Diabetes Mellitus and Cardiovascular Diseases: Nutraceutical Interventions Related to Caloric Restriction
Abstract
:1. Introduction
1.1. Diabetes and Cardiovascular Diseases
1.2. New Dietary Interventions in T2DM/CVD Management
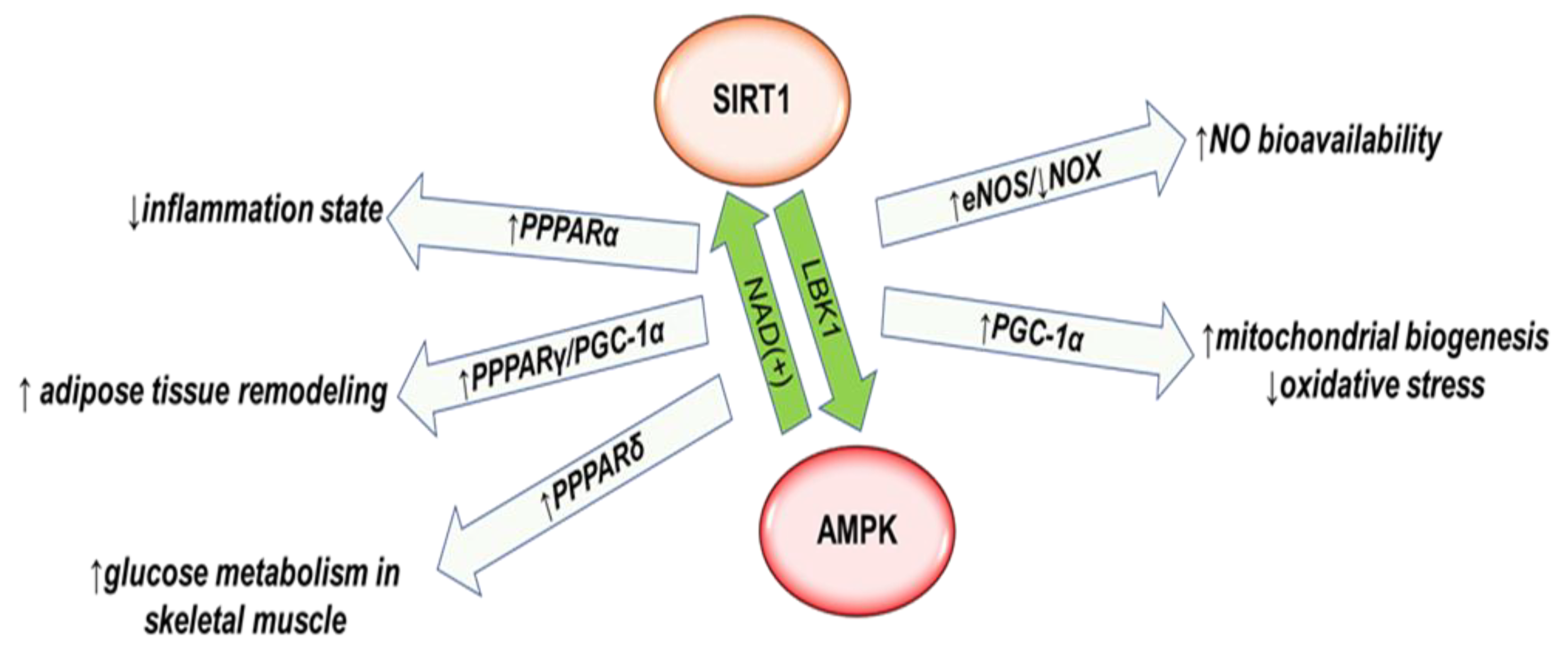
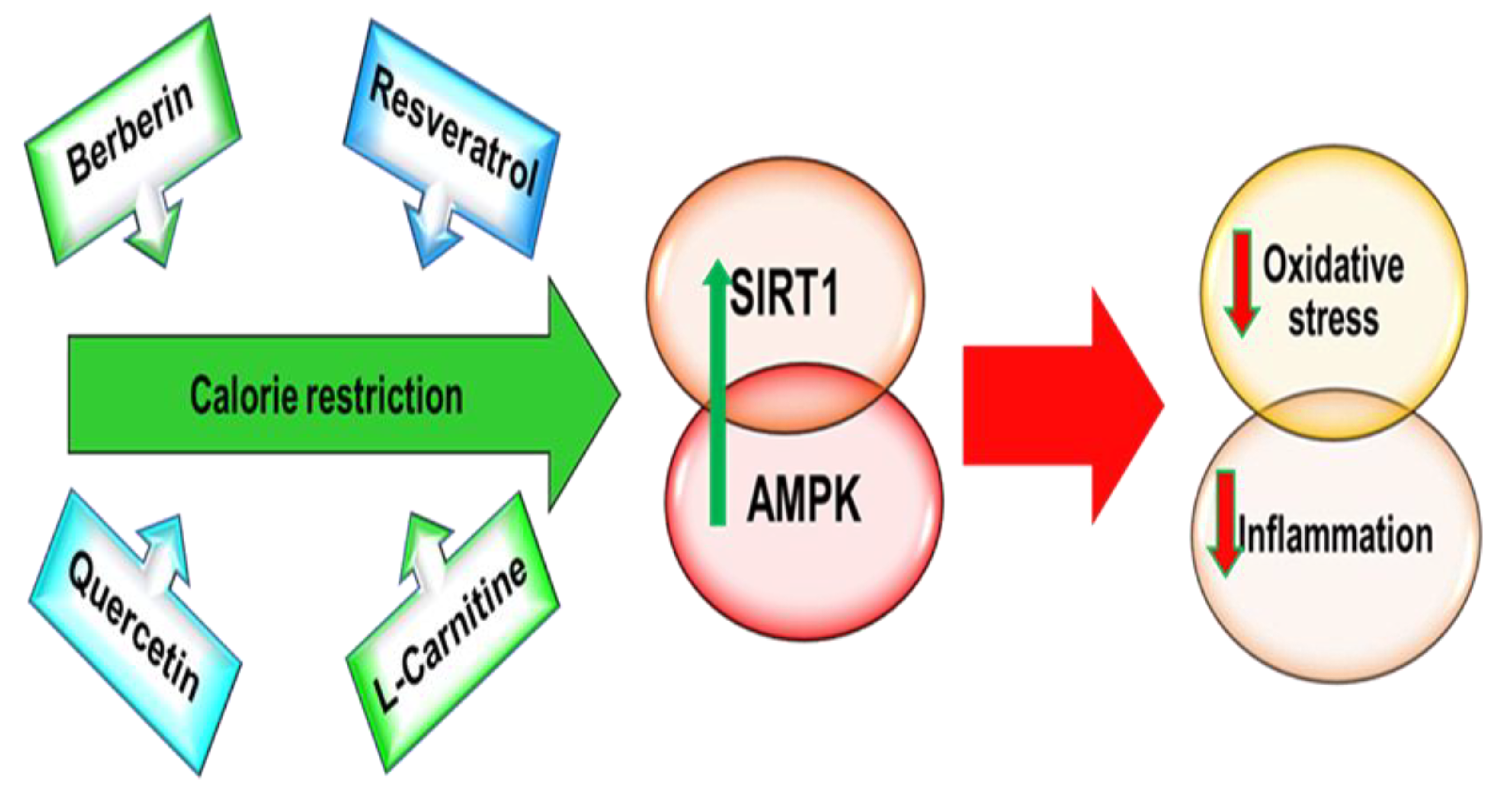
1.3. From Caloric Restriction to Caloric Restriction Mimetics
2. From Caloric Restriction to CR-Related Nutrients: Berberine
3. From Caloric Restriction to CR-Related Nutrients: Resveratrol
4. From Caloric Restriction to CR-Related Nutrients: Quercetin
5. From Caloric Restriction to CR-Related Nutrients: L-Carnitine
6. From Caloric Restriction to CR-Related Nutrients: Bioavailability and Pharmacokinetics
7. Conclusions
Author Contributions
Funding
Conflicts of Interest
Abbreviations
| T2DM | Type 2 diabetes mellitus |
| CVD | Cardiovascular diseases |
| LPS | Lipopolysaccharide |
| NAFLD | Non-alcoholic fatty liver disease |
| ROS | Reactive oxygen species |
| CR | Caloric restriction |
| AMPK | AMP-activated protein kinase |
| SIRT1 | Sirtuin 1 |
| NO | Nitric oxide |
| eNOS | Endothelial nitric oxide synthase |
| NOX | Nicotinamide Adenine Dinucleotide Phosphate-NADPH-Oxidase |
| FOXO | Fork Head Box O1 |
| PPARs | Peroxisome proliferator-activated receptors |
| UCP1 | Uncoupling protein-1 expression |
| BBR | Berberine |
| RSV | Resveratrol |
| QE | Quercetin |
| LC | L-Carnitine |
| TMAO | Trimethylamine–N-oxide |
| TMA | Trimethylamine |
| FMO | Flavin monooxygenase enzymes |
References
- Weitzman, S. The link between diabetes and cardiovascular disease: The epidemiological perspective. Isr. Med. Assoc. J. 2016, 18, 709–711. [Google Scholar]
- Viigimaa, M.; Sachinidis, A.; Toumpourleka, M.; Koutsampasopoulos, K.; Alliksoo, S.; Titma, T. Macrovascular Complications of Type 2 Diabetes Mellitus. Curr. Vasc. Pharmacol. 2020, 18, 110–116. [Google Scholar] [CrossRef]
- Tan, Y.; Zhang, Z.; Zheng, C.; Wintergerst, K.A.; Keller, B.B.; Cai, L. Mechanisms of diabetic cardiomyopathy and potential therapeutic strategies: Preclinical and clinical evidence. Nat. Rev. Cardiol. 2020, 17, 585–607. [Google Scholar] [CrossRef] [PubMed]
- Xia, J.; Yin, C. Glucose variability and coronary artery disease. Hear. Lung Circ. 2019, 28, 553–559. [Google Scholar] [CrossRef] [PubMed]
- Shah, M.S.; Brownlee, M. Molecular and cellular mechanisms of cardiovascular disorders in diabetes. Circ. Res. 2016, 118, 1808–1829. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terruzzi, I.; Allibardi, S.; Bendinelli, P.; Maroni, P.; Piccoletti, R.; Vesco, F.; Samaja, M.; Luzi, L. Amino acid- and lipid-induced insulin resistance in rat heart: Molecular mechanisms. Mol. Cell. Endocrinol. 2002, 190, 135–145. [Google Scholar] [CrossRef]
- Penna, C.; Andreadou, I.; Aragno, M.; Beauloye, C.; Bertrand, L.; Lazou, A.; Falcão-Pires, I.; Bell, R.; Zuurbier, C.J.; Pagliaro, P.; et al. Effect of hyperglycaemia and diabetes on acute myocardial ischaemia-reperfusion injury and cardioprotection by ischaemic conditioning protocols. Br. J. Pharmacol. 2020, 177, 5312–5335. [Google Scholar] [CrossRef]
- Satthenapalli, V.R.; Lamberts, R.R.; Katare, R.G. Concise review: Challenges in regenerating the diabetic heart: A comprehensive review. Stem Cells. 2017, 35, 2009–2026. [Google Scholar] [CrossRef] [Green Version]
- Poznyak, A.; Grechko, A.V.; Poggio, P.; Myasoedova, V.A.; Alfieri, V.; Orekhov, A.N. The diabetes mellitus-atherosclerosis connection: The role of lipid and glucose metabolism and chronic inflammation. Int. J. Mol. Sci. 2020, 21, 1835. [Google Scholar] [CrossRef] [Green Version]
- Chait, A.; Hartigh, L.J.D. Adipose tissue distribution, inflammation and its metabolic consequences, including diabetes and cardiovascular disease. Front. Cardiovasc. Med. 2020, 7, 22. [Google Scholar] [CrossRef] [Green Version]
- Patterson, E.; Ryan, P.M.; Cryan, J.F.; Dinan, T.G.; Ross, R.P.; Fitzgerald, G.F.; Stanton, C. Gut microbiota, obesity and diabetes. Postgrad. Med. J. 2016, 92, 286–300. [Google Scholar] [CrossRef] [PubMed]
- Wen, L.; Duffy, A. Factors influencing the gut microbiota, inflammation, and type 2 diabetes. J. Nutr. 2017, 147, 1468S–1475S. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Senesi, P.; Luzi, L.; Terruzzi, I. Adipokines, myokines, and cardiokines: The role of nutritional interventions. Int. J. Mol. Sci. 2020, 21, 8372. [Google Scholar] [CrossRef] [PubMed]
- Guo, R.; Rogers, O.; Nair, S. Targeting Apelinergic System in Cardiometabolic Disease. Curr. Drug Targets 2017, 18, 1785–1791. [Google Scholar] [CrossRef] [PubMed]
- Ahmad, A.; Yang, W.; Chen, G.; Shafiq, M.; Javed, S.; Zaidi, S.S.A.; Shahid, R.; Liu, C.; Bokhari, H. Analysis of gut microbiota of obese individuals with type 2 diabetes and healthy individuals. PLoS ONE 2019, 14, e0226372. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Greenhill, C. Gut microbiota: Firmicutes and Bacteroidetes involved in insulin resistance by mediating levels of glucagon-like peptide 1. Nat. Rev. Endocrinol. 2015, 11, 254. [Google Scholar] [CrossRef]
- Jiao, N.; Baker, S.S.; Nugent, C.A.; Tsompana, M.; Cai, L.; Wang, Y.; Buck, M.J.; Genco, R.J.; Baker, R.D.; Zhu, R.; et al. Gut microbiome may contribute to insulin resistance and systemic inflammation in obese rodents: A meta-analysis. Physiol. Genom. 2018, 50, 244–254. [Google Scholar] [CrossRef] [Green Version]
- Kim, K.A.; Jeong, J.J.; Yoo, S.Y.; Kim, D.H. Gut microbiota lipopolysaccharide accelerates inflamm-aging in mice. BMC Microbiol. 2016, 16, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Belizário, J.E.; Faintuch, J.; Garay-Malpartida, M. Gut microbiome dysbiosis and immunometabolism: New frontiers for treatment of metabolic diseases. Mediat. Inflamm. 2018, 2018, 1–12. [Google Scholar] [CrossRef]
- Byrne, C.D.; Targher, G. NAFLD: A multisystem disease. J. Hepatol. 2015, 62, S47–S64. [Google Scholar] [CrossRef] [Green Version]
- Safari, Z.; Gérard, P. The links between the gut microbiome and non-alcoholic fatty liver disease (NAFLD). Cell. Mol. Life Sci. 2019, 76, 1541–1558. [Google Scholar] [CrossRef]
- Morrison, D.J.; Preston, T. Formation of short chain fatty acids by the gut microbiota and their impact on human metabolism. Gut Microbes 2016, 7, 189–200. [Google Scholar] [CrossRef] [Green Version]
- Chu, H.; Duan, Y.; Yang, L.; Schnabl, B. Small metabolites, possible big changes: A microbiota-centered view of non-alcoholic fatty liver disease. Gut 2018, 68, 359–370. [Google Scholar] [CrossRef]
- Adams, L.A.; Anstee, Q.M.; Tilg, H.; Targher, G. Non-alcoholic fatty liver disease and its relationship with cardiovascular disease and other extrahepatic diseases. Gut 2017, 66, 1138–1153. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- American Diabetes Association 4. Lifestyle management: Standards of medical care in diabetes-2018. Diabetes Care 2017, 41, S38–S50. [Google Scholar] [CrossRef] [Green Version]
- Herrera, M.C.A.; Subhan, F.B.; Chan, C.B. Dietary patterns and cardiovascular disease risk in people with type 2 diabetes. Curr. Obes. Rep. 2017, 6, 405–413. [Google Scholar] [CrossRef] [PubMed]
- Corella, D.; Ordovás, J.M. Papel de las ómicas en la nutrición de precisión: Fortalezas y debilidades [The role of omics in precision nutrition: Strengths and weaknesses]. Nutr. Hosp. 2018, 35, 10–18. [Google Scholar] [CrossRef] [PubMed]
- Carrasco-Ramiro, F.; Peiró-Pastor, R.; Aguado, B. Human genomics projects and precision medicine. Gene Ther. 2017, 24, 551–561. [Google Scholar] [CrossRef]
- Mathers, J.C. Nutrigenomics in the modern era. Proc. Nutr. Soc. 2016, 76, 265–275. [Google Scholar] [CrossRef]
- Ren, X.; Li, X. Advances in research on diabetes by human nutriomics. Int. J. Mol. Sci. 2019, 20, 5375. [Google Scholar] [CrossRef] [Green Version]
- Lean, M.E.J. Low-calorie diets in the management of type 2 diabetes mellitus. Nat. Rev. Endocrinol. 2019, 15, 251–252. [Google Scholar] [CrossRef]
- Taylor, R. Calorie restriction for long-term remission of type 2 diabetes. Clin. Med. 2019, 19, 37–42. [Google Scholar] [CrossRef]
- Yaribeygi, H.; Atkin, S.L.; Ramezani, M.; Sahebkar, A. A review of the molecular pathways mediating the improvement in diabetes mellitus following caloric restriction. J. Cell. Physiol. 2018, 234, 8436–8442. [Google Scholar] [CrossRef]
- Rickman, A.D.; Williamson, D.A.; Martin, C.K.; Gilhooly, C.H.; Stein, R.I.; Bales, C.W.; Roberts, S.; Das, S.K. The CALERIE Study: Design and methods of an innovative 25% caloric restriction intervention. Contemp. Clin. Trials 2011, 32, 874–881. [Google Scholar] [CrossRef] [Green Version]
- Ma, L.; Wang, R.; Wang, H.; Zhang, Y.; Zhao, Z. Long-term caloric restriction activates the myocardial SIRT1/AMPK/PGC-1α pathway in C57BL/6J male mice. Food Nutr. Res. 2020, 64. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weir, H.J.; Yao, P.; Huynh, F.K.; Escoubas, C.C.; Goncalves, R.L.; Burkewitz, K.; Laboy, R.; Hirschey, M.D.; Mair, W.B. Dietary restriction and AMPK increase lifespan via mitochondrial network and peroxisome remodeling. Cell Metab. 2017, 26, 884–896.e5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Velingkaar, N.; Mezhnina, V.; Poe, A.; Makwana, K.; Tulsian, R.; Kondratov, R.V. Reduced caloric intake and periodic fasting independently contribute to metabolic effects of caloric restriction. Aging Cell 2020, 19, e13138. [Google Scholar] [CrossRef] [PubMed]
- Gensous, N.; Franceschi, C.; Santoro, A.; Milazzo, M.; Garagnani, P.; Bacalini, M.G. The impact of caloric restriction on the epigenetic signatures of aging. Int. J. Mol. Sci. 2019, 20, 2022. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pifferi, F.; Terrien, J.; Perret, M.; Epelbaum, J.; Blanc, S.; Picq, J.L.; Dhenain, M.; Aujard, F. Promoting healthspan and lifespan with caloric restriction in primates. Commun. Biol. 2019, 2, 107. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Martin-Montalvo, A.; Mercken, E.M.; Mitchell, S.J.; Palacios, H.H.; Mote, P.L.; Scheibye-Knudsen, M.; Gomes, A.P.; Ward, T.M.; Minor, R.K.; Blouin, M.J.; et al. Metformin improves healthspan and lifespan in mice. Nat. Commun. 2013, 4, 2192. [Google Scholar] [CrossRef] [PubMed]
- Senesi, P.; Montesano, A.; Luzi, L.; Codella, R.; Benedini, S.; Terruzzi, I. Metformin treatment prevents sedentariness related damages in mice. J. Diabetes Res. 2015, 2016, 1–11. [Google Scholar] [CrossRef] [Green Version]
- Mehrabani, S.; Bagherniya, M.; Askari, G.; Read, M.I.; Sahebkar, A. The effect of fasting or calorie restriction on mitophagy induction: A literature review. J. Cachex- Sarcopenia Muscle 2020, 11, 1447–1458. [Google Scholar] [CrossRef] [PubMed]
- Gutiérrez-Casado, E.; Khraiwesh, H.; López-Domínguez, J.A.; Montero-Guisado, J.; López-Lluch, G.; Navas, P.; de Cabo, R.; Ramsey, J.J.; González-Reyes, J.A.; Villalba, J.M. The impact of aging, calorie restriction and dietary fat on autophagy markers and mitochondrial ultrastructure and dynamics in mouse skeletal muscle. J. Gerontol. A Biol. Sci. Med. Sci. 2018, 74, 760–769. [Google Scholar] [CrossRef]
- Tang, B.L. Sirt1 and the mitochondria. Mol. Cells. 2016, 39, 87–95. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lan, F.; Cacicedo, J.M.; Ruderman, N.; Ido, Y. SIRT1 modulation of the acetylation status, cytosolic localization, and activity of LKB1. Possible role in AMP-activated protein kinase activation. J. Biol. Chem. 2008, 283, 27628–27635. [Google Scholar] [CrossRef] [Green Version]
- Rodríguez, C.; Muñoz, M.; Contreras, C.; Prieto, D. AMPK, metabolism, and vascular function. FEBS J. 2021, 288, 3746–3771. [Google Scholar] [CrossRef]
- Finley, L.W.; Haigis, M.C. The coordination of nuclear and mitochondrial communication during aging and calorie restriction. Ageing Res. Rev. 2009, 8, 173–188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Vanhoutte, P.M. Macro- and microvascular endothelial dysfunction in diabetes. J. Diabetes 2017, 9, 434–449. [Google Scholar] [CrossRef] [Green Version]
- Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; Laviola, L.; Giorgino, F.; Incalza, M.A.; D’Oria, R.; Natalicchio, A.; Perrini, S.; et al. Oxidative stress and reactive oxygen species in endothelial dysfunction associated with cardiovascular and metabolic diseases. Vasc. Pharmacol. 2018, 100, 1–19. [Google Scholar] [CrossRef]
- Grandl, G.; Wolfrum, C. Hemostasis, endothelial stress, inflammation, and the metabolic syndrome. Semin. Immunopathol. 2017, 40, 215–224. [Google Scholar] [CrossRef] [Green Version]
- Ojagbemi, A.; Okekunle, A.P.; Olowoyo, P.; Akpa, O.M.; Akinyemi, R.; Ovbiagele, B.; Owolabi, M. Dietary intakes of green leafy vegetables and incidence of cardiovascular diseases. Cardiovasc. J. Afr. 2021, 32, 1–9. [Google Scholar] [CrossRef]
- Zhao, Y.; Vanhoutte, P.M.; Leung, S.W. Vascular nitric oxide: Beyond eNOS. J. Pharmacol. Sci. 2015, 129, 83–94. [Google Scholar] [CrossRef] [Green Version]
- Vermot, A.; Petit-Härtlein, I.; Smith, S.M.E.; Fieschi, F. NADPH Oxidases (NOX): An overview from discovery, molecular mechanisms to physiology and pathology. Antioxidants 2021, 10, 890. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Higashi, Y.; Nakagawa, K.; Kimura, M.; Noma, K.; Sasaki, S.; Hara, K.; Matsuura, H.; Goto, C.; Oshima, T.; et al. A low-calorie diet improves endothelium-dependent vasodilation in obese patients with essential hypertension. Am. J. Hypertens. 2002, 15, 302–309. [Google Scholar] [CrossRef]
- Dolinsky, V.W.; Morton, J.S.; Oka, T.; Robillard-Frayne, I.; Bagdan, M.; Lopaschuk, G.D.; Des Rosiers, C.; Walsh, K.; Davidge, S.T.; Dyck, J.R. Calorie restriction prevents hypertension and cardiac hypertrophy in the spontaneously hypertensive rat. Hypertension 2010, 56, 412–421. [Google Scholar] [CrossRef] [PubMed]
- Kröller-Schön, S.; Daiber, A.; Schulz, E. Modulation of vascular function by AMPK: Assessment of NO bioavailability and surrogates of oxidative stress. Methods Mol. Biol. 2018, 1732, 495–506. [Google Scholar] [CrossRef] [PubMed]
- García-Prieto, C.F.; Pulido-Olmo, H.; Ruiz-Hurtado, G.; Gil-Ortega, M.; Aranguez, I.; Rubio, M.A.; Ruiz-Gayo, M.; Somoza, B.; Fernández-Alfonso, M.S. Mild caloric restriction reduces blood pressure and activates endothelial AMPK-PI3K-Akt-eNOS pathway in obese Zucker rats. Vasc. Pharmacol. 2015, 65-66, 3–12. [Google Scholar] [CrossRef] [PubMed]
- García-Prieto, C.F.; Gil-Ortega, M.; Plaza, A.; Manzano-Lista, F.J.; González-Blázquez, R.; Alcalá, M.; Rodríguez-Rodríguez, P.; Viana, M.; Aránguez, I.; Gollasch, M.; et al. Caloric restriction induces H2O2 formation as a trigger of AMPK-eNOS-NO pathway in obese rats: Role for CAMKII. Free. Radic. Biol. Med. 2019, 139, 35–45. [Google Scholar] [CrossRef] [PubMed]
- Rodríguez, C.; Contreras, C.; Sáenz-Medina, J.; Muñoz, M.; Corbacho, C.; Carballido, J.; García-Sacristán, A.; Hernandez, M.; López, M.; Rivera, L.; et al. Activation of the AMP-related kinase (AMPK) induces renal vasodilatation and downregulates Nox-derived reactive oxygen species (ROS) generation. Redox Biol. 2020, 34, 101575. [Google Scholar] [CrossRef]
- Hasan, R.; Lasker, S.; Hasan, A.; Zerin, F.; Zamila, M.; Chowdhury, F.I.; Nayan, S.I.; Rahman, M.M.; Khan, F.; Subhan, N.; et al. Canagliflozin attenuates isoprenaline-induced cardiac oxidative stress by stimulating multiple antioxidant and anti-inflammatory signaling pathways. Sci. Rep. 2020, 10, 1–19. [Google Scholar] [CrossRef]
- Mattagajasingh, I.; Kim, C.S.; Naqvi, A.; Yamamori, T.; Hoffman, T.A.; Jung, S.B.; DeRicco, J.; Kasuno, K.; Irani, K. SIRT1 promotes endothelium-dependent vascular relaxation by activating endothelial nitric oxide synthase. Proc. Natl. Acad. Sci. USA 2007, 104, 14855–14860. [Google Scholar] [CrossRef] [Green Version]
- Lempiäinen, J.; Finckenberg, P.; Mervaala, E.E.; Sankari, S.; Levijoki, J.; Mervaala, E.M. Caloric restriction ameliorates kidney ischaemia/reperfusion injury through PGC-1α-eNOS pathway and enhanced autophagy. Acta Physiol. 2013, 208, 410–421. [Google Scholar] [CrossRef]
- Luo, X.; Hu, Y.; He, S.; Ye, Q.; Lv, Z.; Liu, J.; Chen, X. Dulaglutide inhibits high glucose- induced endothelial dysfunction and NLRP3 inflammasome activation. Arch. Biochem. Biophys. 2019, 671, 203–209. [Google Scholar] [CrossRef]
- Villena, J.A. New insights into PGC-1 coactivators: Redefining their role in the regulation of mitochondrial function and beyond. FEBS J. 2015, 282, 647–672. [Google Scholar] [CrossRef] [PubMed]
- Yap, K.H.; Yee, G.S.; Candasamy, M.; Tan, S.C.; Md, S.; Majeed, A.B.A.; Bhattamisra, S.K. Catalpol ameliorates insulin sensitivity and mitochondrial respiration in skeletal muscle of Type-2 diabetic mice through insulin signaling pathway and AMPK/SIRT1/PGC-1α/PPAR-γ activation. Biomolecules 2020, 10, 1360. [Google Scholar] [CrossRef] [PubMed]
- Waldman, M.; Nudelman, V.; Shainberg, A.; Zemel, R.; Kornwoski, R.; Aravot, D.; Peterson, S.J.; Arad, M.; Hochhauser, E. The role of heme oxygenase 1 in the protective effect of caloric restriction against diabetic cardiomyopathy. Int. J. Mol. Sci. 2019, 20, 2427. [Google Scholar] [CrossRef] [Green Version]
- Singh, V.; Ubaid, S. Role of silent information regulator 1 (SIRT1) in regulating oxidative stress and inflammation. Inflamm. 2020, 43, 1589–1598. [Google Scholar] [CrossRef] [PubMed]
- Kobayashi, Y.; Furukawa-Hibi, Y.; Chen, C.; Horio, Y.; Isobe, K.; Ikeda, K.; Motoyama, N. SIRT1 is critical regulator of FOXO-mediated transcription in response to oxidative stress. Int. J. Mol. Med. 2005, 16, 237–243. [Google Scholar] [CrossRef]
- Greer, E.L.; Banko, M.R.; Brunet, A. AMP-activated protein kinase and FoxO transcription factors in dietary restriction-induced longevity. Ann. N. Y. Acad. Sci. 2009, 1170, 688–692. [Google Scholar] [CrossRef] [PubMed]
- Kauppinen, A.; Suuronen, T.; Ojala, J.; Kaarniranta, K.; Salminen, A. Antagonistic crosstalk between NF-κB and SIRT1 in the regulation of inflammation and metabolic disorders. Cell. Signal. 2013, 25, 1939–1948. [Google Scholar] [CrossRef]
- Jordan, S.; Tung, N.; Casanova-Acebes, M.; Chang, C.; Cantoni, C.; Zhang, D.; Wirtz, T.H.; Naik, S.; Rose, S.A.; Brocker, C.N.; et al. Dietary intake regulates the circulating inflammatory monocyte pool. Cell 2019, 178, 1102–1114.e17. [Google Scholar] [CrossRef]
- Cohen, K.; Waldman, M.; Abraham, N.G.; Laniado-Schwartzman, M.; Gurfield, D.; Aravot, D.; Arad, M.; Hochhauser, E. Caloric restriction ameliorates cardiomyopathy in animal model of diabetes. Exp. Cell Res. 2017, 350, 147–153. [Google Scholar] [CrossRef]
- Corrales, P.; Vidal-Puig, A.; Medina-Gómez, G. PPARs and metabolic disorders associated with challenged adipose tissue plasticity. Int. J. Mol. Sci. 2018, 19, 2124. [Google Scholar] [CrossRef] [Green Version]
- 74. Bargut, T.C.L.; Souza-Mello, V.; Aguila, M.B.; Mandarim-de-Lacerda, C.A. Browning of white adipose tissue: Lessons from experimental models. Horm. Mol. Biol. Clin. Investig. 2017, 31. [Google Scholar] [CrossRef]
- Aquilano, K.; Sciarretta, F.; Turchi, R.; Li, B.-H.; Rosina, M.; Ceci, V.; Guidobaldi, G.; Arena, S.; D’Ambrosio, C.; Audano, M.; et al. Low-protein/high-carbohydrate diet induces AMPK-dependent canonical and non-canonical thermogenesis in subcutaneous adipose tissue. Redox Biol. 2020, 36, 101633. [Google Scholar] [CrossRef]
- Qiang, L.; Wang, L.; Kon, N.; Zhao, W.; Lee, S.; Zhang, Y.; Rosenbaum, M.; Zhao, Y.; Gu, W.; Farmer, S.; et al. Brown remodeling of white adipose tissue by SirT1-dependent deacetylation of Pparγ. Cell 2012, 150, 620–632. [Google Scholar] [CrossRef] [Green Version]
- Boström, P.; Wu, J.; Jedrychowski, M.P.; Korde, A.; Ye, L.; Lo, J.C.; Rasbach, K.A.; Boström, E.A.; Choi, J.H.; Long, J.Z.; et al. A PGC1-α-dependent myokine that drives brown-fat-like development of white fat and thermogenesis. Nat. Cell Biol. 2012, 481, 463–468. [Google Scholar] [CrossRef] [PubMed]
- Yang, X.; Liu, Q.; Li, Y.; Tang, Q.; Wu, T.; Chen, L.; Pu, S.; Zhao, Y.; Zhang, G.; Huang, C.; et al. The diabetes medication canagliflozin promotes mitochondrial remodelling of adipocyte via the AMPK-Sirt1-Pgc-1α signalling pathway. Adipocyte 2020, 9, 484–494. [Google Scholar] [CrossRef] [PubMed]
- Jamwal, S.; Blackburn, J.K.; Elsworth, J.D. PPARγ/PGC1α signaling as a potential therapeutic target for mitochondrial biogenesis in neurodegenerative disorders. Pharmacol. Ther. 2021, 219, 107705. [Google Scholar] [CrossRef] [PubMed]
- Fujii, N.; Uta, S.; Kobayashi, M.; Sato, T.; Okita, N.; Higami, Y. Impact of aging and caloric restriction on fibroblast growth factor 21 signaling in rat white adipose tissue. Exp. Gerontol. 2019, 118, 55–64. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.B.; Wu, X.L.; Ke, B.; Huang, Y.J.; Chen, S.Q.; Su, Y.Q.; Qin, J. Effects of caloric restriction on peroxisome proliferator-activated receptors and positive transcription elongation factor b expression in obese rats. Eur. Rev. Med. Pharmacol. Sci. 2017, 21, 4369–4378. [Google Scholar]
- Medina-Gomez, G.; Gray, S.; Vidal-Puig, A. Adipogenesis and lipotoxicity: Role of peroxisome proliferator-activated receptor gamma (PPARgamma) and PPARgammacoactivator-1 (PGC1). Public Heal. Nutr. 2007, 10, 1132–1137. [Google Scholar] [CrossRef]
- Liu, F.; Fang, S.; Liu, X.; Li, J.; Wang, X.; Cui, J.; Chen, T.; Li, Z.; Yang, F.; Tian, J.; et al. Omentin-1 protects against high glucose-induced endothelial dysfunction via the AMPK/PPARδ signaling pathway. Biochem Pharmacol. 2020, 174, 113830. [Google Scholar] [CrossRef]
- Okazaki, M.; Iwasaki, Y.; Nishiyama, M.; Taguchi, T.; Tsugita, M.; Nakayama, S.; Kambayashi, M.; Hashimoto, K.; Terada, Y. PPARbeta/delta regulates the human SIRT1 gene transcription via Sp1. Endocr. J. 2010, 57, 403–413. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Y.; Colby, J.K.; Zuo, X.; Jaoude, J.; Wei, D.; Shureiqi, I. The role of PPAR-δ in metabolism, inflammation, and cancer: Many characters of a critical transcription factor. Int. J. Mol. Sci. 2018, 19, 3339. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gan, Z.; Burkart-Hartman, E.M.; Han, D.H.; Finck, B.; Leone, T.C.; Smith, E.Y.; Ayala, J.E.; Holloszy, J.; Kelly, D.P. The nuclear receptor PPARβ/δ programs muscle glucose metabolism in cooperation with AMPK and MEF2. Genes Dev. 2011, 25, 2619–2630. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paoli, A.; Tinsley, G.; Bianco, A.; Moro, T. The influence of meal frequency and timing on health in humans: The role of fasting. Nutrients 2019, 11, 719. [Google Scholar] [CrossRef] [Green Version]
- Zheng, X.; Wang, S.; Jia, W. Calorie restriction and its impact on gut microbial composition and global metabolism. Front. Med. 2018, 12, 634–644. [Google Scholar] [CrossRef] [Green Version]
- Banini, B.A.; Sanyal, A.J. Current and future pharmacologic treatment of nonalcoholic steatohepatitis. Curr. Opin. Gastroenterol. 2017, 33, 134–141. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Huang, M.; You, X.; Zhao, J.; Chen, L.; Wang, L.; Luo, Y.; Chen, Y. Gut microbiota mediates the anti-obesity effect of calorie restriction in mice. Sci. Rep. 2018, 8, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Moreno-Navarrete, J.M.; Fernandez-Real, J.M. The gut microbiota modulates both browning of white adipose tissue and the activity of brown adipose tissue. Rev. Endocr. Metab. Disord. 2019, 20, 387–397. [Google Scholar] [CrossRef] [PubMed]
- Corrales, P.; Vivas-García, Y.; Izquierdo-Lahuerta, A.; Horrillo, D.; Seoane-Collazo, P.; Velasco, I.; Torres, L.; Lopez, Y.; Martínez, C.; López, M.; et al. Long-term caloric restriction ameliorates deleterious effects of aging on white and brown adipose tissue plasticity. Aging Cell 2019, 18, e12948. [Google Scholar] [CrossRef] [Green Version]
- Harper, C.; Maher, J.; Grunseit, A.; Seimon, R.V.; Sainsbury, A. Experiences of using very low energy diets for weight loss by people with overweight or obesity: A review of qualitative research. Obes. Rev. 2018, 19, 1412–1423. [Google Scholar] [CrossRef] [PubMed]
- Martens, C.R.; Seals, D.R. Practical alternatives to chronic caloric restriction for optimizing vascular function with ageing. J. Physiol. 2016, 594, 7177–7195. [Google Scholar] [CrossRef]
- Mariño, G.; Pietrocola, F.; Madeo, F.; Kroemer, G. Caloric restriction mimetics: Natural/physiological pharmacological autophagy inducers. Autophagy 2014, 10, 1879–1882. [Google Scholar] [CrossRef] [Green Version]
- Madeo, F.; Pietrocola, F.; Eisenberg, T.; Kroemer, G. Caloric restriction mimetics: Towards a molecular definition. Nat. Rev. Drug Discov. 2014, 13, 727–740. [Google Scholar] [CrossRef]
- Pietrocola, F.; Castoldi, F.; Maiuri, M.C.; Kroemer, G. Aspirin-another caloric-restriction mimetic. Autophagy 2018, 14, 1162–1163. [Google Scholar] [CrossRef]
- Cicero, A.F.; Baggioni, A. Berberine and its role in chronic disease. Adv. Exp. Med. Biol. 2016, 928, 27–45. [Google Scholar] [CrossRef] [PubMed]
- Hu, X.; Zhang, Y.; Xue, Y.; Zhang, Z.; Wang, J. Berberine is a potential therapeutic agent for metabolic syndrome via brown adipose tissue activation and metabolism regulation. Am. J. Transl. Res. 2018, 10, 3322–3329. [Google Scholar] [PubMed]
- Tabeshpour, J.; Imenshahidi, M.; Hosseinzadeh, H. A review of the effects of Berberis vulgaris and its major component, berberine, in metabolic syndrome. Iran. J. Basic. Med. Sci. 2017, 20, 557–568. [Google Scholar] [CrossRef]
- Chang, W.; Chen, L.; Hatch, G.M. Berberine as a therapy for type 2 diabetes and its complications: From mechanism of action to clinical studies. Biochem. Cell Biol. 2015, 93, 479–486. [Google Scholar] [CrossRef] [PubMed]
- Ma, X.; Chen, Z.; Wang, L.; Wang, G.; Wang, Z.; Dong, X.; Wen, B.; Zhang, Z. The pathogenesis of diabetes mellitus by oxidative stress and inflammation: Its inhibition by berberine. Front. Pharmacol. 2018, 9, 782. [Google Scholar] [CrossRef] [Green Version]
- Yao, S.; Yuan, Y.; Zhang, H.; Meng, X.; Jin, L.; Yang, J.; Wang, W.; Ning, G.; Zhang, Y.; Zhang, Z. Berberine attenuates the abnormal ectopic lipid deposition in skeletal muscle. Free. Radic. Biol. Med. 2020, 159, 66–75. [Google Scholar] [CrossRef]
- Ren, G.; Guo, J.H.; Qian, Y.Z.; Kong, W.J.; Jiang, J.D. Berberine improves glucose and lipid metabolism in HepG2 cells through AMPKα1 activation. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef]
- Wu, Y.S.; Li, Z.M.; Chen, Y.T.; Dai, S.J.; Zhou, X.J.; Yang, Y.X.; Lou, J.S.; Ji, L.T.; Bao, Y.T.; Xuan, L.; et al. Berberine improves inflammatory responses of diabetes mellitus in zucker diabetic fatty rats and insulin-resistant HepG2 cells through the PPM1B pathway. J. Immunol. Res. 2020, 2020, 1–32. [Google Scholar] [CrossRef]
- Wang, L.; Ye, X.; Hua, Y.; Song, Y. Berberine alleviates adipose tissue fibrosis by inducing AMP-activated kinase signaling in high-fat diet-induced obese mice. Biomed. Pharmacother. 2018, 105, 121–129. [Google Scholar] [CrossRef]
- Shan, Y.; Zhang, S.; Gao, B.; Liang, S.; Zhang, H.; Yu, X.; Zhao, J.; Ye, L.; Yang, Q.; Shang, W. Adipose tissue SIRT1 regulates insulin sensitizing and anti-inflammatory effects of berberine. Front. Pharmacol. 2020, 11. [Google Scholar] [CrossRef] [PubMed]
- Wu, L.; Xia, M.; Duan, Y.; Zhang, L.; Jiang, H.; Hu, X.; Yan, H.; Zhang, Y.; Gu, Y.; Shi, H.; et al. Berberine promotes the recruitment and activation of brown adipose tissue in mice and humans. Cell Death Dis. 2019, 10, 468. [Google Scholar] [CrossRef] [PubMed]
- Hang, W.; He, B.; Chen, J.; Xia, L.; Wen, B.; Liang, T.; Wang, X.; Zhang, Q.; Wu, Y.; Chen, Q.; et al. Berberine ameliorates high glucose-induced cardiomyocyte injury via AMPK signaling activation to stimulate mitochondrial biogenesis and restore autophagic flux. Front. Pharmacol. 2018, 9. [Google Scholar] [CrossRef]
- Chang, W.; Li, K.; Guan, F.; Yao, F.; Yu, Y.; Zhang, M.; Hatch, G.M.; Chen, L. Berberine pretreatment confers cardioprotection against ischemia-reperfusion injury in a rat model of type 2 diabetes. J. Cardiovasc. Pharmacol. Ther. 2016, 21, 486–494. [Google Scholar] [CrossRef]
- Wang, Y.; Huang, Y.; Lam, K.S.; Li, Y.; Wong, W.T.; Ye, H.; Lau, C.-W.; Vanhoutte, P.M.; Xu, A. Berberine prevents hyperglycemia-induced endothelial injury and enhances vasodilatation via adenosine monophosphate-activated protein kinase and endothelial nitric oxide synthase. Cardiovasc. Res. 2009, 82, 484–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, D.; Zhang, Y.; Liu, Y.; Hou, L.; Li, S.; Tian, H.; Zhao, T. Berberine modulates gut microbiota and reduces insulin resistance via the TLR4 signaling pathway. Exp. Clin. Endocrinol. Diabetes 2018, 126, 513–520. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Shou, J.-W.; Li, X.-Y.; Zhao, Z.-X.; Fu, J.; He, C.-Y.; Feng, R.; Ma, C.; Wen, B.-Y.; Guo, F.; et al. Berberine-induced bioactive metabolites of the gut microbiota improve energy metabolism. Metabolism 2017, 70, 72–84. [Google Scholar] [CrossRef] [PubMed]
- Tian, Y.; Cai, J.; Gui, W.; Nichols, R.; Koo, I.; Zhang, J.; Anitha, M.; Patterson, A.D. Berberine directly affects the gut microbiota to promote intestinal farnesoid X receptor activation. Drug Metab. Dispos. 2018, 47, 86–93. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shi, Y.; Hu, J.; Geng, J.; Hu, T.; Wang, B.; Yan, W.; Jiang, Y.; Li, J.; Liu, S. Berberine treatment reduces atherosclerosis by mediating gut microbiota in apoE-/- mice. Biomed. Pharmacother. 2018, 107, 1556–1563. [Google Scholar] [CrossRef]
- Xiong, P.; Niu, L.; Talaei, S.; Kord-Varkaneh, H.; Clark, C.; Găman, M.-A.; Rahmani, J.; Dorosti, M.; Mousavi, S.M.; Zarezadeh, M.; et al. The effect of berberine supplementation on obesity indices: A dose- response meta-analysis and systematic review of randomized controlled trials. Complement. Ther. Clin. Pr. 2020, 39, 101113. [Google Scholar] [CrossRef] [PubMed]
- Beba, M.; Djafarian, K.; Shab-Bidar, S. Effect of Berberine on C-reactive protein: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2019, 46, 81–86. [Google Scholar] [CrossRef]
- Asbaghi, O.; Ghanbari, N.; Shekari, M.; Reiner, Ž.; Amirani, E.; Hallajzadeh, J.; Mirsafaei, L.; Asemi, Z. The effect of berberine supplementation on obesity parameters, inflammation and liver function enzymes: A systematic review and meta-analysis of randomized controlled trials. Clin. Nutr. ESPEN 2020, 38, 43–49. [Google Scholar] [CrossRef]
- Nawaz, W.; Zhou, Z.; Deng, S.; Ma, X.; Ma, X.; Li, C.; Shu, X. Therapeutic versatility of resveratrol derivatives. Nutrients 2017, 9, 1188. [Google Scholar] [CrossRef] [Green Version]
- Castaldo, L.; Narváez, A.; Izzo, L.; Graziani, G.; Gaspari, A.; Di Minno, G.; Ritieni, A. Red wine consumption and cardiovascular health. Molecules 2019, 24, 3626. [Google Scholar] [CrossRef] [Green Version]
- Breuss, J.M.; Atanasov, A.G.; Uhrin, P. Resveratrol and its effects on the vascular system. Int. J. Mol. Sci. 2019, 20, 1523. [Google Scholar] [CrossRef] [Green Version]
- Elgebaly, A.; Radwan, I.; AboElnas, M.; Ibrahim, H.H.; Eltoomy, M.F.M.; Atta, A.A.; Mesalam, H.A.; Sayed, A.A.; Othman, A.A. Resveratrol supplementation in patients with non-alcoholic fatty liver disease: Systematic review and meta-analysis. J. Gastrointest. Liver Dis. 2017, 26, 59–67. [Google Scholar] [CrossRef]
- Jeyaraman, M.M.; Al-Yousif, N.S.H.; Mann, A.S.; Dolinsky, V.W.; Rabbani, R.; Zarychanski, R.; Abou-Setta, A.M. Resveratrol for adults with type 2 diabetes mellitus. Cochrane Database Syst. Rev. 2020, 1, CD011919. [Google Scholar] [CrossRef]
- Pan, M.-H.; Wu, J.-C.; Ho, C.-T.; Lai, C.-S. Antiobesity molecular mechanisms of action: Resveratrol and pterostilbene. BioFactors 2018, 44, 50–60. [Google Scholar] [CrossRef] [PubMed]
- Chaplin, A.; Carpéné, C.; Mercader, J. Resveratrol, metabolic syndrome, and gut microbiota. Nutrients 2018, 10, 1651. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Akar, F.; Uludağ, O.; Aydın, A.; Aytekin, Y.A.; Elbeg, S.; Tuzcu, M.; Sahin, K. High-fructose corn syrup causes vascular dysfunction associated with metabolic disturbance in rats: Protective effect of resveratrol. Food Chem. Toxicol. 2012, 50, 2135–2141. [Google Scholar] [CrossRef]
- Cheng, P.-W.; Lee, H.-C.; Lu, P.-J.; Chen, H.-H.; Lai, C.-C.; Sun, G.-C.; Yeh, T.-C.; Hsiao, M.; Lin, Y.-T.; Liu, C.-P.; et al. Resveratrol inhibition of Rac1-derived reactive oxygen species by AMPK decreases blood pressure in a fructose-induced rat model of hypertension. Sci. Rep. 2016, 6, 25342. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bhatt, S.R.; Lokhandwala, M.F.; Banday, A.A. Resveratrol prevents endothelial nitric oxide synthase uncoupling and attenuates development of hypertension in spontaneously hypertensive rats. Eur. J. Pharmacol. 2011, 667, 258–264. [Google Scholar] [CrossRef] [PubMed]
- Ding, S.; Jiang, J.; Zhang, G.; Bu, Y.; Zhang, G.; Zhao, X. Resveratrol and caloric restriction prevent hepatic steatosis by regulating SIRT1-autophagy pathway and alleviating endoplasmic reticulum stress in high-fat diet-fed rats. PLoS ONE 2017, 12, e0183541. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shang, J.; Chen, L.-L.; Xiao, F.-X.; Sun, H.; Ding, H.-C.; Xiao, H. Resveratrol improves non-alcoholic fatty liver disease by activating AMP-activated protein kinase. Acta Pharmacol. Sin. 2008, 29, 698–706. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Huang, Y.; Lang, H.; Chen, K.; Zhang, Y.; Gao, Y.; Ran, L.; Yi, L.; Mi, M.; Zhang, Q. Resveratrol protects against nonalcoholic fatty liver disease by improving lipid metabolism and redox homeostasis via the PPARα pathway. Appl. Physiol. Nutr. Metab. 2020, 45, 227–239. [Google Scholar] [CrossRef] [PubMed]
- Teng, W.; Zhao, L.; Yang, S.; Zhang, C.; Liu, M.; Luo, J.; Jin, J.; Zhang, M.; Bao, C.; Li, D.; et al. The hepatic-targeted, resveratrol loaded nanoparticles for relief of high fat diet-induced nonalcoholic fatty liver disease. J. Control. Release 2019, 307, 139–149. [Google Scholar] [CrossRef] [PubMed]
- Chen, S.; Zhao, Z.; Ke, L.; Li, Z.; Li, W.; Zhang, Z.; Zhou, Y.; Feng, X.; Zhu, W. Resveratrol improves glucose uptake in insulin-resistant adipocytes via Sirt1. J. Nutr. Biochem. 2018, 55, 209–218. [Google Scholar] [CrossRef] [PubMed]
- Vlavcheski, F.; Hartogh, D.J.D.; Giacca, A.; Tsiani, E. Amelioration of high-insulin-induced skeletal muscle cell insulin resistance by resveratrol is linked to activation of AMPK and restoration of GLUT4 translocation. Nutrients 2020, 12, 914. [Google Scholar] [CrossRef] [Green Version]
- Shu, L.; Zhao, H.; Huang, W.; Hou, G.; Song, G.; Ma, H. Resveratrol upregulates mmu-miR-363-3p via the PI3K-Akt pathway to improve insulin resistance induced by a high-fat diet in mice. Diabetes, Metab. Syndr. Obesity: Targets Ther. 2020; ume 13, 391–403. [Google Scholar] [CrossRef] [Green Version]
- Andrade, J.M.O.; Barcala-Jorge, A.S.; Batista-Jorge, G.C.; Paraíso, A.F.; Freitas, K.M.; Lelis, D.F.; Guimarães, A.L.S.; de Paula, A.M.B.; Santos, S.H.S. Effect of resveratrol on expression of genes involved thermogenesis in mice and humans. Biomed. Pharmacother. 2019, 112, 108634. [Google Scholar] [CrossRef] [PubMed]
- Wang, S.; Liang, X.; Yang, Q.; Fu, X.; Rogers, C.J.; Zhu, M.; Rodgers, B.D.; Jiang, Q.; Dodson, M.V.; Du, M. Resveratrol induces brown-like adipocyte formation in white fat through activation of AMP-activated protein kinase (AMPK) α1. Int. J. Obes. 2015, 39, 967–976. [Google Scholar] [CrossRef] [Green Version]
- Hui, S.; Liu, Y.; Huang, L.; Zheng, L.; Zhou, M.; Lang, H.; Wang, X.; Yi, L.; Mi, M. Resveratrol enhances brown adipose tissue activity and white adipose tissue browning in part by regulating bile acid metabolism via gut microbiota remodeling. Int. J. Obes. 2020, 44, 1678–1690. [Google Scholar] [CrossRef]
- Liao, W.; Yin, X.; Li, Q.; Zhang, H.; Liu, Z.; Zheng, X.; Zheng, L.; Feng, X. Resveratrol-induced white adipose tissue browning in obese mice by remodeling fecal microbiota. Molecules 2018, 23, 3356. [Google Scholar] [CrossRef] [Green Version]
- Campbell, C.L.; Yu, R.; Li, F.; Zhou, Q.; Chen, D.; Qi, C.; Yin, Y.; Sun, J. Modulation of fat metabolism and gut microbiota by resveratrol on high-fat diet-induced obese mice. Diabetes Metab. Syndr. Obes. 2019, 12, 97–107. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.-L.; Yi, L.; Zhang, Y.; Zhou, X.; Ran, L.; Yang, J.; Zhu, J.-D.; Zhang, Q.-Y.; Mi, M.-T. Resveratrol attenuates Trimethylamine-N-Oxide (TMAO)-induced atherosclerosis by regulating TMAO synthesis and bile acid metabolism via remodeling of the gut microbiota. mBio 2016, 7, e02210-15. [Google Scholar] [CrossRef] [Green Version]
- Parsamanesh, N.; Asghari, A.; Sardari, S.; Tasbandi, A.; Jamialahmadi, T.; Xu, S.; Sahebkar, A. Resveratrol and endothelial function: A literature review. Pharmacol. Res. 2021, 170, 105725. [Google Scholar] [CrossRef]
- Jakubczyk, K.; Skonieczna-Żydecka, K.; Kałduńska, J.; Stachowska, E.; Gutowska, I.; Janda, K. Effects of resveratrol supplementation in patients with non-alcoholic fatty liver disease-A meta-analysis. Nutrients 2020, 12, 2435. [Google Scholar] [CrossRef]
- Pollack, R.M.; Barzilai, N.; Anghel, V.; Kulkarni, A.; Golden, A.; Broin, P.; Sinclair, D.; Bonkowski, M.; Coleville, A.J.; Powell, D.; et al. Resveratrol improves vascular function and mitochondrial number but not glucose metabolism in older adults. J. Gerontol. A Biol. Sci. Med. Sci. 2017, 72, 1703–1709. [Google Scholar] [CrossRef] [PubMed]
- Kang, W.; Hong, H.J.; Guan, J.; Kim, D.G.; Yang, E.-J.; Koh, G.; Park, D.; Han, C.H.; Lee, Y.-J.; Lee, D.-H.; et al. Resveratrol improves insulin signaling in a tissue-specific manner under insulin-resistant conditions only: In vitro and in vivo experiments in rodents. Metabolism 2012, 61, 424–433. [Google Scholar] [CrossRef] [PubMed]
- Montesano, A.; Luzi, L.; Senesi, P.; Mazzocchi, N.; Terruzzi, I. Resveratrol promotes myogenesis and hypertrophy in murine myoblasts. J. Transl. Med. 2013, 11, 310. [Google Scholar] [CrossRef] [Green Version]
- Wang, P.; Sang, S. Metabolism and pharmacokinetics of resveratrol and pterostilbene. Biofactors. 2018, 44, 16–25. [Google Scholar] [CrossRef] [PubMed]
- Batiha, G.E.-S.; Beshbishy, A.M.; Ikram, M.; Mulla, Z.S.; El-Hack, M.E.A.; Taha, A.E.; Algammal, A.M.; Elewa, Y.H.A. The pharmacological activity, biochemical properties, and pharmacokinetics of the major natural polyphenolic flavonoid: Quercetin. Foods 2020, 9, 374. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferenczyova, K.; Kalocayova, B.; Bartekova, M. Potential implications of quercetin and its derivatives in cardioprotection. Int. J. Mol. Sci. 2020, 21, 1585. [Google Scholar] [CrossRef] [Green Version]
- Chen, S.; Jiang, H.; Wu, X.; Fang, J. Therapeutic effects of quercetin on inflammation, obesity, and type 2 diabetes. Mediat. Inflamm. 2016, 2016, 1–5. [Google Scholar] [CrossRef]
- Murota, K.; Nakamura, Y.; Uehara, M. Flavonoid metabolism: The interaction of metabolites and gut microbiota. Biosci. Biotechnol. Biochem. 2018, 82, 600–610. [Google Scholar] [CrossRef] [Green Version]
- Calabró, V.; Litterio, M.C.; Fraga, C.G.; Galleano, M.; Piotrkowski, B. Effects of quercetin on heart nitric oxide metabolism in l-NAME treated rats. Arch. Biochem. Biophys. 2018, 647, 47–53. [Google Scholar] [CrossRef] [PubMed]
- Kim, S.G.; Kim, J.-R.; Choi, H.C.; Kim, S.G.; Kim, J.-R.; Choi, H.C. Quercetin-induced AMP-activated protein kinase activation attenuates vasoconstriction through LKB1-AMPK signaling pathway. J. Med. Food 2018, 21, 146–153. [Google Scholar] [CrossRef] [PubMed]
- Yan, L.; Zhang, J.D.; Wang, B.; Lv, Y.J.; Jiang, H.; Liu, G.L.; Qiao, Y.; Ren, M.; Guo, X.F. Quercetin inhibits left ventricular hypertrophy in spontaneously hypertensive rats and inhibits angiotensin II-induced H9C2 cells hypertrophy by enhancing PPAR-γ expression and suppressing AP-1 activity. PLoS ONE 2013, 8, e72548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ulasova, E.; Perez, J.; Hill, B.; Bradley, W.E.; Garber, D.W.; Landar, A.; Barnes, S.; Prasain, J.; Parks, D.A.; Dell’Italia, L.J.; et al. Quercetin prevents left ventricular hypertrophy in the Apo E knockout mouse. Redox Biol. 2013, 1, 381–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Guo, G.; Gong, L.; Sun, L.; Xu, H. Quercetin supports cell viability and inhibits apoptosis in cardiocytes by down-regulating miR-199a. Artif. Cells, Nanomedicine, Biotechnol. 2019, 47, 2909–2916. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Roslan, J.; Giribabu, N.; Karim, K.; Salleh, N. Quercetin ameliorates oxidative stress, inflammation and apoptosis in the heart of streptozotocin-nicotinamide-induced adult male diabetic rats. Biomed. Pharmacother. 2017, 86, 570–582. [Google Scholar] [CrossRef]
- Zhang, Y.; Dong, H.; Wang, M.; Zhang, J. Quercetin isolated from toona sinensis leaves attenuates hyperglycemia and protects hepatocytes in high-carbohydrate/high-fat diet and alloxan induced experimental diabetic mice. J. Diabetes Res. 2016, 2016, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Jeong, S.-M.; Kang, M.-J.; Choi, H.-N.; Kim, J.-H.; Kim, J.-I. Quercetin ameliorates hyperglycemia and dyslipidemia and improves antioxidant status in type 2 diabetic db/db mice. Nutr. Res. Pr. 2012, 6, 201–207. [Google Scholar] [CrossRef] [Green Version]
- Zhu, X.; Xiong, T.; Liu, P.; Guo, X.; Xiao, L.; Zhou, F.; Tang, Y.; Yao, P.; Zhu, X.; Xiong, T.; et al. Quercetin ameliorates HFD-induced NAFLD by promoting hepatic VLDL assembly and lipophagy via the IRE1a/XBP1s pathway. Food Chem. Toxicol. 2018, 114, 52–60. [Google Scholar] [CrossRef]
- Qin, G.; Ma, J.; Huang, Q.; Yin, H.; Han, J.; Li, M.; Deng, Y.; Wang, B.; Hassan, W.; Shang, J. Isoquercetin improves hepatic lipid accumulation by activating AMPK pathway and suppressing TGF-β signaling on an HFD-induced nonalcoholic fatty liver disease rat model. Int. J. Mol. Sci. 2018, 19, 4126. [Google Scholar] [CrossRef] [Green Version]
- Zhou, J.; Yoshitomi, H.; Liu, T.; Zhou, B.; Sun, W.; Qin, L.; Guo, X.; Huang, L.; Wu, L.; Gao, M. Isoquercitrin activates the AMP-activated protein kinase (AMPK) signal pathway in rat H4IIE cells. BMC Complement. Altern. Med. 2014, 14, 42. [Google Scholar] [CrossRef] [Green Version]
- Haddad, P.S.; Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G.; Haddad, P.S.; Eid, H.M.; Nachar, A.; Thong, F.; Sweeney, G. The molecular basis of the antidiabetic action of quercetin in cultured skeletal muscle cells and hepatocytes. Pharmacogn. Mag. 2015, 11, 74–81. [Google Scholar] [CrossRef] [Green Version]
- Liu, K.; Mei, F.; Wang, Y.; Xiao, N.; Yang, L.; Wang, Y.; Li, J.; Huang, F.; Kou, J.; Liu, B.; et al. Quercetin oppositely regulates insulin-mediated glucose disposal in skeletal muscle under normal and inflammatory conditions: The dual roles of AMPK activation. Mol. Nutr. Food Res. 2015, 60, 551–565. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Li, Q.; Li, K.; Zhu, L.; Lin, X.; Lin, X.; Shen, Q.; Li, G.; Xie, X. Quercetin improves glucose and lipid metabolism of diabetic rats: Involvement of Akt signaling and SIRT1. J. Diabetes Res. 2017, 2017, 1–10. [Google Scholar] [CrossRef] [Green Version]
- Guo, X.-D.; Zhang, D.-Y.; Gao, X.-J.; Parry, J.; Liu, K.; Liu, B.-L.; Wang, M. Quercetin and quercetin-3-O-glucuronide are equally effective in ameliorating endothelial insulin resistance through inhibition of reactive oxygen species-associated inflammation. Mol. Nutr. Food Res. 2013, 57, 1037–1045. [Google Scholar] [CrossRef]
- Li, X.; Wang, R.; Zhou, N.; Wang, X.; Liu, Q.; Bai, Y.; Bai, Y.; Liu, Z.; Yang, H.; Zou, J.; et al. Quercetin improves insulin resistance and hepatic lipid accumulation in vitro in a NAFLD cell model. Biomed. Rep. 2012, 1, 71–76. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Forney, L.A.; Lenard, N.R.; Stewart, L.K.; Henagan, T.M. Dietary quercetin attenuates adipose tissue expansion and inflammation and alters adipocyte morphology in a tissue-specific manner. Int. J. Mol. Sci. 2018, 19, 895. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seo, M.-J.; Lee, Y.-J.; Hwang, J.-H.; Kim, K.-J.; Lee, B.-Y. The inhibitory effects of quercetin on obesity and obesity-induced inflammation by regulation of MAPK signaling. J. Nutr. Biochem. 2015, 26, 1308–1316. [Google Scholar] [CrossRef]
- Kobori, M.; Takahashi, Y.; Sakurai, M.; Akimoto, Y.; Tsushida, T.; Oike, H.; Ippoushi, K.; Kobori, M.; Takahashi, Y.; Sakurai, M.; et al. Quercetin suppresses immune cell accumulation and improves mitochondrial gene expression in adipose tissue of diet-induced obese mice. Mol. Nutr. Food Res. 2015, 60, 300–312. [Google Scholar] [CrossRef] [Green Version]
- Porras, D.; Nistal, E.; Martínez-Flórez, S.; Pisonero-Vaquero, S.; Olcoz, J.L.; Jover, R.; González-Gallego, J.; García-Mediavilla, M.V.; Sánchez-Campos, S. Protective effect of quercetin on high-fat diet-induced non-alcoholic fatty liver disease in mice is mediated by modulating intestinal microbiota imbalance and related gut-liver axis activation. Free. Radic. Biol. Med. 2017, 102, 188–202. [Google Scholar] [CrossRef]
- Nie, J.; Zhang, L.; Zhao, G.; Du, X. Quercetin reduces atherosclerotic lesions by altering the gut microbiota and reducing atherogenic lipid metabolites. J. Appl. Microbiol. 2019, 127, 1824–1834. [Google Scholar] [CrossRef]
- Wu, D.-N.; Guan, L.; Jiang, Y.-X.; Ma, S.-H.; Sun, Y.-N.; Lei, H.-T.; Yang, W.-F.; Wang, Q.-F. Microbiome and metabonomics study of quercetin for the treatment of atherosclerosis. Cardiovasc. Diagn. Ther. 2019, 9, 545–560. [Google Scholar] [CrossRef]
- Zhang, F.; Feng, J.; Zhang, J.; Kang, X.; Qian, D. Quercetin modulates AMPK/SIRT1/NF-κB signaling to inhibit inflammatory/oxidative stress responses in diabetic high fat diet-induced atherosclerosis in the rat carotid artery. Exp. Ther. Med. 2020, 20, 1. [Google Scholar] [CrossRef]
- Ghorbani, A. Mechanisms of antidiabetic effects of flavonoid rutin. Biomed. Pharmacother. 2017, 96, 305–312. [Google Scholar] [CrossRef]
- Chalet, C.; Rubbens, J.; Tack, J.; Duchateau, G.S.; Augustijns, P. Intestinal disposition of quercetin and its phase-II metabolites after oral administration in healthy volunteers. J. Pharm. Pharmacol. 2018, 70, 1002–1008. [Google Scholar] [CrossRef] [PubMed]
- Adeva-Andany, M.M.; Calvo-Castro, I.; Fernández-Fernández, C.; Donapetry-García, C.; Pedre-Piñeiro, A.M. Significance of l-carnitine for human health. IUBMB Life 2017, 69, 578–594. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Janeiro, M.H.; Ramírez, M.J.; Milagro, F.I.; Martínez, J.A.; Solas, M. Implication of Trimethylamine N-Oxide (TMAO) in disease: Potential biomarker or new therapeutic target. Nutrients 2018, 10, 1398. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Seldin, M.M.; Meng, Y.; Qi, H.; Zhu, W.; Wang, Z.; Hazen, S.L.; Lusis, A.J.; Shih, D.M. Trimethylamine N-Oxide promotes vascular inflammation through signaling of mitogen-activated protein kinase and nuclear factor-κB. J. Am. Hear. Assoc. 2016, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ma, G.; Pan, B.; Chen, Y.; Guo, C.; Zhao, M.; Zheng, L.; Chen, B. Trimethylamine N-oxide in atherogenesis: Impairing endothelial self-repair capacity and enhancing monocyte adhesion. Biosci. Rep. 2017, 37. [Google Scholar] [CrossRef] [Green Version]
- Chen, M.; Zhu, X.; Ran, L.; Lang, H.; Yi, L.; Mi, M. Trimethylamine-N-Oxide induces vascular inflammation by activating the NLRP3 inflammasome through the SIRT3-SOD2-mtROS signaling pathway. J. Am. Hear. Assoc. 2017, 6, e006347. [Google Scholar] [CrossRef]
- Canyelles, M.; Tondo, M.; Cedó, L.; Farràs, M.; Escolà-Gil, J.C.; Blanco-Vaca, F. Trimethylamine N-Oxide: A link among diet, gut microbiota, gene regulation of liver and intestine cholesterol homeostasis and HDL function. Int. J. Mol. Sci. 2018, 19, 3228. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Warrier, M.; Shih, D.M.; Burrows, A.C.; Ferguson, D.; Gromovsky, A.D.; Brown, A.L.; Marshall, S.; McDaniel, A.; Schugar, R.C.; Wang, Z.; et al. The TMAO-generating enzyme flavin monooxygenase 3 is a central regulator of cholesterol balance. Cell Rep. 2015, 10, 326–338. [Google Scholar] [CrossRef] [Green Version]
- Koeth, R.A.; Lam-Galvez, B.R.; Kirsop, J.; Wang, Z.; Levison, B.S.; Gu, X.; Copeland, M.F.; Bartlett, D.; Cody, D.B.; Dai, H.J.; et al. l-Carnitine in omnivorous diets induces an atherogenic gut microbial pathway in humans. J. Clin. Investig. 2018, 129, 373–387. [Google Scholar] [CrossRef]
- Koeth, R.A.; Wang, Z.; Levison, B.; Buffa, J.A.; Org, E.; Sheehy, B.T.; Britt, E.B.; Fu, X.; Wu, Y.; Li, L.; et al. Intestinal microbiota metabolism of L-carnitine, a nutrient in red meat, promotes atherosclerosis. Nat. Med. 2013, 19, 576–585. [Google Scholar] [CrossRef] [Green Version]
- Collins, H.L.; Drazul-Schrader, D.; Sulpizio, A.C.; Koster, P.D.; Williamson, Y.; Adelman, S.J.; Owen, K.; Sanli, T.; Bellamine, A. L-Carnitine intake and high trimethylamine N-oxide plasma levels correlate with low aortic lesions in ApoE(-/-) transgenic mice expressing CETP. Atherosclerosis 2016, 244, 29–37. [Google Scholar] [CrossRef] [Green Version]
- Bordoni, L.; Sawicka, A.; Szarmach, A.; Winklewski, P.J.; Olek, R.A.; Gabbianelli, R. A pilot study on the effects of l-Carnitine and Trimethylamine-N-Oxide on platelet mitochondrial DNA methylation and CVD biomarkers in aged women. Int. J. Mol. Sci. 2020, 21, 1047. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vallance, H.; Koochin, A.; Branov, J.; Rosen-Heath, A.; Bosdet, T.; Wang, Z.; Hazen, S.; Horvath, G. Marked elevation in plasma trimethylamine-N-oxide (TMAO) in patients with mitochondrial disorders treated with oral l-carnitine. Mol. Genet. Metab. Rep. 2018, 15, 130–133. [Google Scholar] [CrossRef]
- Wang, Z.-Y.; Liu, Y.-Y.; Liu, G.-H.; Lu, H.-B.; Mao, C.-Y. l-Carnitine and heart disease. Life Sci. 2018, 194, 88–97. [Google Scholar] [CrossRef] [PubMed]
- Strilakou, A.A.; Lazaris, A.C.; Perelas, A.I.; Mourouzis, I.; Douzis, I.C.; Karkalousos, P.L.; Stylianaki, A.T.; Pantos, C.I.; Liapi, C.A. Heart dysfunction induced by choline-deficiency in adult rats: The protective role of L-carnitine. Eur. J. Pharmacol. 2013, 709, 20–27. [Google Scholar] [CrossRef] [PubMed]
- O’Brien, D.; Chunduri, P.; Iyer, A.; Brown, L. L-carnitine attenuates cardiac remodelling rather than vascular remodelling in deoxycorticosterone acetate-salt hypertensive rats. Basic Clin. Pharmacol. Toxicol. 2009, 106, 296–301. [Google Scholar] [CrossRef] [Green Version]
- Li, M.; Xue, L.; Sun, H.; Xu, S. Myocardial protective effects of L-Carnitine on ischemia-reperfusion injury in patients with rheumatic valvular heart disease undergoing cardiac surgery. J. Cardiothorac. Vasc. Anesthesia 2016, 30, 1485–1493. [Google Scholar] [CrossRef]
- Xue, M.; Chen, X.; Guo, Z.; Liu, X.; Bi, Y.; Yin, J.; Hu, H.; Zhu, P.; Zhuang, J.; Cates, C.; et al. L-Carnitine attenuates cardiac dysfunction by ischemic insults through Akt signaling pathway. Toxicol. Sci. 2017, 160, 341–350. [Google Scholar] [CrossRef]
- Vacante, F.; Senesi, P.; Montesano, A.; Frigerio, A.; Luzi, L.; Terruzzi, I. L-Carnitine: An antioxidant remedy for the survival of cardiomyocytes under hyperglycemic condition. J. Diabetes Res. 2018, 2018, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.; Liu, Y.; Zhang, C.; Song, Q. Protective effect of L-carnitine on myocardial injury in rats with heatstroke. Acta Cir. Bras. 2020, 35, e351206. [Google Scholar] [CrossRef] [PubMed]
- Zhao, T.; Chen, S.; Wang, B.; Cai, D. L-Carnitine reduces myocardial oxidative stress and alleviates myocardial ischemia-reperfusion injury by activating nuclear transcription-related factor 2 (Nrf2)/Heme Oxygenase-1 (HO-1) signaling pathway. Med. Sci. Monit. 2020, 26, e923251. [Google Scholar] [CrossRef] [PubMed]
- Fathizadeh, H.; Milajerdi, A.; Reiner, Ž.; Amirani, E.; Asemi, Z.; Mansournia, M.A.; Hallajzadeh, J. The effects of L-carnitine supplementation on indicators of inflammation and oxidative stress: A systematic review and meta-analysis of randomized controlled trials. J. Diabetes Metab. Disord. 2020, 19, 1879–1894. [Google Scholar] [CrossRef]
- Li, M.; Xu, S.; Geng, Y.; Sun, L.; Wang, R.; Yan, Y.; Wang, H.; Li, Y.; Yi, Q.; Zhang, Y.; et al. The protective effects of L-carnitine on myocardial ischaemia-reperfusion injury in patients with rheumatic valvular heart disease undergoing CPB surgery are associated with the suppression of NF-κB pathway and the activation of Nrf2 pathway. Clin. Exp. Pharmacol. Physiol. 2019, 46, 1001–1012. [Google Scholar] [CrossRef]
- Samir, S.M.; Abbas, A.M.; Safwat, S.M.; Elserougy, H.G. Effect of L-carnitine on diabetes-induced changes of skeletal muscles in rats. J. Basic Clin. Physiol. Pharmacol. 2017, 29, 47–59. [Google Scholar] [CrossRef]
- Mollica, G.; Senesi, P.; Codella, R.; Vacante, F.; Montesano, A.; Luzi, L.; Terruzzi, I. L-carnitine supplementation attenuates NAFLD progression and cardiac dysfunction in a mouse model fed with methionine and choline-deficient diet. Dig. Liver Dis. 2020, 52, 314–323. [Google Scholar] [CrossRef] [Green Version]
- Abolfathi, M.; Mohd-Yusof, B.-N.; Hanipah, Z.N.; Redzwan, S.M.; Yusof, L.M.; Khosroshahi, M.Z. The effects of carnitine supplementation on clinical characteristics of patients with non-alcoholic fatty liver disease: A systematic review and meta-analysis of randomized controlled trials. Complement. Ther. Med. 2020, 48, 102273. [Google Scholar] [CrossRef]
- Montesano, A.; Senesi, P.; Vacante, F.; Mollica, G.; Benedini, S.; Mariotti, M.; Luzi, L.; Terruzzi, I. L-Carnitine counteracts in vitro fructose-induced hepatic steatosis through targeting oxidative stress markers. J. Endocrinol. Investig. 2019, 43, 493–503. [Google Scholar] [CrossRef] [Green Version]
- Sayed-Ahmed, M.M.; Alrufaiq, B.I.; AlRikabi, A.; Abdullah, M.L.; Hafez, M.M.; Al-Shabanah, O.A. Carnitine supplementation attenuates sunitinib-induced inhibition of AMP-activated protein kinase downstream signals in cardiac tissues. Cardiovasc. Toxicol. 2019, 19, 344–356. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Yang, N.; Gao, J.; Li, H.; Cai, W.; Zhang, X.; Ma, Y.; Niu, X.; Yang, G.; Zhou, X.; et al. The effect of different l-Carnitine administration routes on the development of atherosclerosis in ApoE knockout mice. Mol. Nutr. Food Res. 2018, 62. [Google Scholar] [CrossRef]
- Shih, D.M.; Zhu, W.; Schugar, R.C.; Meng, Y.; Jia, X.; Miikeda, A.; Wang, Z.; Zieger, M.; Lee, R.; Graham, M.; et al. Genetic deficiency of Flavin-Containing Monooxygenase 3 ( Fmo3) protects against thrombosis but has only a minor effect on plasma lipid levels-brief report. Arter. Thromb. Vasc. Biol. 2019, 39, 1045–1054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bennett, B.J.; Vallim, T.Q.D.A.; Wang, Z.; Shih, D.M.; Meng, Y.; Gregory, J.; Allayee, H.; Lee, R.; Graham, M.; Crooke, R.; et al. Trimethylamine-N-oxide, a metabolite associated with atherosclerosis, exhibits complex genetic and dietary regulation. Cell Metab. 2013, 17, 49–60. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Annunziata, G.; Maisto, M.; Schisano, C.; Ciampaglia, R.; Narciso, V.; Tenore, G.C.; Novellino, E. Effects of Grape Pomace Polyphenolic Extract (Taurisolo®) in Reducing TMAO Serum Levels in Humans: Preliminary Results from a Randomized, Placebo-Controlled, Cross-Over Study. Nutrients. 2019, 11, 139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gautam, A.; Paudel, Y.N.; Abidin, S.; Bhandari, U. Guggulsterone, a farnesoid X receptor antagonist lowers plasma trimethylamine-N-oxide levels: An evidence from in vitro and in vivo studies. Hum. Exp. Toxicol. 2018, 38, 356–370. [Google Scholar] [CrossRef] [PubMed]
- Baksi, A.; Kraydashenko, O.; Zalevkaya, A.; Stets, R.; Elliott, P.; Haddad, J.; Hoffmann, E.; Vlasuk, G.P.; Jacobson, E.W. A phase II, randomized, placebo-controlled, double-blind, multi-dose study of SRT2104, a SIRT1 activator, in subjects with type 2 diabetes. Br. J. Clin. Pharmacol. 2014, 78, 69–77. [Google Scholar] [CrossRef]
- Noh, R.M.; Venkatasubramanian, S.; Daga, S.; Langrish, J.; Mills, N.L.; Lang, N.N.; Hoffmann, E.; Waterhouse, B.; Newby, D.E.; Frier, B.M. Cardiometabolic effects of a novel SIRT1 activator, SRT2104, in people with type 2 diabetes mellitus. Open Hear. 2017, 4. [Google Scholar] [CrossRef]
- Venkatasubramanian, S.; Noh, R.M.; Daga, S.; Langrish, J.P.; Mills, N.; Waterhouse, B.R.; Hoffmann, E.; Jacobson, E.W.; Lang, N.; Frier, B.M.; et al. Effects of the small molecule SIRT1 activator, SRT2104 on arterial stiffness in otherwise healthy cigarette smokers and subjects with type 2 diabetes mellitus. Open Hear. 2016, 3, e000402. [Google Scholar] [CrossRef] [Green Version]
- Mautone, N.; Zwergel, C.; Mai, A.; Rotili, D. Sirtuin modulators: Where are we now? A review of patents from 2015 to 2019. Expert Opin. Ther. Patents 2020, 30, 389–407. [Google Scholar] [CrossRef]
- Chen, J.; Cao, J.; Fang, L.; Liu, B.; Zhou, Q.; Sun, Y.; Wang, Y.; Li, Y.; Meng, S.; Chen, J.; et al. Berberine derivatives reduce atherosclerotic plaque size and vulnerability in apoE(-/-) mice. J. Transl. Med. 2014, 12, 326. [Google Scholar] [CrossRef] [Green Version]
- Wang, J.-T.; Peng, J.-G.; Zhang, J.-Q.; Wang, Z.-X.; Zhang, Y.; Zhou, X.-R.; Miao, J.; Tang, L. Novel berberine-based derivatives with potent hypoglycemic activity. Bioorganic Med. Chem. Lett. 2019, 29, 126709. [Google Scholar] [CrossRef]
- Li, A.; Zhang, S.; Li, J.; Liu, K.; Huang, F.; Liu, B.; Li, A.; Zhang, S.; Li, J.; Liu, K.; et al. Metformin and resveratrol inhibit Drp1-mediated mitochondrial fission and prevent ER stress-associated NLRP3 inflammasome activation in the adipose tissue of diabetic mice. Mol. Cell. Endocrinol. 2016, 434, 36–47. [Google Scholar] [CrossRef]
- Frendo-Cumbo, S.; MacPherson, R.; Wright, D.C. Beneficial effects of combined resveratrol and metformin therapy in treating diet-induced insulin resistance. Physiol. Rep. 2016, 4, e12877. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhu, X.; Yang, J.; Zhu, W.; Yin, X.; Yang, B.; Wei, Y.; Guo, X. Combination of berberine with resveratrol improves the lipid-lowering efficacy. Int. J. Mol. Sci. 2018, 19, 3903. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhao, L.; Zhang, Q.; Ma, W.; Tian, F.; Shen, H.; Zhou, M. A combination of quercetin and resveratrol reduces obesity in high-fat diet-fed rats by modulation of gut microbiota. Food Funct. 2017, 8, 4644–4656. [Google Scholar] [CrossRef] [PubMed]
- Mattson, M.P.; Longo, V.D.; Harvie, M. Impact of intermittent fasting on health and disease processes. Ageing Res. Rev. 2017, 39, 46–58. [Google Scholar] [CrossRef]
- Longo, V.D.; Panda, S. Fasting, circadian rhythms, and time-restricted feeding in healthy lifespan. Cell Metab. 2016, 23, 1048–1059. [Google Scholar] [CrossRef] [Green Version]
- McGee, S.L.; Hargreaves, M. Exercise adaptations: Molecular mechanisms and potential targets for therapeutic benefit. Nat. Rev. Endocrinol. 2020, 16, 495–505. [Google Scholar] [CrossRef]
- Montesano, A.; Senesi, P.; Luzi, L.; Benedini, S.; Terruzzi, I. Potential therapeutic role of L-carnitine in skeletal muscle oxidative stress and atrophy conditions. Oxidative Med. Cell. Longev. 2015, 2015, 1–13. [Google Scholar] [CrossRef] [PubMed]

| Type of Studies | Tissue Molecular Mechanisms | Effects | References |
|---|---|---|---|
| In vitro and in vivo (obese mice) | Skeletal muscle: ↑ AMPK/PGC-1α pathway activation | ↓ lipid deposition in skeletal muscle ↑ glucose metabolism ↑ mitochondrial biogenesis and function | Yao et al. [103] |
| In vitro study | Hepatocytes (HepG2): ↑ AMPKα1 activation in | ↑ glucose and lipid metabolism | Ren et al. [104] |
| In vitro and in vivo (diabetic rats) | Liver: ↑ PKA activation | ↓ inflammatory response | Wu et al. [105] |
| In vivo (obese rats) | Adipose tissue: ↑ AMPK activation | ↓ body weight ↑ glucose metabolism ↓ fibrosis response in adipose tissue | Wang et al. [106] |
| In vitro and in vivo (obese rats) | Adipose tissue: ↑ AMPK/SIRT1/PGC-1α activation | ↑ insulin sensitizing ↓ inflammation state ↓ macrophage infiltration | Shan et al. [107] |
| In vivo (obese mice) and clinical study (overweight NAFLD patients) | Brown adipose tissue: ↑ AMPK/PRDM16 signaling cascade | ↑ activation of brown adipose tissue | Wu et al. [108] |
| In vitro | Cardiomyocytes grown in high glucose: ↑ AMPK/ activation | ↑ mitochondrial biogenesis | Hang et al. [109] |
| In vivo (diabetic rats with cardiac ischemia) | Non-ischemic areas of the diabetic heart: ↑ AMPK activity | ↓ damages induced by ischemia–reperfusion injury | Chang et al. [110] |
| In vitro | Cultured endothelial cells and blood vessels isolated from rat aorta: ↑ AMPK/eNOS signaling | ↑ improved endothelial dysfunction ↑ vasodilatation | Wang et al. [111] |
| In vivo (obese rats) | Liver: ↓ Toll-like receptor 4 (TLR4)/tumor necrosis factor (TNF)-α pathway | ↑ improved insulin resistance ↓ hepatic steatosis and LPS release | Liu et al. [112] |
| In vivo (Sprague–Dawley rats and hamsters, obese mice) | ↑ Butyrate production by gut microbiota | ↓ blood lipid and glucose levels | Wang et al. [113] |
| In vitro and in vivo (mice) | Gut microbiota: ↓ Clostridium species | activation of intestinal FXR | Tian et al. [114] |
| In vivo (obese male apoE−/− mice) | Modification of gut composition | ↓ atherosclerosis development, inflammatory cytokine expression, hepatic FMO3 expression and TMAO | Shi et al. [115] |
| Type of Studies | Tissue Molecular Mechanisms | Effects | References |
|---|---|---|---|
| In vivo (obese rats) | aortas: ↓ NOS signaling pathway | ↓ endothelial dysfunction and vascular insulin resistance | Akar et al. [126] |
| In vivo (hypertensive rats) | rostral ventrolateral medulla (RVLM): ↑ AMPK activation | ↓ blood pressure and ROS generation ↑ ERK1/2–RSK–nNOS pathway | Cheng et al. [127] |
| In vivo (hypertensive rats) | endothelium: ↑ superoxide dismutase activity | ↓ oxidative stress induced by altered nitrite/nitrate levels ↓ development of hypertension | Bhatt et al. [128] |
| In vivo (obese rats) | liver: ↑ activation of SIRT1 signaling ↑ autophagy | ↓ endoplasmic reticulum stress ↓ hepatic lipid accumulation | Ding et al. [129] |
| In vitro and in vivo (obese rats) | Liver and hepatocytes treated with high concentration of glucose and insulin: ↑ AMPK activation | ↓ triacylglycerol (TG) accumulation ↑ improved insulin resistance | Shang et al. [130] |
| In vitro and in vivo (obese rats) | Liver: ↑ PKA/AMPK/PPARα signaling pathway activation | ↓ redox homeostasis and lipid accumulation | Huang et al. [131] |
| In vitro and in vivo (NAFLD mice model) | Liver: ↑ AMPK/SIRT1/FAS/ SREBP1c signaling pathway activation | ↓ triglyceride accumulation ↑ improved insulin resistance | Teng et al. [132] |
| In vitro | 3T3 L1 adipocytes: ↑ SIRT1–AMPK signalling activation ↑ FOXO nuclear translocation | ↑ glucose metabolism ↑ improved insulin resistance | Chen et al. [133] |
| In vitro | Skeletal muscle cells: ↑ AMPK activation | ↑ GLUT4 translocation ↑ improved insulin resistance | Vlavcheski et al. [134] |
| In vitro and in vivo (obese mice) | Liver and hepatocytes: ↑ PI3K–Akt signalling activation | ↑ improved insulin resistance | Shu et al. [135] |
| In vivo (obese mice) and human study (obese volunteers aged 30–55 years) | Adipose tissue: ↑ SIRT signalling activation | ↑ improved glycemic and lipid profiles ↑ expression of genes (UCP1, PRDM16, PGC1α) involved in adipose tissue thermogenesis | Andrade et al. [136] |
| In vivo (obese female mice) | Adipose tissue: ↑AMPK activation | ↑ brown-like adipocyte formation in inguinal white adipose tissue | Wang et al. [137] |
| In vivo (obese mice) | Adipose tissue and gut: ↑ gut microbiota–bile acid–TGR5/UCP1 pathway | ↑ brown adipose tissue activation and white adipose tissue browning | Hui et al. [138] |
| In vivo (obese mice) | Adipose tissue and gut: ↑ SIRT1 signalling activation | ↓ fat accumulation ↓ gut microbiota dysbiosis ↑ white adipose tissue browning | Liao et al. [139] |
| In vivo (obese mice) | Adipose tissue: ↑ antioxidative mitochondrial pathway | ↓ body weight gain ↓ oxidative and inflammatory condition ↓ gut microbiota alterations | Campbell et al. [140] |
| In vivo (atherosclerotic mice model) | ↓ enterohepatic farnesoid X receptor-fibroblast growth factor 15 axis | ↑ gut microbiota remodeling ↑ hepatic bile acid neosynthesis ↓ TMAO production | Chen et al. [141] |
| Type of Studies | Tissue Molecular Mechanisms | Effects | References |
|---|---|---|---|
| In vivo (rats) | Heart: ↓ NADPH oxidase (NOX)-dependent superoxide anion production | ↓ blood pressure ↑ activities of oxidant detoxifying enzymes | Calabrò et al. [152] |
| In vitro | vascular smooth muscle cells: ↑ AMPK activation | ↓ myosin light chain kinase (MLCK) expression ↓ phosphorylated myosin light chain | Kim et al. [153] |
| In vitro and in vivo (hypertensive rats) | Heart and hypertrophic cardiomyocytes: ↑ PPAR-γ expression ↓ AP-1 signaling pathway | ↓ blood pressure ↓ reduced the ratio of left ventricular to body weight | Yan et al. [154] |
| In vivo (hypercholesterolemic mice) | Blood sample | ↓ total cholesterol and very low-density lipoprotein ↓ maladaptive myocardial remodeling | Ulasova et al. [155] |
| In vitro | Cardiomyocytes ↑ SIRT1–AMPK signaling pathway activation after hypoxia damages | ↓ apoptosis | Guo et al. [156] |
| In vivo (diabetic rats) | Heart: ↑ activity level of cardiac anti-oxidative enzymes | ↓ cardiac injury ↑ hemodynamic parameters ↑ metabolic profile | Roslan et al. [157] |
| In vivo (obese diabetic mice) | Liver: ↓ p65/NF-κB and ERK1-2/MAPK signaling pathways | ↓ body weight gain, oxidative state, and liver injury ↑ metabolic profile | Zhang et al. [158] |
| In vivo (obese diabetic mice) | Liver: ↑ activity level of hepatic anti-oxidative enzymes | ↑ metabolic profile and adiponectin serum level ↓ oxidative state and dyslipidaemia | Jeong et al. [159] |
| In vitro and in vivo (obese rats) | Liver and hepatocytes: ↑ IRE1a/XBP1s pathway signaling activation ↓ lipophagy | ↓ hepatic steatosis | Zhu et al. [160] |
| In vivo (obese rats) | Liver: ↑AMPK activation ↓ TGF-β signalling | ↓ lipid accumulation ↓ inflammation state ↓ oxidative stress | Qin et al. [161] |
| In vitro | Rat hepatoma cells (H4IIE): ↑ AMPK activation and AdipoR1 expression ↓ SREBP-1 and FAS expression | ↓ lipid accumulation | Zhou et al. [162] |
| In vitro | Skeletal muscle cells, murine and human hepatocytes: ↑ AMPK activation ↑ GLUT4 translocation | ↑ glucose metabolism | Eid et al. [163] |
| In vitro | Skeletal muscle cells: ↑ AMPK activation | ↓ insulin-mediated glucose disposal in normal condition ↑ insulin resistance correlated to inflammatory condition | Liu et al. [164] |
| In vivo (obese diabetic rats) | Liver: ↑ SIRT1 expression ↑ AKT activation | ↑ glucose and lipid metabolism ↓ hepatic histomorphological injury | Peng et al. [165] |
| In vitro | Endothelial cells: ↑ IRS1/PI3K signaling pathway activation ↑ Akt/eNOS signaling pathway activation | ↓ inflammation state ↓ oxidative stress | Guo et al. [166] |
| In vitro | Hepatocytes: ↓ SREBP-1c and fatty acid synthase FAS | ↓ hepatic lipid accumulation | Li et al. [167] |
| In vivo (obese mice) | Adipose tissue: ↓ inflammatory mediators | ↓ adipocyte size and number in subcutaneous and visceral white adipose tissue | Forney et al. [168] |
| In vitro and in vivo (zebrafish and mouse) | Adipocytes and macrophages: ↓ adipogenic factors (C/EBPs and PPARγ) ↓ MAPK signaling pathway ↓ inflammatory cytokines | ↓ weight gain ↓ lipid accumulation ↓ inflammatory state | Seo et al. [169] |
| In vivo (obese mice) | Adipose tissue: ↓ NFκB activity ↑ mitochondrial function | ↓ inflammatory state in adipose tissue | Kobori et al. [170] |
| In vivo (obese mice) | Gut-liver: ↓ (TLR-4)-NF-κB signaling pathway | ↓ intrahepatic lipid accumulation ↓ insulin resistance ↓ gut dysbiosis | Porras et al. [171] |
| In vivo (obese mice) | aortic sinus and gut microbiota | ↓ atherosclerotic lesions and gut dysbiosis | Nie et al. [172] |
| In vivo (obese mice) | aortic sinus | ↓ atherosclerotic lesions ↓ lipid accumulation ↑ microbiome diversity | Wu et al. [173] |
| In vivo (obese diabetic rats) | carotid artery: ↑ AMPK/SIRT1 activation ↓ NF-kB signaling pathway | ↑ lipid profile ↓ atherosclerotic lesions ↓ oxidative stress | Zhang et al. [174] |
| Type of Studies | Tissue Molecular Mechanisms | Effects | References |
|---|---|---|---|
| In vivo (rats fed with choline deficient diet) | Heart | ↑ cardiac function ↓ cardiac inflammation | Strilakou et al. [190] |
| In vivo (hypertensive rats) | Heart | ↑ cardiac function ↓ blood pressure ↓ cardiac inflammation and fibrotic process | O’Brien et al. [191] |
| Human study (patients undergoing valve replacement) | Heart: ↑ Bcl-2 anti-apoptotic factor ↓ Bax pro-apoptotic factor | ↓ cardiac cells apoptosis | Li et al. [192] |
| In vivo (mice with I/R injury) | Heart: ↑ PI3K/Akt activation ↑ Bcl-2 anti-apoptotic factor ↓ Bax pro-apoptotic factor | ↑ myocardial contractile function ↓ myocardial apoptosis | Xue et al. [193] |
| In vitro | Cardiac cells (H9c2) grown in hyperglycemic condition: ↑ AMPK and STAT3 activation ↑ anti-oxidative factors | ↓ oxidative stress | Vacante et al. [194] |
| In vivo (Sprague–Dawley rats with heatstroke-induced cardiac injury) | Heart: ↑ anti-oxidative factors | ↓ inflammatory response ↓ oxidative stress ↓ cardiomyocytes apoptosis | Wang et al. [195] |
| In vitro and in vivo (rats with I/R injury) | Heart and cardiomyocytes: ↓ nuclear transcription-related factor 2/heme oxygenase-1 (Nrf2/HO-1) | ↓ oxidative stress ↓ cardiomyocytes apoptosis | Zhao et al. [196] |
| Human study (meta-analysis) | ↓ serum inflammatory mediators ↑ superoxide dismutase level | ↓ inflammatory cytokines ↑ antioxidant mitochondrial enzymes | Fathizadeh et al. [197] |
| Human study | Heart: ↓ NF-κB signaling pathway ↑ Nrf2 levels | ↓ inflammatory cytokines ↑ antioxidant mitochondrial enzymes | Li et al. [198] |
| In vivo (diabetic rats) | Skeletal muscle: ↑ anti-oxidative factors | ↑ insulin sensitivity index ↑ metabolic profile ↑ contractile properties | Samir et al. [199] |
| In vivo (NAFLD model mice) | Heart and liver: ↓ hepatic NF-kB signaling ↑ hepatic PPARƔ ↓ myocardial ERK/STAT3 pathway | ↓ hepatic steatosis ↓ hepatic fibrosis ↓ hepatic and myocardial oxidative stress | Mollica et al. [200] |
| Human study (meta-analysis in patients with NAFLD) | Liver | ↑ hepatic function ↓ insulin resistance condition | Abolfathi et al. [201] |
| In vitro | Hepatic cells treated with fructose: ↑ AMPK activation | ↓ lipid accumulation ↓ oxidative stress ↑ mitochondrial function | Montesano et al. [202] |
| In vivo (rats treated with sunitinib) | Heart: ↑ AMPK activation | ↓ induced-sunitinib cardiotoxicity ↑ mitochondrial transport of LCFA | Sayed-Ahmed et al. [203] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Senesi, P.; Ferrulli, A.; Luzi, L.; Terruzzi, I. Diabetes Mellitus and Cardiovascular Diseases: Nutraceutical Interventions Related to Caloric Restriction. Int. J. Mol. Sci. 2021, 22, 7772. https://doi.org/10.3390/ijms22157772
Senesi P, Ferrulli A, Luzi L, Terruzzi I. Diabetes Mellitus and Cardiovascular Diseases: Nutraceutical Interventions Related to Caloric Restriction. International Journal of Molecular Sciences. 2021; 22(15):7772. https://doi.org/10.3390/ijms22157772
Chicago/Turabian StyleSenesi, Pamela, Anna Ferrulli, Livio Luzi, and Ileana Terruzzi. 2021. "Diabetes Mellitus and Cardiovascular Diseases: Nutraceutical Interventions Related to Caloric Restriction" International Journal of Molecular Sciences 22, no. 15: 7772. https://doi.org/10.3390/ijms22157772
APA StyleSenesi, P., Ferrulli, A., Luzi, L., & Terruzzi, I. (2021). Diabetes Mellitus and Cardiovascular Diseases: Nutraceutical Interventions Related to Caloric Restriction. International Journal of Molecular Sciences, 22(15), 7772. https://doi.org/10.3390/ijms22157772








