Suppression of Pain in the Late Phase of Chronic Trigeminal Neuropathic Pain Failed to Rescue the Decision-Making Deficits in Rats
Abstract
:1. Introduction
2. Results
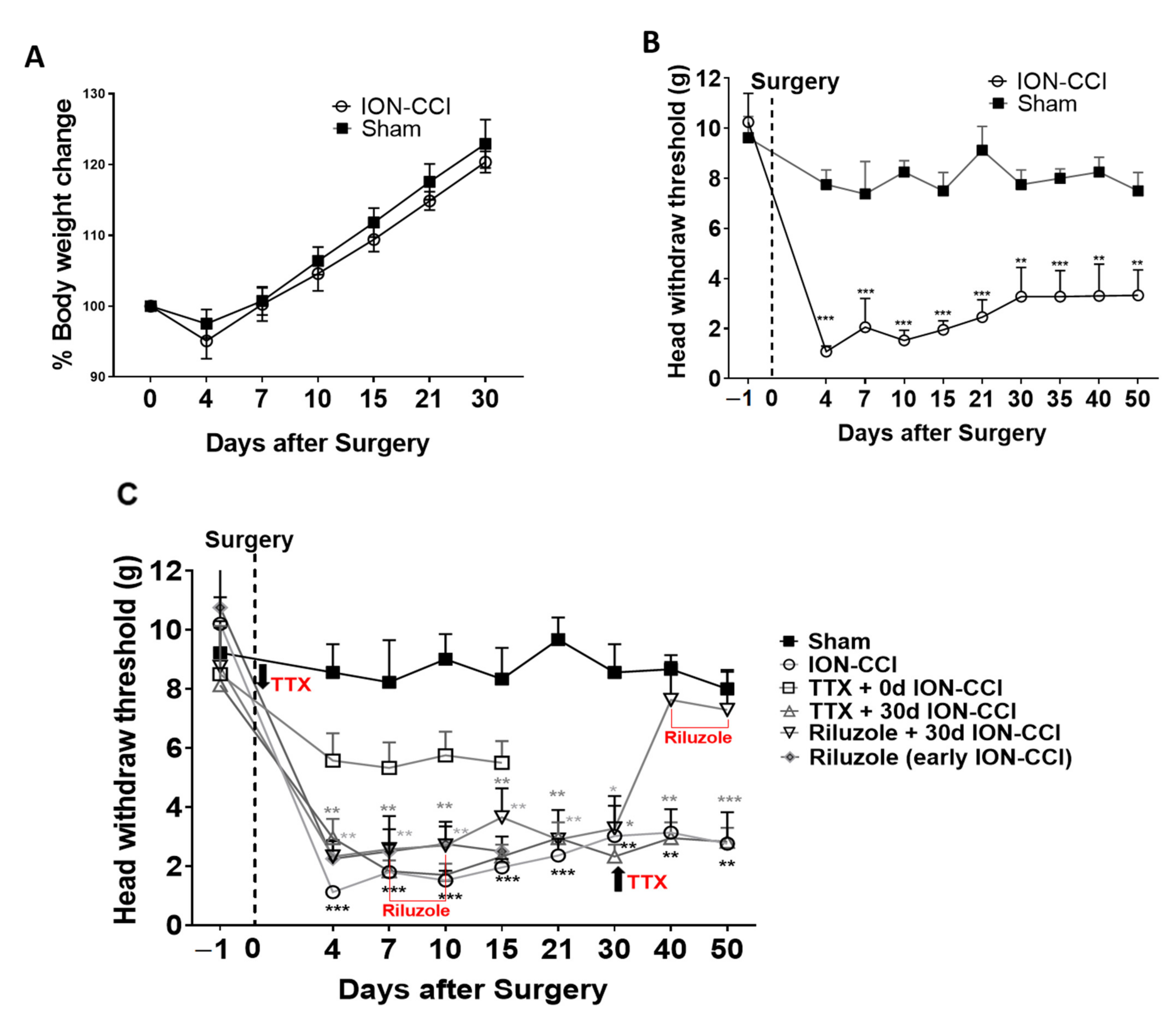
2.1. Mechanical Nociceptive Behavior
2.2. Effects of Drug Treatment on Pain Perception
2.3. Decision-Making Deficit in ION-CCI Rats
2.4. Early TTX Treatment Prevented Decision-Making Impairment in ION-CCI Rats
2.5. Riluzole Treatment in Early and Late Phase of TNP
2.6. Riluzole Treatment Did Not Rescue Decision-Making Impairments
2.7. Riluzole Suppressed the Neural Activity in ION-CCI Rats
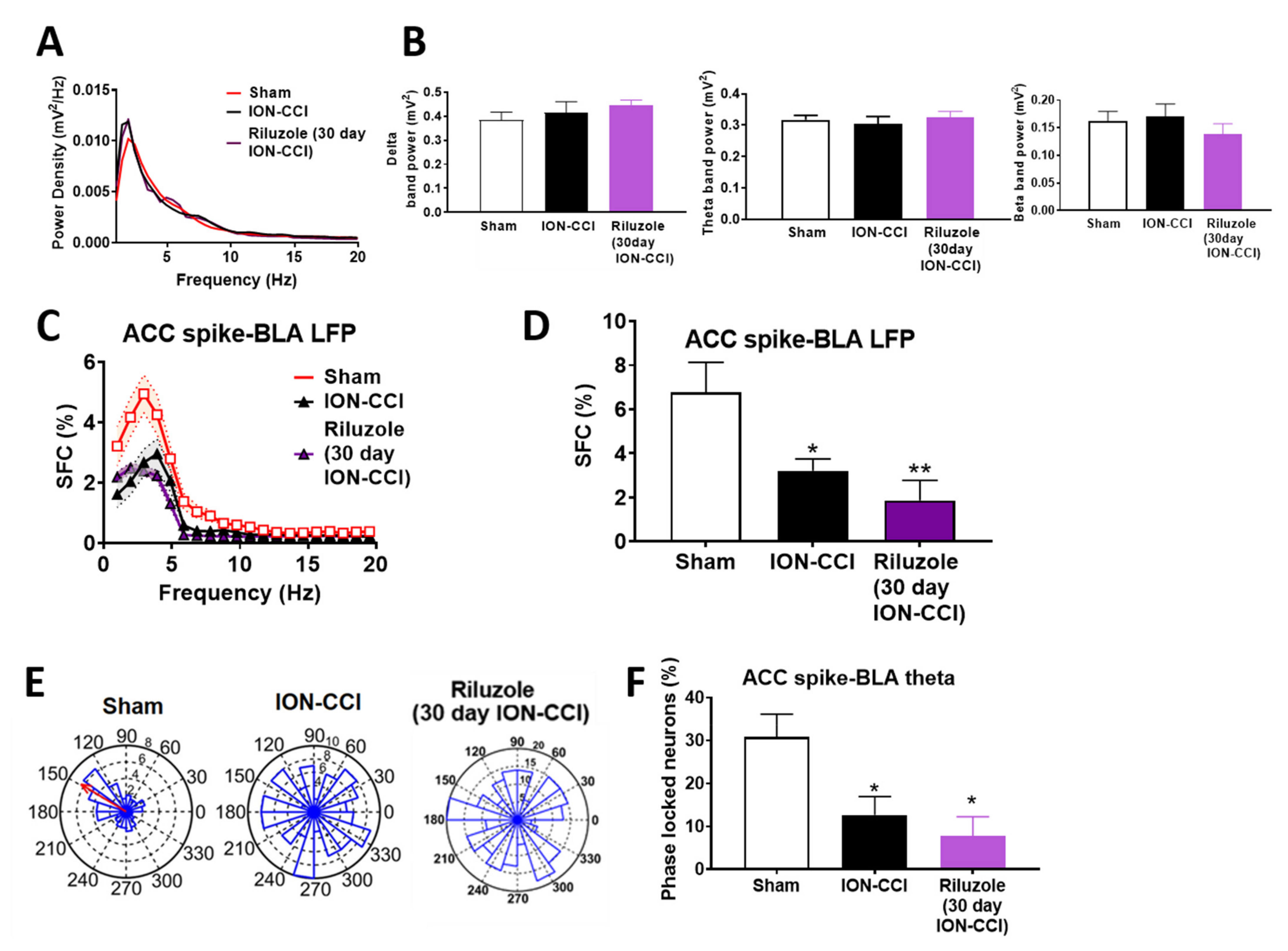
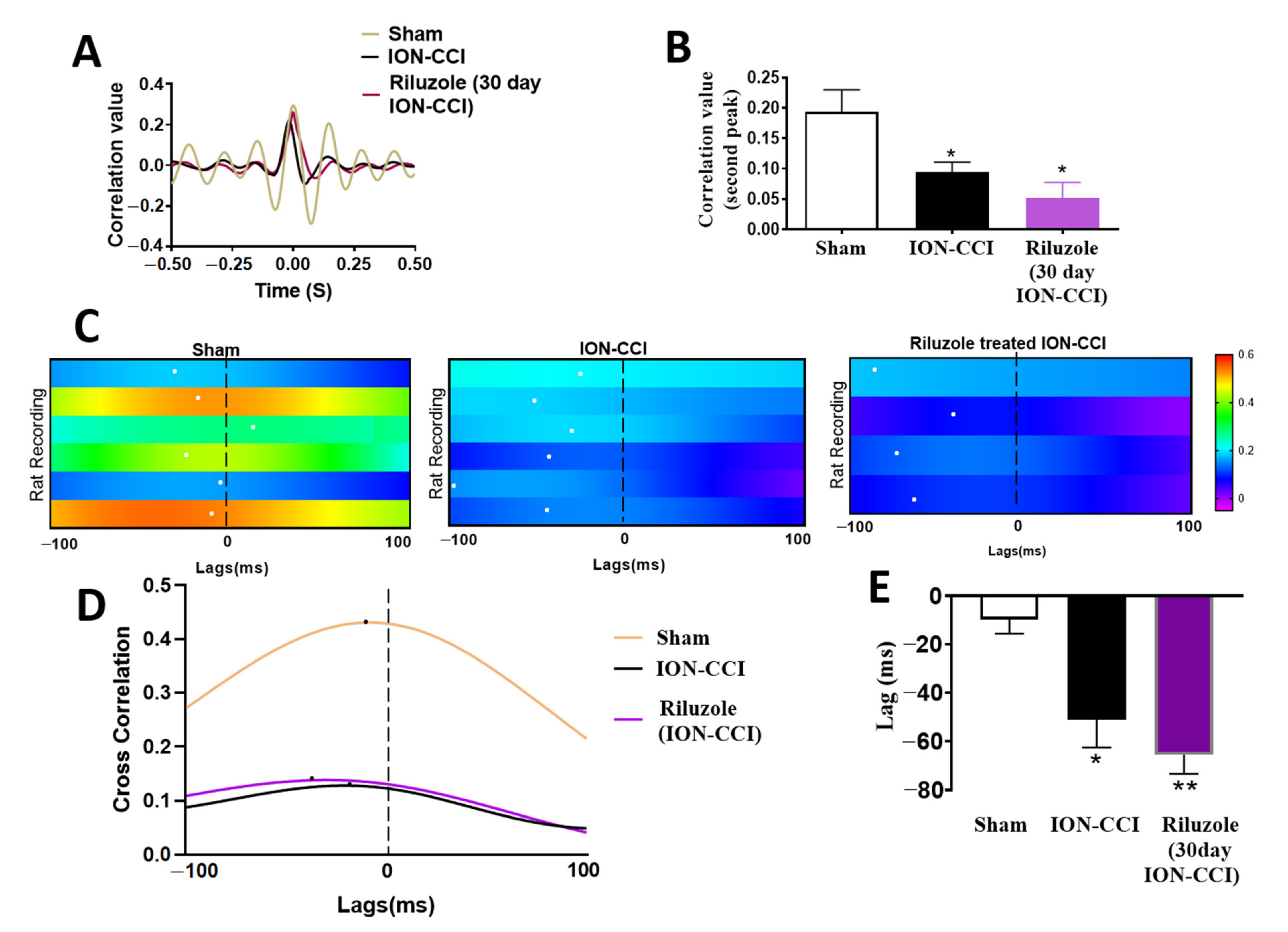
2.8. Impaired ACC-BLA Spike-Field Phase Synchrony during the Late Stages of Chronic Neuropathic Pain
2.9. Riluzole Treatment Did Not Restore the ACC-BLA Spike-Field Phase Synchrony during the Late Stages of Chronic Neuropathic Pain
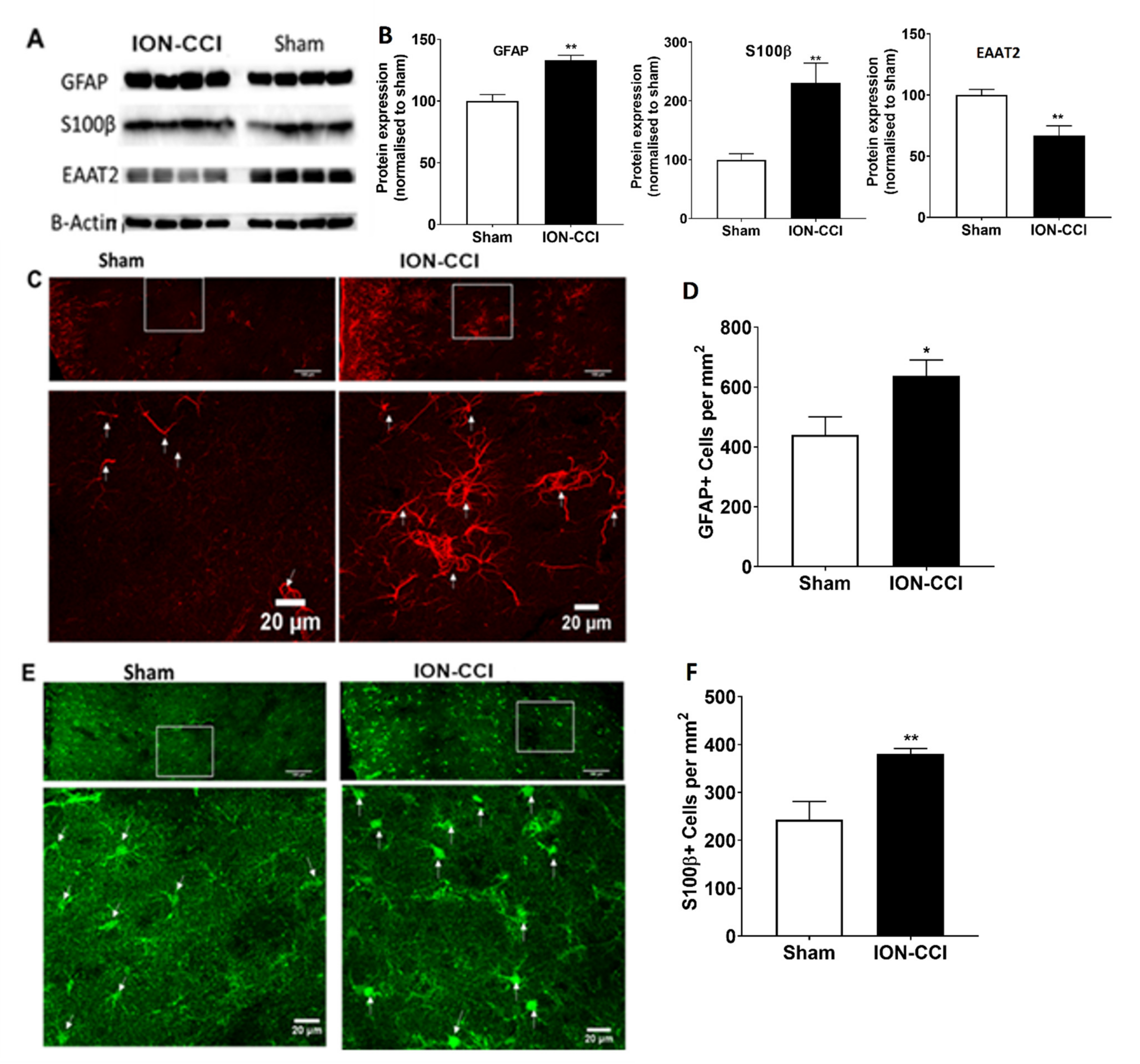
2.10. Dysfunction of Astrocytic Glutamate Reuptake and Astrocyte Neuron Metabolic Coupling in Chronic Neuropathic Pain
3. Materials and Methods
3.1. Animals
3.2. Chronic Trigeminal Neuropathic Pain Model
3.3. Allodynia and Hypersensitivity Testing
3.4. Rat Gambling Task (RGT)
3.5. TTX-Elvax Preparation and Implantation
3.6. Riluzole Administration
3.7. Multiple-Channel Electrophysiological Data Analyses
3.7.1. Power Spectral Analysis
3.7.2. Spike Sorting
3.7.3. Spike-Field Coherence
3.7.4. Phase-Locking of Single Neuron Spikes to the Theta Oscillation
3.7.5. Synchronized Theta Oscillations between ACC and BLA by Cross-Correlation Analyses
3.8. Experimental Design and Statistical Analysis
4. Discussion
5. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Latremoliere, A.; Woolf, C.J. Central sensitization: A generator of pain hypersensitivity by central neural plasticity. J. Pain 2009, 10, 895–926. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sessle, B.J. Acute and chronic craniofacial pain: Brainstem mechanisms of nociceptive transmission and neuroplasticity, and their clinical correlates. Crit. Rev. Oral Biol. Med. 2000, 11, 57–91. [Google Scholar] [CrossRef]
- Sessle, B.J. Trigeminal Central Sensitization. Rev. Analg. 2005, 8, 85–102. [Google Scholar] [CrossRef]
- Becerra, L.; Morris, S.; Bazes, S.; Gostic, R.; Sherman, S.; Gostic, J.; Pendse, G.; Moulton, E.; Scrivani, S.; Keith, D.; et al. Trigeminal neuropathic pain alters responses in CNS circuits to mechanical (brush) and thermal (cold and heat) stimuli. J. Neurosci. 2006, 26, 10646–10657. [Google Scholar] [CrossRef] [PubMed]
- Witting, N.; Kupers, R.C.; Svensson, P.; Jensen, T.S. A PET activation study of brush-evoked allodynia in patients with nerve injury pain. Pain 2006, 120, 145–154. [Google Scholar] [CrossRef] [PubMed]
- Murugappan, S.K.; Hasan, M.; Lei, Z.; Iqbal, Z.; Ramkrishnan, A.S.; Wong, H.Y.; Li, Y. Trigeminal neuropathy causes hypomyelination in the anterior cingulate cortex, disrupts the synchrony of neural circuitry and impairs decision-making in male rats. J. Neurosci. Res. 2021. in Press. [Google Scholar]
- Sun, Q.; Tu, H.; Xing, G.G.; Han, J.S.; Wan, Y. Ectopic discharges from injured nerve fibers are highly correlated with tactile allodynia only in early, but not late, stage in rats with spinal nerve ligation. Exp. Neurol. 2005, 191, 128–136. [Google Scholar] [CrossRef]
- Porreca, F.; Ossipov, M.H.; Gebhart, G.F. Chronic pain and medullary descending facilitation. Trends Neurosci. 2002, 25, 319–325. [Google Scholar] [CrossRef]
- Willoch, F.; Gamringer, U.; Medele, R.; Steude, U.; Tölle, T.R. Analgesia by electrostimulation of the trigeminal ganglion in patients with trigeminopathic pain: A PET activation study. Pain 2003, 103, 119–130. [Google Scholar] [CrossRef]
- Zhuo, M. Cortical excitation and chronic pain. Trends Neurosci. 2008, 31, 199–207. [Google Scholar] [CrossRef]
- Iwata, K.; Miyachi, S.; Imanishi, M.; Tsuboi, Y.; Kitagawa, J.; Teramoto, K.; Hitomi, S.; Shinoda, M.; Kondo, M.; Takada, M. Ascending multisynaptic pathways from the trigeminal ganglion to the anterior cingulate cortex. Exp. Neurol. 2011, 227, 69–78. [Google Scholar] [CrossRef]
- Cao, B.; Wang, J.; Mu, L.; Poon, D.C.; Li, Y. Impairment of decision making associated with disruption of phase-locking in the anterior cingulate cortex in viscerally hypersensitive rats. Exp. Neurol. 2016, 286, 21–31. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, J.; Shahed, M.; Jelfs, B.; Chan, R.H.M.; Li, Y. Vagus nerve stimulation alters phase synchrony of the anterior cingulate cortex and facilitates decision making in rats. Sci. Rep. 2016, 6, 35135. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vogt, B.A.; Sikes, R.W.; Vogt, L.J. Anterior cingulate cortex and the medial pain system. In Neurobiology of Cingulate Cortex and Limbic Thalamus; Vogt, B.A., Gabriel, M., Eds.; Birkhäuser: Boston, MA, USA, 1993. [Google Scholar] [CrossRef]
- Cao, Z.; Wu, X.; Chen, S.; Fan, J.; Zhang, R.; Owyang, C.; Li, Y. Anterior cingulate cortex modulates visceral pain as measured by visceromotor responses in viscerally hypersensitive rats. Gastroenterology 2008, 134, 535–543. [Google Scholar] [CrossRef]
- Fan, J.; Wu, X.; Cao, Z.; Chen, S.; Owyang, C.; Li, Y. Up-regulation of anterior cingulate cortex NR2B receptors contributes to visceral pain responses in rats. Gastroenterology 2009, 136, 1732–1740. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yan, N.; Cao, B.; Xu, J.; Hao, C.; Zhang, X.; Li, Y. Glutamatergic activation of anterior cingulate cortex mediates the affective component of visceral pain memory in rats. Neurobiol. Learn. Mem. 2012, 97, 156–164. [Google Scholar] [CrossRef] [PubMed]
- Gao, J.; Wu, X.; Owyang, C.; Li, Y. Enhanced responses of the anterior cingulate cortex neurones to colonic distension in viscerally hypersensitive rats. J. Physiol. 2006, 570(Pt. 1), 169–183. [Google Scholar] [CrossRef]
- Hart, R.P.; Martelli, M.F.; Zasler, N.D. Chronic pain and neuropsychological functioning. Neuropsychol. Rev. 2000, 10, 131–149. [Google Scholar] [CrossRef]
- Villemure, C.; Bushnell, C.M. Cognitive modulation of pain: How do attention and emotion influence pain processing? Pain 2002, 95, 195–199. [Google Scholar] [CrossRef]
- Rivalan, M.; Ahmed, S.H.; Dellu-Hagedorn, F. Risk-prone individuals prefer the wrong options on a rat version of the Iowa Gambling Task. Biol Psychiatry 2009, 66, 743–749. [Google Scholar] [CrossRef]
- Colgin, L.L. Mechanisms and functions of theta rhythms. Annu. Rev. Neurosci. 2013, 36, 295–312. [Google Scholar] [CrossRef] [Green Version]
- Rutishauser, U.; Ross, I.B.; Mamelak, A.N.; Schuman, E.M. Human memory strength is predicted by theta-frequency phase-locking of single neurons. Nature 2010, 464, 903–907. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mu, L.; Wang, J.; Cao, B.; Jelfs, B.; Chan, R.H.; Xu, X.; Hasan, M.; Zhang, X.; Li, Y. Impairment of cognitive function by chemotherapy: Association with the disruption of phase-locking and synchronization in anterior cingulate cortex. Mol. Brain 2015, 8, 32. [Google Scholar] [CrossRef] [Green Version]
- Vos, B.P.; Strassman, A.M.; Maciewicz, R.J. Behavioral evidence of trigeminal neuropathic pain following chronic constriction injury to the rat’s infraorbital nerve. J. Neurosci. 1994, 14, 2708–2723. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Urban, R.; Scherrer, G.; Goulding, E.H.; Tecott, L.H.; Basbaum, A.I. Behavioral indices of ongoing pain are largely unchanged in male mice with tissue or nerve injury-induced mechanical hypersensitivity. Pain 2011, 152, 990–1000. [Google Scholar] [CrossRef] [Green Version]
- Seidenbecher, T.; Laxmi, T.R.; Stork, O.; Pape, H.C. Amygdalar and hippocampal theta rhythm synchronization during fear memory retrieval. Science 2003, 301, 846–850. [Google Scholar] [CrossRef] [Green Version]
- Burda, J.E.; Sofroniew, M.V. Reactive gliosis and the multicellular response to CNS damage and disease. Neuron 2014, 81, 229–248. [Google Scholar] [CrossRef] [Green Version]
- Eng, L.F.; Ghirnikar, R.S.; Lee, Y.L. Glial fibrillary acidic protein: GFAP-thirty-one years (1969–2000). Neurochem. Res. 2000, 25, 1439–1451. [Google Scholar] [CrossRef]
- Pellerin, L.; Magistretti, P.J. Glutamate uptake into astrocytes stimulates aerobic glycolysis: A mechanism coupling neuronal activity to glucose utilization. Proc. Natl. Acad. Sci. USA 1994, 91, 10625–10629. [Google Scholar] [CrossRef] [Green Version]
- Uehara, T.; Sumiyoshi, T.; Itoh, H.; Kurachi, M. Role of glutamate transporters in the modulation of stress-induced lactate metabolism in the rat brain. Psychopharmacology 2007, 195, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Kernisant, M.; Gear, R.W.; Jasmin, L.; Vit, J.P.; Ohara, P.T. Chronic constriction injury of the infraorbital nerve in the rat using modified syringe needle. J. Neurosci. Methods 2008, 172, 43–47. [Google Scholar] [CrossRef] [Green Version]
- Kawasaki, Y.; Xu, Z.Z.; Wang, X.; Park, J.Y.; Zhuang, Z.Y.; Tan, P.H.; Gao, Y.J.; Roy, K.; Corfas, G.; Lo, E.H.; et al. Distinct roles of matrix metalloproteases in the early- and late-phase development of neuropathic pain. Nat. Med. 2008, 14, 331–336. [Google Scholar] [CrossRef] [PubMed]
- Leiser, S.; Moxon, K. Responses of Trigeminal Ganglion Neurons during Natural Whisking Behaviors in the Awake Rat. Neuron 2007, 53, 117–133. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Cao, B.; Yang, X.; Wu, J.; Chan, L.L.; Li, Y. Chronic ciguatoxin poisoning causes emotional and cognitive dysfunctions in rats. Toxicol. Res. 2017, 6, 179–187. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, J.; Tu, J.; Cao, B.; Mu, L.; Yang, X.; Cong, M.; Ramkrishnan, A.S.; Chan, R.H.M.; Wang, L.; Li, Y. Astrocytic l-Lactate Signaling Facilitates Amygdala-Anterior Cingulate Cortex Synchrony and Decision Making in Rats. Cell Rep. 2017, 21, 2407–2418. [Google Scholar] [CrossRef]
- Silberstein, G.B.; Daniel, C.W. Elvax 40P implants: Sustained, local release of bioactive molecules influencing mammary ductal development. Dev. Biol. 1982, 93, 272–278. [Google Scholar] [CrossRef]
- Echegoyen, J.; Neu, A.; Graber, K.D.; Soltesz, I. Homeostatic plasticity studied using in vivo hippocampal activity-blockade: Synaptic scaling, intrinsic plasticity and age-dependence. PLoS ONE 2007, 2, e700. [Google Scholar] [CrossRef] [Green Version]
- Kim, S.K.; Nabekura, J. Rapid synaptic remodeling in the adult somatosensory cortex following peripheral nerve injury and its association with neuropathic pain. J. Neurosci. 2011, 31, 5477–5482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kakizawa, S.; Miyazaki, T.; Yanagihara, D.; Iino, M.; Watanabe, M.; Kano, M. Maintenance of presynaptic function by AMPA receptor-mediated excitatory postsynaptic activity in adult brain. Proc. Natl. Acad. Sci. USA 2005, 102, 19180–19185. [Google Scholar] [CrossRef] [Green Version]
- Talbot, J.D.; Marrett, S.; Evans, A.C.; Meyer, E.; Bushnell, M.C.; Duncan, G.H. Multiple representations of pain in human cerebral cortex. Science 1991, 251, 1355. [Google Scholar] [CrossRef] [PubMed]
- Cao, B.; Wang, J.; Zhang, X.; Yang, X.; Poon, D.C.; Jelfs, B.; Chan, R.H.; Wu, J.C.; Li, Y. Impairment of decision making and disruption of synchrony between basolateral amygdala and anterior cingulate cortex in the maternally separated rat. Neurobiol. Learn. Mem. 2016, 136, 74–85. [Google Scholar] [CrossRef] [PubMed]
- Gebhart, G.F. Descending modulation of pain. Neurosci. Biobehav. Rev. 2004, 27, 729–737. [Google Scholar] [CrossRef]
- Marbach, J.J. Is phantom tooth pain a deafferentation (neuropathic) syndrome? Part I: Evidence derived from pathophysiology and treatment. Oral Surg. Oral Med. Oral Pathol. 1993, 75, 95–105. [Google Scholar] [CrossRef]
- Cui, Y.; Yang, Y.; Ni, Z.; Dong, Y.; Cai, G.; Foncelle, A.; Ma, S.; Sang, K.; Tang, S.; Li, Y.; et al. Astroglial Kir4.1 in the lateral habenula drives neuronal bursts in depression. Nature 2018, 554, 323–327. [Google Scholar] [CrossRef] [Green Version]
- Yang, Y.; Wang, H.; Hu, J.; Hu, H. Lateral habenula in the pathophysiology of depression. Curr. Opin. Neurobiol. 2018, 48, 90–96. [Google Scholar] [CrossRef] [PubMed]
- Dubin, A.E.; Patapoutian, A. Nociceptors: The sensors of the pain pathway. J. Clin. Invest. 2010, 120, 3760–3772. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Komagata, S.; Chen, S.; Suzuki, A.; Yamashita, H.; Hishida, R.; Maeda, T.; Shibata, M.; Shibuki, K. Initial phase of neuropathic pain within a few hours after nerve injury in mice. J. Neurosci. 2011, 31, 4896. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lyu, Y.S.; Park, S.K.; Chung, K.; Chung, J.M. Low dose of tetrodotoxin reduces neuropathic pain behaviors in an animal model. Brain Res. 2000, 871, 98–103. [Google Scholar] [CrossRef]
- Sung, B.; Lim, G.; Mao, J. Altered expression and uptake activity of spinal glutamate transporters after nerve injury contribute to the pathogenesis of neuropathic pain in rats. J. Neurosci. 2003, 23, 2899–2910. [Google Scholar] [CrossRef] [Green Version]
- Coderre, T.J.; Kumar, N.; Lefebvre, C.D.; Yu, J.S. A comparison of the glutamate release inhibition and anti-allodynic effects of gabapentin, lamotrigine, and riluzole in a model of neuropathic pain. J. Neurochem. 2007, 100, 1289–1299. [Google Scholar] [CrossRef]
- Volterra, A.; Meldolesi, J. Astrocytes, from brain glue to communication elements: The revolution continues. Nat. Rev. Neurosci. 2005, 6, 626–640. [Google Scholar] [CrossRef]
- Chiang, C.Y.; Wang, J.; Xie, Y.F.; Zhang, S.; Hu, J.W.; Dostrovsky, J.O.; Sessle, B.J. Astroglial glutamate-glutamine shuttle is involved in central sensitization of nociceptive neurons in rat medullary dorsal horn. J. Neurosci. 2007, 27, 9068–9076. [Google Scholar] [CrossRef]
- Haydon, P.G.; Carmignoto, G. Astrocyte control of synaptic transmission and neurovascular coupling. Physiol. Rev. 2006, 86, 1009–1031. [Google Scholar] [CrossRef] [Green Version]
- Moon, E.S.; Karadimas, S.K.; Yu, W.R.; Austin, J.W.; Fehlings, M.G. Riluzole attenuates neuropathic pain and enhances functional recovery in a rodent model of cervical spondylotic myelopathy. Neurobiol. Dis. 2014, 62, 394–406. [Google Scholar] [CrossRef]
- Wu, Y.; Satkunendrarajah, K.; Teng, Y.; Chow, D.S.; Buttigieg, J.; Fehlings, M.G. Delayed post-injury administration of riluzole is neuroprotective in a preclinical rodent model of cervical spinal cord injury. J. Neurotrauma 2013, 30, 441–452. [Google Scholar] [CrossRef] [Green Version]
- Gosselin, R.D.; O’Mahony, S.; Gibney, S.; Fitzgerald, P.; O’Malley, D.; Dinan, T.G.; Cryan, J.F. Altered spinal glial activation in genetically anxious Wistar Kyoto rats: Implications for visceral pain in irritable bowel syndrome (IBS). In Proceedings of the Society for Neuroscience, Neuroscience Meeting Planner, San Diego, CA, USA, 3–7 November 2007. [Google Scholar]
- Gold, J.I.; Shadlen, M.N. The neural basis of decision making. Annu. Rev. Neurosci. 2007, 30, 535–574. [Google Scholar] [CrossRef] [Green Version]
- Lee, D.; Kim, S.; Seo, H. Role of prefrontal cortex in reinforcement learning and decision making. In Principles of Frontal Lobe Functions, 2nd ed.; Stuss, D.T., Knight, R.T., Eds.; Oxford University Press: Oxford, UK, 2013; pp. 259–272. [Google Scholar]
- May, A. Chronic pain may change the structure of the brain. Pain 2008, 137, 7–15. [Google Scholar] [CrossRef] [PubMed]
- Moriarty, O.; McGuire, B.E.; Finn, D.P. The effect of pain on cognitive function: A review of clinical and preclinical research. Prog. Neurobiol. 2011, 93, 385–404. [Google Scholar] [CrossRef] [Green Version]
- Wu, X.; Gao, J.; Yan, J.; Fan, J.; Owyang, C.; Li, Y. Role for NMDA receptors in visceral nociceptive transmission in the anterior cingulate cortex of viscerally hypersensitive rats. Am. J. Physiol. Gastrointest. Liver Physiol. 2008, 294, G918–G927. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhuo, M. A synaptic model for pain: Long-term potentiation in the anterior cingulate cortex. Mol. Cells 2007, 23, 259–271. [Google Scholar] [PubMed]
- Buzsáki, G.; Draguhn, A. Neuronal oscillations in cortical networks. Science 2004, 304, 1926–1929. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Floresco, S.B.; Ghods-Sharifi, S. Amygdala-prefrontal cortical circuitry regulates effort-based decision making. Cereb. Cortex 2007, 17, 251–260. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Murugappan, S.K.; Xie, L.; Wong, H.Y.; Iqbal, Z.; Lei, Z.; Ramkrishnan, A.S.; Li, Y. Suppression of Pain in the Late Phase of Chronic Trigeminal Neuropathic Pain Failed to Rescue the Decision-Making Deficits in Rats. Int. J. Mol. Sci. 2021, 22, 7846. https://doi.org/10.3390/ijms22157846
Murugappan SK, Xie L, Wong HY, Iqbal Z, Lei Z, Ramkrishnan AS, Li Y. Suppression of Pain in the Late Phase of Chronic Trigeminal Neuropathic Pain Failed to Rescue the Decision-Making Deficits in Rats. International Journal of Molecular Sciences. 2021; 22(15):7846. https://doi.org/10.3390/ijms22157846
Chicago/Turabian StyleMurugappan, Suresh Kanna, Li Xie, Heung Yan Wong, Zafar Iqbal, Zhuogui Lei, Aruna Surendran Ramkrishnan, and Ying Li. 2021. "Suppression of Pain in the Late Phase of Chronic Trigeminal Neuropathic Pain Failed to Rescue the Decision-Making Deficits in Rats" International Journal of Molecular Sciences 22, no. 15: 7846. https://doi.org/10.3390/ijms22157846
APA StyleMurugappan, S. K., Xie, L., Wong, H. Y., Iqbal, Z., Lei, Z., Ramkrishnan, A. S., & Li, Y. (2021). Suppression of Pain in the Late Phase of Chronic Trigeminal Neuropathic Pain Failed to Rescue the Decision-Making Deficits in Rats. International Journal of Molecular Sciences, 22(15), 7846. https://doi.org/10.3390/ijms22157846





