation increases nitric oxide-mediated vasodilation in rat uterine arteries. Am. J. Obstet. Gynecol. 1997, 176, 856–864. [Google Scholar] [CrossRef]
- Xiao, D.; Liu, Y.; Pearce, W.J.; Zhang, L. Endothelial nitric oxide release in isolated perfused ovine uterine arteries: Effect of pregnancy. Eur. J. Pharmacol. 1999, 367, 223–230. [Google Scholar] [CrossRef]
- Hale, S.A.; Weger, L.; Mandala, M.; Osol, G. Reduced NO signaling during pregnancy attenuates outward uterine artery remodeling by altering MMP expression and collagen and elastin deposition. Am. J. Physiol. Heart Circ. Physiol. 2011, 301, H1266–H1275. [Google Scholar] [CrossRef] [Green Version]
- Osol, G.; Barron, C.; Gokina, N.; Mandala, M. Inhibition of nitric oxide synthases abrogates pregnancy-induced uterine vascular expansive remodeling. J. Vasc. Res. 2009, 46, 478–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Possomato-Vieira, J.S.; Khalil, R.A. Mechanisms of Endothelial Dysfunction in Hypertensive Pregnancy and Preeclampsia. Adv. Pharmacol. 2016, 77, 361–431. [Google Scholar]
- Bai, J.; Qi, Q.-R.; Li, Y.; Day, R.; Makhoul, J.; Magness, R.R.; Chen, D.-B. Estrogen Receptors and Estrogen-Induced Uterine Vasodilation in Pregnancy. Int. J. Mol. Sci. 2020, 21, 4349. [Google Scholar] [CrossRef]
- Veille, J.C.; Li, P.; Eisenach, J.C.; Massmann, A.G.; Figueroa, J.P. Effects of estrogen on nitric oxide biosynthesis and vasorelaxant activity in sheep uterine and renal arteries in vitro. Am. J. Obstet. Gynecol. 1996, 174, 1043–1049. [Google Scholar] [CrossRef]
- Chen, D.B.; Bird, I.M.; Zheng, J.; Magness, R.R. Membrane estrogen receptor-dependent extracellular signal-regulated kinase pathway mediates acute activation of endothelial nitric oxide synthase by estrogen in uterine artery endothelial cells. Endocrinology 2004, 145, 113–125. [Google Scholar] [CrossRef] [PubMed]
- Mandalà, M. Influence of Estrogens on Uterine Vascular Adaptation in Normal and Preeclamptic Pregnancies. Int. J. Mol. Sci. 2020, 21, 2592. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Polikandriotis, J.A.; Mazzella, L.J.; Rupnow, H.L.; Hart, C.M. Peroxisome proliferator-activated receptor gamma ligands stimulate endothelial nitric oxide production through distinct peroxisome proliferator-activated receptor gamma-dependent mechanisms. Arter. Thromb. Vasc. Biol. 2005, 25, 1810–1816. [Google Scholar] [CrossRef] [Green Version]
- Duan, S.Z.; Usher, M.G.; Mortensen, R.M. Peroxisome proliferator-activated receptor-gamma-mediated effects in the vasculature. Circ. Res. 2008, 102, 283–294. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, J.; Zhang, Z.; Li, Z.; Feng, X.; He, L.; Liu, S.; Mao, J.; Wang, G.; Wang, X. Peroxisome proliferator-activated receptor-gamma (PPARgamma) agonist improves coronary artery endothelial function in diabetic patients with coronary artery disease. J. Int. Med. Res. 2010, 38, 86–94. [Google Scholar] [CrossRef]
- Grummer, M.A.; Sullivan, J.A.; Magness, R.R.; Bird, I.M. Vascular endothelial growth factor acts through novel, pregnancy-enhanced receptor signalling pathways to stimulate endothelial nitric oxide synthase activity in uterine artery endothelial cells. Biochem. J. 2009, 417, 501–511. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keller, H.; Givel, F.; Perroud, M.; Wahli, W. Signaling cross-talk between peroxisome proliferator-activated receptor/retinoid X receptor and estrogen receptor through estrogen response elements. Mol. Endocrinol. 1995, 9, 794–804. [Google Scholar]
- Soares, R.; Reis-Filho, J.S.; Gartner, F.; Schmitt, F.C. Vascular endothelial growth factor, transforming growth factor-alpha, and estrogen receptors: Possible cross-talks and interactions. Am. J. Pathol. 2002, 160, 381–382. [Google Scholar] [CrossRef]
- Masaharu, M.; Kang, J.H. Bisphenol A (BPA) and cell signaling pathways. Biotechnol. Adv. 2018, 36, 311–327. [Google Scholar]
- Gokina, N.I.; Chan, S.-L.; Chapman, A.C.; Oppenheimer, K.; Jetton, T.L.; Cipolla, M.J. Inhibition of PPARγ during rat pregnancy causes intrauterine growth restriction and attenuation of uterine vasodilation. Front. Physiol. 2013, 23, 4–187. [Google Scholar] [CrossRef] [Green Version]
- Yin, G.; Zhu, X.; Guo, C.; Yang, Y.; Han, T.; Chen, L.; Yin, W.; Gao, P.; Zhang, H.; Geng, J.; et al. Differential expression of estradiol and estrogen receptor α in severe preeclamptic pregnancies compared with normal pregnancies. Mol. Med. Rep. 2013, 7, 981–985. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Widlansky, M.E.; Price, D.T.; Gokce, N.; Eberhardt, R.T.; Duffy, S.J.; Holbrook, M.; Maxwell, C.; Palmisano, J.; Keaney, J.F., Jr.; Morrow, J.D.; et al. Short- and long-term COX-2 inhibition reverses endothelial dysfunction in patients with hypertension. Hypertension 2003, 42, 310–315. [Google Scholar] [CrossRef] [Green Version]
- Blais, V.; Rivest, S. Inhibitory action of nitric oxide on circulating tumor necrosis factor-induced NF-kappaB activity and COX-2 transcription in the endothelium of the brain capillaries. J. Neuropathol. Exp. Neurol. 2001, 60, 893–905. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sordelli, M.S.; Beltrame, J.S.; Cella, M.; Franchi, A.M.; Riberio, M.L. Cyclooxygenase-2 prostaglandins mediate anandamide-inhibitory action on nitric oxide synthase activity in the receptive rat uterus. Eur. J. Pharmacol. 2012, 685, 174–179. [Google Scholar] [CrossRef]
- Pulgar, V.M.; Yamaleyeva, L.M.; Varagic, J.; McGee, C.; Bader, M.; Dechend, R.; Brosnihan, K.B. Functional changes in the uterine artery precede the hypertensive phenotype in a transgenic model of hypertensive pregnancy. Am. J. Physiol. Endocrinol. Metab. 2015, 1, E811–E817. [Google Scholar] [CrossRef] [Green Version]
- Morris, E.A.; Mandalà, M.; Ko, N.L.; Osolo, G. Postpartum Persistence of Maternal Uterine Vascular Gestational Adaptation in Rodents. Reprod. Sci. 2020, 27, 611–620. [Google Scholar] [CrossRef] [PubMed]
- Buhimschi, I.A.; Hall, G.; Thompson, L.P.; Weiner, C.P. Pregnancy and estradiol decrease GTPase activity in the guinea pig uterine artery. Am. J. Physiol. Heart Circ. Physiol. 2001, 281, H2168–H2175. [Google Scholar] [CrossRef] [PubMed]
- Colton, I.; Mandalà, M.; Morton, J.; Davidge, S.T.; Osol, G. Influence of constriction, wall tension, smooth muscle activation and cellular deformation on rat resistance artery vasodilatod reactivity. Cell. Physiol. Biochem. 2012, 29, 883–892. [Google Scholar] [CrossRef] [PubMed]
- Grasselli, E.; Voci, A.; Demori, I.; Vecchione, G.; Compalati, A.D.; Gallo, G.; Goglia, F.; De Matteis, R.; Silvestri, E.; Vergani, L. Triglyceride mobilization from lipid droplets sustains the anti-ateatotic action of lodothyronines in cultured rat hepatocytes. Front. Physiol. 2015, 6, 418. [Google Scholar] [PubMed]
- Pfaffl, M.W. A new mathematical model for relative quantification in real- time RT-PCR. Nucleic Acids Res. 2001, 29, e45. [Google Scholar] [CrossRef] [PubMed]






| Control (n = 7) | BPA 2.5 µg/kg/day (n = 6) | BPA 25 µg/kg/die (n = 8) | BPA 250 µg/kg/die (n = 7) | p Value | |
|---|---|---|---|---|---|
| Fetal Number (n) | 13.0 ± 0.79 | 13.0 ± 0.89 | 13.1 ± 0.81 | 13.1 ± 0.94 | NS |
| Fetal Weight (g) | 2.15 ± 0.02 | 2.34 ± 0.11 * | 2.13 ± 0.03 | 2.19 ± 0.02 | BPA2.5 vs. Control * p = 0.0158 |
| Placental Weight (g) | 0.50 ± 0.01 | 0.48 ± 0.01 ** | 0.51 ± 0.01 | 0.45 ± 0.003 ** | BPA2.5 vs. Control ** p = 0.0066 BPA250 vs. Control ** p = 0.0050 |
| Ratio Placental Fetal Weight | 0.23 ± 0.004 | 0.20 ± 0.006 *** | 0.24 ± 0.008 | 0.21 ± 0.003 *** | BPA2.5 vs. Control *** p = 0.0008 BPA250 vs. Control *** p= 0.0002 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Barberio, L.; Paulesu, L.; Canesi, L.; Grasselli, E.; Mandalà, M. Bisphenol a Interferes with Uterine Artery Features and Impairs Rat Feto-Placental Growth. Int. J. Mol. Sci. 2021, 22, 6912. https://doi.org/10.3390/ijms22136912
Barberio L, Paulesu L, Canesi L, Grasselli E, Mandalà M. Bisphenol a Interferes with Uterine Artery Features and Impairs Rat Feto-Placental Growth. International Journal of Molecular Sciences. 2021; 22(13):6912. https://doi.org/10.3390/ijms22136912
Chicago/Turabian StyleBarberio, Laura, Luana Paulesu, Laura Canesi, Elena Grasselli, and Maurizio Mandalà. 2021. "Bisphenol a Interferes with Uterine Artery Features and Impairs Rat Feto-Placental Growth" International Journal of Molecular Sciences 22, no. 13: 6912. https://doi.org/10.3390/ijms22136912