Impact of P2X7 Purinoceptors on Goblet Cell Function: Implications for Dry Eye
Abstract
:1. Introduction
1.1. Adaptive Role of Conjunctival Goblet Cells
1.2. Impact of Ion Channels
1.3. Channel-Mediated Responses
1.4. Research Objective
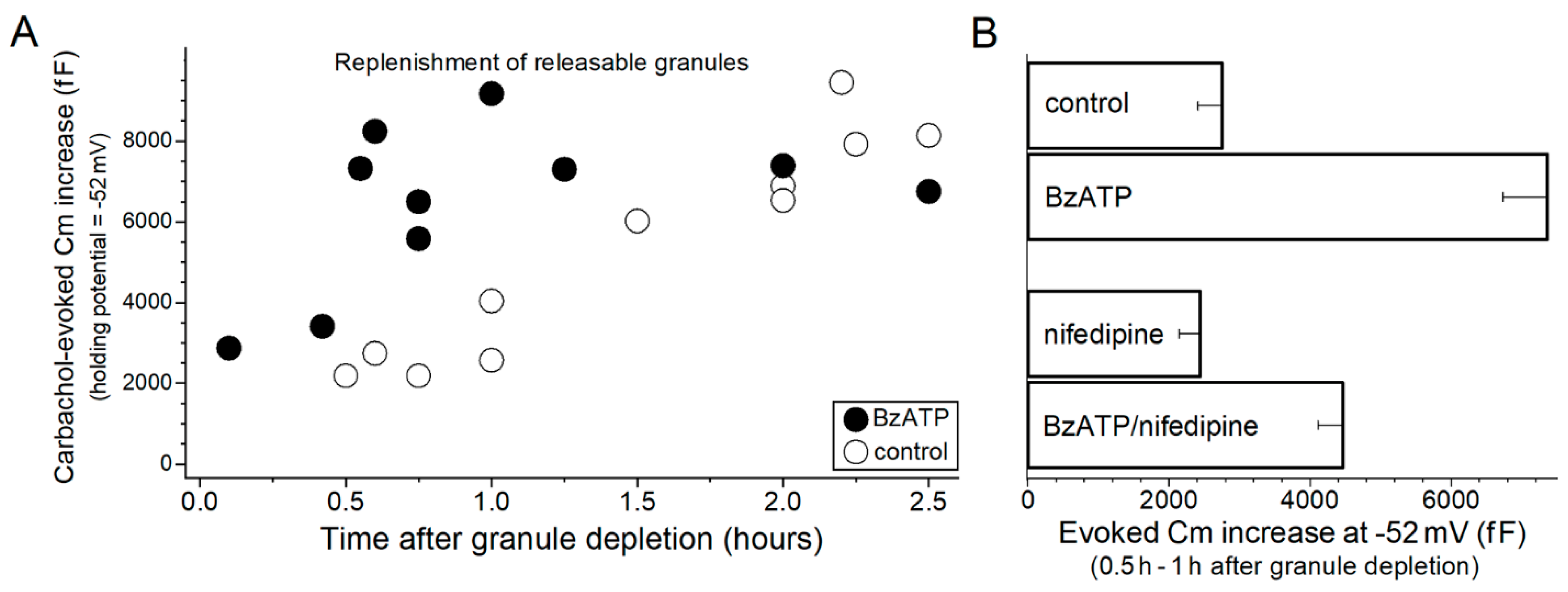
2. Results
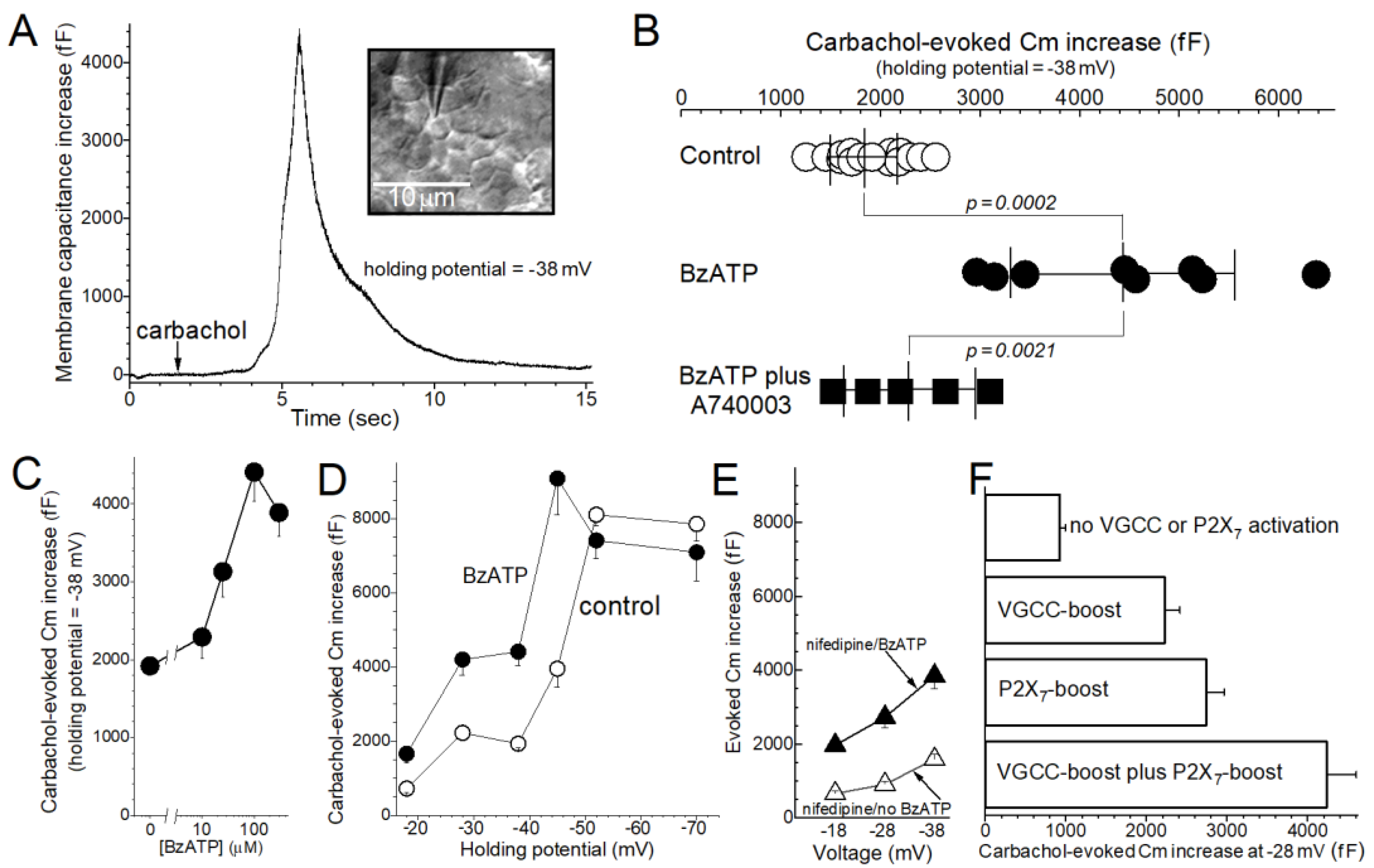
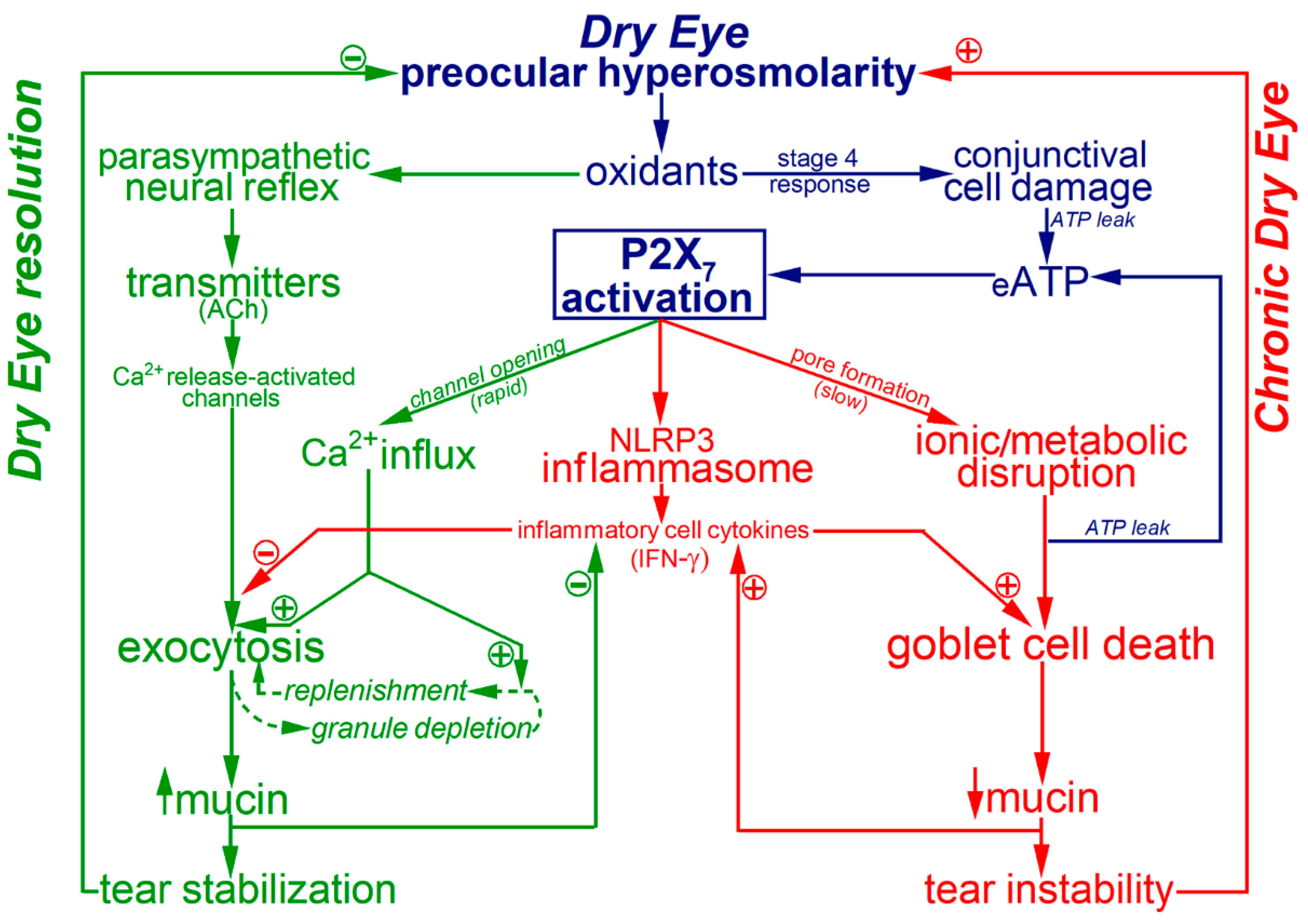
3. Discussion
4. Materials and Methods
4.1. Experimental Preparation
4.2. Electrophysiology
4.3. Chemicals
4.4. Statistics
Funding
Institutional Review Board Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Courville, C.B.; Smolek, M.K.; Klyce, S.D. Contribution of the ocular surface to visual optics. Exp. Eye Res. 2004, 78, 417–425. [Google Scholar] [CrossRef]
- Willcox, M.D.P.; Argueso, P.; Georgiev, G.A.; Holopainen, J.M.; Laurie, G.W.; Millar, T.J.; Papas, E.B.; Rolland, J.P.; Schmidt, T.A.; Stahl, U.; et al. TFOS DEWS II Tear Film Report. Ocul. Surf. 2017, 15, 366–403. [Google Scholar] [CrossRef] [Green Version]
- Clayton, J.A. Dry Eye. N. Engl. J. Med. 2018, 378, 2212–2223. [Google Scholar] [CrossRef]
- Bron, A.J.; De Paiva, C.S.; Chauhan, S.K.; Bonini, S.; Gabison, E.E.; Jain, S.; Knop, E.; Markoulli, M.; Ogawa, Y.; Perez, V.; et al. TFOS DEWS II pathophysiology report. Ocul. Surf. 2017, 15, 438–510. [Google Scholar] [CrossRef] [PubMed]
- Dartt, D.A. Regulation of mucin and fluid secretion by conjunctival epithelial cells. Prog. Retin. Eye Res. 2002, 21, 555–576. [Google Scholar] [CrossRef]
- Garcia-Posadas, L.; Hodges, R.R.; Li, D.; Shatos, M.A.; Storr-Paulsen, T.; Diebold, Y.; Dartt, D.A. Interaction of IFN-gamma with cholinergic agonists to modulate rat and human goblet cell function. Mucosal. Immunol. 2016, 9, 206–217. [Google Scholar] [CrossRef]
- Hodges, R.R.; Bair, J.A.; Carozza, R.B.; Li, D.; Shatos, M.A.; Dartt, D.A. Signaling pathways used by EGF to stimulate conjunctival goblet cell secretion. Exp. Eye Res. 2012, 103, 99–113. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, D.; Jiao, J.; Shatos, M.A.; Hodges, R.R.; Dartt, D.A. Effect of VIP on intracellular [Ca2+], extracellular regulated kinase 1/2, and secretion in cultured rat conjunctival goblet cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 2872–2884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Puro, D.G. Role of ion channels in the functional response of conjunctival goblet cells to dry eye. Am. J. Physiol. Cell Physiol. 2018, 315, C236–C246. [Google Scholar] [CrossRef] [Green Version]
- Puro, D.G. How goblet cells respond to dry eye: Adaptive and pathological roles of voltage-gated calcium channels and P2X7 purinoceptors. Am. J. Physiol. Cell Physiol. 2020, 318, C1305–C1315. [Google Scholar] [CrossRef]
- Puro, D.G. Bioelectric responses of conjunctival goblet cells to dry eye: Impact of ion channels on exocytotic function and viability. Int. J. Mol. Sci. 2020, 21, 9415. [Google Scholar] [CrossRef]
- Li, D.; Shatos, M.A.; Hodges, R.R.; Dartt, D.A. Role of PKCalpha activation of Src, PI-3K/AKT, and ERK in EGF-stimulated proliferation of rat and human conjunctival goblet cells. Investig. Ophthalmol. Vis. Sci. 2013, 54, 5661–5674. [Google Scholar] [CrossRef]
- Guzman-Aranguez, A.; Perez de Lara, M.J.; Pintor, J. Hyperosmotic stress induces ATP release and changes in P2X7 receptor levels in human corneal and conjunctival epithelial cells. Purinergic Signal. 2017, 13, 249–258. [Google Scholar] [CrossRef] [Green Version]
- McGilligan, V.E.; Gregory-Ksander, M.S.; Li, D.; Moore, J.E.; Hodges, R.R.; Gilmore, M.S.; Moore, T.C.; Dartt, D.A. Staphylococcus aureus activates the NLRP3 inflammasome in human and rat conjunctival goblet cells. PLoS ONE 2013, 8, e74010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coursey, T.G.; Tukler Henriksson, J.; Barbosa, F.L.; De Paiva, C.S.; Pflugfelder, S.C. Interferon-gamma-induced unfolded protein response in conjunctival goblet cells as a cause of mucin deficiency in sjogren syndrome. Am. J. Pathol. 2016, 186, 1547–1558. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pflugfelder, S.C.; de Paiva, C.S. The pathophysiology of dry eye disease: What we know and future directions for research. Ophthalmology 2017, 124, S4–S13. [Google Scholar] [CrossRef]
- Zhang, X.; Chen, W.; De Paiva, C.S.; Corrales, R.M.; Volpe, E.A.; McClellan, A.J.; Farley, W.J.; Li, D.Q.; Pflugfelder, S.C. Interferon-gamma exacerbates dry eye-induced apoptosis in conjunctiva through dual apoptotic pathways. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6279–6285. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; De Paiva, C.S.; Su, Z.; Volpe, E.A.; Li, D.Q.; Pflugfelder, S.C. Topical interferon-gamma neutralization prevents conjunctival goblet cell loss in experimental murine dry eye. Exp. Eye Res. 2014, 118, 117–124. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pflugfelder, S.C.; De Paiva, C.S.; Moore, Q.L.; Volpe, E.A.; Li, D.Q.; Gumus, K.; Zaheer, M.L.; Corrales, R.M. Aqueous tear deficiency increases conjunctival interferon-gamma (IFN-gamma) expression and goblet cell loss. Investig. Ophthalmol. Vis. Sci. 2015, 56, 7545–7550. [Google Scholar] [CrossRef] [Green Version]
- Nelson, J.D.; Wright, J.C. Conjunctival goblet cell densities in ocular surface disease. Arch. Ophthalmol. 1984, 102, 1049–1051. [Google Scholar] [CrossRef]
- Hessen, M.; Akpek, E.K. Dry eye: An inflammatory ocular disease. J. Ophthalmic Vis. Res. 2014, 9, 240–250. [Google Scholar]
- North, R.A.; Surprenant, A. Pharmacology of cloned P2X receptors. Annu. Rev. Pharmacol. Toxicol. 2000, 40, 563–580. [Google Scholar] [CrossRef]
- Rituper, B.; Gucek, A.; Jorgacevski, J.; Flašker, A.; Kreft, M.; Zorec, R. High-resolution membrane capacitance measurements for the study of exocytosis and endocytosis. Nat. Protoc. 2013, 8, 1169–1183. [Google Scholar] [CrossRef]
- Moore, J.E.; Vasey, G.T.; Dartt, D.A.; McGilligan, V.E.; Atkinson, S.D.; Grills, C.; Lamey, P.J.; Leccisotti, A.; Frazer, D.G.; Moore, T.C. Effect of tear hyperosmolarity and signs of clinical ocular surface pathology upon conjunctival goblet cell function in the human ocular surface. Investig. Ophthalmol. Vis. Sci. 2011, 52, 6174–6180. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pflugfelder, S.C.; Stern, M.E. Biological functions of tear film. Exp. Eye Res. 2020, 197, 108115. [Google Scholar] [CrossRef] [PubMed]
- Gipson, I.K. Goblet cells of the conjunctiva: A review of recent findings. Prog. Retin. Eye Res. 2016, 54, 49–63. [Google Scholar] [CrossRef] [Green Version]
- Horikawa, Y.; Shatos, M.A.; Hodges, R.R.; Zoukhri, D.; Rios, J.D.; Chang, E.L.; Bernardino, C.R.; Rubin, P.A.; Dartt, D.A. Activation of mitogen-activated protein kinase by cholinergic agonists and EGF in human compared with rat cultured conjunctival goblet cells. Investig. Ophthalmol. Vis. Sci. 2003, 44, 2535–2544. [Google Scholar] [CrossRef] [Green Version]
- Setzer, P.Y.; Nichols, B.A.; Dawson, C.R. Unusual structure of rat conjunctival epithelium. Light and electron microscopy. Investig. Ophthalmol. Vis. Sci. 1987, 28, 531–537. [Google Scholar]
- Gipson, I.K.; Tisdale, A.S. Visualization of conjunctival goblet cell actin cytoskeleton and mucin content in tissue whole mounts. Exp. Eye Res. 1997, 65, 407–415. [Google Scholar] [CrossRef] [PubMed]
- Smith, C.B.; Betz, W.J. Simultaneous independent measurement of endocytosis and exocytosis. Nature 1996, 380, 531–534. [Google Scholar] [CrossRef] [PubMed]
- Mann, A.; Tighe, B. Contact lens interactions with the tear film. Exp. Eye Res. 2013, 117, 88–98. [Google Scholar] [CrossRef] [PubMed]
- Marques, D.L.; Alves, M.; Modulo, C.M.; Mendes da Silva, L.E.C.; Reinach, P.; Rocha, E.M. Lacrimal osmolarity and ocular surface in experimental model of dry eye caused by toxicity. Rev. Bras. Oftalmol. 2015, 74, 68–72. [Google Scholar] [CrossRef] [Green Version]
- Rismondo, V.; Osgood, T.B.; Leering, P.; Hattenhauer, M.G.; Ubels, J.L.; Edelhauser, H.F. Electrolyte composition of lacrimal gland fluid and tears of normal and vitamin A-deficient rabbits. CLAO J. 1989, 15, 222–228. [Google Scholar] [PubMed]
- Lamkin, I.D.; Zimmerman, K.L.; Smith Fleming, K.M.; Martins, B.C. Osmolarity of basal and reflex tears of normal dogs. Vet. Ophthalmol. 2020, 23, 747–753. [Google Scholar] [CrossRef] [PubMed]
- Lindau, M.; Neher, E. Patch-clamp techniques for time-resolved capacitance measurements in single cells. Pflug. Arch. 1988, 411, 137–146. [Google Scholar] [CrossRef] [PubMed]
- Gillis, K.D. Admittance-based measurement of membrane capacitance using the EPC-9 patch-clamp amplifier. Pflug. Arch. 2000, 439, 655–664. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the author. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Puro, D.G. Impact of P2X7 Purinoceptors on Goblet Cell Function: Implications for Dry Eye. Int. J. Mol. Sci. 2021, 22, 6935. https://doi.org/10.3390/ijms22136935
Puro DG. Impact of P2X7 Purinoceptors on Goblet Cell Function: Implications for Dry Eye. International Journal of Molecular Sciences. 2021; 22(13):6935. https://doi.org/10.3390/ijms22136935
Chicago/Turabian StylePuro, Donald G. 2021. "Impact of P2X7 Purinoceptors on Goblet Cell Function: Implications for Dry Eye" International Journal of Molecular Sciences 22, no. 13: 6935. https://doi.org/10.3390/ijms22136935





