The Knockout of Enterobactin-Related Gene in Pectobacterium atrosepticum Results in Reduced Stress Resistance and Virulence towards the Primed Plants
Abstract
1. Introduction
2. Results
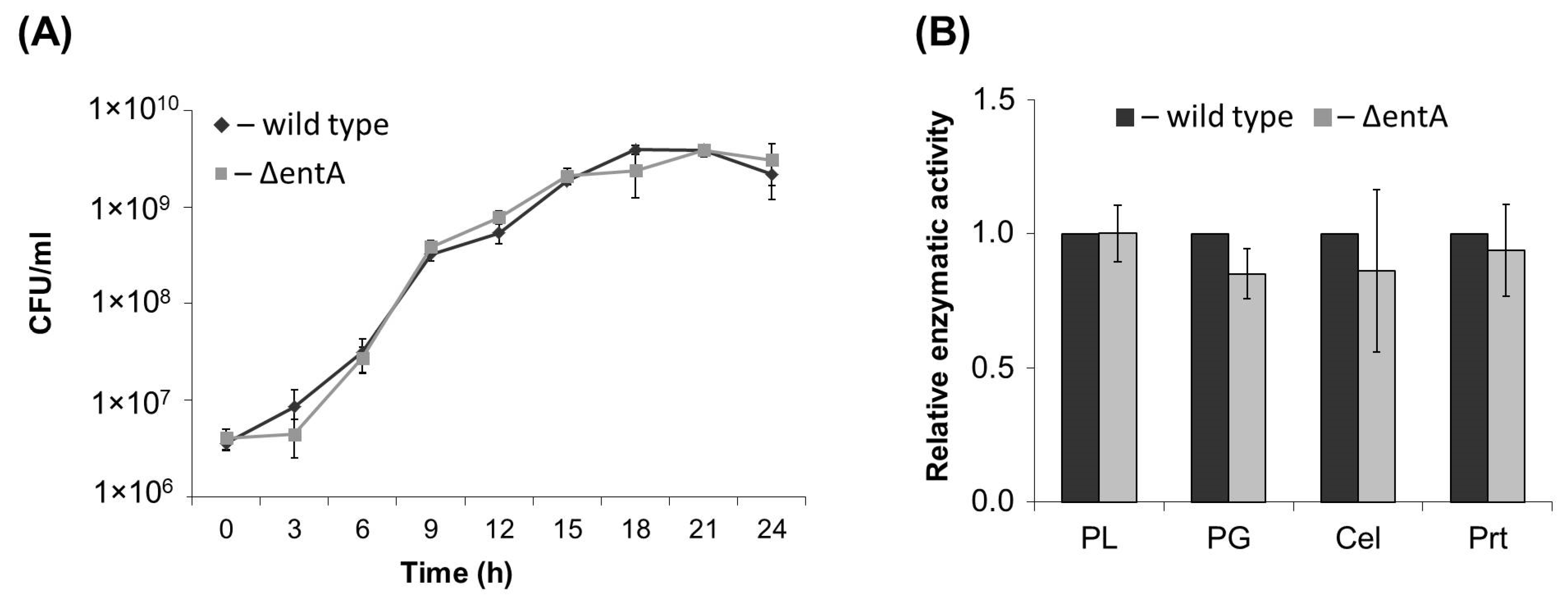
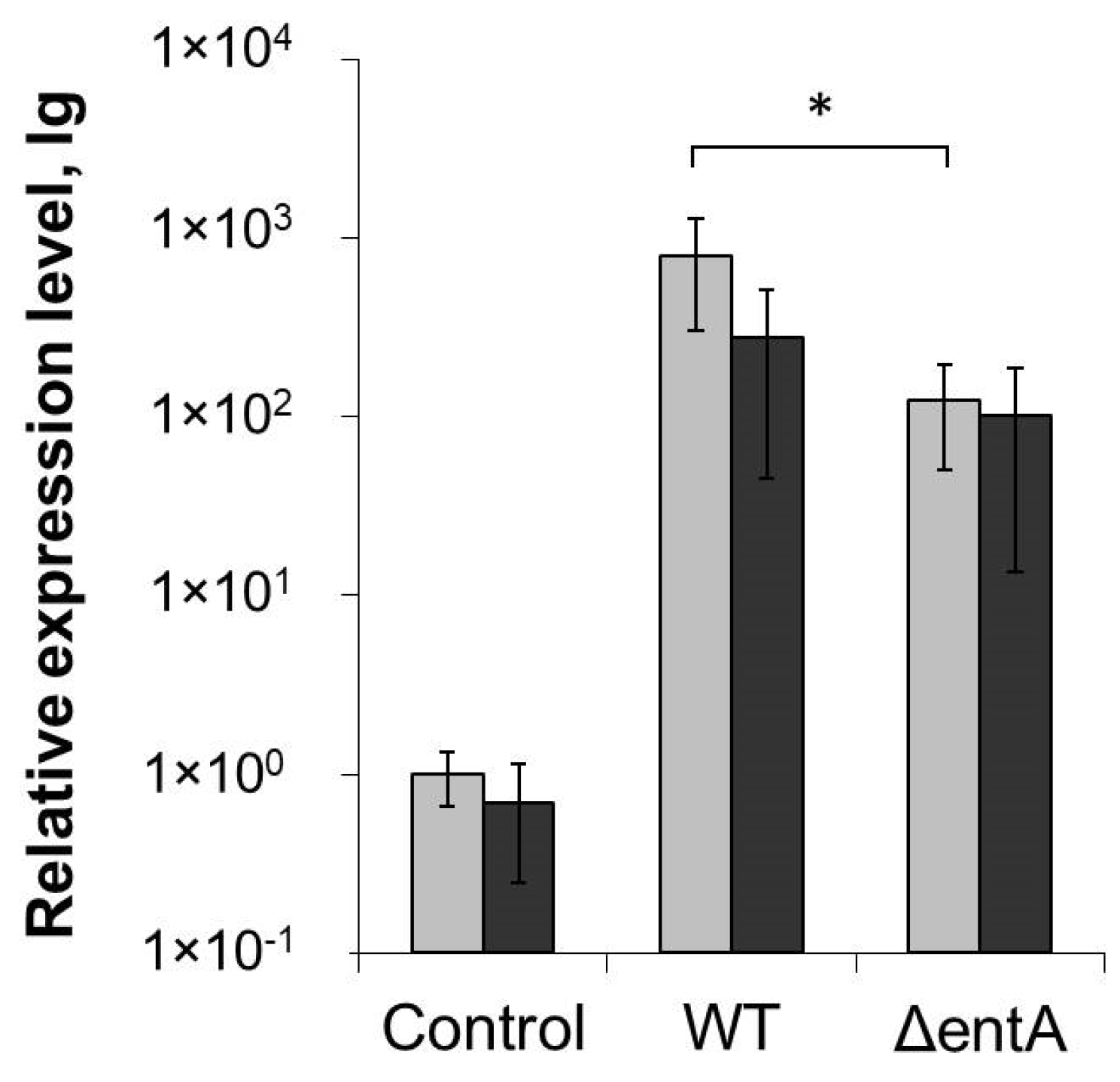
2.1. Primary Characteristics of Enterobactin-Deficient Pba Mutant
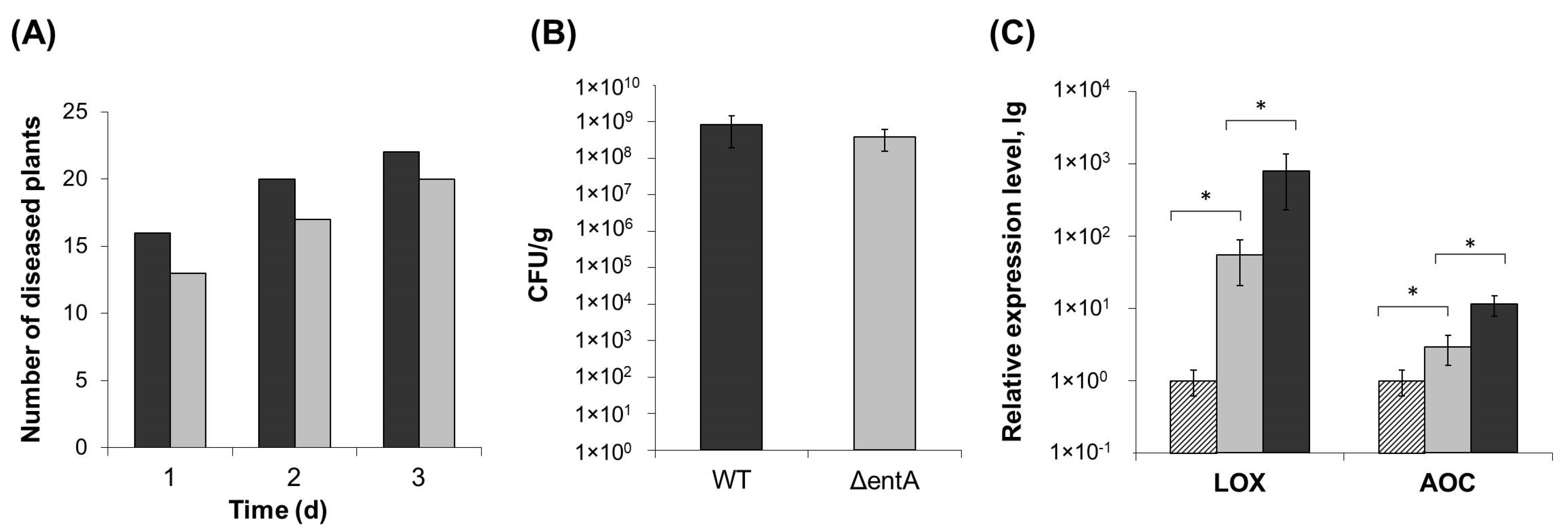
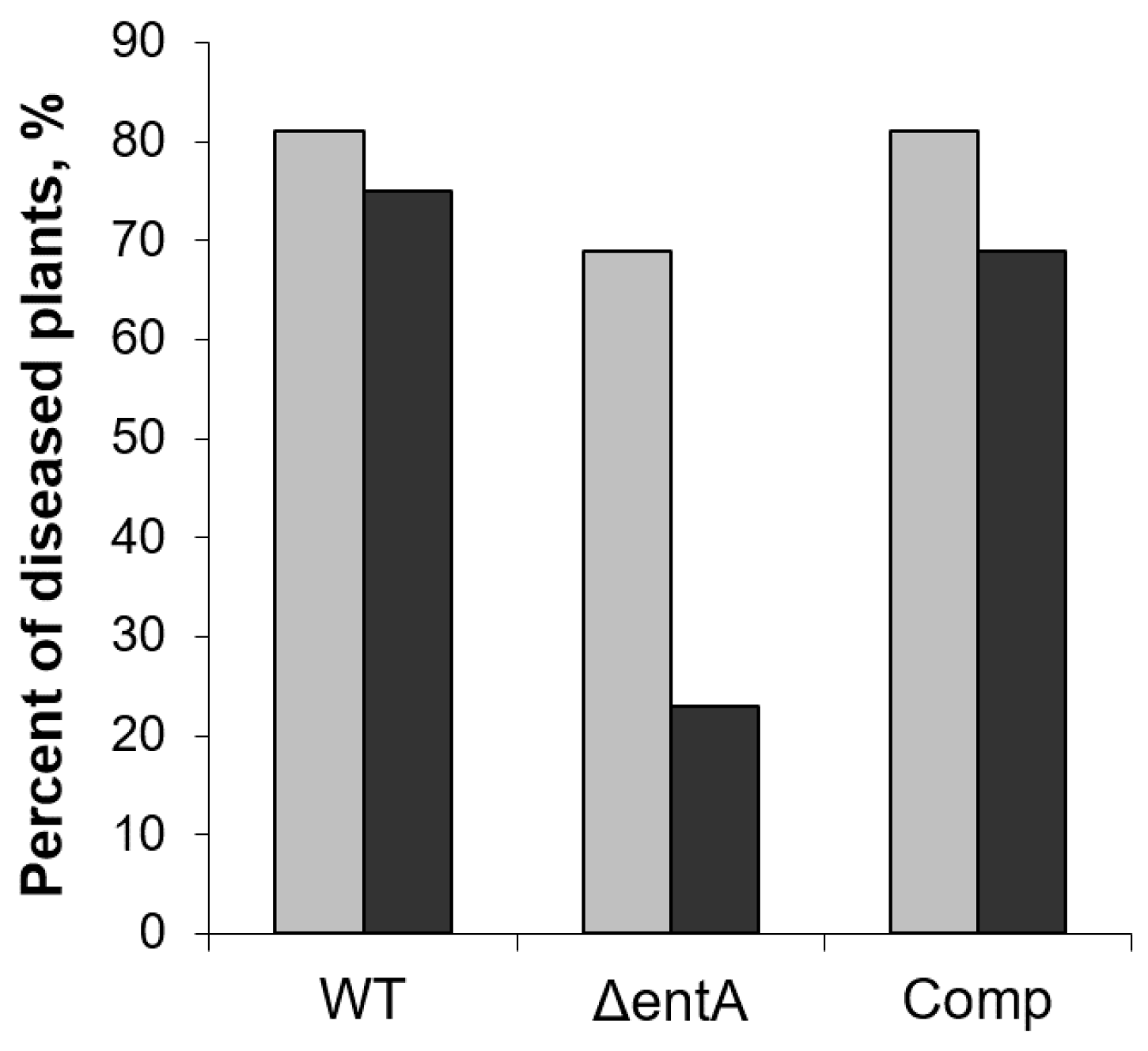
2.2. Virulence of ΔentA Mutant
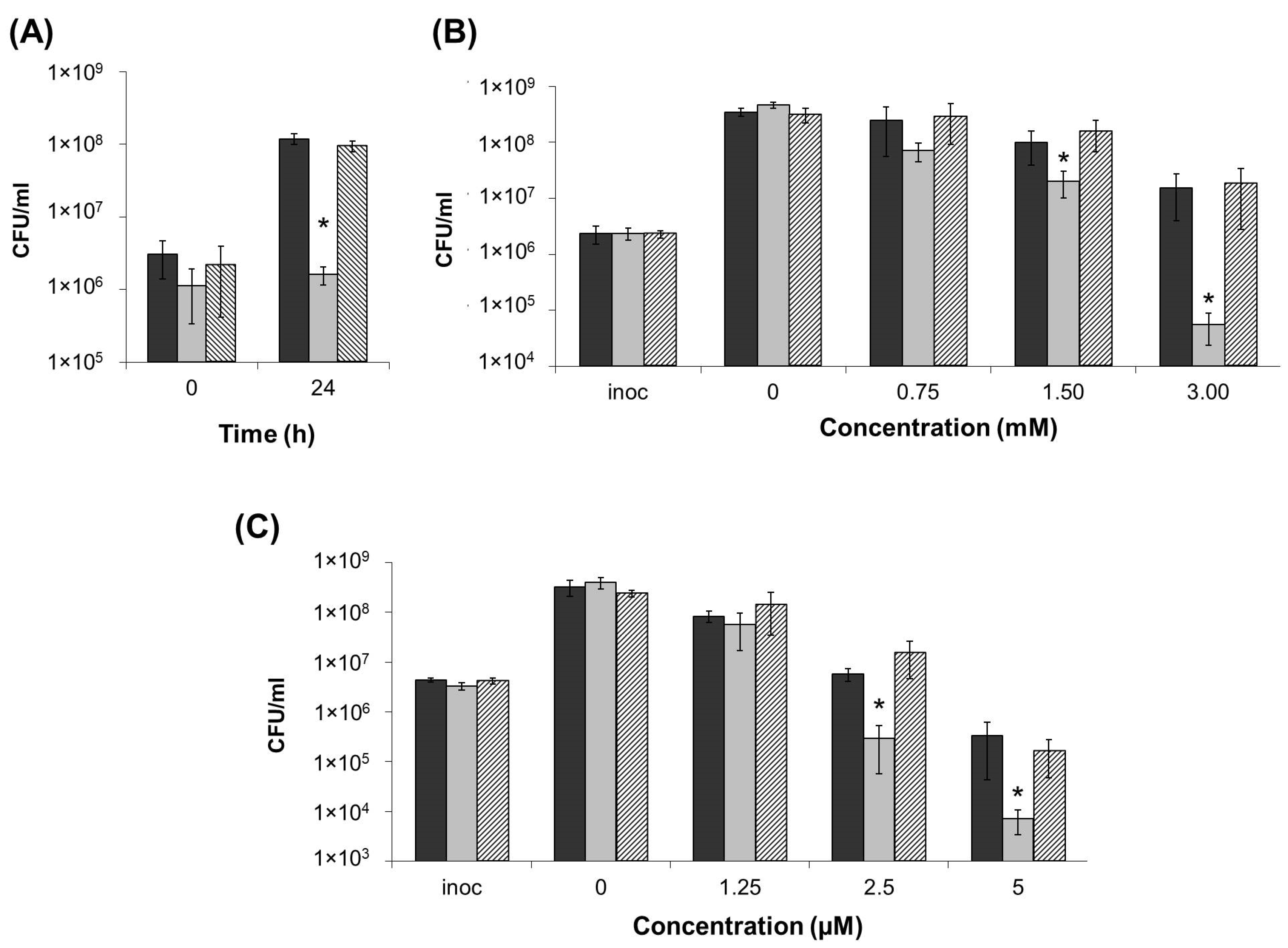
2.3. Stress Resistance of ΔentA Mutant
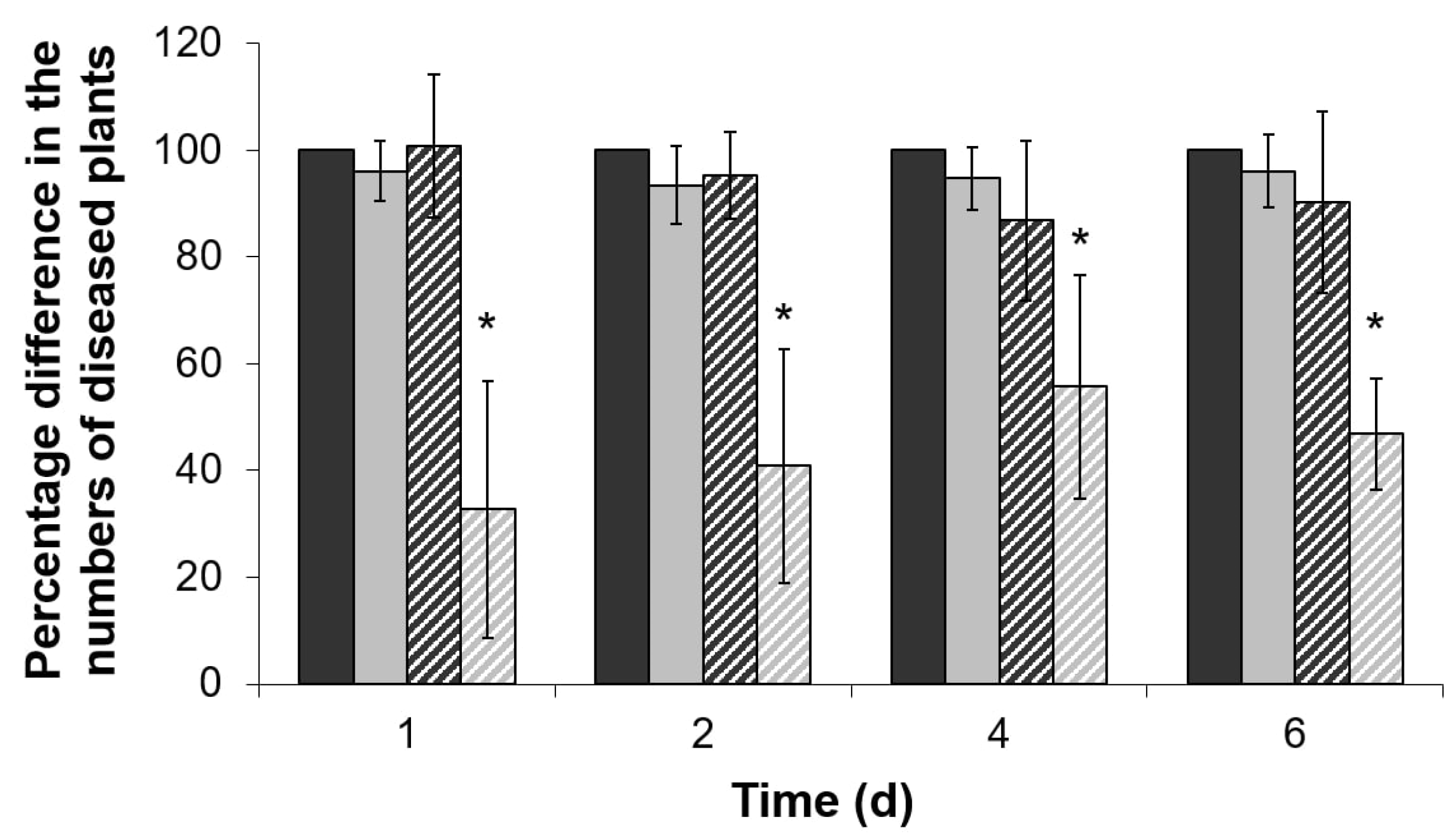
2.4. Virulence of ΔentA Mutant towards Salicylic Acid-Primed Plants
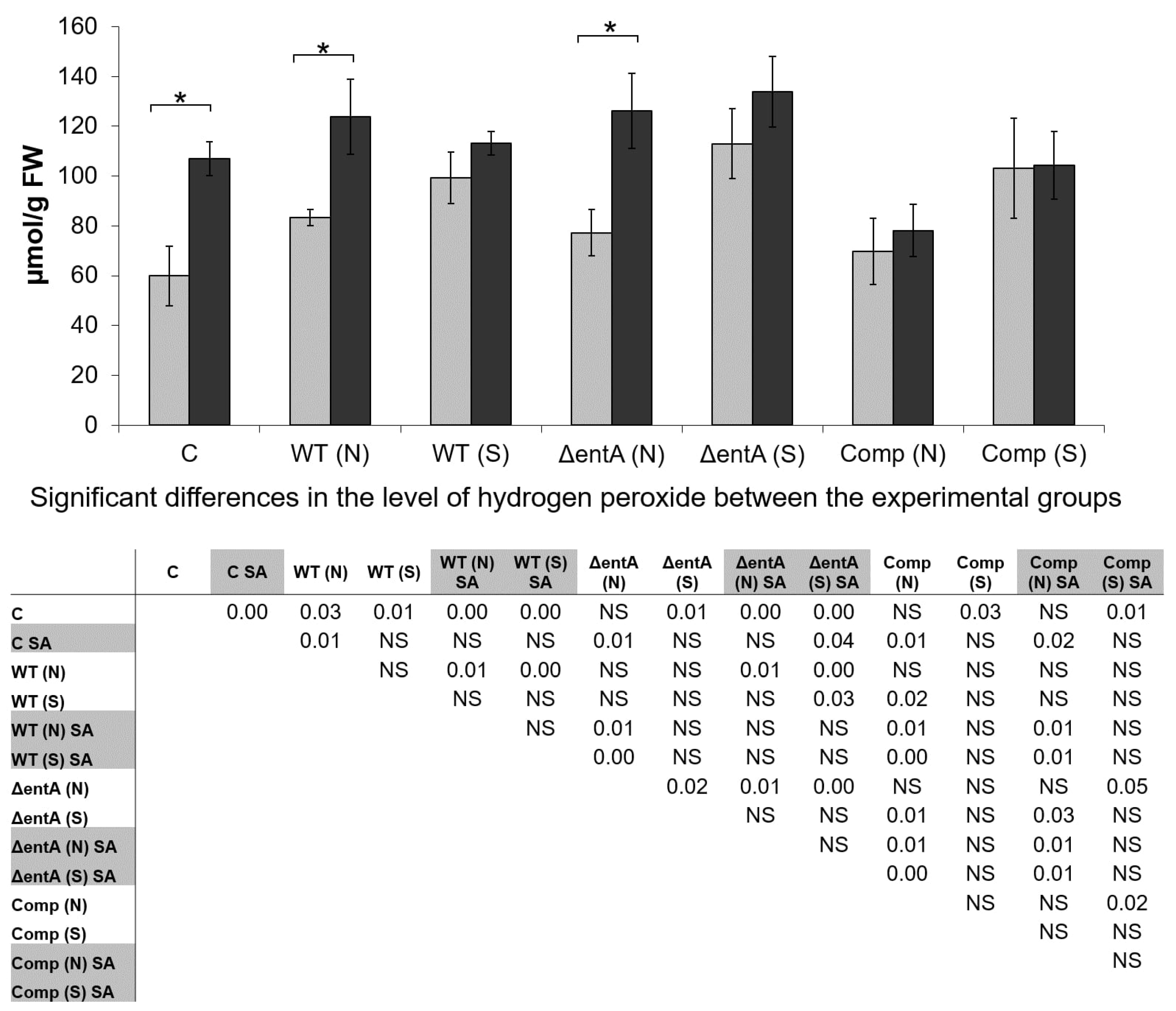
2.5. H2O2-Level in Infected and Non-Infected Tobacco Plants Pretreated or Not with Salicylic Acid
3. Discussion
4. Materials and Methods
4.1. Bacterial Strains, Media and Culture Conditions
4.2. Construction of entA Deletion Mutant
4.3. Construction of the Complementation ΔentA Mutant Strain Carring the entA Gene within the Plasmid
4.4. Plant Cultivation and Infection
4.5. Stress Tolerance Assay
4.6. Enzymatic Activity Assays
4.7. Gene Expression Analysis
4.8. Determination of the Hydrogen Peroxide Level
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Charkowski, A.O. The changing face of bacterial soft-rot diseases. Annu. Rev. Phytopathol. 2018, 56, 269–288. [Google Scholar] [CrossRef]
- Charkowski, A.; Blanco, C.; Condemine, G.; Expert, D.; Franza, T.; Hayes, C.; Hugouvieux-Cotte-Pattat, N.; Solanilla, E.L.; Low, D.; Moleleki, L.; et al. The role of secretion systems and small molecules in soft-rot Enterobacteriaceae pathogenicity. Annu. Rev. Phytopathol. 2012, 50, 425–449. [Google Scholar] [CrossRef]
- Corbett, M.; Virtue, S.; Bell, K.; Birch, P.; Burr, T.; Hyman, L.; Lilley, K.; Poock, S.; Toth, I.; Salmond, G. Identification of a new quorum-sensing-controlled virulence factor in Erwinia carotovora subsp. atroseptica secreted via the type II targeting pathway. Mol. Plant Microbe Interact. 2005, 18, 334–342. [Google Scholar] [CrossRef]
- Pemberton, C.L.; Whitehead, N.A.; Sebaihia, M.; Bell, K.S.; Hyman, L.J.; Harris, S.J.; Matlin, A.J.; Robson, N.D.; Birch, P.R.J.; Carr, J.P.; et al. Novel quorum-sensing-controlled genes in Erwinia carotovora subsp. carotovora: Identification of a fungal elicitor homologue in a soft-rotting bacterium. Mol. Plant Microbe Interact. 2005, 18, 343–353. [Google Scholar] [CrossRef]
- Ageichik, A.V.; Evtushenkov, A.N.; Nikolaichik, E.A. The role of type III secretion system in Erwinia carotovora subsp. atroseptica virulence. Plant Protect. Sci. 2002, 38, 523–527. [Google Scholar] [CrossRef]
- Holeva, M.C.; Bell, K.S.; Hyman, L.J.; Avrova, A.O.; Whisson, S.C.; Birch, P.R.; Toth, I.K. Use of a pooled transposon mutation grid to demonstrate roles in disease development for Erwinia carotovora subsp. atroseptica putative type III secreted effector (DspE/A) and helper (HrpN) proteins. Mol. Plant Microbe Interact. 2004, 17, 943–950. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Liu, H.; Coulthurst, S.J.; Pritchard, L.; Hedley, P.E.; Ravensdale, M.; Humphris, S.; Burr, T.; Takle, G.; Brurberg, M.B.; Birch, P.R.; et al. Quorum sensing coordinates brute force and stealth modes of infection in the plant pathogen Pectobacterium atrosepticum. PLoS Pathog. 2008, 4, e1000093. [Google Scholar] [CrossRef] [PubMed]
- Mattinen, L.; Nissinen, R.; Riipi, T.; Kalkkinen, N.; Pirhonen, M. Host-extract induced changes in the secretome of the plant pathogenic bacterium Pectobacterium atrosepticum. Proteomics 2007, 7, 3527–3537. [Google Scholar] [CrossRef]
- Bell, K.S.; Sebaihia, M.; Pritchard, L.; Holden, M.T.G.; Hyman, L.J.; Holeva, M.C.; Thomson, N.R.; Bentley, S.D.; Churcher, L.J.C.; Mungall, K.; et al. Genome sequence of the enterobacterial phytopathogen Erwinia carotovora subsp. atroseptica and characterization of virulence factors. Proc. Natl. Acad. Sci. USA 2004, 101, 11105–11110. [Google Scholar] [CrossRef] [PubMed]
- Gorshkov, V.; Gubaev, R.; Petrova, O.; Daminova, A.; Gogoleva, N.; Ageeva, M.; Parfirova, O.; Prokchorchik, M.; Nikolaichik, Y.; Gogolev, Y. Transcriptome profiling helps to identify potential and true molecular switches of stealth to brute force behavior in Pectobacterium atrosepticum during systemic colonization of tobacco plants. Eur. J. Plant Pathol. 2018, 152, 957–976. [Google Scholar] [CrossRef]
- Panda, P.; Vanga, B.R.; Lu, A.; Fiers, M.; Fineran, P.C.; Butler, R.; Armstrong, K.; Ronson, C.W.; Pitman, A.R. Pectobacterium atrosepticum and Pectobacterium carotovorum harbor distinct, independently acquired integrative and conjugative elements encoding coronafacic acid that enhance virulence on potato stems. Front. Microbiol. 2016, 7, 397. [Google Scholar] [CrossRef]
- Aznar, A.; Dellagi, A. New insights into the role of siderophores as triggers of plant immunity: What can we learn from animals? J. Exp. Bot. 2015, 66, 3001–3010. [Google Scholar] [CrossRef]
- Kramer, J.; Özkaya, Ö.; Kümmerli, R. Bacterial siderophores in community and host interactions. Nat. Rev. Microbiol. 2019, 18, 152–163. [Google Scholar] [CrossRef] [PubMed]
- Holden, V.I.; Bachman, M.A. Diverging roles of bacterial siderophores during infection. Metallomics 2015, 7, 986–995. [Google Scholar] [CrossRef] [PubMed]
- Franza, T.; Expert, D. Role of iron homeostasis in the virulence of phytopathogenic bacteria: An ‘a la carte’menu. Mol. Plant Pathol. 2013, 14, 429–438. [Google Scholar] [CrossRef] [PubMed]
- Marchetti, M.; De Bei, O.; Bettati, S.; Campanini, B.; Kovachka, S.; Gianquinto, E.; Spyrakis, F.; Ronda, L. Iron Metabolism at the Interface between Host and Pathogen: From Nutritional Immunity to Antibacterial Development. Int. J. Mol. Sci. 2020, 21, 2145. [Google Scholar] [CrossRef] [PubMed]
- Bakker, P.A.; Pieterse, C.M.; Van Loon, L.C. Induced systemic resistance by fluorescent Pseudomonas spp. Phytopathology 2007, 97, 239–243. [Google Scholar] [CrossRef]
- Oide, S.; Moeder, W.; Krasnoff, S.; Gibson, D.; Haas, H.; Yoshioka, K.; Turgeon, B.G. NPS6, encoding a nonribosomal peptide synthetase involved in siderophore-mediated iron metabolism, is a conserved virulence determinant of plant pathogenic ascomycetes. Plant Cell 2006, 18, 2836–2853. [Google Scholar] [CrossRef] [PubMed]
- Dellagi, A.; Brisset, M.N.; Paulin, J.P.; Expert, D. Dual role of desferrioxamine in Erwinia amylovora pathogenicity. Mol. Plant Microbe Interact. 1998, 11, 734–742. [Google Scholar] [CrossRef]
- Taguchi, F.; Suzuki, T.; Inagaki, Y.; Toyoda, K.; Shiraishi, T.; Ichinose, Y. The siderophore pyoverdine of Pseudomonas syringae pv. tabaci 6605 is an intrinsic virulence factor in host tobacco infection. J. Bacteriol. 2010, 192, 117–126. [Google Scholar] [CrossRef]
- Ong, S.A.; Peterson, T.; Neilands, J.B. Agrobactin, a siderophore from Agrobacterium tumefaciens. J. Biol. Chem. 1979, 254, 1860–1865. [Google Scholar] [CrossRef]
- Münzinger, M.; Budzikiewicz, H.; Expert, D.; Enard, C.; Meyer, J.M. Achromobactin, a new citrate siderophore of Erwinia chrysanthemi. Z. Naturforsch. C. J. Biosci. 2000, 55, 328–332. [Google Scholar] [CrossRef]
- Persmark, M.; Expert, D.; Neilands, J.B. Isolation, characterization, and synthesis of chrysobactin, a compound with siderophore activity from Erwinia chrysanthemi. J. Biol. Chem. 1989, 264, 3187–3193. [Google Scholar] [CrossRef]
- Enard, C.; Diolez, A.; Expert, D. Systemic virulence of Erwinia chrysanthemi 3937 requires a functional iron assimilation system. J. Bacteriol. 1988, 170, 2419–2426. [Google Scholar] [CrossRef] [PubMed]
- Franza, T.; Mahé, B.; Expert, D. Erwinia chrysanthemi requires a second iron transport route dependent of the siderophore achromobactin for extracellular growth and plant infection. Mol. Microbiol. 2005, 55, 261–275. [Google Scholar] [CrossRef]
- Aznar, A.; Chen, N.W.; Rigault, M.; Riache, N.; Joseph, D.; Desmaële, D.; Mouille, G.; Boutet, S.; Soubigou-Taconnat, L.; Renou, J.P.; et al. Scavenging iron: A novel mechanism of plant immunity activation by microbial siderophores. Plant Physiol. 2014, 164, 2167–2183. [Google Scholar] [CrossRef] [PubMed]
- Neema, C.; Laulhère, J.P.; Expert, D. Iron deficiency induced by chrysobactin in Saintpaulia leaves inoculated with Erwinia chrysanthemi. Plant Physiol. 1993, 102, 967–973. [Google Scholar] [CrossRef]
- Dellagi, A.; Rigault, M.; Segond, D.; Roux, C.; Kraepiel, Y.; Cellier, F.; Briat, J.F.; Gaymard, F.; Expert, D. Siderophore-mediated upregulation of Arabidopsis ferritin expression in response to Erwinia chrysanthemi infection. Plant J. 2005, 43, 262–272. [Google Scholar] [CrossRef] [PubMed]
- Dellagi, A.; Segond, D.; Rigault, M.; Fagard, M.; Simon, C.; Saindrenan, P.; Expert, D. Microbial siderophores exert a subtle role in Arabidopsis during infection by manipulating the immune response and the iron status. Plant Physiol. 2009, 150, 1687–1696. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Segond, D.; Dellagi, A.; Lanquar, V.; Rigault, M.; Patrit, O.; Thomine, S.; Expert, D. NRAMP genes function in Arabidopsis thaliana resistance to Erwinia chrysanthemi infection. Plant J. 2009, 58, 195–207. [Google Scholar] [CrossRef]
- Albarouki, E.; Schafferer, L.; Ye, F.; von Wirén, N.; Haas, H.; Deising, H.B. Biotrophy-specific downregulation of siderophore biosynthesis in Colletotrichum graminicola is required for modulation of immune responses of maize. Mol. Microbiol. 2014, 92, 338–355. [Google Scholar] [CrossRef] [PubMed]
- Ran, L.X.; Li, Z.N.; Wu, G.J.; Van Loon, L.C.; Bakker, P.H. Induction of systemic resistance against bacterial wilt in Eucalyptus urophylla by fluorescent Pseudomonas spp. Eur. J. Plant Pathol. 2005, 113, 59–70. [Google Scholar] [CrossRef]
- Kieu, N.P.; Aznar, A.; Segond, D.; Rigault, M.; Simond-Cote, E.; Kunz, C.; Soulie, M.C.; Expert, D.; Dellagi, A. Iron deficiency affects plant defence responses and confers resistance to Dickeya dadantii and Botrytis cinerea. Molec. Plant Pathol. 2012, 13, 816–827. [Google Scholar] [CrossRef] [PubMed]
- Khan, A.; Singh, P.; Srivastava, A. Synthesis, nature and utility of universal iron chelator–Siderophore: A review. Microbiol. Res. 2018, 212, 103–111. [Google Scholar] [CrossRef] [PubMed]
- Tsers, I.; Gorshkov, V.; Gogoleva, N.; Parfirova, O.; Petrova, O.; Gogolev, Y. Plant Soft Rot Development and Regulation from the Viewpoint of Transcriptomic Profiling. Plants 2020, 9, 1176. [Google Scholar] [CrossRef]
- Achard, M.E.; Chen, K.W.; Sweet, M.J.; Watts, R.E.; Schroder, K.; Schembri, M.A.; McEwan, A.G. An antioxidant role for catecholate siderophores in Salmonella. Biochem. J. 2013, 454, 543–549. [Google Scholar] [CrossRef] [PubMed]
- Adler, C.; Corbalan, N.S.; Peralta, D.R.; Pomares, M.F.; de Cristóbal, R.E.; Vincent, P.A. The alternative role of enterobactin as an oxidative stress protector allows Escherichia coli colony development. PLoS ONE 2014, 9, e84734. [Google Scholar] [CrossRef] [PubMed]
- Boughammoura, A.; Franza, T.; Dellagi, A.; Roux, C.; Matzanke-Markstein, B.; Expert, D. Ferritins, bacterial virulence and plant defence. Biometals 2007, 20, 347. [Google Scholar] [CrossRef]
- Peralta, D.R.; Adler, C.; Corbalán, N.S.; Paz García, E.C.; Pomares, M.F.; Vincent, P.A. Enterobactin as part of the oxidative stress response repertoire. PLoS ONE 2016, 11, e0157799. [Google Scholar] [CrossRef] [PubMed]
- Cody, Y.S.; Gross, D.C. Outer membrane protein mediating iron uptake via pyoverdinpss, the fluorescent siderophore produced by Pseudomonas syringae pv. syringae. J. Bacteriol. 1987, 169, 2207–2214. [Google Scholar] [CrossRef]
- Jones, A.M.; Wildermuth, M.C. The phytopathogen Pseudomonas syringae pv. tomato DC3000 has three high-affinity iron-scavenging systems functional under iron limitation conditions but dispensable for pathogenesis. J. Bacteriol. 2011, 193, 2767–2775. [Google Scholar] [CrossRef]
- Rondon, M.R.; Ballering, K.S.; Thomas, M.G. Identification and analysis of a siderophore biosynthetic gene cluster from Agrobacterium tumefaciens C58. Microbiology 2004, 150, 3857–3866. [Google Scholar] [CrossRef]
- Hassan, S.; Shevchik, V.E.; Robert, X.; Hugouvieux-Cotte-Pattat, N. PelN is a new pectate lyase of Dickeya dadantii with unusual characteristics. J. Bacteriol. 2013, 95, 2197–2206. [Google Scholar] [CrossRef] [PubMed]
- Vlot, A.C.; Dempsey, D.M.A.; Klessig, D.F. Salicylic acid, a multifaceted hormone to combat disease. Annu. Rev. Phytopathol. 2009, 47, 177–206. [Google Scholar] [CrossRef]
- Czajkowski, R.; van der Wolf, J.M.; Krolicka, A.; Ozymko, Z.; Narajczyk, M.; Kaczynska, N.; Lojkowska, E. Salicylic acid can reduce infection symptoms caused by Dickeya solani in tissue culture grown potato (Solanum tuberosum L.) plants. Europ. J. Plant Pathol. 2015, 141, 545–558. [Google Scholar] [CrossRef][Green Version]
- Palva, T.K.; Hurtig, M.; Saindrenan, P.; Palva, E.T. Salicylic acid induced resistance to Erwinia carotovora subsp. carotovora in tobacco. Mol. Plant Microbe Interact. 1994, 7, 356–363. [Google Scholar] [CrossRef]
- Quan, J.; Tian, J. Circular polymerase extension cloning of complex gene libraries and pathways. PLoS ONE 2009, 4, e6441. [Google Scholar] [CrossRef] [PubMed]
- Shevchik, V.E.; Robert-Baudouy, J.; Hugouvieux-Cotte-Pattat, N. Pectate lyase PelI of Erwinia chrysanthemi 3937 belongs to a new family. J. Bacteriol. 1997, 179, 7321–7330. [Google Scholar] [CrossRef]
- Miller, G.L. Use of dinitrosalicylic acid reagent for determination of reducing sugar. Anal. Chem. 1959, 31, 426–428. [Google Scholar] [CrossRef]
- Seneesrisakul, K.; Guralp, S.A.; Gulari, E.; Chavadej, S. Escherichia coli expressing endoglucanase gene from Thai higher termite bacteria for enzymatic and microbial hydrolysis of cellulosic materials. Electro. J. Biotechnol. 2017, 27, 70–79. [Google Scholar] [CrossRef]
- Kühnel, S.; Schols, H.A.; Gruppen, H. Aiming for the complete utilization of sugar-beet pulp: Examination of the effects of mild acid and hydrothermal pretreatment followed by enzymatic digestion. Biotechnol. Biofuels 2011, 4, 1–14. [Google Scholar] [CrossRef] [PubMed]
- Wrolstad, R.E. Current Protocols in Food Analytical Chemistry; Wiley: Hoboken, NJ, USA, 2001. [Google Scholar]
- Bellincampi, D.; Dipierro, N.; Salvi, G.; Cervone, F.; De Lorenzo, G. Extracellular H2O2 induced by oligogalacturonides is not involved in the inhibition of the auxin-regulated rolB gene expression in tobacco leaf explants. Plant Physiol. 2000, 122, 1379–1386. [Google Scholar] [CrossRef] [PubMed]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gorshkov, V.; Parfirova, O.; Petrova, O.; Gogoleva, N.; Kovtunov, E.; Vorob’ev, V.; Gogolev, Y. The Knockout of Enterobactin-Related Gene in Pectobacterium atrosepticum Results in Reduced Stress Resistance and Virulence towards the Primed Plants. Int. J. Mol. Sci. 2021, 22, 9594. https://doi.org/10.3390/ijms22179594
Gorshkov V, Parfirova O, Petrova O, Gogoleva N, Kovtunov E, Vorob’ev V, Gogolev Y. The Knockout of Enterobactin-Related Gene in Pectobacterium atrosepticum Results in Reduced Stress Resistance and Virulence towards the Primed Plants. International Journal of Molecular Sciences. 2021; 22(17):9594. https://doi.org/10.3390/ijms22179594
Chicago/Turabian StyleGorshkov, Vladimir, Olga Parfirova, Olga Petrova, Natalia Gogoleva, Evgeny Kovtunov, Vladimir Vorob’ev, and Yuri Gogolev. 2021. "The Knockout of Enterobactin-Related Gene in Pectobacterium atrosepticum Results in Reduced Stress Resistance and Virulence towards the Primed Plants" International Journal of Molecular Sciences 22, no. 17: 9594. https://doi.org/10.3390/ijms22179594
APA StyleGorshkov, V., Parfirova, O., Petrova, O., Gogoleva, N., Kovtunov, E., Vorob’ev, V., & Gogolev, Y. (2021). The Knockout of Enterobactin-Related Gene in Pectobacterium atrosepticum Results in Reduced Stress Resistance and Virulence towards the Primed Plants. International Journal of Molecular Sciences, 22(17), 9594. https://doi.org/10.3390/ijms22179594




