Interruptions of the FXN GAA Repeat Tract Delay the Age at Onset of Friedreich’s Ataxia in a Location Dependent Manner
Abstract
:1. Introduction
2. Results
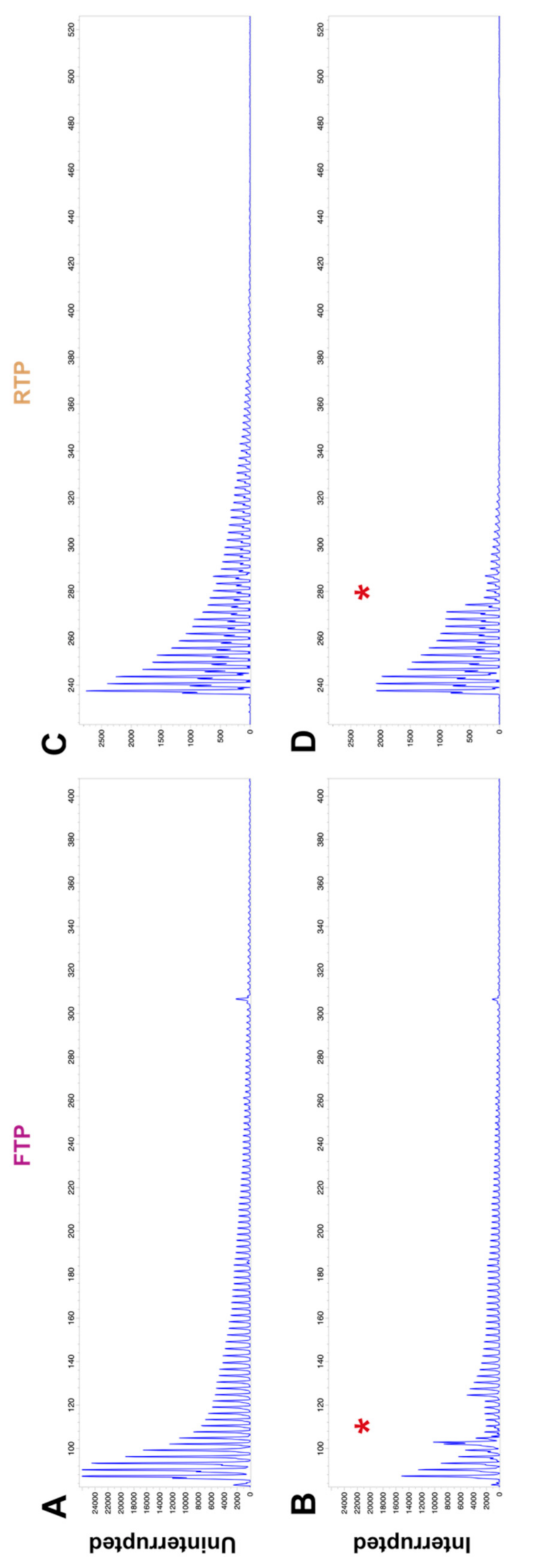
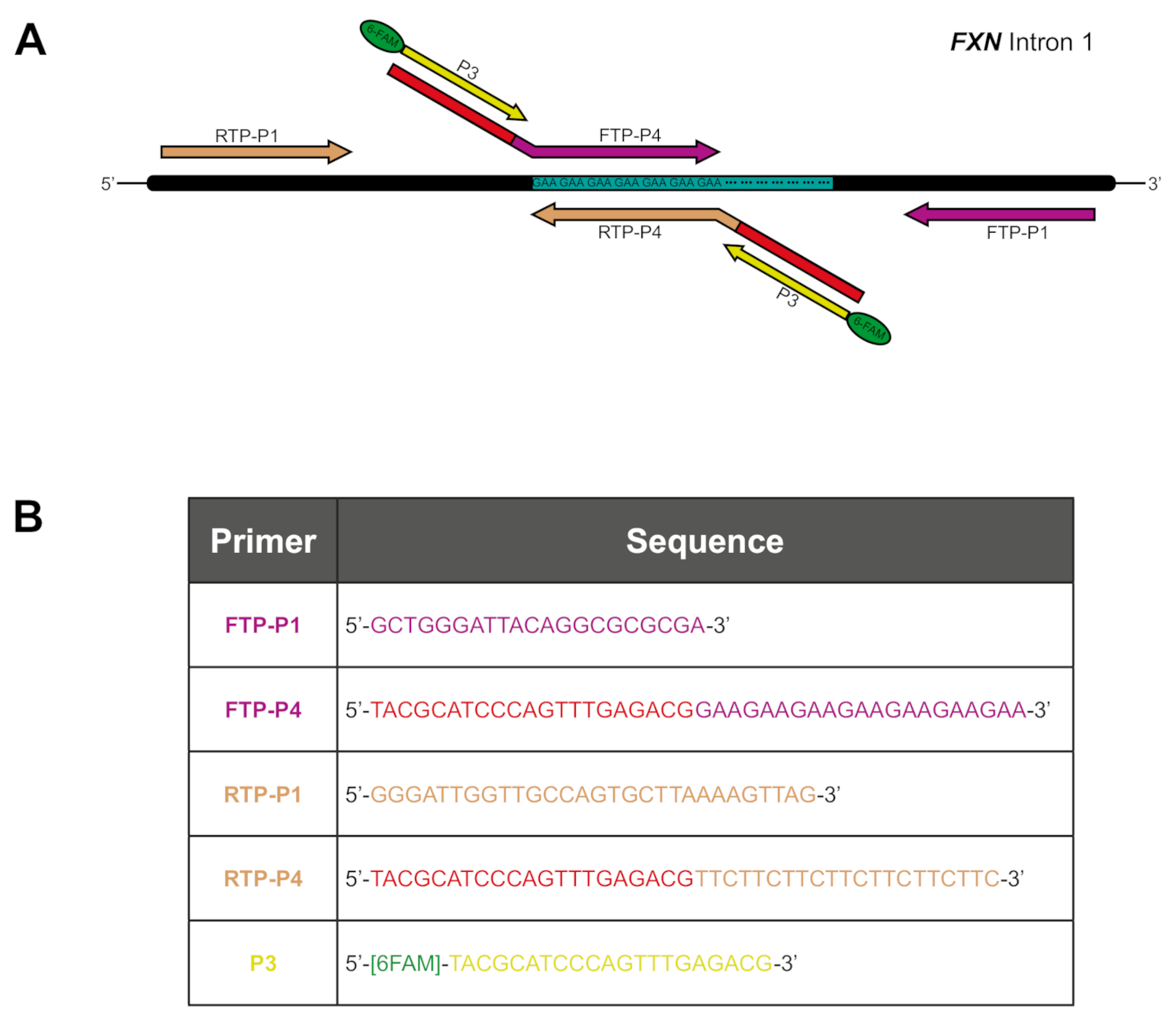
2.1. GAA Repeat Tract Interruptions Can Be Detected through Alteration of TP PCR Electropherograms
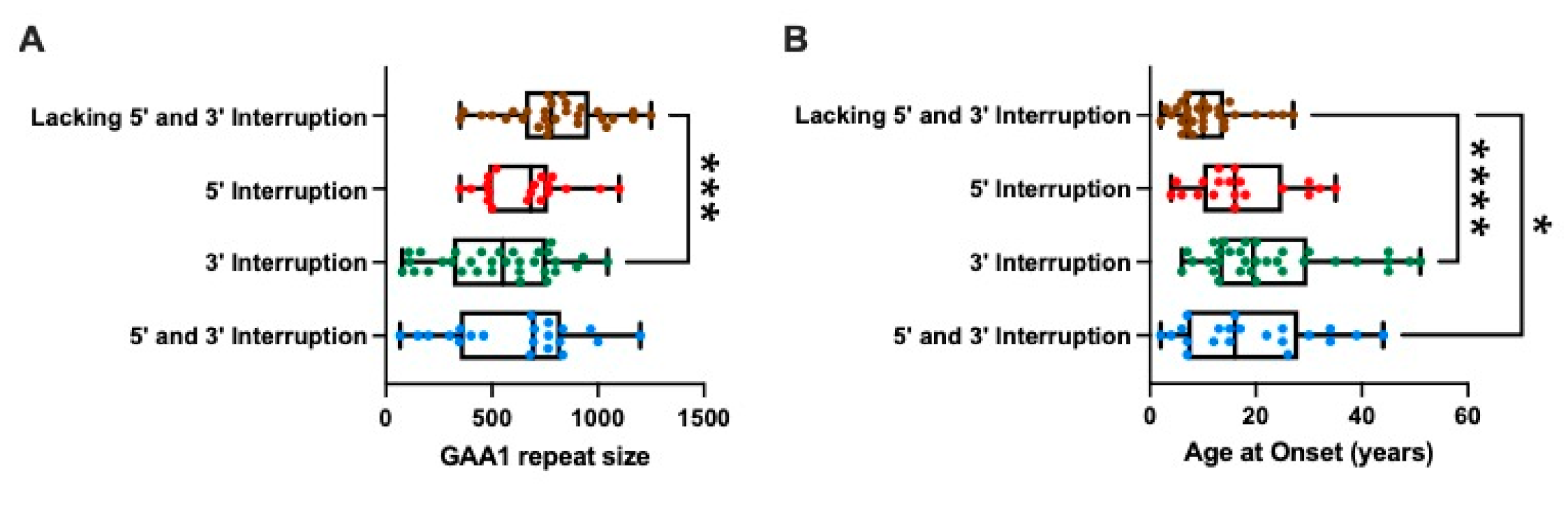
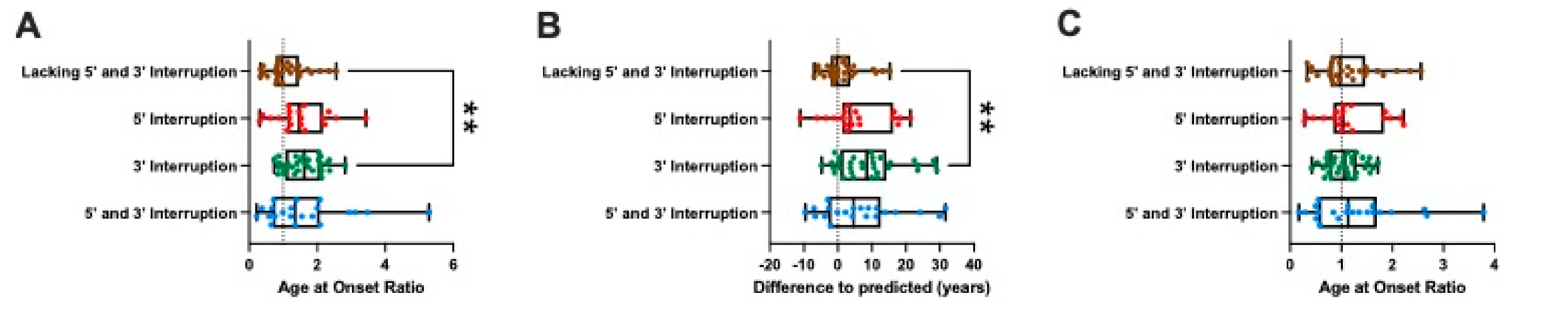
2.2. Interruption at the 3′ End of the GAA Repeat Tract Is Associated with a Shorter GAA1 Repeat Size and a Later Age at Onset
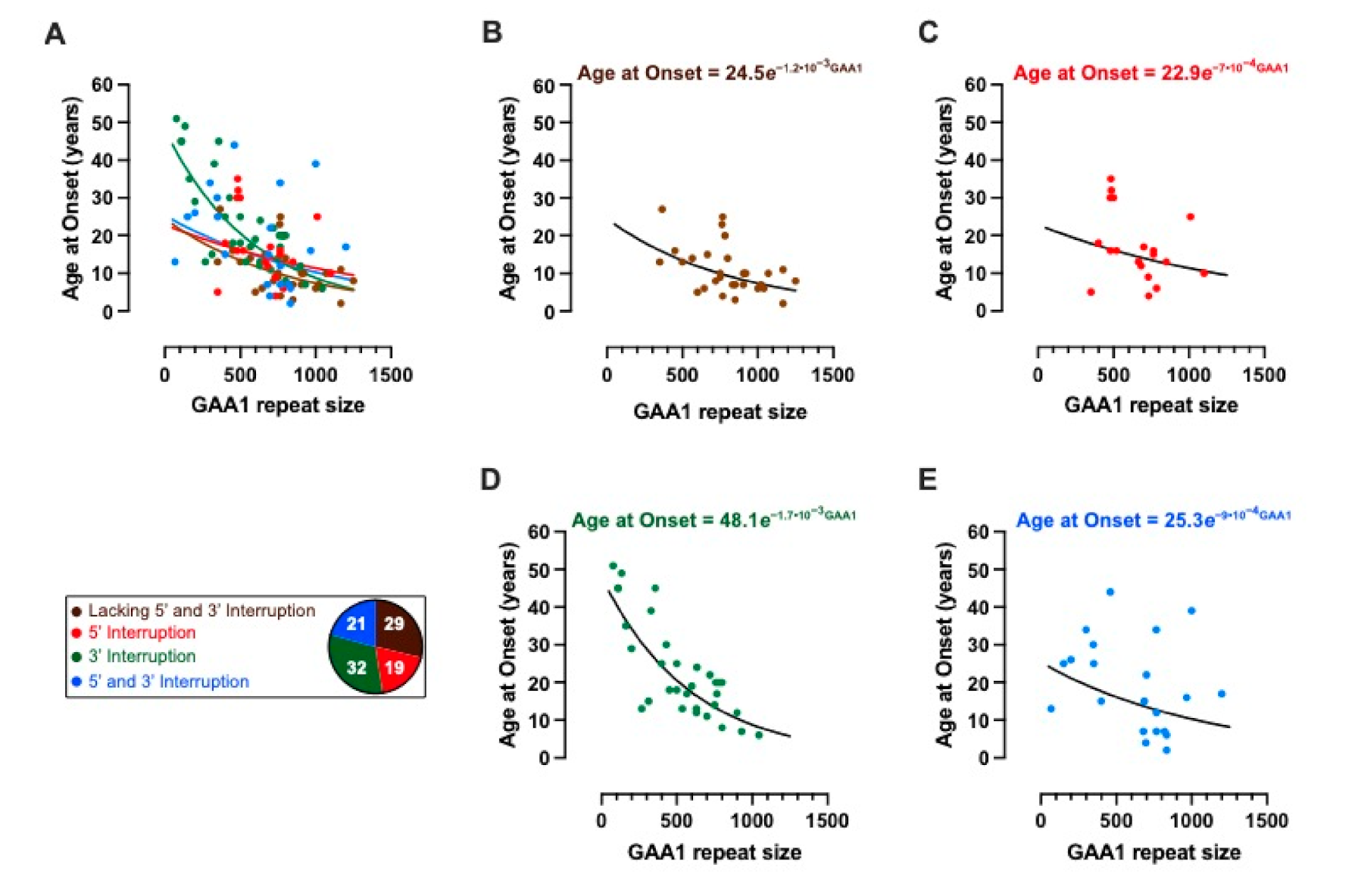
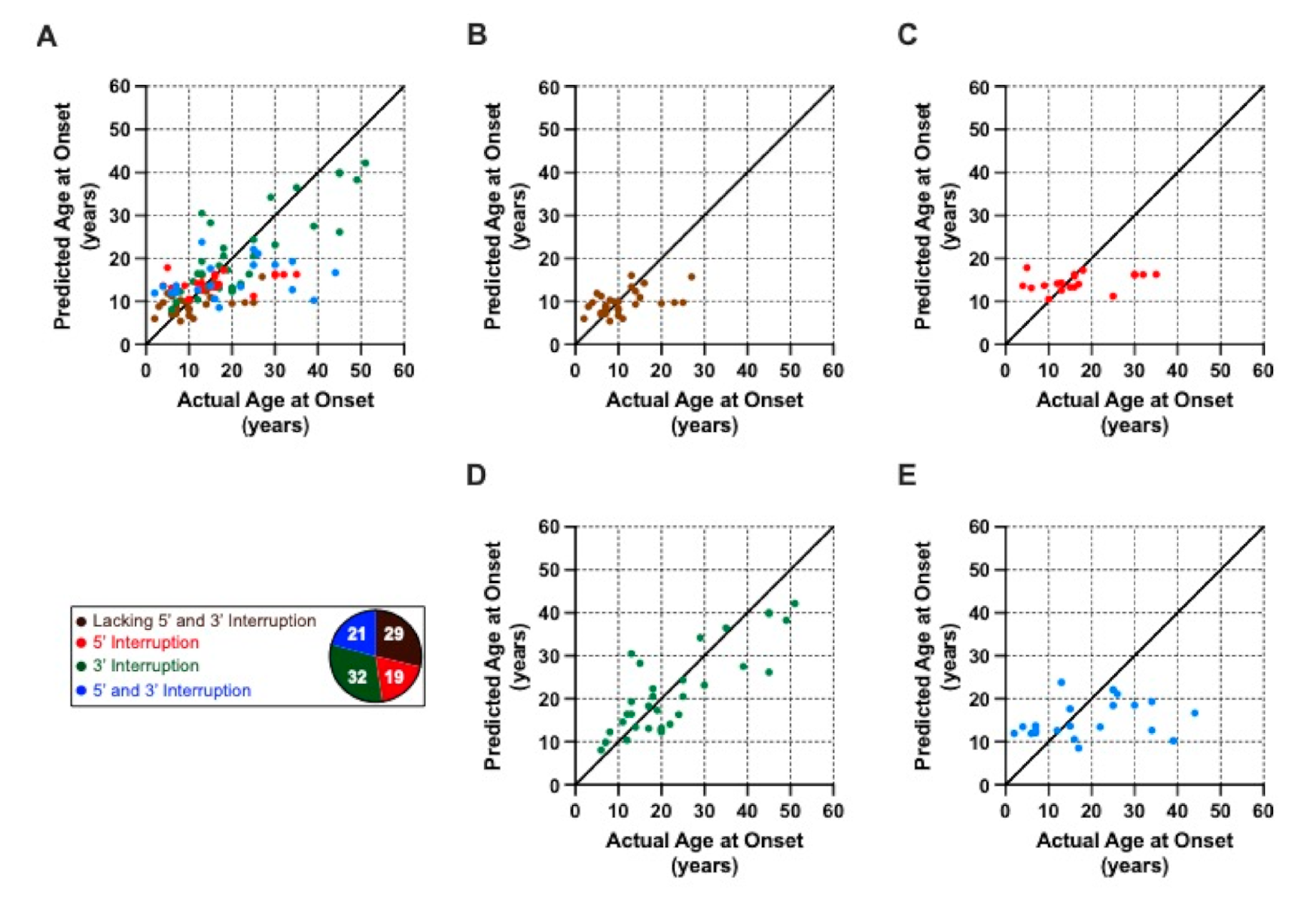
2.3. Modelling the Impact of Interruptions by Subgrouping
3. Discussion
4. Materials and Methods
4.1. Patient Cohort and Ethical Statement
4.2. Triplet Repeat Primed PCR (TP PCR)
4.3. Assessing and Modelling the Impact of Interruptions
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Winter, R.M.; Harding, A.E.; Baraitser, M.; Bravery, M.B. Intrafamilial correlation in Friedreich’s ataxia. Clin. Genet. 2008, 20, 419–427. [Google Scholar] [CrossRef]
- Reetz, K.; Dogan, I.; Costa, A.S.; Dafotakis, M.; Fedosov, K.; Giunti, P.; Parkinson, M.H.; Sweeney, M.G.; Mariotti, C.; Panzeri, M.; et al. Biological and clinical characteristics of the European Friedreich’s Ataxia Consortium for Translational Studies (EFACTS) cohort: A cross-sectional analysis of baseline data. Lancet Neurol. 2015, 14, 174–182. [Google Scholar] [CrossRef]
- Harding, A.E. Friedreich’s ataxia: A clinical and genetic study of 90 families with an analysis of early diagnostic criteria and intrafamilial clustering of clinical features. Brain 1981, 104, 589–620. [Google Scholar] [CrossRef]
- Hewer, R.L.; Robinson, N. Diabetes mellitus in Friedreich’s ataxia. J. Neurol. Neurosurg. Psychiatry 1968, 31, 226–231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thoren, C. Diabetes mellitus in Friedreich’s ataxia. Acta Paediatr. Suppl. 1962, 135, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Dürr, A.; Cossee, M.; Agid, Y.; Campuzano, V.; Mignard, C.; Penet, C.; Mandel, J.-L.; Brice, A.; Koenig, M. Clinical and Genetic Abnormalities in Patients with Friedreich’s Ataxia. N. Engl. J. Med. 1996, 335, 1169–1175. [Google Scholar] [CrossRef]
- Cossée, M.; Schmitt, M.; Campuzano, V.; Reutenauer, L.; Moutou, C.; Mandel, J.-L.; Koenig, M. Evolution of the Friedreich’s ataxia trinucleotide repeat expansion: Founder effect and premutations. Proc. Natl. Acad. Sci. USA 1997, 94, 7452–7457. [Google Scholar] [CrossRef] [Green Version]
- Montermini, L.; Andermann, E.; Labuda, M.; Richter, A.; Pandolfo, M.; Cavalcanti, F.; Pianese, L.; Iodice, L.; Farina, G.; Monticelli, A.; et al. The Friedreich ataxia GAA triplet repeat: Premutation and normal alleles. Hum. Mol. Genet. 1997, 6, 1261–1266. [Google Scholar] [CrossRef] [Green Version]
- Sharma, R.; De Biase, I.; Gómez, M.; Delatycki, M.B.; Ashizawa, T.; Bidichandani, S.I. Friedreich ataxia in carriers of unstable borderline GAA triplet-repeat alleles. Ann. Neurol. 2004, 56, 898–901. [Google Scholar] [CrossRef]
- Campuzano, V.; Montermini, L.; Moltò, M.D.; Pianese, L.; Cossée, M.; Cavalcanti, F.; Monros, E.; Rodius, F.; Duclos, F.; Monticelli, A.; et al. Friedreich’s Ataxia: Autosomal Recessive Disease Caused by an Intronic GAA Triplet Repeat Expansion. Science 1996, 271, 1423–1427. [Google Scholar] [CrossRef]
- Filla, A.; de Michele, G.; Cavalcanti, F.; Pianese, L.; Monticelli, A.; Campanella, G.; Cocozza, S. The Relationship between Trinucleotide (GAA) Repeat Length and Clinical Features in Friedreich Ataxia. Am. J. Hum. Genet. 1996, 59, 554–560. [Google Scholar] [CrossRef]
- Epplen, C.; Frank, G.; Miterski, B.; Santos, E.J.M. Differential stability of the (GAA) n tract in the Friedreich ataxia (STM7) gene. Qual. Life Res. 1997, 99, 834–836. [Google Scholar] [CrossRef]
- Tai, G.; Yiu, E.M.; Corben, L.A.; Delatycki, M.B. A longitudinal study of the Friedreich Ataxia Impact Scale. J. Neurol. Sci. 2015, 352, 53–57. [Google Scholar] [CrossRef] [PubMed]
- Monrós, E.; Molto, M.D.; Martínez, F.; Canizares, J.; Blanca, J.; Vílchez, J.J.; Prieto, F.; de Frutos, R.; Palau, F. Phenotype Correlation and Intergenerational Dynamics of the Friedreich Ataxia GAA Trinucleotide Repeat. Am. J. Hum. Genet. 1997, 61, 101–110. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galea, C.A.; Huq, A.; Lockhart, P.; Tai, G.; Corben, L.A.; Yiu, E.M.; Gurrin, L.C.; Lynch, D.R.; Gelbard, S.; Durr, A.; et al. Compound heterozygousFXNmutations and clinical outcome in friedreich ataxia. Ann. Neurol. 2016, 79, 485–495. [Google Scholar] [CrossRef]
- Ohshima, K.; Sakamoto, N.; Labuda, M.; Poirier, J.; Moseley, M.L.; Montermini, L.; Ranum, L.P.W.; Wells, R.D.; Pandolfo, M. A nonpathogenic GAAGGA repeat in the Friedreich gene: Implications for pathogenesis. Neurology 1999, 53, 1854. [Google Scholar] [CrossRef] [PubMed]
- Moseley, M.L.; Benzow, K.A.; Schut, L.J.; Bird, T.D.; Gomez, C.M.; Barkhaus, P.E.; Blindauer, K.A.; Labuda, M.; Pandolfo, M.; Koob, M.D.; et al. Incidence of dominant spinocerebellar and Friedreich triplet repeats among 361 ataxia families. Neurology 1998, 51, 1666–1671. [Google Scholar] [CrossRef]
- McDaniel, D.O.; Keats, B.; Vedanarayanan, V.; Subramony, S. Sequence variation in GAA repeat expansions may cause differential penotype display in Friedreich’s ataxia. Mov. Disord. 2001, 16, 1153–1158. [Google Scholar] [CrossRef]
- Stolle, C.A.; Frackelton, E.C.; McCallum, J.; Farmer, J.M.; Tsou, A.; Wilson, R.B.; Lynch, D.R. Novel, complex interruptions of the GAA repeat in small, expanded alleles of two affected siblings with late-onset Friedreich ataxia. Mov. Disord. 2008, 23, 1303–1306. [Google Scholar] [CrossRef]
- Matsuura, T.; Fang, P.; Pearson, C.E.; Jayakar, P.; Ashizawa, T.; Roa, B.B.; Nelson, D.L. Interruptions in the Expanded ATTCT Repeat of Spinocerebellar Ataxia Type 10: Repeat Purity as a Disease Modifier? Am. J. Hum. Genet. 2006, 78, 125–129. [Google Scholar] [CrossRef] [Green Version]
- Menon, R.P.; Nethisinghe, S.; Faggiano, S.; Vannocci, T.; Rezaei, H.; Pemble, S.; Sweeney, M.G.; Wood, N.W.; Davis, M.B.; Pastore, A.; et al. The Role of Interruptions in polyQ in the Pathology of SCA1. PLoS Genet. 2013, 9, e1003648. [Google Scholar] [CrossRef] [Green Version]
- Sakamoto, N.; Larson, J.E.; Iyer, R.R.; Montermini, L.; Pandolfo, M.; Wells, R.D. GGA·TCC-interrupted Triplets in Long GAA·TTC Repeats Inhibit the Formation of Triplex and Sticky DNA Structures, Alleviate Transcription Inhibition, and Reduce Genetic Instabilities. J. Biol. Chem. 2001, 276, 27178–27187. [Google Scholar] [CrossRef] [Green Version]
- Holloway, T.P.; Rowley, S.M.; Delatycki, M.B.; Sarsero, J.P. Detection of interruptions in the GAA trinucleotide repeat expansion in the FXN gene of Friedreich ataxia. BioTechniques 2011, 50, 182–186. [Google Scholar] [CrossRef] [Green Version]
- Al-Mahdawi, S.; Ging, H.; Bayot, A.; Cavalcanti, F.; La Cognata, V.; Cavallaro, S.; Giunti, P.; Pook, M.A. Large Interruptions of GAA Repeat Expansion Mutations in Friedreich Ataxia Are Very Rare. Front. Cell. Neurosci. 2018, 12, 443. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciotti, P.; Di Maria, E.; Bellone, E.; Ajmar, F.; Mandich, P. Triplet Repeat Primed PCR (TP PCR) in Molecular Diagnostic Testing for Friedreich Ataxia. J. Mol. Diagn. 2004, 6, 285–289. [Google Scholar] [CrossRef] [Green Version]
- Warner, J.P.; Barron, L.H.; Goudie, D.; Kelly, K.; Dow, D.; Fitzpatrick, D.R.; Brock, D.J. A general method for the detection of large CAG repeat expansions by fluorescent PCR. J. Med. Genet. 1996, 33, 1022–1026. [Google Scholar] [CrossRef] [Green Version]
- Ranum, L.P.W.; Chung, M.-Y.; Banfi, S.; Bryer, A.; Schut, L.J.; Ramesar, R.; Duvick, L.A.; McCall, A.; Subramony, S.H.; Goldfarb, L.; et al. Molecular and Clinical Correlations in Spinocerebellar Ataxia Type I: Evidence for Familial Effects on the Age at Onset. Am. J. Hum. Genet. 1994, 55, 244–252. [Google Scholar]
- Goldfarb, L.G.; Vasconcelos, O.; Platonov, F.A.; Lunkes, A.; Kipnis, V.; Kononova, S.; Chabrashvili, T.; Vladimirtsev, V.A.; Alexeev, V.P.; Gajdusek, D.C. Unstable triplet repeat and phenotypic variability of spinocerebellar ataxia type 1. Ann. Neurol. 1996, 39, 500–506. [Google Scholar] [CrossRef] [PubMed]
- Pulst, S.-M.; Nechiporuk, A.; Nechiporuk, T.; Gispert, S.; Chen, X.-N.; Lopes-Cendes, I.; Pearlman, S.; Starkman, S.; Orozco-Diaz, G.; Lunkes, A.; et al. Moderate expansion of a normally biallelic trinucleotide repeat in spinocerebellar ataxia type 2. Nat. Genet. 1996, 14, 269–276. [Google Scholar] [CrossRef] [PubMed]
- Van De Warrenburg, B.P.C.; Hendriks, H.; Dürr, A.; Van Zuijlen, M.C.A.; Stevanin, G.; Camuzat, A.; Sinke, R.J.; Brice, A.; Kremer, B.P.H. Age at onset variance analysis in spinocerebellar ataxias: A study in a Dutch-French cohort. Ann. Neurol. 2005, 57, 505–512. [Google Scholar] [CrossRef] [PubMed]
- Langbehn, D.R.; Brinkman, R.R.; Falush, D.; Paulsen, J.; Hayden, M.R.; On behalf of an International Huntington’s Disease Collaborative Group. A new model for prediction of the age of onset and penetrance for Huntington’s disease based on CAG length. Clin. Genet. 2004, 65, 267–277. [Google Scholar] [CrossRef] [PubMed]
- Genetic Modifiers of Huntington’s Disease (GeM-HD) Consortium. CAG Repeat Not Polyglutamine Length Determines Timing of Huntington’s Disease Onset. Cell 2019, 178, 887–900.e14. [Google Scholar] [CrossRef] [Green Version]
- Wright, G.E.; Collins, J.A.; Kay, C.; McDonald, C.; Dolzhenko, E.; Xia, Q.; Bečanović, K.; Drögemöller, B.I.; Semaka, A.; Nguyen, C.M.; et al. Length of Uninterrupted CAG, Independent of Polyglutamine Size, Results in Increased Somatic Instability, Hastening Onset of Huntington Disease. Am. J. Hum. Genet. 2019, 104, 1116–1126. [Google Scholar] [CrossRef] [Green Version]
- Shishkin, A.A.; Voineagu, I.; Matera, R.; Cherng, N.; Chernet, B.T.; Krasilnikova, M.M.; Narayanan, V.; Lobachev, K.S.; Mirkin, S.M. Large-Scale Expansions of Friedreich’s Ataxia GAA Repeats in Yeast. Mol. Cell 2009, 35, 82–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerhardt, J.; Bhalla, A.D.; Butler, J.S.; Puckett, J.W.; Dervan, P.B.; Rosenwaks, Z.; Napierala, M. Stalled DNA Replication Forks at the Endogenous GAA Repeats Drive Repeat Expansion in Friedreich’s Ataxia Cells. Cell Rep. 2016, 16, 1218–1227. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Lacking 5′ and 3′ Interruption (n = 29) | 5′ Interruption (n = 19) | 3′ Interruption (n = 32) | 5′ and 3′ Interruption (n = 21) | |
|---|---|---|---|---|
| Age at Onset (years) | 10 (7–14.0) | 16 (10–25) | 20 (13–30) | 16 (7–28) |
| Number of FXN GAA repeats | ||||
| Shorter Allele (GAA1) | 782 (656–960) | 683 (483–765) | 552 (316–758) | 696 (349–827) |
| Longer Allele (GAA2) | 1000 (842–1144) | 1040 (800–1100) | 974 (765–1040) | 900 (783–1301) |
| 24.5 | 22.9 | 48.1 | 25.3 | |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Nethisinghe, S.; Kesavan, M.; Ging, H.; Labrum, R.; Polke, J.M.; Islam, S.; Garcia-Moreno, H.; Callaghan, M.F.; Cavalcanti, F.; Pook, M.A.; et al. Interruptions of the FXN GAA Repeat Tract Delay the Age at Onset of Friedreich’s Ataxia in a Location Dependent Manner. Int. J. Mol. Sci. 2021, 22, 7507. https://doi.org/10.3390/ijms22147507
Nethisinghe S, Kesavan M, Ging H, Labrum R, Polke JM, Islam S, Garcia-Moreno H, Callaghan MF, Cavalcanti F, Pook MA, et al. Interruptions of the FXN GAA Repeat Tract Delay the Age at Onset of Friedreich’s Ataxia in a Location Dependent Manner. International Journal of Molecular Sciences. 2021; 22(14):7507. https://doi.org/10.3390/ijms22147507
Chicago/Turabian StyleNethisinghe, Suran, Maheswaran Kesavan, Heather Ging, Robyn Labrum, James M. Polke, Saiful Islam, Hector Garcia-Moreno, Martina F. Callaghan, Francesca Cavalcanti, Mark A. Pook, and et al. 2021. "Interruptions of the FXN GAA Repeat Tract Delay the Age at Onset of Friedreich’s Ataxia in a Location Dependent Manner" International Journal of Molecular Sciences 22, no. 14: 7507. https://doi.org/10.3390/ijms22147507






