Quantitative Analysis of the Potency of Equimolar Two-Drug Combinations and Combi-Molecules Involving Kinase Inhibitors In Vitro: The Concept of Balanced Targeting
Abstract
1. Introduction
2. Results
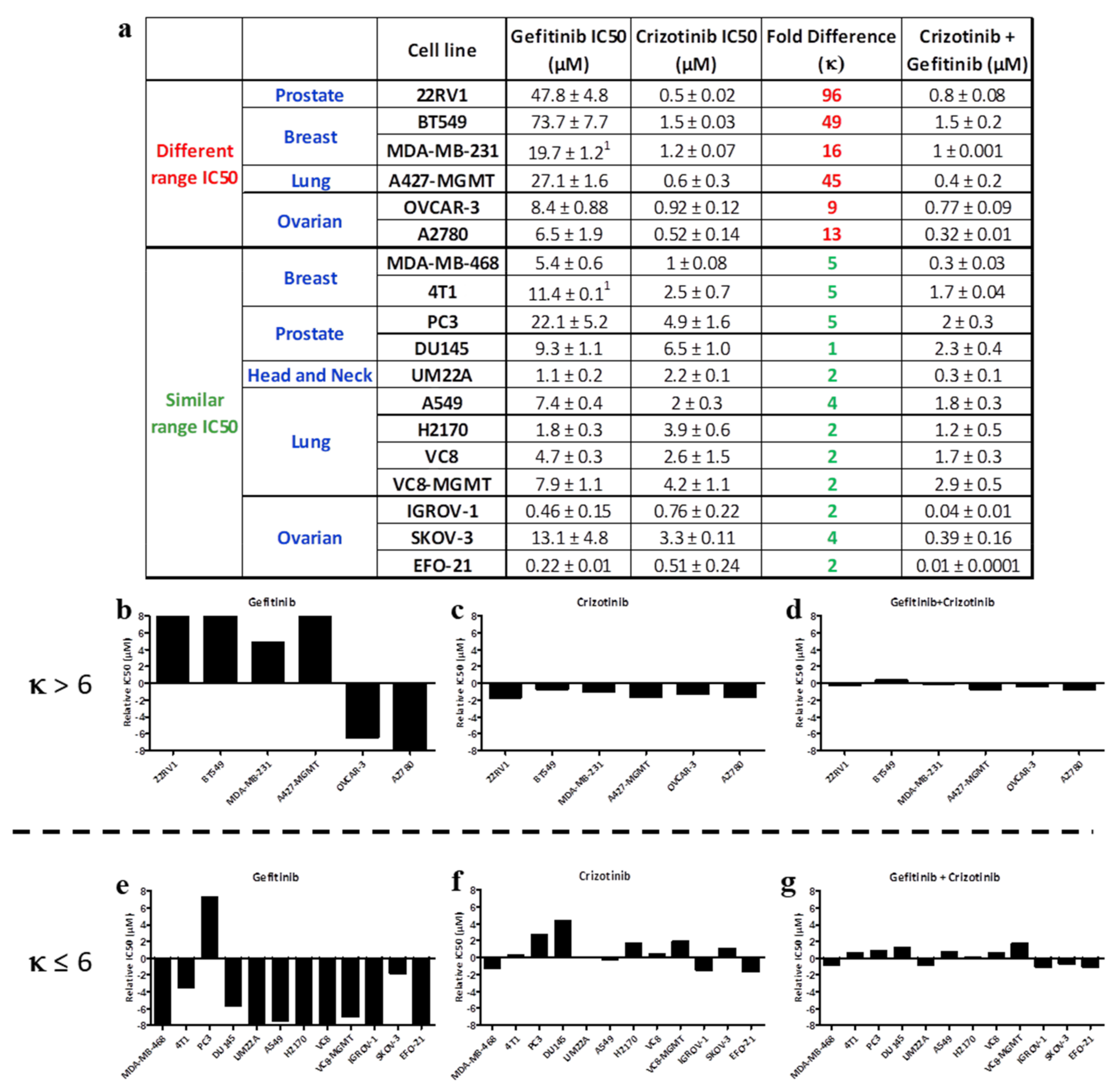
2.1. Growth Inhibitory Potency of Single Versus Equimolar Combinations of Clinical Inhibitors on a Panel of Cancer Cell Lines
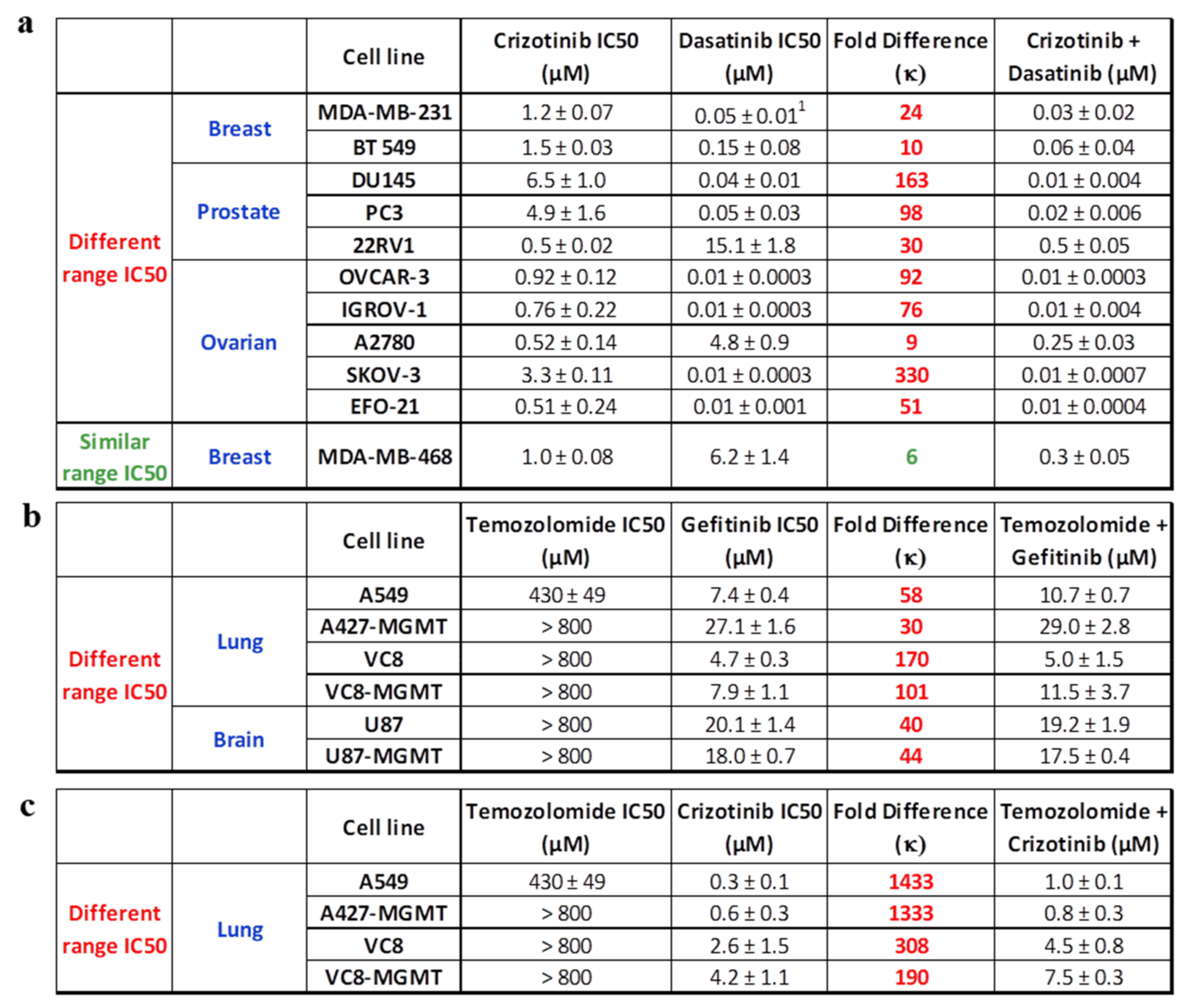
2.1.1. EGFR-c-Src Targeting
2.1.2. EGFR-c-Met Targeting
2.1.3. c-Met-c-Src Targeting
2.1.4. EGFR- or c-Met-DNA Targeting
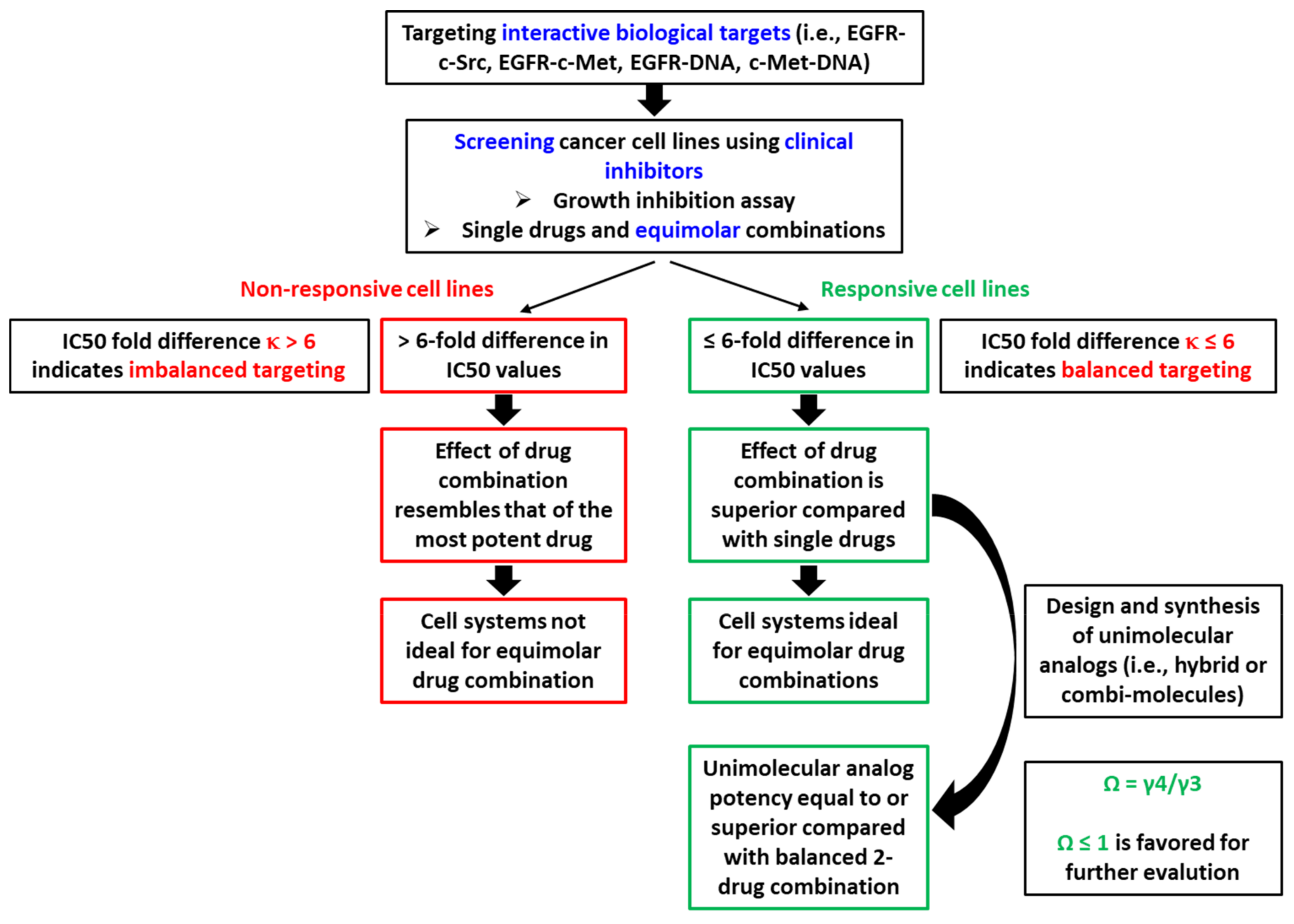
2.2. Parameterization of the Response Profiles
2.3. Unimolecular Combinations
2.3.1. EGFR-c-Src Targeting Combi-Molecules
2.3.2. Design, Synthesis, and Biological Potency of LP121, an EGFR-c-Met-Targeting Combi-Molecule
2.4. Parameterization of Potency of Combi-Molecules: A New Parameter Ω as a Potency Index
3. Discussion
- If two drugs are combined at an equimolar ratio, and one drug shows a 6-fold greater IC50 than the other (i.e., κ > 6), the overall effect will resemble the IC50 of the combination.
- If two drugs are combined at an equimolar ratio, and the IC50 of one drug is 6-fold or less than that of the other (i.e., κ ≤ 6), then the overall effect is superior to that of each individual drug, leading to balanced targeting.
- Under conditions of balanced targeting, a unimolecular combination (e.g., combi-molecule) is said to be effective if the IC50 of the combi-molecule is equal to or a fraction of the IC50 of the equimolar 2-drug combination (i.e., Ω ≤ 1).
4. Materials and Methods
4.1. Combi-Molecule Synthesis
4.2. Cell Culture
4.3. Drug Treatment
4.4. Growth Inhibition Assay
4.5. In Vitro Kinase Assay
4.6. Western Blot Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Krause, D.S.; Van Etten, R.A. Tyrosine Kinases as Targets for Cancer Therapy. N. Engl. J. Med. 2005, 353, 172–187. [Google Scholar] [CrossRef] [PubMed]
- Martin, G.S. Cell Signaling and Cancer. Cancer Cell 2003, 4, 167–174. [Google Scholar] [CrossRef]
- Paul, M.K.; Mukhopadhyay, A.K. Tyrosine Kinase—Role and Significance in Cancer. Int. J. Med. Sci. 2004, 1, 101–115. [Google Scholar] [CrossRef] [PubMed]
- Petrelli, A.; Giordano, S. From Single- to Multi-Target Drugs in Cancer Therapy: When Aspecificity Becomes an Advantage. Curr. Med. Chem. 2008, 15, 422–432. [Google Scholar]
- Kerr, D.J.; La Thangue, N.B. Signal Transduction Blockade and Cancer: Combination Therapy or Multi-Targeted Inhibitors? Ann. Oncol. 2004, 15, 1727–1729. [Google Scholar] [CrossRef]
- Banerjee, R.; Rachid, Z.; McNamee, J.; Jean-Claude, B.J. Synthesis of a Prodrug Designed to Release Multiple Inhibitors of the Epidermal Growth Factor Receptor Tyrosine Kinase and an Alkylating Agent: A Novel Tumor Targeting Concept. J. Med. Chem. 2003, 46, 5546–5551. [Google Scholar] [CrossRef] [PubMed]
- Huang, Y.; Rachid, Z.; Jean-Claude, B.J. MGMT Is a Molecular Determinant for Potency of the DNA-EGFR-Combi-Molecule ZRS1. Mol. Cancer Res. 2011, 9, 320–331. [Google Scholar] [CrossRef]
- Banerjee, R.; Huang, Y.; Qiu, Q.; McNamee, J.P.; Belinsky, G.; Jean-Claude, B.J. The Combi-Targeting Concept: Mechanism of Action of the Pleiotropic Combi-Molecule RB24 and Discovery of a Novel Cell Signaling-Based Combination Principle. Cell Signal. 2011, 23, 630–640. [Google Scholar] [CrossRef]
- MacPhee, M.; Rachid, Z.; Todorova, M.; Qiu, Q.; Belinsky, G.; Jean-Claude, B.J. Characterization of the Potency of Epidermal Growth Factor (EGFR)-DNA Targeting Combi-Molecules Containing a Hydrolabile Carbamate at the 3-Position of the Triazene Chain. Investig. New Drugs 2011, 29, 833–845. [Google Scholar] [CrossRef]
- Goodfellow, E.; Senhaji Mouhri, Z.; Williams, C.; Jean-Claude, B.J. Design, Synthesis and Biological Activity of Novel Molecules Designed to Target PARP and DNA. Bioorg. Med. Chem. Lett. 2017, 27, 688–694. [Google Scholar] [CrossRef][Green Version]
- Heravi, M.; Kumala, S.; Rachid, Z.; Jean-Claude, B.J.; Radzioch, D.; Muanza, T.M. ZRBA1, a Mixed EGFR/DNA Targeting Molecule, Potentiates Radiation Response Through Delayed DNA Damage Repair Process in a Triple Negative Breast Cancer Model. Int. J. Radiat. Oncol. Biol. Phys. 2015, 92, 399–406. [Google Scholar] [CrossRef]
- Rupp, M.; Mouhri, Z.S.; Williams, C.; Jean-Claude, B.J. Molecular Analysis of the Dual Targeting of the Epidermal Growth Factor Receptor and the O6-Methylguanine-DNA Methyltransferase with a Double Arm Hybrid Molecule. Oncotarget 2018, 9, 35041–35055. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Sharifi, Z.; Abdulkarim, B.; Meehan, B.; Rak, J.; Daniel, P.; Schmitt, J.; Lauzon, N.; Eppert, K.; Duncan, H.M.; Petrecca, K.; et al. Mechanisms and Antitumor Activity of a Binary EGFR/DNA-Targeting Strategy Overcomes Resistance of Glioblastoma Stem Cells to Temozolomide. Clin. Cancer Res. 2019, 25, 7594–7608. [Google Scholar] [CrossRef] [PubMed]
- Schmitt, J.; Goodfellow, E.; Huang, S.; Williams, C.; Gomes, I.N.F.; Rosa, M.N.; Reis, R.M.; Yang, R.; Titi, H.M.; Jean-Claude, B.J. Comparative Analysis of the Dual EGFR-DNA Targeting and Growth Inhibitory Properties of 6-Mono-Alkylamino- and 6,6-Dialkylaminoquinazoline-Based Type II Combi-Molecules. Eur. J. Med. Chem. 2020, 192, 112185. [Google Scholar] [CrossRef]
- Schmitt, J.; Huang, S.; Goodfellow, E.; Williams, C.; Jean-Claude, B.J. Design and Synthesis of a Trifunctional Molecular System “Programmed” to Block Epidermal Growth Factor Receptor Tyrosine Kinase, Induce High Levels of DNA Damage, and Inhibit the DNA Repair Enzyme (Poly(ADP-Ribose) Polymerase) in Prostate Cancer Cells. J. Med. Chem. 2020, 63, 5752–5762. [Google Scholar] [CrossRef] [PubMed]
- Larroque-Lombard, A.-L.; Chatelut, E.; Delord, J.-P.; Imbs, D.-C.; Rochaix, P.; Jean-Claude, B.; Allal, B. Design and Mechanism of Action of a New Prototype of Combi-Molecule “Programed” to Release Bioactive Species at a PH Range Akin to That of the Tumor Microenvironment. Pharmaceuticals 2021, 14, 160. [Google Scholar] [CrossRef]
- Barchéchath, S.; Williams, C.; Saade, K.; Lauwagie, S.; Jean-Claude, B. Rational Design of Multitargeted Tyrosine Kinase Inhibitors: A Novel Approach. Chem. Biol. Drug Des. 2009, 73, 380–387. [Google Scholar] [CrossRef]
- Larroque-Lombard, A.-L.; Ning, N.; Rao, S.; Lauwagie, S.; Halaoui, R.; Coudray, L.; Huang, Y.; Jean-Claude, B.J. Biological Effects of AL622, a Molecule Rationally Designed to Release an EGFR and a c-Src Kinase Inhibitor. Chem. Biol. Drug Des. 2012, 80, 981–991. [Google Scholar] [CrossRef] [PubMed]
- Rao, S.; Larroque-Lombard, A.-L.; Peyrard, L.; Thauvin, C.; Rachid, Z.; Williams, C.; Jean-Claude, B.J. Target Modulation by a Kinase Inhibitor Engineered to Induce a Tandem Blockade of the Epidermal Growth Factor Receptor (EGFR) and c-Src: The Concept of Type III Combi-Targeting. PLoS ONE 2015, 10, e0117215. [Google Scholar] [CrossRef]
- Matheson, S.L.; McNamee, J.; Jean-Claude, B.J. Design of a Chimeric 3-Methyl-1,2,3-Triazene with Mixed Receptor Tyrosine Kinase and DNA Damaging Properties: A Novel Tumor Targeting Strategy. J. Pharmacol. Exp. Ther. 2001, 296, 832–840. [Google Scholar]
- Banerjee, R.; Rachid, Z.; Qiu, Q.; McNamee, J.P.; Tari, A.M.; Jean-Claude, B.J. Sustained Antiproliferative Mechanisms by RB24, a Targeted Precursor of Multiple Inhibitors of Epidermal Growth Factor Receptor and a DNA Alkylating Agent in the A431 Epidermal Carcinoma of the Vulva Cell Line. Br. J. Cancer 2004, 91, 1066–1073. [Google Scholar] [CrossRef][Green Version]
- Ait-Tihyaty, M.; Rachid, Z.; Larroque-Lombard, A.-L.; Jean-Claude, B.J. ZRX1, the First EGFR Inhibitor-Capecitabine Based Combi-Molecule, Requires Carboxylesterase-Mediated Hydrolysis for Optimal Activity. Investig. New Drugs 2013, 31, 1409–1423. [Google Scholar] [CrossRef]
- Qiu, Q.; Domarkas, J.; Banerjee, R.; Katsoulas, A.; McNamee, J.P.; Jean-Claude, B.J. Type II Combi-Molecules: Design and Binary Targeting Properties of the Novel Triazolinium-Containing Molecules JDD36 and JDE05. Anticancer Drugs 2007, 18, 171–177. [Google Scholar] [CrossRef]
- Huang, Y.; Rachid, Z.; Peyrard, L.; Senhaji Mouhri, Z.; Williams, C.; Jean-Claude, B.J. Positional Isomerization of a Non-Cleavable Combi-Molecule Dramatically Altered Tumor Cell Response Profile. Chem. Biol. Drug Des. 2015, 85, 153–162. [Google Scholar] [CrossRef]
- Fortin, S.; Bérubé, G. Advances in the Development of Hybrid Anticancer Drugs. Expert Opin. Drug Discov. 2013, 8, 1029–1047. [Google Scholar] [CrossRef] [PubMed]
- Gediya, L.K.; Njar, V.C. Promise and Challenges in Drug Discovery and Development of Hybrid Anticancer Drugs. Expert Opin. Drug Discov. 2009, 4, 1099–1111. [Google Scholar] [CrossRef] [PubMed]
- El Meskini, R.; Iacovelli, A.J.; Kulaga, A.; Gumprecht, M.; Martin, P.L.; Baran, M.; Householder, D.B.; Van Dyke, T.; Weaver Ohler, Z. A Preclinical Orthotopic Model for Glioblastoma Recapitulates Key Features of Human Tumors and Demonstrates Sensitivity to a Combination of MEK and PI3K Pathway Inhibitors. Dis. Model. Mech. 2015, 8, 45–56. [Google Scholar] [CrossRef]
- Effenberger-Neidnicht, K.; Schobert, R. Combinatorial Effects of Thymoquinone on the Anti-Cancer Activity of Doxorubicin. Cancer Chemother. Pharmacol. 2011, 67, 867–874. [Google Scholar] [CrossRef]
- Garcia, A.G.; Nedev, H.; Bijian, K.; Su, J.; Alaoui-Jamali, M.A.; Saragovi, H.U. Reduced in Vivo Lung Metastasis of a Breast Cancer Cell Line after Treatment with Herceptin MAb Conjugated to Chemotherapeutic Drugs. Oncogene 2013, 32, 2527–2533. [Google Scholar] [CrossRef]
- Lödige, M.; Hiersch, L. Design and Synthesis of Novel Hybrid Molecules against Malaria. Int. J. Med. Chem. 2015, 2015, 458319. [Google Scholar] [CrossRef] [PubMed]
- Lödige, M.; Lewis, M.D.; Paulsen, E.S.; Esch, H.L.; Pradel, G.; Lehmann, L.; Brun, R.; Bringmann, G.; Mueller, A.-K. A Primaquine-Chloroquine Hybrid with Dual Activity against Plasmodium Liver and Blood Stages. Int. J. Med. Microbiol. 2013, 303, 539–547. [Google Scholar] [CrossRef]
- Chou, T.C.; Talalay, P. Quantitative Analysis of Dose-Effect Relationships: The Combined Effects of Multiple Drugs or Enzyme Inhibitors. Adv. Enzyme Regul. 1984, 22, 27–55. [Google Scholar] [CrossRef]
- Chou, T.-C. Theoretical Basis, Experimental Design, and Computerized Simulation of Synergism and Antagonism in Drug Combination Studies. Pharmacol. Rev. 2006, 58, 621–681. [Google Scholar] [CrossRef]
- Chou, T.-C. Drug Combination Studies and Their Synergy Quantification Using the Chou-Talalay Method. Cancer Res. 2010, 70, 440–446. [Google Scholar] [CrossRef]
- Capela, R.; Cabal, G.G.; Rosenthal, P.J.; Gut, J.; Mota, M.M.; Moreira, R.; Lopes, F.; Prudêncio, M. Design and Evaluation of Primaquine-Artemisinin Hybrids as a Multistage Antimalarial Strategy. Antimicrob. Agents Chemother. 2011, 55, 4698–4706. [Google Scholar] [CrossRef] [PubMed]
- Amrein, L.; Rachid, Z.; Jean-Claude, B.; Soulières, D.; Aloyz, R.; Panasci, L. ZRF4, a Combi-Molecule with Increased Efficacy as Compared with the Individual Components in Chronic Lymphocytic Leukemia Lymphocytes in Vitro. Leukemia 2011, 25, 1512–1516. [Google Scholar] [CrossRef] [PubMed]
- Leese, M.P.; Jourdan, F.; Kimberley, M.R.; Cozier, G.E.; Thiyagarajan, N.; Stengel, C.; Regis-Lydi, S.; Foster, P.A.; Newman, S.P.; Acharya, K.R.; et al. Chimeric Microtubule Disruptors. Chem. Commun. 2010, 46, 2907–2909. [Google Scholar] [CrossRef] [PubMed]
- Seo, S.-Y. Multi-Targeted Hybrids Based on HDAC Inhibitors for Anti-Cancer Drug Discovery. Arch. Pharm. Res. 2012, 35, 197–200. [Google Scholar] [CrossRef] [PubMed]
- Uecker, A.; Sicker, M.; Beckers, T.; Mahboobi, S.; Hägerstrand, D.; Ostman, A.; Böhmer, F.-D. Chimeric Tyrosine Kinase-HDAC Inhibitors as Antiproliferative Agents. Anticancer Drugs 2010, 21, 759–765. [Google Scholar] [CrossRef] [PubMed]
- Canellos, G.P.; Anderson, J.R.; Propert, K.J.; Nissen, N.; Cooper, M.R.; Henderson, E.S.; Green, M.R.; Gottlieb, A.; Peterson, B.A. Chemotherapy of Advanced Hodgkin’s Disease with MOPP, ABVD, or MOPP Alternating with ABVD. N. Engl. J. Med. 1992, 327, 1478–1484. [Google Scholar] [CrossRef] [PubMed]
- Fisher, R.I.; Gaynor, E.R.; Dahlberg, S.; Oken, M.M.; Grogan, T.M.; Mize, E.M.; Glick, J.H.; Coltman, C.A.; Miller, T.P. Comparison of a Standard Regimen (CHOP) with Three Intensive Chemotherapy Regimens for Advanced Non-Hodgkin’s Lymphoma. N. Engl. J. Med. 1993, 328, 1002–1006. [Google Scholar] [CrossRef]
- Dong, H.; Yin, H.; Zhao, C.; Cao, J.; Xu, W.; Zhang, Y. Design, Synthesis and Biological Evaluation of Novel Osimertinib-Based HDAC and EGFR Dual Inhibitors. Molecules 2019, 24, 2407. [Google Scholar] [CrossRef] [PubMed]
- Katsoulas, A.; Rachid, Z.; McNamee, J.P.; Williams, C.; Jean-Claude, B.J. Combi-Targeting Concept: An Optimized Single-Molecule Dual-Targeting Model for the Treatment of Chronic Myelogenous Leukemia. Mol. Cancer Ther. 2008, 7, 1033–1043. [Google Scholar] [CrossRef] [PubMed]
- Ait-Tihyaty, M.; Rachid, Z.; Mihalcioiu, C.; Jean-Claude, B.J. Inhibition of EGFR Phosphorylation in a Panel of Human Breast Cancer Cells Correlates with Synergistic Interactions between Gefitinib and 5’-DFUR, the Bioactive Metabolite of Xeloda. Breast Cancer Res. Treat. 2012, 133, 217–226. [Google Scholar] [CrossRef] [PubMed]
- Todorova, M.I.; Larroque, A.-L.; Dauphin-Pierre, S.; Fang, Y.-Q.; Jean-Claude, B.J. Subcellular Distribution of a Fluorescence-Labeled Combi-Molecule Designed to Block Epidermal Growth Factor Receptor Tyrosine Kinase and Damage DNA with a Green Fluorescent Species. Mol. Cancer Ther. 2010, 9, 869–882. [Google Scholar] [CrossRef] [PubMed]
- Rix, U.; Hantschel, O.; Dürnberger, G.; Remsing Rix, L.L.; Planyavsky, M.; Fernbach, N.V.; Kaupe, I.; Bennett, K.L.; Valent, P.; Colinge, J.; et al. Chemical Proteomic Profiles of the BCR-ABL Inhibitors Imatinib, Nilotinib, and Dasatinib Reveal Novel Kinase and Nonkinase Targets. Blood 2007, 110, 4055–4063. [Google Scholar] [CrossRef]
- Heppner, G.H.; Miller, F.R.; Shekhar, P.M. Nontransgenic Models of Breast Cancer. Breast Cancer Res. 2000, 2, 331–334. [Google Scholar] [CrossRef]
- Skehan, P.; Storeng, R.; Scudiero, D.; Monks, A.; McMahon, J.; Vistica, D.; Warren, J.T.; Bokesch, H.; Kenney, S.; Boyd, M.R. New Colorimetric Cytotoxicity Assay for Anticancer-Drug Screening. J. Natl. Cancer Inst. 1990, 82, 1107–1112. [Google Scholar] [CrossRef]



Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Rao, S.; Thibault, B.; Peyrard, L.; Larroque-Lombard, A.-L.; Rupp, M.; Thauvin, C.; Jean-Claude, B.J. Quantitative Analysis of the Potency of Equimolar Two-Drug Combinations and Combi-Molecules Involving Kinase Inhibitors In Vitro: The Concept of Balanced Targeting. Int. J. Mol. Sci. 2021, 22, 9569. https://doi.org/10.3390/ijms22179569
Rao S, Thibault B, Peyrard L, Larroque-Lombard A-L, Rupp M, Thauvin C, Jean-Claude BJ. Quantitative Analysis of the Potency of Equimolar Two-Drug Combinations and Combi-Molecules Involving Kinase Inhibitors In Vitro: The Concept of Balanced Targeting. International Journal of Molecular Sciences. 2021; 22(17):9569. https://doi.org/10.3390/ijms22179569
Chicago/Turabian StyleRao, Suman, Benoît Thibault, Lisa Peyrard, Anne-Laure Larroque-Lombard, Martin Rupp, Cédric Thauvin, and Bertrand J. Jean-Claude. 2021. "Quantitative Analysis of the Potency of Equimolar Two-Drug Combinations and Combi-Molecules Involving Kinase Inhibitors In Vitro: The Concept of Balanced Targeting" International Journal of Molecular Sciences 22, no. 17: 9569. https://doi.org/10.3390/ijms22179569
APA StyleRao, S., Thibault, B., Peyrard, L., Larroque-Lombard, A.-L., Rupp, M., Thauvin, C., & Jean-Claude, B. J. (2021). Quantitative Analysis of the Potency of Equimolar Two-Drug Combinations and Combi-Molecules Involving Kinase Inhibitors In Vitro: The Concept of Balanced Targeting. International Journal of Molecular Sciences, 22(17), 9569. https://doi.org/10.3390/ijms22179569





