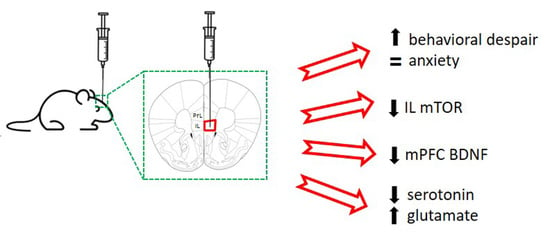
mTOR Knockdown in the Infralimbic Cortex Evokes A Depressive-like State in Mouse
Abstract
1. Introduction
2. Results
2.1. Acute Unilateral mTOR Silencing in the Infralimbic Cortex Induces a Depressive- but Not Anxiety-like Behavior
2.2. Intra-Infralimbic mTOR-siRNA Infusion Reduces mTOR mRNA Expression and Protein Levels
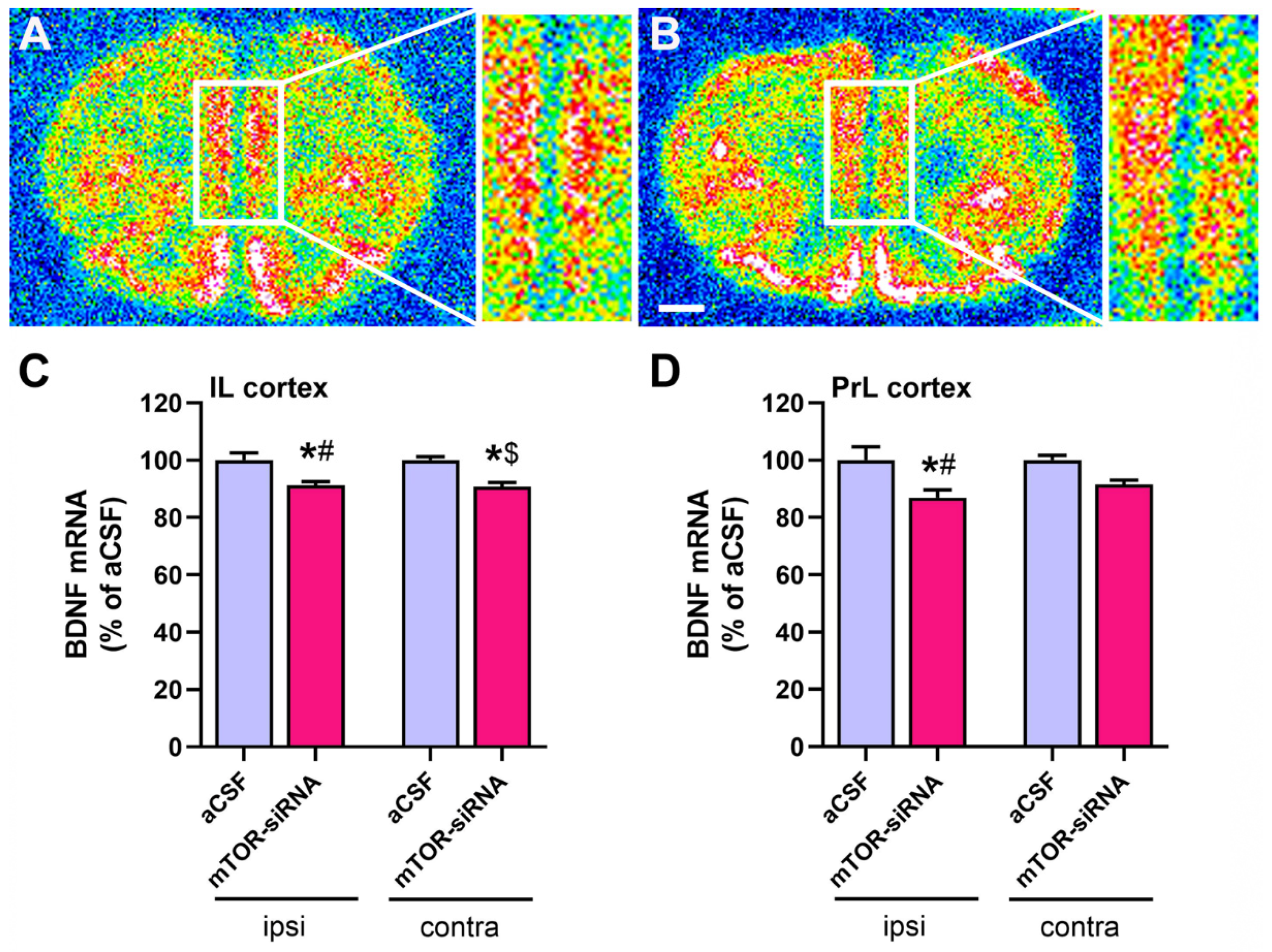
2.3. Reduced BDNF mRNA Expression in Medial Prefrontal Cortex after mTOR Knockdown in Infralimbic Cortex
2.4. Extracellular Serotonin and Glutamate Levels in Dorsal Raphe Nucleus after mTOR Knockdown in Infralimbic Cortex
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. siRNAs
4.3. Drugs and Reagents
4.4. Acute Intracerebral siRNA Infusion
4.5. Behavioral Studies
4.5.1. Forced Swimming Test (FST)
4.5.2. Tail Suspension Test (TST)
4.5.3. Open-Field Test (OF)
4.6. In situ Hybridization
4.7. mTOR Immunofluorescence
4.8. Intracerebral Microdialysis
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- WHO. 2018. Available online: https://www.who.int/news-room/fact-sheets/detail/depression (accessed on 30 July 2019).
- Duman, R.S.; Heninger, G.R.; Nestler, E.J. A molecular and cellular theory of depression. Arch. Gen. Psychiatry 1997, 54, 597–606. [Google Scholar] [CrossRef]
- Duman, R.S.; Monteggia, L.M. A neurotrophic model for stress-related mood disorders. Biol. Psychiatry 2006, 59, 1116–1127. [Google Scholar] [CrossRef]
- Duman, R.S.; Aghajanian, G.K.; Sanacora, G.; Krystal, J.H. Synaptic plasticity and depression: New insights from stress and rapid-acting antidepressants. Nat. Med. 2016, 22, 238–249. [Google Scholar] [CrossRef] [PubMed]
- Chen, B.; Dowlatshahi, D.; MacQueen, G.M.; Wang, J.F.; Young, L.T. Increased hippocampal BDNF immunoreactivity in subjects treated with antidepressant medication. Biol. Psychiatry 2001, 50, 260–265. [Google Scholar] [CrossRef]
- Karege, F.; Vaudan, G.; Schwald, M.; Perroud, N.; La Harpe, R. Neurotrophin levels in postmortem brains of suicide victims and the effects of antemortem diagnosis and psychotropic drugs. Brain Res. Mol. Brain Res. 2005, 136, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Nibuya, M.; Morinobu, S.; Duman, R.S. Regulation of BDNF and trkB mRNA in rat brain by chronic electroconvulsive seizure and antidepressant drug treatments. J. Neurosci. 1995, 15, 7539–7547. [Google Scholar] [CrossRef]
- Nibuya, M.; Nestler, E.J.; Duman, R.S. Chronic antidepressant administration increases the expression of cAMP response element binding protein (CREB) in rat hippocampus. J. Neurosci. 1996, 16, 2365–2372. [Google Scholar] [CrossRef]
- Dwivedi, Y.; Rizavi, H.S.; Pandey, G.N. Antidepressants reverse corticosterone-mediated decrease in brain-derived neurotrophic factor expression: Differential regulation of specific exons by antidepressants and corticosterone. Neuroscience 2006, 139, 1017–1029. [Google Scholar] [CrossRef]
- Calabrese, F.; Molteni, R.; Maj, P.F.; Cattaneo, A.; Gennarelli, M.; Racagni, G.; Riva, M.A. Chronic duloxetine treatment induces specific changes in the expression of BDNF transcripts and in the subcellular localization of the neurotrophin protein. Neuropsychopharmacology 2007, 32, 2351–2359. [Google Scholar] [CrossRef][Green Version]
- Balu, D.T.; Hoshaw, B.A.; Malberg, J.E.; Rosenzweig-Lipson, S.; Schechter, L.E.; Lucki, I. Differential regulation of central BDNF protein levels by antidepressant and non-antidepressant drug treatments. Brain Res. 2008, 1211, 37–43. [Google Scholar] [CrossRef]
- Zhang, Y.; Gu, F.; Chen, J.; Dong, W. Chronic antidepressant administration alleviates frontal and hippocampal BDNF deficits in CUMS rat. Brain Res. 2010, 1366, 141–148. [Google Scholar] [CrossRef]
- Bath, K.G.; Jing, D.Q.; Dincheva, I.; Neeb, C.C.; Pattwell, S.S.; Chao, M.V.; Lee, F.S.; Ninan, I. BDNF Val66Met impairs fluoxetine-induced enhancement of adult hippocampus plasticity. Neuropsychopharmacology 2012, 37, 1297–1304. [Google Scholar] [CrossRef]
- Li, N.; Lee, B.; Liu, R.J.; Banasr, M.; Dwyer, J.M.; Iwata, M.; Li, X.Y.; Aghajanian, G.; Duman, R.S. mTOR-dependent synapse formation underlies the rapid antidepressant effects of NMDA antagonists. Science 2010, 329, 959–964. [Google Scholar] [CrossRef] [PubMed]
- Liu, R.J.; Lee, F.S.; Li, X.Y.; Bambico, F.; Duman, R.S.; Aghajanian, G.K. Brain-derived neurotrophic factor Val66Met allele impairs basal and ketamine-stimulated synaptogenesis in prefrontal cortex. Biol. Psychiatry 2012, 71, 996–1005. [Google Scholar] [CrossRef]
- Lepack, A.E.; Fuchikami, M.; Dwyer, J.M.; Banasr, M.; Duman, R.S. BDNF release is required for the behavioral actions of ketamine. Int. J. Neuropsychopharmacol. 2014, 18, 33. [Google Scholar] [CrossRef] [PubMed]
- Choi, M.; Lee, S.H.; Chang, H.L.; Son, H. Hippocampal VEGF is necessary for antidepressant-like behaviors but not sufficient for antidepressant-like effects of ketamine in rats. Biochim. Biophys. Acta 2016, 1862, 1247–1254. [Google Scholar] [CrossRef]
- Lepack, A.E.; Bang, E.; Lee, B.; Dwyer, J.M.; Duman, R.S. Fast-acting antidepressants rapidly stimulate ERK signaling and BDNF release in primary neuronal cultures. Neuropharmacology 2016, 111, 242–252. [Google Scholar] [CrossRef] [PubMed]
- Li, N.; Liu, R.J.; Dwyer, J.M.; Banasr, M.; Lee, B.; Son, H.; Li, X.Y.; Aghajanian, G.; Duman, R.S. Glutamate N-methyl-D-aspartate receptor antagonists rapidly reverse behavioral and synaptic deficits caused by chronic stress exposure. Biol. Psychiatry 2011, 69, 754–761. [Google Scholar] [CrossRef] [PubMed]
- Heitman, J.; Movva, N.R.; Hall, M.N. Targets for cell cycle arrest by the immunosuppressant rapamycin in yeast. Science 1991, 253, 905–909. [Google Scholar] [CrossRef] [PubMed]
- Guertin, D.A.; Sabatini, D.M. Defining the role of mTOR in cancer. Cancer Cell 2007, 12, 9–22. [Google Scholar] [CrossRef]
- Hoeffer, C.A.; Klann, E. mTOR signaling: At the crossroads of plasticity, memory and disease. Trends Neurosci. 2010, 33, 67–75. [Google Scholar] [CrossRef] [PubMed]
- Tang, S.J.; Reis, G.; Kang, H.; Gingras, A.C.; Sonenberg, N.; Schuman, E.M. A rapamycin-sensitive signaling pathway contributes to long-term synaptic plasticity in the hippocampus. Proc. Natl. Acad. Sci. USA 2002, 99, 467–472. [Google Scholar] [CrossRef] [PubMed]
- Gong, R.; Park, C.S.; Abbassi, N.R.; Tang, S.J. Roles of glutamate receptors and the mammalian target of rapamycin (mTOR) signaling pathway in activity-dependent dendritic protein synthesis in hippocampal neurons. J. Biol. Chem. 2006, 281, 18802–18815. [Google Scholar] [CrossRef]
- Duman, R.S.; Voleti, B. Signaling pathways underlying the pathophysiology and treatment of depression: Novel mechanisms for rapid-acting agents. Trends Neurosci. 2012, 35, 47–56. [Google Scholar] [CrossRef]
- Cammalleri, M.; Lutjens, R.; Berton, F.; King, A.R.; Simpson, C.; Francesconi, W.; Sanna, P.P. Time-restricted role for dendritic activation of the mTOR-p70S6K pathway in the induction of late-phase long-term potentiation in the CA1. Proc. Natl. Acad. Sci. USA 2003, 100, 14368–14373. [Google Scholar] [CrossRef] [PubMed]
- Bargmann, C.I.; Lieberman, J.A. What the BRAIN Initiative means for psychiatry. Am. J. Psychiatry 2014, 171, 1038–1040. [Google Scholar] [CrossRef]
- Mayberg, H.S. Targeted electrode-based modulation of neural circuits for depression. J. Clin. Investig. 2009, 119, 717–725. [Google Scholar] [CrossRef]
- Savitz, J.B.; Drevets, W.C. Imaging phenotypes of major depressive disorder: Genetic correlates. Neuroscience 2009, 164, 300–330. [Google Scholar] [CrossRef]
- Mayberg, H.S.; Lozano, A.M.; Voon, V.; McNeely, H.E.; Seminowicz, D.; Hamani, C.; Schwalb, J.M.; Kennedy, S.H. Deep brain stimulation for treatment-resistant depression. Neuron 2005, 45, 651–660. [Google Scholar] [CrossRef]
- Fullana, M.N.; Ruiz-Bronchal, E.; Ferrés-Coy, A.; Juárez-Escoto, E.; Artigas, F.; Bortolozzi, A. Regionally selective knockdown of astroglial glutamate transporters in infralimbic cortex induces a depressive phenotype in mice. Glia 2019, 67, 1122–1137. [Google Scholar] [CrossRef]
- Fullana, N.; Gasull-Camós, J.; Tarrés-Gatius, M.; Castañé, A.; Bortolozzi, A.; Artigas, F. Astrocyte control of glutamatergic activity: Downstream effects on serotonergic function and emotional behavior. Neuropharmacology 2020, 166, 107914. [Google Scholar] [CrossRef]
- Hamani, C.; Diwan, M.; Macedo, C.E.; Brandão, M.L.; Shumake, J.; Gonzalez-Lima, F.; Raymond, R.; Lozano, A.M.; Fletcher, P.J.; Nobrega, J.N. Antidepressant-like effects of medial prefrontal cortex deep brain stimulation in rats. Biol. Psychiatry 2010, 67, 117–124. [Google Scholar] [CrossRef] [PubMed]
- Veerakumar, A.; Challis, C.; Gupta, P.; Da, J.; Upadhyay, A.; Beck, S.G.; Berton, O. Antidepressant-like effects of cortical deep brain stimulation coincide with pro-neuroplastic adaptations of serotonin systems. Biol. Psychiatry 2014, 76, 203–212. [Google Scholar] [CrossRef] [PubMed]
- Jiménez-Sánchez, L.; Castañé, A.; Pérez-Caballero, L.; Grifoll-Escoda, M.; López-Gil, X.; Campa, L.; Galofré, M.; Berrocoso, E.; Adell, A. Activation of AMPA Receptors Mediates the Antidepressant Action of Deep Brain Stimulation of the Infralimbic Prefrontal Cortex. Cereb. Cortex 2016, 26, 2778–2789. [Google Scholar] [CrossRef]
- Jiménez-Sánchez, L.; Linge, R.; Campa, L.; Valdizán, E.M.; Pazos, Á.; Díaz, Á.; Adell, A. Behavioral, neurochemical and molecular changes after acute deep brain stimulation of the infralimbic prefrontal cortex. Neuropharmacology 2016, 108, 91–102. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Fogaça, M.V.; Liu, R.J.; Duman, C.; Kato, T.; Li, X.Y.; Duman, R.S. Activity-dependent brain-derived neurotrophic factor signalling is required for the antidepressant actions of (2R,6R)-hydroxynorketamine. Proc. Natl. Acad. Sci. USA 2019, 116, 297–302. [Google Scholar] [CrossRef]
- Fuchikami, M.; Thomas, A.; Liu, R.; Wohleb, E.S.; Land, B.B.; DiLeone, R.J.; Aghajanian, G.K.; Duman, R.S. Optogenetic stimulation of infralimbic PFC reproduces ketamine’s rapid and sustained antidepressant actions. Proc. Natl. Acad. Sci. USA 2015, 112, 8106–8111. [Google Scholar] [CrossRef]
- Jernigan, C.S.; Goswami, D.B.; Austin, M.C.; Iyo, A.H.; Chandran, A.; Stockmeier, C.A.; Karolewicz, B. The mTOR signaling pathway in the prefrontal cortex is compromised in major depressive disorder. Prog. Neuropsychopharmacol. Biol. Psychiatry 2011, 35, 1774–1779. [Google Scholar] [CrossRef]
- Chandran, A.; Iyo, A.H.; Jernigan, C.S.; Legutko, B.; Austin, M.C.; Karolewicz, B. Reduced phosphorylation of the mTOR signaling pathway components in the amygdala of rats exposed to chronic stress. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 40, 240–245. [Google Scholar] [CrossRef]
- Ota, K.T.; Liu, R.J.; Voleti, B.; Maldonado-Aviles, J.G.; Duric, V.; Iwata, M.; Dutheil, S.; Duman, C.; Boikess, S.; Lewis, D.A.; et al. REDD1 is essential for stress-induced synaptic loss and depressive behavior. Nat. Med. 2014, 20, 531–535. [Google Scholar] [CrossRef]
- Wu, R.; Zhang, H.; Xue, W.; Zou, Z.; Lu, C.; Xia, B.; Wang, W.; Chen, G. Transgenerational impairment of hippocampal Akt-mTOR signaling and behavioral deficits in the offspring of mice that experience postpartum depression-like illness. Prog. Neuropsychopharmacol. Biol. Psychiatry 2017, 73, 11–18. [Google Scholar] [CrossRef]
- Wang, X.; Zou, Z.; Shen, Q.; Huang, Z.; Chen, J.; Tang, J.; Xue, W.; Tao, W.; Wu, H.; Wang, D.; et al. Involvement of NMDA-AKT-mTOR Signaling in Rapid Antidepressant-like Activity of Chaihu-jia-Longgu-Muli-tang on Olfactory Bulbectomized Mice. Front. Pharmacol. 2019, 9, 1537. [Google Scholar] [CrossRef]
- Olescowicz, G.; Sampaio, T.B.; de Paula Nascimento-Castro, C.; Brocardo, P.S.; Gil-Mohapel, J.; Rodrigues, A.L.S. Protective Effects of Agmatine Against Corticosterone-Induced Impairment on Hippocampal mTOR Signaling and Cell Death. Neurotox. Res. 2020, 38, 319–329. [Google Scholar] [CrossRef]
- Molendijk, M.L.; Bus, B.A.; Spinhoven, P.; Penninx, B.W.; Kenis, G.; Prickaerts, J.; Voshaar, R.C.; Elzinga, B.M. Serum levels of brain-derived neurotrophic factor in major depressive disorder: State-trait issues, clinical features and pharmacological treatment. Mol. Psychiatry 2011, 16, 1088–1095. [Google Scholar] [CrossRef]
- Kreinin, A.; Lisson, S.; Nesher, E.; Schneider, J.; Bergman, J.; Farhat, K.; Farah, J.; Lejbkowicz, F.; Yadid, G.; Raskin, L.; et al. Blood BDNF level is gender specific in severe depression. PLoS ONE 2015, 10, e0127643. [Google Scholar] [CrossRef] [PubMed]
- Challis, C.; Berton, O. Top-Down Control of Serotonin Systems by the Prefrontal Cortex: A Path toward Restored Socioemotional Function in Depression. ACS Chem. Neurosci. 2015, 6, 1040–1054. [Google Scholar] [CrossRef]
- Popoli, M.; Yan, Z.; McEwen, B.S.; Sanacora, G. The stressed synapse: The impact of stress and glucocorticoids on glutamate transmission. Nat. Rev. Neurosci. 2011, 13, 22–37. [Google Scholar] [CrossRef] [PubMed]
- Garro-Martínez, E.; Vidal, R.; Adell, A.; Díaz, Á.; Castro, E.; Amigó, J.; Gutiérrez-Lanza, R.; Florensa-Zanuy, E.; Gómez-Acero, L.; Taketo, M.M.; et al. β-Catenin Role in the Vulnerability/Resilience to Stress-Related Disorders Is Associated to Changes in the Serotonergic System. Mol. Neurobiol. 2020, 57, 1704–1715. [Google Scholar] [CrossRef] [PubMed]
- Malhi, G.S.; Mann, J.J. Depression. Lancet 2018, 392, 2299–2312. [Google Scholar] [CrossRef]
- Russo, E.; Citraro, R.; Donato, G.; Camastra, C.; Iuliano, R.; Cuzzocrea, S.; Constanti, A.; De Sarro, G. mTOR inhibition modulates epileptogenesis, seizures and depressive behavior in a genetic rat model of absence epilepsy. Neuropharmacology 2013, 69, 25–36. [Google Scholar] [CrossRef]
- Russo, E.; Leo, A.; Crupi, R.; Aiello, R.; Lippiello, P.; Spiga, R.; Chimirri, S.; Citraro, R.; Cuzzocrea, S.; Constanti, A.; et al. Everolimus improves memory and learning while worsening depressive- and anxiety-like behavior in an animal model of depression. J. Psychiatr. Res. 2016, 78, 1–10. [Google Scholar] [CrossRef]
- Cleary, C.; Linde, J.A.; Hiscock, K.M.; Hadas, I.; Belmaker, R.H.; Agam, G.; Flaisher-Grinberg, S.; Einat, H. Antidepressive-like effects of rapamycin in animal models: Implications for mTOR inhibition as a new target for treatment of affective disorders. Brain Res. Bull. 2008, 76, 469–473. [Google Scholar] [CrossRef] [PubMed]
- Russo, E.; Andreozzi, F.; Iuliano, R.; Dattilo, V.; Procopio, T.; Fiume, G.; Mimmi, S.; Perrotti, N.; Citraro, R.; Sesti, G.; et al. Early molecular and behavioral response to lipopolysaccharide in the WAG/Rij rat model of absence epilepsy and depressive-like behavior, involves interplay between AMPK, AKT/mTOR pathways and neuroinflammatory cytokine release. Brain Behav. Immun. 2014, 42, 157–168. [Google Scholar] [CrossRef]
- Cambiaghi, M.; Cursi, M.; Magri, L.; Castoldi, V.; Comi, G.; Minicucci, F.; Galli, R.; Leocani, L. Behavioural and EEG effects of chronic rapamycin treatment in a mouse model of tuberous sclerosis complex. Neuropharmacology 2013, 67, 1–7. [Google Scholar] [CrossRef]
- Masini, D.; Bonito-Oliva, A.; Bertho, M.; Fisone, G. Inhibition of mTORC1 Signaling Reverts Cognitive and Affective Deficits in a Mouse Model of Parkinson’s Disease. Front. Neurol. 2018, 9, 208. [Google Scholar] [CrossRef] [PubMed]
- Tsai, P.T.; Greene-Colozzi, E.; Goto, J.; Anderl, S.; Kwiatkowski, D.J.; Sahin, M. Prenatal rapamycin results in early and late behavioral abnormalities in wildtype C57BL/6 mice. Behav. Genet. 2013, 43, 51–59. [Google Scholar] [CrossRef]
- Yang, C.; Ren, Q.; Qu, Y.; Zhang, J.C.; Ma, M.; Dong, C.; Hashimoto, K. Mechanistic Target of Rapamycin-Independent Antidepressant Effects of (R)-Ketamine in a Social Defeat Stress Model. Biol. Psychiatry 2017, 83, 18–28. [Google Scholar] [CrossRef] [PubMed]
- Berman, R.M.; Cappiello, A.; Anand, A.; Oren, D.A.; Heninger, G.R.; Charney, D.S.; Krystal, J.H. Antidepressant effects of ketamine in depressed patients. Biol. Psychiatry 2000, 47, 351–354. [Google Scholar] [CrossRef]
- Zarate, C.A., Jr.; Singh, J.B.; Carlson, P.J.; Brutsche, N.E.; Ameli, R.; Luckenbaugh, D.A.; Charney, D.S.; Manji, H.K. A randomized trial of an N-methyl-D-aspartate antagonist in treatment-resistant major depression. Arch. Gen. Psychiatry 2006, 63, 856–864. [Google Scholar] [CrossRef]
- Murrough, J.W.; Perez, A.M.; Pillemer, S.; Stern, J.; Parides, M.K.; aan het Rot, M.; Collins, K.A.; Mathew, S.J.; Charney, D.S.; Iosifescu, D.V. Rapid and Longer-Term Antidepressant Effects of Repeated Ketamine Infusions in Treatment-Resistant Major Depression. Biol. Psychiatry 2013, 74, 250–256. [Google Scholar] [CrossRef]
- Autry, A.E.; Adachi, M.; Nosyreva, E.; Na, E.S.; Los, M.F.; Cheng, P.F.; Kavalali, E.T.; Monteggia, L.M. NMDA receptor blockade at rest triggers rapid behavioural antidepressant responses. Nature 2011, 475, 91–95. [Google Scholar] [CrossRef] [PubMed]
- Abdallah, C.G.; Averill, L.A.; Gueorguieva, R.; Goktas, S.; Purohit, P.; Ranganathan, M.; Sherif, M.; Ahn, K.H.; D’Souza, D.C.; Formica, R.; et al. Modulation of the antidepressant effects of ketamine by the mTORC1 inhibitor rapamycin. Neuropsychopharmacology 2020, 45, 990–997. [Google Scholar] [CrossRef] [PubMed]
- Jones, B.F.; Groenewegen, H.J.; Witter, M.P. Intrinsic connections of the cingulate cortex in the rat suggest the existence of multiple functionally segregated networks. Neuroscience 2005, 133, 193–207. [Google Scholar] [CrossRef] [PubMed]
- Van Aerde, K.I.; Heistek, T.S.; Mansvelder, H.D. Prelimbic and infralimbic prefrontal cortex interact during fast network oscillations. PLoS ONE 2008, 3, e2725. [Google Scholar] [CrossRef]
- Deyama, S.; Duman, R.S. Neurotrophic mechanisms underlying the rapid and sustained antidepressant actions of ketamine. Pharmacol. Biochem. Behav. 2020, 188, 172837. [Google Scholar] [CrossRef]
- Dwivedi, Y.; Rizavi, H.S.; Conley, R.R.; Roberts, R.C.; Tamminga, C.A.; Pandey, G.N. Altered gene expression of brain-derived neurotrophic factor and receptor tyrosine kinase B in postmortem brain of suicide subjects. Arch. Gen. Psychiatry 2003, 60, 804–815. [Google Scholar] [CrossRef]
- Qi, X.R.; Zhao, J.; Liu, J.; Fang, H.; Swaab, D.F.; Zhou, J.N. Abnormal retinoid and TrkB signaling in the prefrontal cortex in mood disorders. Cereb. Cortex 2015, 25, 75–83. [Google Scholar] [CrossRef]
- Krishnan, V.; Nestler, E.J. Linking molecules to mood: New insight into the biology of depression. Am. J. Psychiatry 2010, 167, 1305–1320. [Google Scholar] [CrossRef]
- Amat, J.; Baratta, M.V.; Paul, E.; Bland, S.T.; Watkins, L.R.; Maier, S.F. Medial prefrontal cortex determines how stressor controllability affects behavior and dorsal raphe nucleus. Nat. Neurosci. 2005, 8, 365–371. [Google Scholar] [CrossRef]
- Challis, C.; Boulden, J.; Veerakumar, A.; Espallergues, J.; Vassoler, F.M.; Pierce, R.C.; Beck, S.G.; Berton, O. Raphe GABAergic neurons mediate the acquisition of avoidance after social defeat. J. Neurosci. 2013, 33, 13978–13988. [Google Scholar] [CrossRef]
- Challis, C.; Beck, S.G.; Berton, O. Optogenetic modulation of descending prefrontocortical inputs to the dorsal raphe bidirectionally bias socioaffective choices after social defeat. Front. Behav. Neurosci. 2014, 17, 43. [Google Scholar] [CrossRef]
- Linge, R.; Jiménez-Sánchez, L.; Campa, L.; Pilar-Cuéllar, F.; Vidal, R.; Pazos, A.; Adell, A.; Díaz, Á. Cannabidiol induces rapid-acting antidepressant-like effects and enhances cortical 5-HT/glutamate neurotransmission: Role of 5-HT1A receptors. Neuropharmacology 2016, 103, 16–26. [Google Scholar] [CrossRef] [PubMed]
- Hernandez, D.; Torres, C.A.; Setlik, W.; Cebrián, C.; Mosharov, E.V.; Tang, G.; Cheng, H.C.; Kholodilov, N.; Yarygina, O.; Burke, R.E.; et al. Regulation of presynaptic neurotransmission by macroautophagy. Neuron 2012, 74, 277–284. [Google Scholar] [CrossRef] [PubMed]
- Saitoh, A.; Yamaguchi, K.; Tatsumi, Y.; Murasawa, H.; Nakatani, A.; Hirose, N.; Yamada, M.; Yamada, M.; Kamei, J. Effects of milnacipran and fluvoxamine on hyperemotional behaviors and the loss of tryptophan hydroxylase-positive cells in olfactory bulbectomized rats. Psychopharmacology (Berl.) 2007, 191, 857–865. [Google Scholar] [CrossRef] [PubMed]
- Aksoz, E.; Aksoz, T.; Bilge, S.S.; Ilkaya, F.; Celik, S.; Diren, H.B. Antidepressant-like effects of echo-planar magnetic resonance imaging in mice determined using the forced swimming test. Brain Res. 2008, 1236, 194–199. [Google Scholar] [CrossRef] [PubMed]
- Bagley, J.; Moghaddam, B. Temporal dynamics of glutamate efflux in the prefrontal cortex and in the hippocampus following repeated stress: Effects of pretreatment with saline or diazepam. Neuroscience 1997, 77, 65–73. [Google Scholar] [CrossRef]
- Treccani, G.; Musazzi, L.; Perego, C.; Milanese, M.; Nava, N.; Bonifacino, T.; Lamanna, J.; Malgaroli, A.; Drago, F.; Racagni, G.; et al. Acute stress rapidly increases the readily releasable pool of glutamate vesicles in prefrontal and frontal cortex through non-genomic action of corticosterone. Mol. Psychiatry 2014, 19, 401. [Google Scholar] [CrossRef]
- Reznikov, L.R.; Grillo, C.A.; Piroli, G.G.; Pasumarthi, R.K.; Reagan, L.P.; Fadel, J. Acute stress-mediated increases in extracellular glutamate levels in the rat amygdala: Differential effects of antidepressant treatment. Eur. J. Neurosci. 2007, 25, 3109–3114. [Google Scholar] [CrossRef]
- McCabe, M.P.; Cullen, E.R.; Barrows, C.M.; Shore, A.N.; Tooke, K.I.; Laprade, K.A.; Stafford, J.M.; Weston, M.C. Genetic inactivation of mTORC1 or mTORC2 in neurons reveals distinct functions in glutamatergic synaptic transmission. Elife 2019, 9, e51440. [Google Scholar] [CrossRef]
- Nishitani, N.; Nagayasu, K.; Asaoka, N.; Yamashiro, M.; Shirakawa, H.; Nakagawa, T.; Kaneko, S. Raphe AMPA receptors and nicotinic acetylcholine receptors mediate ketamine-induced serotonin release in the rat prefrontal cortex. Int. J. Neuropsychopharmacol. 2014, 17, 1321–1326. [Google Scholar] [CrossRef]
- Satake, S.; Saitow, F.; Yamada, J.; Konishi, S. Synaptic activation of AMPA receptors inhibits GABA release from cerebellar interneurons. Nat. Neurosci. 2000, 3, 551–558. [Google Scholar] [CrossRef]
- Pavia-Collado, R.; Cóppola-Segovia, V.; Miquel-Rio, L.; Alarcón-Aris, D.; Rodríguez-Aller, R.; Torres-López, M.; Paz, V.; Ruiz-Bronchal, E.; Campa, L.; Artigas, F.; et al. Intracerebral Administration of a Ligand-ASO Conjugate Selectively Reduces α-Synuclein Accumulation in Monoamine Neurons of Double Mutant Human A30P*A53T*α-Synuclein Transgenic Mice. Int. J. Mol. Sci. 2021, 22, 2939. [Google Scholar] [CrossRef] [PubMed]
- Fukumoto, K.; Iijima, M.; Chaki, S. The Antidepressant Effects of an mGlu2/3 Receptor Antagonist and Ketamine Require AMPA Receptor Stimulation in the mPFC and Subsequent Activation of the 5-HT Neurons in the DRN. Neuropsychopharmacology 2016, 41, 1046–1056. [Google Scholar] [CrossRef]
- Hettinger, J.C.; Lee, H.; Bu, G.; Holtzman, D.M.; Cirrito, J.R. AMPA-ergic regulation of amyloid-β levels in an Alzheimer’s disease mouse model. Mol. Neurodegener. 2018, 13, 22. [Google Scholar] [CrossRef]
- Ferrés-Coy, A.; Pilar-Cuellar, F.; Vidal, R.; Paz, V.; Masana, M.; Cortés, R.; Carmona, M.C.; Campa, L.; Pazos, A.; Montefeltro, A.; et al. RNAi-mediated serotonin transporter suppression rapidly increases serotonergic neurotransmission and hippocampal neurogenesis. Transl. Psychiatry 2013, 3, e211. [Google Scholar] [CrossRef]
- Ferrés-Coy, A.; Galofré, M.; Pilar-Cuéllar, F.; Vidal, R.; Paz, V.; Ruiz-Bronchal, E.; Campa, L.; Pazos, Á.; Caso, J.R.; Leza, J.C.; et al. Therapeutic antidepressant potential of a conjugated siRNA silencing the serotonin transporter after intranasal administration. Mol. Psychiatry 2016, 21, 328–338. [Google Scholar] [CrossRef]
- Vidal, R.; Garro-Martínez, E.; Díaz, Á.; Castro, E.; Florensa-Zanuy, E.; Taketo, M.M.; Pazos, Á.; Pilar-Cuéllar, F. Targeting β-Catenin in GLAST-Expressing Cells: Impact on Anxiety and Depression-Related Behavior and Hippocampal Proliferation. Mol. Neurobiol. 2019, 56, 553–566. [Google Scholar] [CrossRef]
- Bortolozzi, A.; Castañé, A.; Semakova, J.; Santana, N.; Alvarado, G.; Cortés, R.; Ferrés-Coy, A.; Fernández, G.; Carmona, M.C.; Toth, M.; et al. Selective siRNA-mediated suppression of 5-HT1A autoreceptors evokes strong anti-depressant-like effects. Mol. Psychiatry 2012, 17, 612–623. [Google Scholar] [CrossRef]
- Pilar-Cuéllar, F.; Vidal, R.; Díaz, Á.; Garro-Martínez, E.; Linge, R.; Castro, E.; Haberzettl, R.; Fink, H.; Bert, B.; Brosda, J.; et al. Enhanced Stress Response in 5-HT1AR Overexpressing Mice: Altered HPA Function and Hippocampal Long-Term Potentiation. ACS Chem. Neurosci. 2017, 8, 2393–2401. [Google Scholar] [CrossRef]
- López-Gil, X.; Babot, Z.; Amargós-Bosch, M.; Suñol, C.; Artigas, F.; Adell, A. Clozapine and haloperidol differently suppress the MK-801-increased glutamatergic and serotonergic transmission in the medial prefrontal cortex of the rat. Neuropsychopharmacology 2007, 32, 2087–2097. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Garro-Martínez, E.; Fullana, M.N.; Florensa-Zanuy, E.; Senserrich, J.; Paz, V.; Ruiz-Bronchal, E.; Adell, A.; Castro, E.; Díaz, Á.; Pazos, Á.; et al. mTOR Knockdown in the Infralimbic Cortex Evokes A Depressive-like State in Mouse. Int. J. Mol. Sci. 2021, 22, 8671. https://doi.org/10.3390/ijms22168671
Garro-Martínez E, Fullana MN, Florensa-Zanuy E, Senserrich J, Paz V, Ruiz-Bronchal E, Adell A, Castro E, Díaz Á, Pazos Á, et al. mTOR Knockdown in the Infralimbic Cortex Evokes A Depressive-like State in Mouse. International Journal of Molecular Sciences. 2021; 22(16):8671. https://doi.org/10.3390/ijms22168671
Chicago/Turabian StyleGarro-Martínez, Emilio, Maria Neus Fullana, Eva Florensa-Zanuy, Julia Senserrich, Verónica Paz, Esther Ruiz-Bronchal, Albert Adell, Elena Castro, Álvaro Díaz, Ángel Pazos, and et al. 2021. "mTOR Knockdown in the Infralimbic Cortex Evokes A Depressive-like State in Mouse" International Journal of Molecular Sciences 22, no. 16: 8671. https://doi.org/10.3390/ijms22168671
APA StyleGarro-Martínez, E., Fullana, M. N., Florensa-Zanuy, E., Senserrich, J., Paz, V., Ruiz-Bronchal, E., Adell, A., Castro, E., Díaz, Á., Pazos, Á., Bortolozzi, A., & Pilar-Cuéllar, F. (2021). mTOR Knockdown in the Infralimbic Cortex Evokes A Depressive-like State in Mouse. International Journal of Molecular Sciences, 22(16), 8671. https://doi.org/10.3390/ijms22168671