Adjunctive Thymosin Beta-4 Treatment Influences MΦ Effector Cell Function to Improve Disease Outcome in Pseudomonas aeruginosa-Induced Keratitis
Abstract
:1. Introduction
2. Results
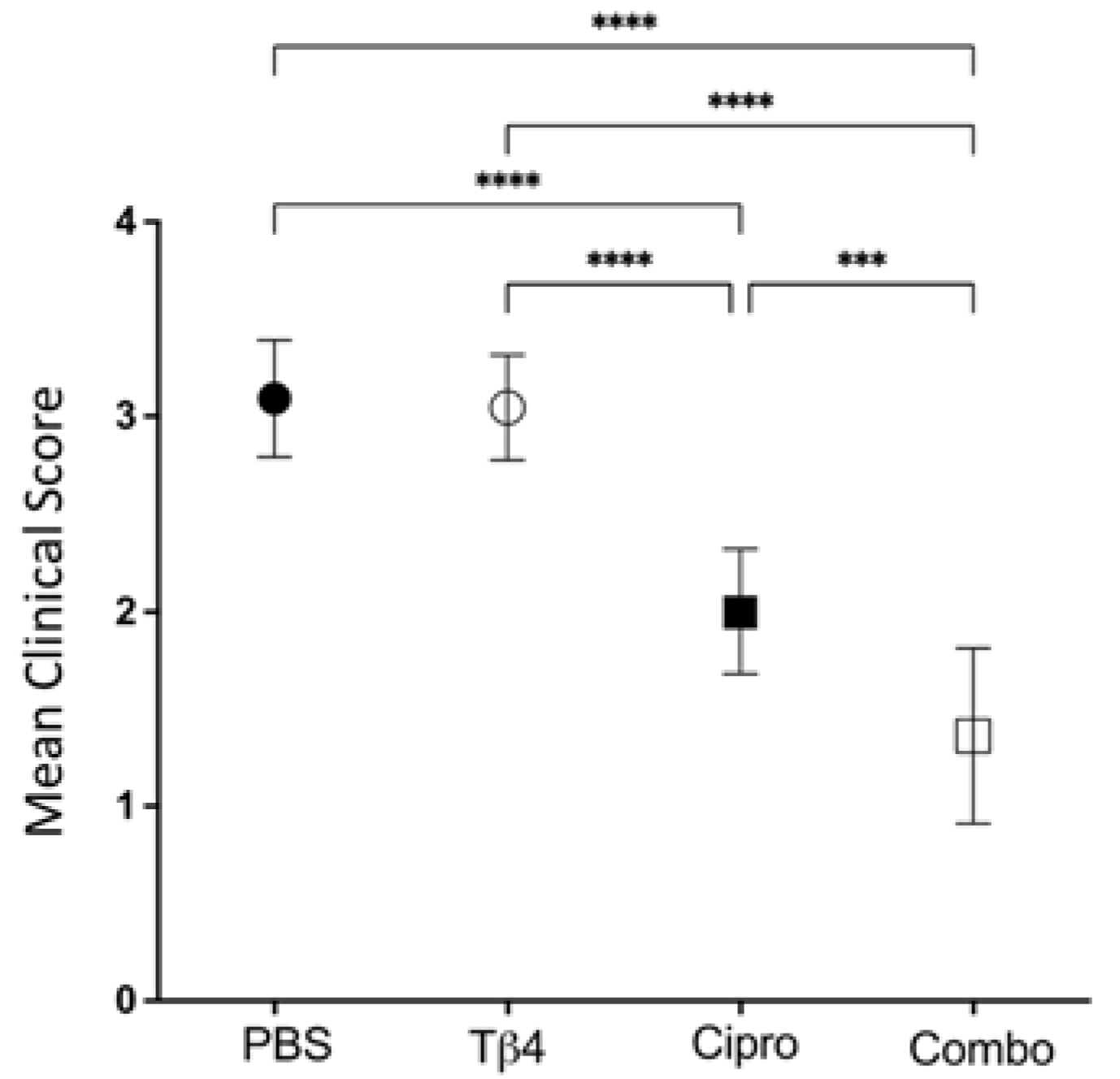
2.1. Disease Response following P. aeruginosa-Induced Infection in B6 Mice
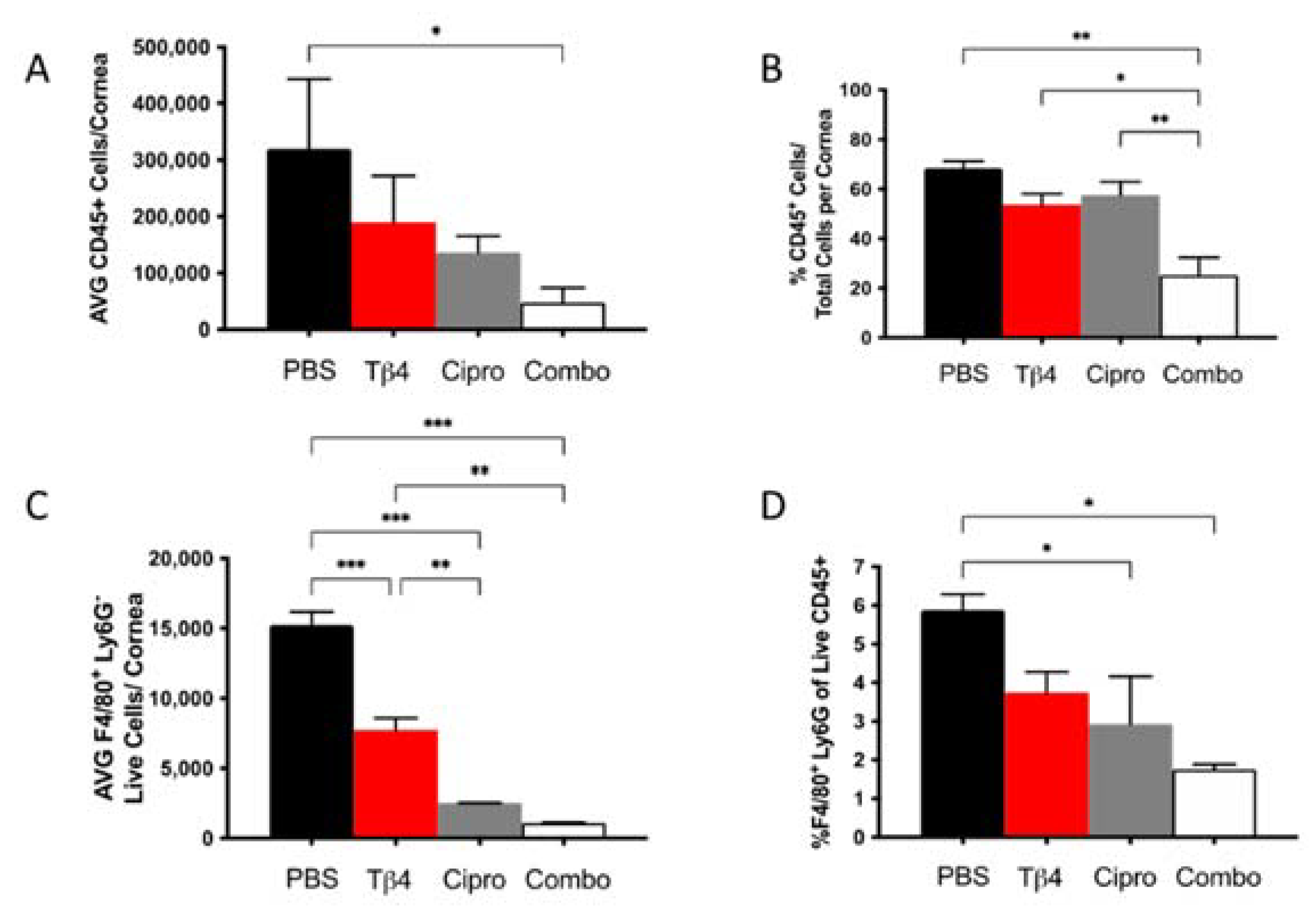
2.2. Flow Cytometric Analyses of MΦ Infiltrates in P. aeruginosa-Infected B6 Mice
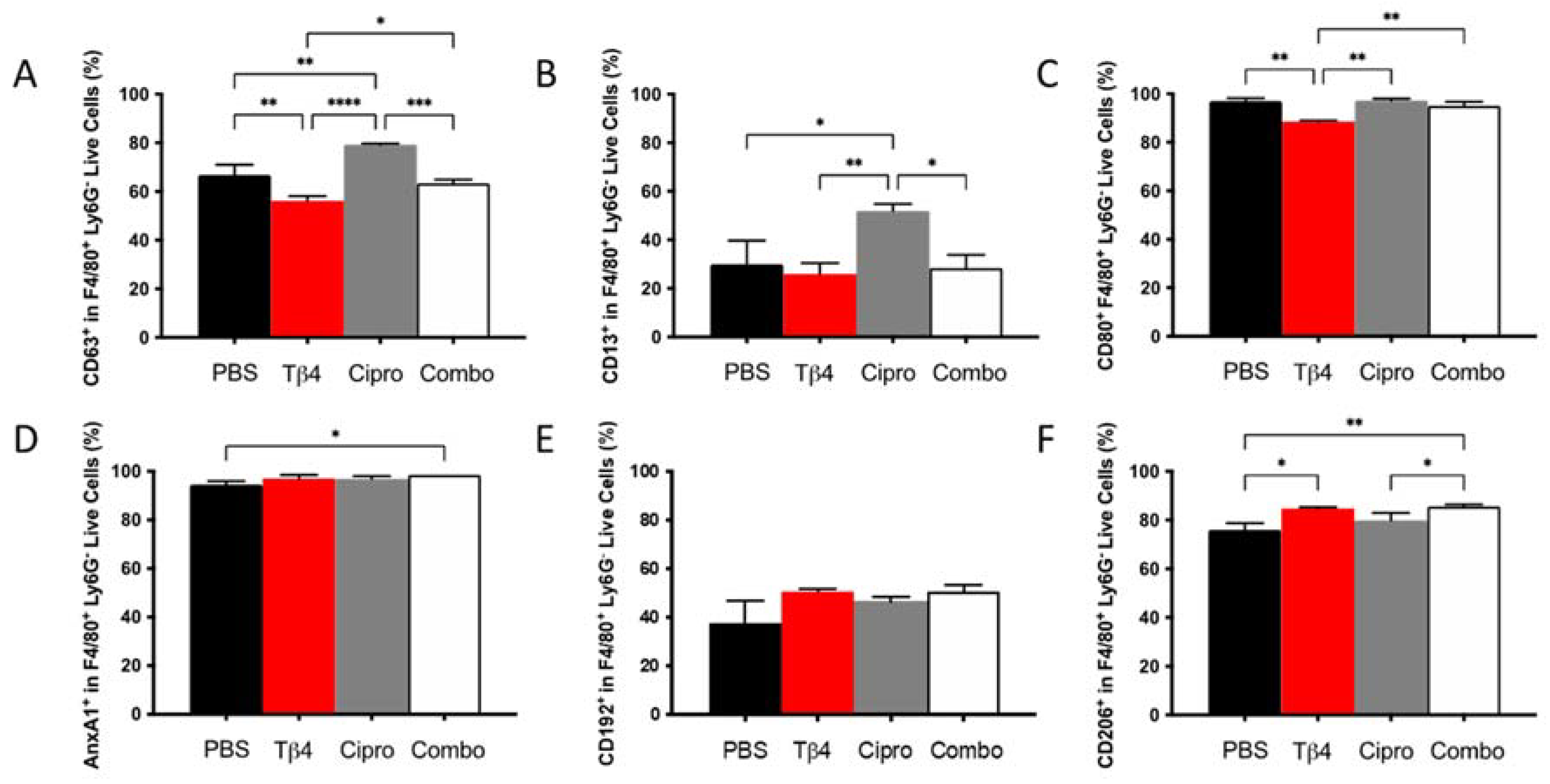
2.3. Adjunctive Tβ4 Treatment Influences Phenotypic Profiles of MΦ Infiltrates in the Infected Cornea
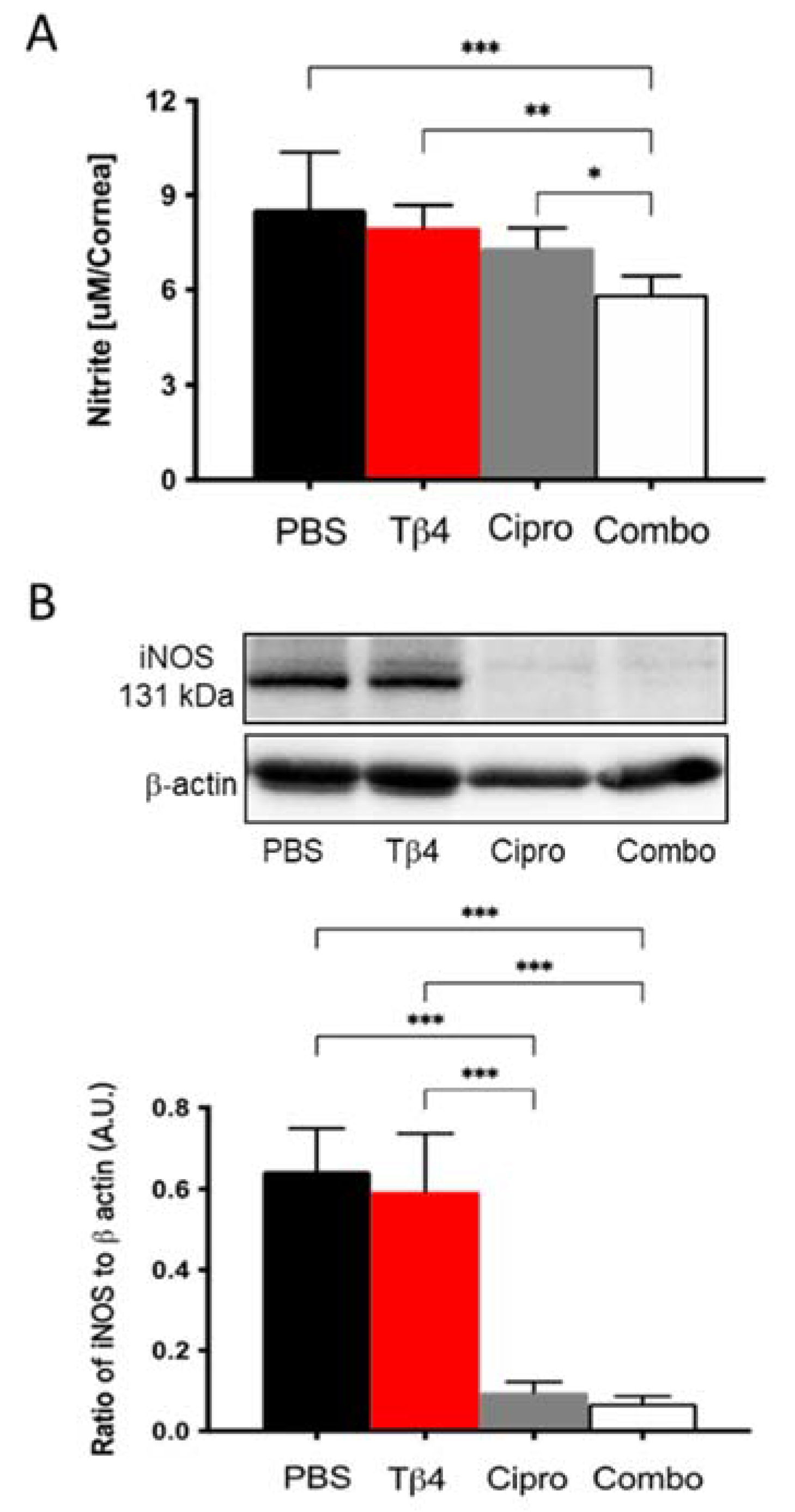
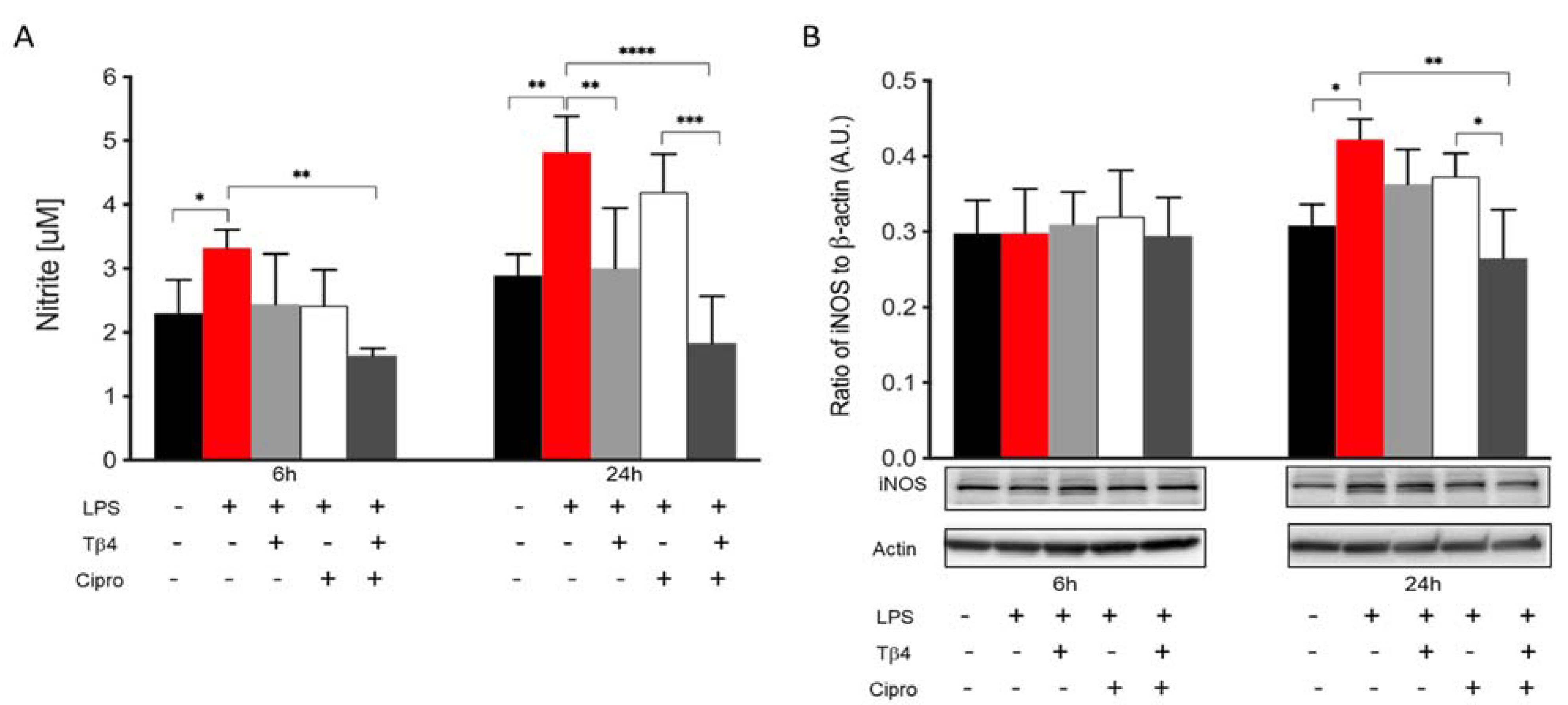
2.4. Adjunctive Tβ4 Treatment Modulates the Inflammatory Response by Inhibiting RNS Generation
2.5. Tβ4 Inhibits NO and iNOS Generation In Vitro
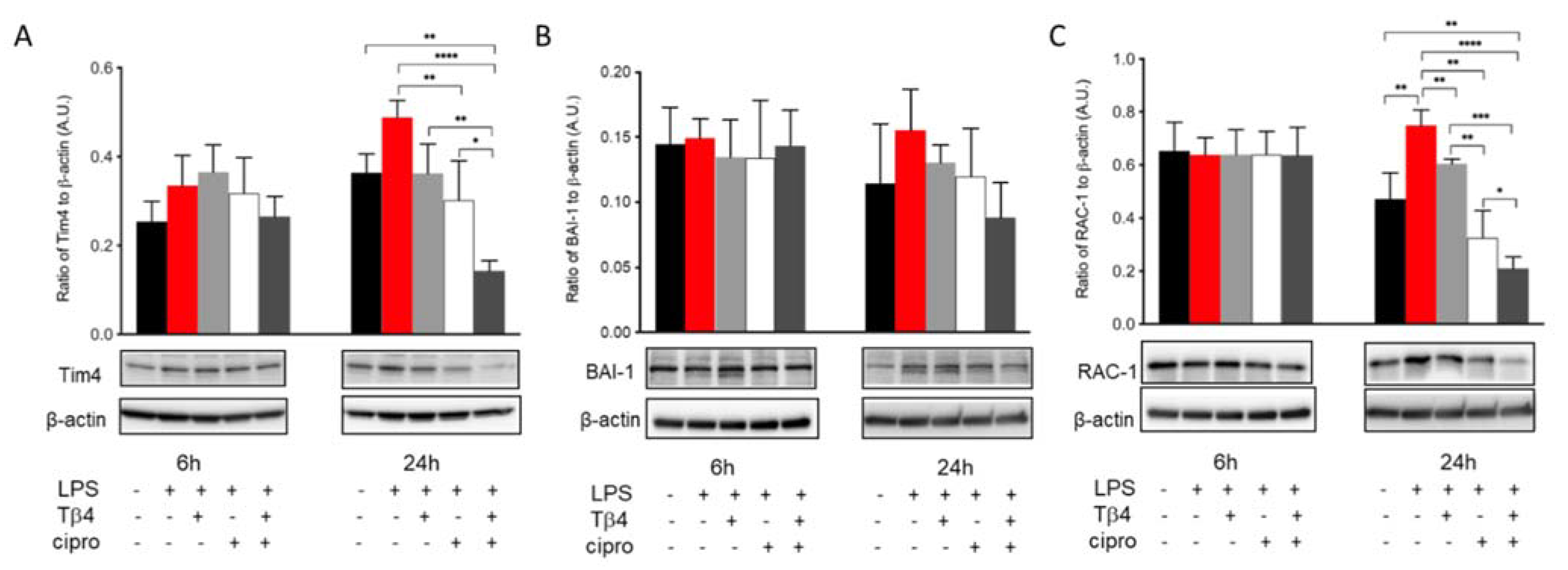
2.6. Adjunctive Tβ4 Treatment Influences Markers of Efferocytosis to Improve Immune Response
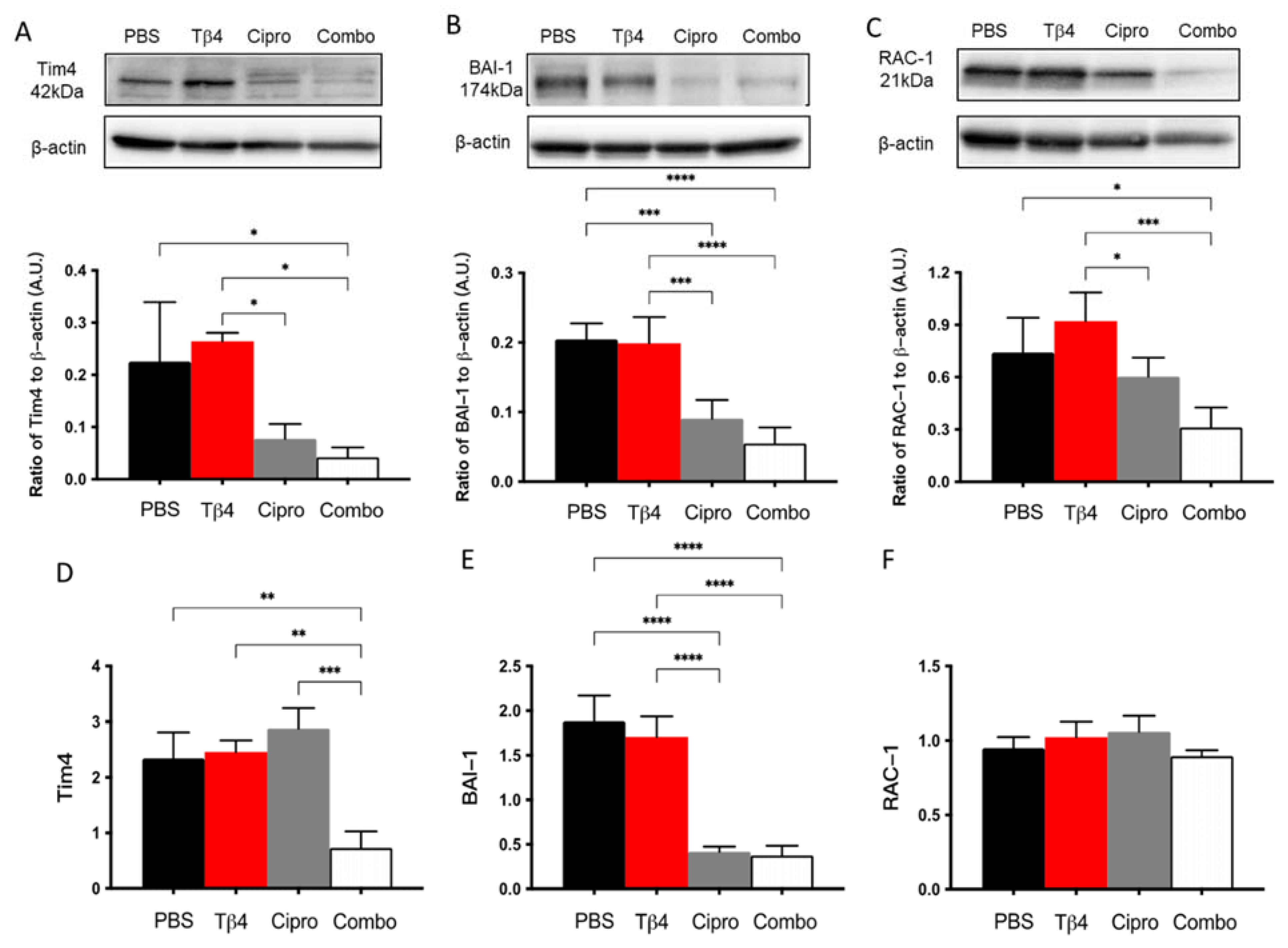
2.7. Tβ4 Treatment Suppresses Markers of MΦ Efferocytosis Activity In Vitro
3. Discussion
4. Materials and Methods
4.1. Experimental Animal Protocol
4.2. Clinical Scoring
4.3. Cell Culture and Treatment
4.4. Flow Cytometric Analyses
4.5. Griess Reaction
4.6. Western Blot
4.7. Real-Time RT-PCR
4.8. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Acknowledgments
Conflicts of Interest
References
- Lakhundi, S.; Siddiqui, R.; Khan, N.A. Pathogenesis of microbial keratitis. Microb. Pathog. 2017, 104, 97–109. [Google Scholar] [CrossRef] [Green Version]
- Chang, V.S.; Dhaliwal, D.K.; Raju, L.; Kowalski, R.P. Antibiotic resistance in the treatment of staphylococcus aureus keratitis: A 20-year review. Cornea 2015, 34, 698–703. [Google Scholar] [CrossRef] [Green Version]
- Lichtinger, A.; Yeung, S.N.; Kim, P.; Amiran, M.D.; Iovieno, A.; Elbaz, U.; Ku, J.Y.; Wolff, R.; Rootman, D.S.; Slomovic, A.R. Shifting trends in bacterial keratitis in Toronto: An 11-year review. Ophthalmology 2012, 119, 1785–1790. [Google Scholar] [CrossRef]
- Burton, M.J.; Ramke, J.; Marques, A.P.; Bourne, R.R.A.; Congdon, N.; Jones, I.; Tong, B.A.M.A.; Arunga, S.; Bachani, D.; Bascaran, C.; et al. The lancet global health commission on global eye health: Vision beyond 2020. Lancet Glob. Health 2021, 9, e489–e551. [Google Scholar] [CrossRef]
- Flaxman, S.R.; Bourne, R.R.; Resnikoff, S.; Ackland, P.; Braithwaite, T.; Cicinelli, M.V.; Das, A.; Jonas, J.B.; Keeffe, J.; Kempen, J.H.; et al. Global causes of blindness and distance vision impairment 1990–2020: A systematic review and meta-analysis. Lancet Glob Health 2017, 5, e1221–e1234. [Google Scholar] [CrossRef] [Green Version]
- Sosa, A.B.; Epstein, S.P.; Asbell, P. Evaluation of Toxicity of Commercial Ophthalmic Fluoroquinolone Antibiotics as Assessed on Immortalized Corneal and Conjunctival Epithelial Cells. Cornea 2008, 27, 930–934. [Google Scholar] [CrossRef]
- Oum, B.S.; Kim, N.M.; Lee, J.S.; Park, Y.M. Effects of Fluoroquinolone Eye Solutions without Preservatives on Human Corneal Epithelial Cells In Vitro. Ophthalmic Res. 2014, 51, 216–223. [Google Scholar] [CrossRef]
- Berger, E.A.; McClellan, S.A.; Vistisen, K.S.; Hazlett, L.D. HIF-1α Is Essential for Effective PMN Bacterial Killing, Antimicrobial Peptide Production and Apoptosis in Pseudomonas aeruginosa Keratitis. PLoS Pathog. 2013, 9, e1003457. [Google Scholar] [CrossRef] [PubMed]
- Tallab, R.T.; Stone, D.U. Corticosteroids as a therapy for bacterial keratitis: An evidence-based review of ‘who, when and why’. Br. J. Ophthalmol. 2016, 100, 731–735. [Google Scholar] [CrossRef] [PubMed]
- Hirano, K.; Tanaka, H.; Kato, K.; Araki-Sasaki, K. Topical Corticosteroids for Infectious Keratitis before Culture-Proven Diagnosis. Clin. Ophthalmol. 2021, 15, 609–616. [Google Scholar] [CrossRef] [PubMed]
- Goldstein, A.L.; Hannappel, E.; Sosne, G.; Kleinman, H.K. Thymosin beta4: A multi-functional regenerative peptide. Basic properties and clinical applications. Expert Opin. Biol. Ther. 2012, 12, 37–51. [Google Scholar] [CrossRef]
- Sosne, G.; Kleinman, H.K. Primary mechanisms of thymosin beta4 repair activity in dry eye disorders and other tissue injuries. Invest. Ophthalmol. Vis. Sci. 2015, 56, 5110–5117. [Google Scholar] [CrossRef] [Green Version]
- Qiu, P.; Wheater, M.K.; Qiu, Y.; Sosne, G. Thymosin beta4 inhibits TNF-alpha-induced NF-kappaB activation, IL-8 expression, and the sensitizing effects by its partners PINCH-1 and ILK. FASEB J. 2011, 25, 1815–1826. [Google Scholar] [CrossRef] [Green Version]
- Sosne, G.; Qiu, P.; Kurpakus-Wheater, M. Thymosin beta 4: A novel corneal wound healing and anti-inflammatory agent. Clin. Ophthalmol. 2007, 1, 201–207. [Google Scholar] [PubMed]
- Dunn, S.P.; Heidemann, D.G.; Chow, C.Y.C.; Crockford, D.; Turjman, N.; Angel, J.; Allan, C.B.; Sosne, G. Treatment of Chronic Nonhealing Neurotrophic Corneal Epithelial Defects with Thymosin Beta 4. Arch. Ophthalmol. 2010, 128, 636–638. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sosne, G.; Dunn, S.P.; Kim, C. Thymosin beta4 significantly improves signs and symptoms of severe dry eye in a phase 2 randomized trial. Cornea 2015, 34, 491–496. [Google Scholar] [CrossRef]
- Sosne, G.; Ousler, G.W. Thymosin beta 4 ophthalmic solution for dry eye: A randomized, placebo-controlled, Phase II clinical trial conducted using the controlled adverse environment (CAE) model. Clin. Ophthalmol. 2015, 9, 877–884. [Google Scholar] [PubMed] [Green Version]
- Sosne, G.; Rimmer, D.; Kleinman, H.K.; Ousler, G. Thymosin Beta 4: A Potential Novel Therapy for Neurotrophic Keratopathy, Dry Eye, and Ocular Surface Diseases. Vitam. Horm. 2016, 102, 277–306. [Google Scholar] [PubMed]
- Freire, M.O.; van Dyke, T.E. Natural resolution of inflammation. Periodontology 2000, 63, 149–164. [Google Scholar] [CrossRef] [Green Version]
- Hume, D.A. The mononuclear phagocyte system. Curr. Opin. Immunol. 2006, 18, 49–53. [Google Scholar] [CrossRef]
- Ma, W.-T.; Gao, F.; Gu, K.; Chen, D.-K. The Role of Monocytes and Macrophages in Autoimmune Diseases: A Comprehensive Review. Front. Immunol. 2019, 10, 1140. [Google Scholar] [CrossRef] [Green Version]
- Fujiwara, N.; Kobayashi, K. Macrophages in inflammation. Curr. Drug Targets Inflamm. Allergy 2005, 4, 281–286. [Google Scholar] [CrossRef] [PubMed]
- Greenlee-Wacker, M.C. Clearance of apoptotic neutrophils and resolution of inflammation. Immunol. Rev. 2016, 273, 357–370. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Daheshia, M.; Kanangat, S.; Rouse, B.T. Production of Key Molecules by Ocular Neutrophils Early after Herpetic Infection of the Cornea. Exp. Eye Res. 1998, 67, 619–624. [Google Scholar] [CrossRef]
- Thomas, J.; Gangappa, S.; Kanangat, S.; Rouse, B.T. On the essential involvement of neutrophils in the immunopathologic disease: Herpetic stromal keratitis. J. Immunol. 1997, 158, 1383–1391. [Google Scholar] [PubMed]
- Rutkowski, R.; Pancewicz, S.A.; Rutkowski, K.; Rutkowska, J. Reactive oxygen and nitrogen species in inflammatory process. Pol. Merkur. Lekarski. 2007, 23, 131–136. [Google Scholar] [PubMed]
- Carion, T.W.; Ebrahim, A.S.; Alluri, S.; Ebrahim, T.; Parker, T.; Burns, J.; Sosne, G.; Berger, E.A. Antimicrobial Effects of Thymosin Beta-4 and Ciprofloxacin Adjunctive Therapy in Pseudomonas aeruginosa Induced Keratitis. Int. J. Mol. Sci. 2020, 21, 6840. [Google Scholar] [CrossRef]
- Carion, T.W.; Ebrahim, A.S.; Kracht, D.; Agrawal, A.; Strand, E.; Kaddurah, O.; McWhirter, C.R.; Sosne, G.; Berger, E.A. Thymosin Beta-4 and Ciprofloxacin Adjunctive Therapy Improves Pseudomonas aeruginosa-Induced Keratitis. Cells 2018, 7, 145. [Google Scholar] [CrossRef] [Green Version]
- Hazlett, L.D.; McClellan, S.; Goshgarian, C.; Huang, X.; Thakur, A.; Barrett, R. The role of nitric oxide in resistance to P. aeruginosa ocular infection. Ocul. Immunol. Inflamm. 2005, 13, 279–288. [Google Scholar] [CrossRef]
- Lemke, G. How macrophages deal with death. Nat. Rev. Immunol. 2019, 19, 539–549. [Google Scholar] [CrossRef]
- Hayat, S.M.G.; Bianconi, V.; Pirro, M.; Sahebkar, A. Efferocytosis: Molecular mechanisms and pathophysiological perspectives. Immunol. Cell Biol. 2018, 97, 124–133. [Google Scholar] [CrossRef]
- Filiberti, A.; Gmyrek, G.B.; Berube, A.N.; Royer, D.J.; Carr, D.J.J. An intact complement system dampens cornea inflammation during acute primary HSV-1 infection. Sci. Rep. 2021, 11, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Sosne, G.; Szliter, E.A.; Barrett, R.; Kernacki, K.A.; Kleinman, H.; Hazlett, L.D. Thymosin Beta 4 Promotes Corneal Wound Healing and Decreases Inflammation In Vivo Following Alkali Injury. Exp. Eye Res. 2002, 74, 293–299. [Google Scholar] [CrossRef]
- Hu, J.; Wang, Y.; Xie, L. Potential Role of Macrophages in Experimental Keratomycosis. Investig. Opthalmology Vis. Sci. 2009, 50, 2087–2094. [Google Scholar] [CrossRef] [Green Version]
- Sharma, J.N.; Al-Omran, A.; Parvathy, S.S. Role of nitric oxide in inflammatory diseases. Inflammopharmacology 2007, 15, 252–259. [Google Scholar] [CrossRef]
- Goureau, O.; Bellot, J.; Thillaye, B.; Courtois, Y.; De Kozak, Y. Increased nitric oxide production in endotoxin-induced uveitis. Reduction of uveitis by an inhibitor of nitric oxide synthase. J. Immunol. 1995, 154, 6518–6523. [Google Scholar]
- Duran, N.; Koc, A.; Oksuz, H.; Tamer, C.; Akaydin, Y.; Kozlu, T.; Celik, M. The protective role of topical propolis on experimental keratitis via nitric oxide levels in rabbits. Mol. Cell. Biochem. 2006, 281, 153–161. [Google Scholar] [CrossRef] [PubMed]
- Fialkow, L.; Wang, Y.; Downey, G.P. Reactive oxygen and nitrogen species as signaling molecules regulating neutrophil function. Free. Radic. Biol. Med. 2007, 42, 153–164. [Google Scholar] [CrossRef] [PubMed]
- Gordon, S.; Plüddemann, A. Macrophage Clearance of Apoptotic Cells: A Critical Assessment. Front. Immunol. 2018, 9, 127. [Google Scholar] [CrossRef] [Green Version]
- Hodge, S.; Hodge, G.; Brozyna, S.; Jersmann, H.; Holmes, M.; Reynolds, P.N. Azithromycin increases phagocytosis of apoptotic bronchial epithelial cells by alveolar macrophages. Eur. Respir. J. 2006, 28, 486–495. [Google Scholar] [CrossRef]
- Hodge, S.; Hodge, G.; Ahern, J.; Jersmann, H.; Holmes, M.; Reynolds, P.N. Smoking alters alveolar macrophage recognition and phagocytic ability: Implications in chronic obstructive pulmonary disease. Am. J. Respir. Cell Mol. Biol. 2007, 37, 748–755. [Google Scholar] [CrossRef]
- Hodge, S.; Hodge, G.; Scicchitano, R.; Reynolds, P.N.; Holmes, M. Alveolar macrophages from subjects with chronic obstructive pulmonary disease are deficient in their ability to phagocytose apoptotic airway epithelial cells. Immunol. Cell Biol. 2003, 81, 289–296. [Google Scholar] [CrossRef]
- Grabiec, A.; Hussell, T. The role of airway macrophages in apoptotic cell clearance following acute and chronic lung inflammation. Semin. Immunopathol. 2016, 38, 409–423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yang, H.M.; Kang, S.W.; Sung, J.; Kim, K.; Kleinman, H. Purinergic Signaling Involvement in Thymosin beta4-mediated Corneal Epithelial Cell Migration. Curr. Eye Res. 2020, 45, 1352–1358. [Google Scholar] [CrossRef] [PubMed]
- Di Virgilio, F.; Ben, D.D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- de Torre-Minguela, C.; Barberà-Cremades, M.; Gómez, A.I.; Martín-Sánchez, F.; Pelegrín, P. Macrophage activation and polarization modify P2X7 receptor secretome influencing the inflammatory process. Sci. Rep. 2016, 6, 22586. [Google Scholar] [CrossRef]
- Rudner, X.L.; Kernacki, K.A.; Barrett, R.P.; Hazlett, L.D. Prolonged elevation of IL-1 in Pseudomonas aeruginosa ocular infection regulates macrophage-inflammatory protein-2 production, polymorphonuclear neutrophil persistence, and corneal perforation. J. Immunol. 2000, 164, 6576–6582. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hazlett, L.D.; Moon, M.M.; Strejc, M.; Berk, R.S. Evidence for N-acetylmannosamine as an ocular receptor for P. aeruginosa adherence to scarified cornea. Investig. Ophthalmol. Vis. Sci. 1987, 28, 1978–1985. [Google Scholar]
- Carion, T.W.; Greenwood, M.; Ebrahim, A.S.; Jerome, A.; Suvas, S.; Gronert, K.; Berger, E.A. Immunoregulatory role of 15-lipoxygenase in the pathogenesis of bacterial keratitis. FASEB J. 2018, 32, 5026–5038. [Google Scholar] [CrossRef]
- Roux, K.H. Optimization and troubleshooting in PCR. PCR Methods Appl. 1995, 4, S185–S194. [Google Scholar] [CrossRef] [Green Version]
| Gene | Nucleotide Sequence | Primer |
|---|---|---|
| β-actin | 5′-ACTGGGAGACATGGAGAAG-3′ | F |
| 5′-GTCTCCGGAGTCCATCACAA-3′ | R | |
| Tim4 | 5′-GGGTGTACTGCTGCCGTATA-3′ | F |
| 5′-TCACTGCTGTACTGAAGGCA-3′ | R | |
| BAI-1 | 5′-CACTTGCTTACCCACCCTTG-3′ | F |
| 5′-AGCTCATCCCCAAACTCCTC-3′ | R | |
| RAC-1 | 5′-GCTCATCAGTTACACGACCA-3′ | F |
| 5′-GTAGGAGAGGGGACGCAATC-3′ | R |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wang, Y.; Carion, T.W.; Ebrahim, A.S.; Sosne, G.; Berger, E.A. Adjunctive Thymosin Beta-4 Treatment Influences MΦ Effector Cell Function to Improve Disease Outcome in Pseudomonas aeruginosa-Induced Keratitis. Int. J. Mol. Sci. 2021, 22, 11016. https://doi.org/10.3390/ijms222011016
Wang Y, Carion TW, Ebrahim AS, Sosne G, Berger EA. Adjunctive Thymosin Beta-4 Treatment Influences MΦ Effector Cell Function to Improve Disease Outcome in Pseudomonas aeruginosa-Induced Keratitis. International Journal of Molecular Sciences. 2021; 22(20):11016. https://doi.org/10.3390/ijms222011016
Chicago/Turabian StyleWang, Yuxin, Thomas W. Carion, Abdul Shukkur Ebrahim, Gabriel Sosne, and Elizabeth A. Berger. 2021. "Adjunctive Thymosin Beta-4 Treatment Influences MΦ Effector Cell Function to Improve Disease Outcome in Pseudomonas aeruginosa-Induced Keratitis" International Journal of Molecular Sciences 22, no. 20: 11016. https://doi.org/10.3390/ijms222011016






