Autoinflammatory Diseases and Cytokine Storms—Imbalances of Innate and Adaptative Immunity
Abstract
:1. Introduction
2. Autoinflammatory Disease: Since the Beginning of ’90s a New Branch of Medicine
2.1. Role of the Inflammasome in the Pathogenesis of Autoinflammatory Diseases
2.2. Cytokines in the Auto-Inflammatory Diseases
3. Hyperinflammation and “Cytokine Storm”, Uncontrolled Immune Responses
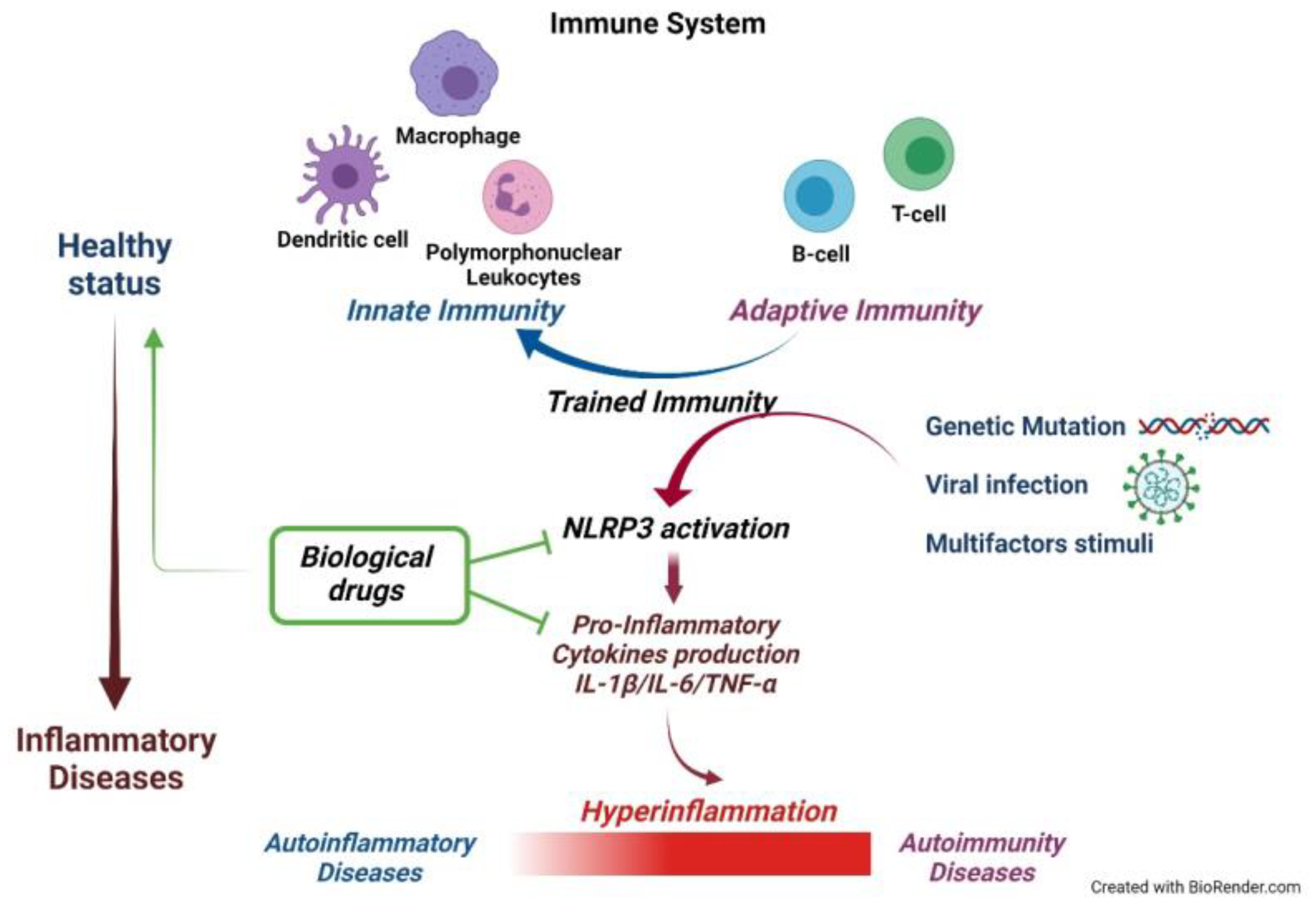
4. A New Immunity Component: Trained Immunity. What Is Its Role in Autoinflammatory Diseases and Cytokine Storms?
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Hedrich, C.M. Shaping the spectrum—From autoinflammation to autoimmunity. Clin Immunol. 2016, 165, 21–28. [Google Scholar] [CrossRef]
- Kanazawa, N.; Tchernev, G.; Wollina, U. Autoimmunity versus autoinflammation—Friend or foe? Wiener Medizinische Wochenschrift 2014, 164, 274–277. [Google Scholar] [CrossRef]
- Place, D.E.; Kanneganti, T.D. The innate immune system and cell death in autoinflammatory and autoimmune disease. Curr. Opin. Immunol. 2020, 67, 95–105. [Google Scholar] [CrossRef]
- Nigrovic, P.A.; Lee, P.Y.; Hoffman, H.M. Monogenic autoinflammatory disorders: Conceptual overview, phenotype, and clinical approach. J. Allergy Clin. Immunol. 2020, 146, 925–937. [Google Scholar] [CrossRef]
- Doria, A.; Zen, M.; Bettio, S.; Gatto, M.; Bassi, N.; Nalotto, L.; Ghirardello, A.; Iaccarino, L.; Punzi, L. Autoinflammation and autoimmunity: Bridging the divide. Autoimmun. Rev. 2012, 12, 22–30. [Google Scholar] [CrossRef]
- Conrad, K.; Shoenfeld, Y.; Fritzler, M.J. Precision health: A pragmatic approach to understanding and addressing key factors in autoimmune diseases. Autoimmun. Rev. 2020, 19, 102508. [Google Scholar] [CrossRef]
- Arakelyan, A.; Nersisyan, L.; Poghosyan, D.; Khondkaryan, L.; Hakobyan, A.; Löf-fler-Wirth, H.; Melanitou, E.; Binder, H. Autoimmunity and autoinflammation: A systems view on signaling pathway dysregulation profiles. PLoS ONE 2017, 12, e0187572. [Google Scholar] [CrossRef] [PubMed]
- Almeida de Jesus, A.; Goldbach-Mansky, R. Monogenic autoinflammatory diseases: Concept and clinical manifestations. Clin. Immunol. 2013, 147, 155–174. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Schnappauf, O.; Chae, J.J.; Kastner, D.; Aksentijevich, I. The Pyrin Inflammasome in health and disease. Front. Immunol. 2019, 10, 1745. [Google Scholar] [CrossRef]
- Bettiol, A.; Lopalco, G.; Emmi, G.; Cantarini, L.; Urban, M.L.; Vitale, A.; Denora, N.; Lopalco, A.; Cutrignelli, A.; Lopedota, A.; et al. Unveiling the Efficacy, Safety, and Tolerability of An-ti-Interleukin-1 Treatment in Monogenic and Multifactorial Autoinflammatory Diseases. Int. J. Mol. Sci. 2019, 20, 1898. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- McGonagle, D.; Savic, S.; McDermott, M.F. The NLR network and the immunologi-cal disease continuum of adaptive and innate immune-mediated inflammation against self. Semin. Immunopathol. 2007, 29, 303–313. [Google Scholar] [CrossRef]
- Ozen, S. What’s new in autoinflammation? Pediatr. Nephrol. 2019, 34, 2449–2456. [Google Scholar] [CrossRef]
- Tuttolomondo, A.; Di Raimondo, D.; Pecoraro, R.; Arnao, V.; Pinto, A.; Licata, G. Atherosclerosis as an inflammatory disease. Curr. Pharm. Des. 2012, 18, 4266–4288. [Google Scholar] [CrossRef]
- Erbel, C.; Akhavanpoor, M.; Okuyucu, D.; Wangler, S.; Dietz, A.; Zhao, L.; Stellos, K.; Little, K.M.; Lasitschka, F.; Doesch, A.; et al. IL-17A influences essential functions of the mono-cyte/macrophage lineage and is involved in advanced murine and human atheroscle-rosis. J. Immunol. 2014, 193, 4344–4355. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wigren, M.; Nilsson, J.; Kolbus, D. Lymphocytes in atherosclerosis. Clin. Chim. Acta 2012, 413, 1562–1568. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- He, X.; Liang, B.; Gu, N. Th17/Treg Imbalance and Atherosclerosis. Dis. Markers 2020, 2020, 8821029. [Google Scholar] [CrossRef]
- Saigusa, R.; Winkels, H.; Ley, K. T cell subsets and functions in atherosclerosis. Nat. Rev. Cardiol. 2020, 17, 387–401. [Google Scholar] [CrossRef]
- Wang, Y.; Li, W.; Zhao, T.; Zou, Y.; Deng, T.; Yang, Z.; Yuan, Z.; Ma, L.; Yu, R.; Wang, T.; et al. Interleukin-17-Producing CD4(+) T Cells Promote Inflammatory Response and Foster Disease Progression in Hyperlipidemic Patients and Atherosclerotic Mice. Front. Cardiovasc. Med. 2021, 8, 66776. [Google Scholar]
- Ali, A.J.; Makings, J.; Ley, K. Regulatory T Cell Stability and Plasticity in Atherosclerosis. Cells 2020, 9, 2665. [Google Scholar] [CrossRef] [PubMed]
- Villar-Fincheira, P.; Sanhueza-Olivares, F.; Norambuena-Soto, I.; Cancino-Arenas, N.; Hernandez-Vargas, F.; Troncoso, R.; Gabrielli, L.; Chiong, M. Role of Interleukin-6 in Vascular Health and Disease. Front. Mol. Biosci. 2021, 8, 641734. [Google Scholar] [CrossRef]
- Biros, E.; Reznik, J.E.; Moran, C.S. Role of inflammatory cytokines in genesis and treatment of atherosclerosis. Trends Cardiovasc. Med. 2021. [Google Scholar] [CrossRef]
- French FMF Consortium. A candidate gene for familial Mediterranean fever. Nat Genet. 1997, 17, 25–31. [Google Scholar] [CrossRef]
- Siegal, S. Benign paroxysmal peritonitis. Gastroenterology 1949, 12, 234–247. [Google Scholar] [CrossRef]
- Cattan, R.; Mamou, H. 14 Cases of periodic disease, 8 of which are complicated by kidney diseases. Bull. Mem. Soc. Med. Hop. Paris 1951, 67, 1104–1107. [Google Scholar]
- Reimann, H.A. Periodic disease; periodic fever, periodic abdominalgia, cyclic neu-tropenia, intermittent arthralgia, angioneurotic edema, anaphylactoid purpura and periodic paralysis. J. Am. Med. Assoc. 1949, 141, 175–183. [Google Scholar] [CrossRef]
- Livneh, A.; Drenth, J.P.; Klasen, I.S.; Langevitz, P.; George, J.; Shelton, D.A.; Gumucio, D.L.; Pras, E.; Kastner, D.L.; Pras, M.; et al. Familial Mediterranean fever and hyperimmunoglobulinemia D syndrome: Two diseases with distinct clinical, serologic, and genetic features. J. Rheumatol. 1997, 24, 1558–1563. [Google Scholar]
- Houten, S.M.; Kuis, W.; Duran, M.; de Koning, T.J.; van Royen-Kerkhof, A.; Romeijn, G.J.; Frenkel, J.; Dorland, L.; de Barse, M.M.; Huijbers, W.A.; et al. Poll-The BT. Mutations in MVK, encoding mevalonate kinase, cause hyperimmunoglobulinaemia D and periodic fever syndrome. Nat. Genet. 1999, 22, 175–177. [Google Scholar] [CrossRef]
- McDermott, M.F.; Aksentijevich, I.; Galon, J.; McDermott, E.M.; Ogunkolade, B.W.; Centola, M.; Mansfield, E.; Gadina, M.; Karenko, L.; Pettersson, T.; et al. Germline mutations in the extracellular domains of the 55 kDa TNF receptor, TNFR1, define a family of dominantly inherited autoinflammatory syndromes. Cell 1999, 97, 133–144. [Google Scholar] [CrossRef]
- Betrains, A.; Staels, F.; Schrijvers, R.; Meyts, I.; Humblet-Baron, S.; De Langhe, E.; Wouters, C.; Blockmans, D.; Vanderschueren, S. Systemic autoinflammatory disease in adults. Autoimmun. Rev. 2021, 20, 102774. [Google Scholar] [CrossRef] [PubMed]
- Georgin-Lavialle, S.; Ducharme-Benard, S.; Sarrabay, G.; Savey, L.; Grateau, G.; Hentgen, V. Systemic autoinflammatory diseases: Clinical state of the art. Best Pract. Res. Clin. Rheumatol. 2020, 34, 101529. [Google Scholar] [CrossRef] [PubMed]
- Papa, R.; Picco, P.; Gattorno, M. The expanding pathways of autoinflammation: A lesson from the first 100 genes related to autoinflammatory manifestations. Adv. Protein Chem. Struct. Biol. 2020, 120, 1–44. [Google Scholar]
- Papa, R.; Penco, F.; Volpi, S.; Gattorno, M. Actin Remodeling Defects Leading to Autoinflammation and Immune Dysregulation. Front. Immunol. 2021, 11, 604206. [Google Scholar] [CrossRef]
- Kallinich, T. Regulating against dysregulation: New treatment options in auto-inflammation. Semin. Immunopathol. 2015, 37, 429–437. [Google Scholar] [CrossRef]
- Gattorno, M.; Hofer, M.; Federici, S.; Vanoni, F.; Bovis, F.; Aksentijevich, I.; Anton, J.; Arostegui, J.I.; Barron, K.; Ben-Cherit, E.; et al. Eurofever Registry and the Paediatric Rheumatology International Trials Organisation (PRINTO). Classification criteria for autoinflammatory recurrent fevers. Ann. Rheum. Dis. 2019, 78, 1025–1032. [Google Scholar] [CrossRef] [Green Version]
- Martinon, F.; Burns, K.; Tschopp, J. The inflammasome: A molecular platform triggering activation of inflammatory caspases and processing of pro IL-beta. Mol. Cell 2002, 10, 417–426. [Google Scholar] [CrossRef]
- Harapas, C.R.; Steiner, A.; Davidson, S.; Masters, S.L. An Update on Autoinflammatory Diseases: Inflammasomopathies. Curr. Rheumatol. Rep. 2018, 20, 40. [Google Scholar] [CrossRef] [PubMed]
- Zheng, D.; Liwinski, T.; Elinav, E. Inflammasome activation and regulation: Toward a better understanding of complex mechanisms. Cell Discov. 2020, 6, 36. [Google Scholar] [CrossRef] [PubMed]
- Savic, S.; Caseley, E.A.; McDermott, M.F. Moving towards a systems-based classifi-cation of innate immune-mediated diseases. Nat. Rev. Rheumatol. 2020, 16, 222–237. [Google Scholar] [CrossRef]
- Song, Y.; Zhao, Y.; Ma, Y.; Wang, Z.; Rong, L.; Wang, B.; Zhang, N. Biological functions of NLRP3 inflammasome: A therapeutic target in inflammatory bowel disease. Cytokine Growth Factor Rev. 2021, 60, 61–75. [Google Scholar] [CrossRef]
- Wei, S.; Ma, W.; Zhang, B.; Li, W. NLRP3 Inflammasome: A Promising Therapeutic Target for Drug-Induced Toxicity. Front. Cell Dev. Biol. 2021, 9, 634607. [Google Scholar] [CrossRef]
- Shao, S.; Chen, C.; Shi, G.; Zhou, Y.; Wei, Y.; Fan, N.; Yang, Y.; Wu, L.; Zhang, T. Therapeutic potential of the target on NLRP3 inflammasome in multiple sclerosis. Pharmacol. Ther. 2021, 227, 107880. [Google Scholar] [CrossRef]
- Holbrook, J.A.; Jarosz-Griffiths, H.H.; Caseley, E.; Lara-Reyna, S.; Poulter, J.A.; Wil-liams-Gray, C.H.; Peckham, D.; McDermott, M.F. Neurodegenerative Disease and the NLRP3 Inflammasome. Front. Pharmacol. 2021, 12, 643254. [Google Scholar] [CrossRef]
- Luzardo-Ocampo, I.; Loarca-Piña, G.; Gonzalez de Mejia, E. Gallic and butyric acids modulated NLRP3 inflammasome markers in a co-culture model of intestinal inflammation. Food Chem. Toxicol. 2020, 146, 111835. [Google Scholar] [CrossRef]
- Yang, X.; Zhan, N.; Jin, Y.; Ling, H.; Xiao, C.; Xie, Z.; Zhong, H.; Yu, X.; Tang, R.; Ma, J.; et al. Tofacitinib restores the balance of γδTreg/γδT17 cells in rheumatoid arthritis by inhibiting the NLRP3 inflammasome. Theranostics 2021, 11, 1446–1457. [Google Scholar] [CrossRef]
- Cordero, M.D.; Alcocer-Gómez, E. Inflammasomes: Clinical and Therapeutic Implications, 1st ed.; Springer: Cham, Switzerland, 2018; Volume VIII, p. 400. [Google Scholar]
- Gabay, C.; Lamacchia, C.; Palmer, G. IL-1 pathways in inflammation and human diseases. Nat. Rev. Rheumatol. 2010, 6, 232–241. [Google Scholar] [CrossRef]
- Man, S.M.; Karki, R.; Kanneganti, T.D. Molecular mechanisms and functions of pyroptosis, inflammatory caspases and inflammasomes in infectious diseases. Immunol. Rev. 2017, 277, 61–75. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yabal, M.; Calleja, D.J.; Simpson, D.S.; Lawlor, K.E. Stressing out the mitochondria: Mechanistic insights into NLRP3 inflammasome activation. J. Leukoc. Biol. 2019, 105, 377–399. [Google Scholar] [CrossRef] [PubMed]
- Liu, Q.; Zhang, D.; Hu, D.; Zhou, X.; Zhou, Y. The role of mitochondria in NLRP3 in-flammasome activation. Mol. Immunol. 2018, 103, 115–124. [Google Scholar] [CrossRef]
- Dan Dunn, J.; Alvarez, L.A.; Zhang, X.; Soldati, T. Reactive oxygen species and mi-tochondria: A nexus of cellular homeostasis. Redox Biol. 2015, 6, 472–485. [Google Scholar] [CrossRef] [PubMed]
- Liu, T.; Zhang, L.; Joo, D.; Sun, S. NF-κB signaling in inflammation. Signal Transduct. Target. Ther. 2017, 2, 17023. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Oeckinghaus, A.; Ghosh, S. The NF-kappaB family of transcription factors and its regulation. Cold Spring Harb. Perspect. Biol. 2009, 1, a000034. [Google Scholar] [CrossRef] [PubMed]
- Lowry, S.F. Cytokine mediators of immunity and inflammation. Arch. Surg. 1993, 128, 1235–1241. [Google Scholar] [CrossRef] [PubMed]
- Oppenheim, J.J. Cytokines: Past, present, and future. Int. J. Hematol. 2001, 74, 3–8. [Google Scholar] [CrossRef] [PubMed]
- Mantovani, A.; Dinarello, C.A.; Molgora, M.; Garlanda, C. Interleukin-1 and Related Cytokines in the Regulation of Inflammation and Immunity. Immunity 2019, 50, 778–795. [Google Scholar] [CrossRef] [Green Version]
- Roe, K An inflammation classification system using cytokine parameters. Scand. J. Immunol. 2021, 93, e12970.
- Pathak, S.; McDermott, M.F.; Savic, S. Autoinflammatory diseases: Update on classification diagnosis and management. J. Clin. Pathol. 2017, 70, 1–8. [Google Scholar] [CrossRef] [Green Version]
- Dinarello, C.A. Interleukin-1 in the pathogenesis and treatment of inflammatory diseases. Blood 2011, 117, 3720–3732. [Google Scholar] [CrossRef] [Green Version]
- Yazdi, A.S.; Ghoreschi, K. The Interleukin-1 Family. Adv. Exp. Med. Biol. 2016, 941, 21–29. [Google Scholar]
- Zhu, J.; Paul, W.E. CD4 T cells: Fates, functions, and faults. Blood 2008, 112, 1557–1569. [Google Scholar] [CrossRef] [Green Version]
- Adinolfi, E.; Giuliani, A.L.; De Marchi, E.; Pegoraro, A.; Orioli, E.; Di Virgilio, F. The P2X7 receptor: A main player in inflammation. Biochem. Pharmacol. 2018, 151, 234–244. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Dal Ben, D.; Sarti, A.C.; Giuliani, A.L.; Falzoni, S. The P2X7 Receptor in Infection and Inflammation. Immunity 2017, 47, 15–31. [Google Scholar] [CrossRef] [Green Version]
- Giuliani, A.L.; Sarti, A.C.; Falzoni, S.; Di Virgilio, F. The P2X7 Receptor-Interleukin-1 Liaison. Front. Pharmacol. 2017, 8, 123. [Google Scholar] [CrossRef] [Green Version]
- Ferrari, D.; Pizzirani, C.; Adinolfi, E.; Lemoli, R.M.; Curti, A.; Idzko, M.; Panther, E.; Di Virgilio, F. The P2X7 receptor: A key player in IL-1 processing and release. J. Immunol. 2006, 176, 3877–3883. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alehashemi, S.; Goldbach-Mansky, R. Human Autoinflammatory Diseases Mediated by NLRP3-, Pyrin-, NLRP1-, and NLRC4-Inflammasome Dysregulation Updates on Diagnosis, Treatment, and the Respective Roles of IL-1 and IL-18. Front. Immunol. 2020, 11, 1840. [Google Scholar] [CrossRef] [PubMed]
- Rathinam, V.A.; Fitzgerald, K.A. Inflammasomes and anti-viral immunity. J. Clin. Immunol. 2010, 30, 632–637. [Google Scholar] [CrossRef] [PubMed]
- Novick, D.; Kim, S.; Kaplanski, G.; Dinarello, C.A. Interleukin-18, more than a Th1 cytokine. Semin. Immunol. 2013, 25, 439–448. [Google Scholar] [CrossRef] [PubMed]
- Sedimbi, S.K.; Hägglöf, T.; Karlsson, M.C. IL-18 in inflammatory and autoimmune disease. Cell Mol. Life Sci. 2013, 70, 4795–4808. [Google Scholar] [CrossRef] [PubMed]
- Gerriets, V.; Bansal, P.; Goyal, A.; Khaddour, K. Tumor Necrosis Factor Inhibitors. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://www.ncbi.nlm.nih.gov/books/NBK482425/ (accessed on 17 October 2021).
- Ellis, C.R.; Azmat, C.E. Adalimumab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/32491812/ (accessed on 5 May 2021).
- Zangrilli, A.; Bavetta, M.; Bianchi, L. Adalimumab in children and adolescents with severe plaque psoriasis: A safety evaluation. Expert Opin. Drug Saf. 2020, 19, 433–438. [Google Scholar] [CrossRef]
- Giunta, A.; Zangrilli, A.; Bavetta, M.; Manfreda, V.; Pensa, C.; Bianchi, L. A single-centre, observational, retrospective, real-life study evaluating adalimumab biosim-ilar ABP 501 in the treatment of plaque-type psoriasis and psoriatic arthritis in origi-nator-naive patients and in patients undergoing non-medical switch from originator. Curr. Med. Res. Opin. 2021, 37, 1099–1102. [Google Scholar]
- Lee, Y.H.; Song, G.G. Comparative efficacy and safety of adalimumab biosimilars and adalimumab in patients with rheumatoid arthritis presenting an insufficient response to methotrexate: A network meta-analysis. Z Rheumatol. 2021. [Google Scholar] [CrossRef]
- Sánchez Martínez, E.M.; Murray, G.; Alfageme Roldán, F.; García Ruiz, R.; Tobin, A.M.; Zouboulis, C.C. Adalimumab dose intensification in hidradenitis suppurativa: Effectiveness and safety results of a multicenter study. Br. J. Dermatol. 2021, 185, 863–865. [Google Scholar] [CrossRef]
- Sejournet, L.; Kerever, S.; Mathis, T.; Kodjikian, L.; Jamilloux, Y.; Seve, P. Therapeutic drug monitoring guides the management of patients with chronic non-infectious uveitis treated with adalimumab: A retrospective study. Br. J. Ophthalmol. 2021, 319072. [Google Scholar] [CrossRef]
- Nagy, A.; Mosdosi, B.; Simon, D.; Dergez, T.; Berki, T. Peripheral Blood Lymphocyte Analysis in Oligo- and Polyarticular Juvenile Idiopathic Arthritis Patients Receiving Methotrexate or Adalimumab Therapy: A Cross-Sectional Study. Front. Pediatr. 2020, 8, 614354. [Google Scholar] [CrossRef]
- Tursi, A.; Mocci, G.; Lorenzetti, R.; Allegretta, L.; Brandimarte, G.; Cassieri, C.; Co-lucci, R.; De Medici, A.; Faggiani, R.; Ferronato, A.; et al. Long-term real-life efficacy and safety of infliximab and adalimumab in the treatment of inflammatory bowel diseases outpatients. Eur. J. Gastroenterol. Hepatol. 2021, 33, 670–679. [Google Scholar] [CrossRef]
- Golimumab. Drugs and Lactation Database (LactMed) [Internet]: Bethesda (MD), National Library of Medicine (US). 2006. Available online: https://pubmed.ncbi.nlm.nih.gov/30000713/ (accessed on 19 April 2021).
- Gatopoulou, A.; Christodoulou, D.K.; Katsanos, K.H.; Bakos, D.; Mouzas, I.; Tzou-vala, M.; Theodoropoulou, A.; Paspatis, G.; Theocharis, G.; Thomopoulos, K.; et al. Effect of golimumab on health-related quality of life, other patient-reported outcomes and healthcare resource utilization in patients with moderate-to-severe ulcerative colitis: A real-world multicenter, non interventional, observational study in Greece. Eur. J. Gastroenterol. Hepatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Serrano-Benavente, B.; Valor, L.; Del Río Blasco, T.; Janta, I.; González Benítez, R.; Nieto-González, J.C.; Martínez-Barrio, J.; Ovalles Bonilla, J.G.; Ariza, A.; López-Longo, F.J.; et al. Long-Term Retention Rate of Golimumab in Patients With Rheumatoid Arthritis, Psoriatic Arthritis, and Spondyloarthritis in a Real-Life Setting. J. Clin. Rheumatol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Pan, A.; Gerriets, V. Etanercept. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/31424836/ (accessed on 25 March 2021).
- Paller, A.S.; Siegfried, E.C.; Langley, R.G.; Gottlieb, A.B.; Pariser, D.; Landells, I.; Hebert, A.A.; Eichenfield, L.F.; Patel, V.; Creamer, K.; et al. Etanercept Pediatric Psoriasis Study Group. Etanercept treatment for children and adolescents with plaque psoriasis. N. Engl. J. Med. 2008, 358, 241–251. [Google Scholar] [CrossRef] [Green Version]
- Atalay, S.; van der Schoot, L.S.; Vandermaesen, L.; van Vugt, L.J.; Eilander, M.; van den Reek, J.M.P.A.; de Jong, E.M.G.J. Evaluation of a One-step Dose Reduction Strategy of Adalimumab, Etanercept and Ustekinumab in Patients with Psoriasis in Daily Practice. Acta Derm. Venereol. 2021, 101, adv00463. [Google Scholar] [CrossRef]
- Klotsche, J.; Klein, A.; Niewerth, M.; Hoff, P.; Windschall, D.; Foeldvari, I.; Haas, J.P.; Horneff, G.; Minden, K. Re-treatment with etanercept is as effective as the initial first-line treatment in patients with juvenile idiopathic arthritis. Arthritis Res. Ther. 2021, 23, 118. [Google Scholar] [CrossRef]
- Mease, P.J.; Kivitz, A.J.; Burch, F.X.; Siegel, E.L.; Cohen, S.B.; Ory, P.; Salonen, D.; Rubenstein, J.; Sharp, J.T.; Tsuji, W. Etanercept treatment of psoriatic arthritis: Safety, efficacy, and effect on disease progression. Arthritis Rheum. 2004, 50, 2264–2272. [Google Scholar] [CrossRef]
- Zhao, S.; Mysler, E.; Moots, R.J. Etanercept for the treatment of rheumatoid arthritis. Immunotherapy 2018, 10, 433–445. [Google Scholar] [CrossRef]
- Fatima, R.; Bittar, K.; Aziz, M. Infliximab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/29763197/ (accessed on 26 January 2021).
- Ge, Y.; Li, S.; Chen, F.; He, L.; Li, C.; Lu, X.; Wang, G. The effects of infliximab in treating idiopathic inflammatory myopathies: A review article. Dermatol. Ther. 2021, 34, e14976. [Google Scholar] [CrossRef]
- Esatoglu, S.N.; Akkoc-Mustafayev, F.N.; Ozguler, Y.; Ozbakır, F.; Nohut, O.K.; Cevirgen, D.; Hamuryudan, V.; Hatemi, I.; Celik, A.F.; Yazici, H.; et al. Immuno-genicity of Infliximab Among Patients With Behcet Syndrome: A Controlled Study. Front. Immunol. 2020, 11, 618973. [Google Scholar] [CrossRef] [PubMed]
- Lim, H.; Lee, S.H.; Lee, H.T.; Lee, J.U.; Son, J.Y.; Shin, W.; Heo, Y.S. Structural Biology of the TNFalpha Antagonists Used in the Treatment of Rheumatoid Arthritis. Int. J. Mol. Sci. 2018, 19, 768. [Google Scholar] [CrossRef] [Green Version]
- Maxwell, L.J.; Zochling, J.; Boonen, A.; Singh, J.A.; Veras, M.M.; Tanjong Ghogomu, E.; Benkhalti Jandu, M.; Tugwell, P.; Wells, G.A. TNF-alpha inhibitors for ankylosing spondylitis. Cochrane Database Syst. Rev. 2015, 4, CD005468. [Google Scholar] [CrossRef] [PubMed]
- Subedi, S.; Gong, Y.; Chen, Y.; Shi, Y. Infliximab and biosimilar infliximab in psoriasis: Efficacy, loss of efficacy, and adverse events. Drug Des. Devel. Ther. 2019, 13, 2491–2502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deeks, E.D. Certolizumab Pegol: A Review in Inflammatory Autoimmune Diseases. BioDrugs 2016, 30, 607–617. [Google Scholar] [CrossRef] [PubMed]
- Patel, V.K.; Ghosh, S. Certolizumab pegol in Crohn’s disease. Drugs Today 2008, 44, 837–844. [Google Scholar] [CrossRef] [PubMed]
- Ruiz Garcia, V.; Burls, A.; Cabello, J.B.; Vela Casasempere, P.; Bort-Marti, S.; Bernal, J.A. Certolizumab pegol (CDP870) for rheumatoid arthritis in adults. Cochrane Database Syst. Rev. 2017, 9, CD007649. [Google Scholar] [CrossRef]
- Lee, A.; Scott, L.J. Certolizumab Pegol: A Review in Moderate to Severe Plaque Psoriasis. BioDrugs 2020, 34, 235–244. [Google Scholar] [CrossRef]
- Dinarello, C.A. Interleukin-1 in disease. Keio J. Med. 1994, 43, 131–136. [Google Scholar] [CrossRef] [Green Version]
- Anakinra. Drugs and Lactation Database (LactMed) [Internet]: Bethesda (MD), National Library of Medicine (US). 2006. Available online: https://pubmed.ncbi.nlm.nih.gov/30000931/ (accessed on 17 May 2021).
- Castañeda, S.; Atienza-Mateo, B.; Martín-Varillas, J.L.; Serra López-Matencio, J.M.; González-Gay, M.A. Anakinra for the treatment of adult-onset Still’s disease. Expert Rev. Clin. Immunol. 2018, 14, 979–992. [Google Scholar] [CrossRef] [Green Version]
- Ramírez, J.; Cañete, J.D. Anakinra for the treatment of rheumatoid arthritis: A safe-ty evaluation. Expert Opin. Drug Saf. 2018, 17, 727–732. [Google Scholar] [CrossRef] [PubMed]
- Koné-Paut, I.; Galeotti, C. Anakinra for cryopyrin-associated periodic syndrome. Expert Rev. Clin. Immunol. 2014, 10, 7–18. [Google Scholar] [CrossRef]
- Lopalco, G.; Rigante, D.; Giannini, M.; Galeazzi, M.; Lapadula, G.; Iannone, F.; Can-tarini, L. Safety profile of anakinra in the management of rheumatologic, metabolic and autoinflammatory disorders. Clin. Exp. Rheumatol. 2016, 34, 531–538. [Google Scholar] [PubMed]
- Jesus, A.A.; Goldbach-Mansky, R. IL-1 blockade in autoinflammatory syndromes. Annu. Rev. Med. 2014, 65, 223–244. [Google Scholar] [CrossRef] [Green Version]
- Canakinumab. Drugs and Lactation Database (LactMed) [Internet]; Bethesda (MD): National Library of Medicine (US). 2006. Available online: https://pubmed.ncbi.nlm.nih.gov/29999640/ (accessed on 19 April 2021).
- Gram, H. Preclinical characterization and clinical development of ILARIS(®) (canakinumab) for the treatment of autoinflammatory diseases. Curr. Opin. Chem. Biol. 2016, 32, 1–9. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-Manubens, J.; Iglesias, E.; Anton, J. Canakinumab for the treatment of hyperimmunoglobulin D syndrome. Expert Rev. Clin. Immunol. 2019, 15, 215–220. [Google Scholar] [CrossRef]
- Kacar, M.; Savic, S.; van der Hilst, J.C.H. The Efficacy, Safety and Tolerability of Cana-kinumab in the Treatment of Familial Mediterranean Fever: A Systematic Review of the Literature. J. Inflamm. Res. 2020, 13, 141–149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Orrock, J.E.; Ilowite, N.T. Canakinumab for the treatment of active systemic juve-nile idiopathic arthritis. Expert Rev. Clin. Pharmacol. 2016, 9, 1015–1024. [Google Scholar] [CrossRef] [PubMed]
- Rilonacept. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]: Bethesda (MD): National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Available online: https://pubmed.ncbi.nlm.nih.gov/31643801/ (accessed on 20 April 2020).
- McDermott, M.F. Rilonacept in the treatment of chronic inflammatory disorders. Drugs Today 2009, 45, 423–430. [Google Scholar] [CrossRef] [PubMed]
- Hoffman, H.M. Rilonacept for the treatment of cryopyrin-associated periodic syn-dromes (CAPS). Expert Opin. Biol. Ther. 2009, 9, 519–531. [Google Scholar] [CrossRef] [PubMed]
- Tombetti, E.; Mulè, A.; Tamanini, S.; Matteucci, L.; Negro, E.; Brucato, A.; Carno-vale, C. Novel Pharmacotherapies for Recurrent Pericarditis: Current Options in 2020. Curr. Cardiol. Rep. 2020, 22, 59. [Google Scholar] [CrossRef] [PubMed]
- Dumusc, A.; So, A. Interleukin-1 as a therapeutic target in gout. Curr. Opin. Rheumatol. 2015, 27, 156–163. [Google Scholar] [CrossRef]
- Kaur, S.; Bansal, Y.; Kumar, R.; Bansal, G. A panoramic review of IL-6: Structure, pathophysiological roles and inhibitors. Bioorg. Med. Chem. 2020, 28, 115327. [Google Scholar] [CrossRef]
- Preuss, C.V.; Anjum, F. Tocilizumab. In StatPearls; StatPearls Publishing: Treasure Island, FL, USA, 2021. Available online: https://pubmed.ncbi.nlm.nih.gov/34033406/ (accessed on 4 May 2021).
- Scott, L.J. Tocilizumab: A Review in Rheumatoid Arthritis. Drugs 2017, 77, 1865–1879. [Google Scholar] [CrossRef] [Green Version]
- Mariano, V.J.; Frishman, W.H. Tocilizumab in Giant Cell Arteritis. Cardiol. Rev. 2018, 26, 321–330. [Google Scholar] [CrossRef]
- Castañeda, S.; Martínez-Quintanilla, D.; Martín-Varillas, J.L.; García-Castañeda, N.; Atienza-Mateo, B.; González-Gay, M.A. Tocilizumab for the treatment of adult-onset Still’s disease. Expert Opin. Biol. Ther. 2019, 19, 273–286. [Google Scholar] [CrossRef]
- Machado, S.H.; Xavier, R.M. Safety of tocilizumab in the treatment of juvenile idiopathic arthritis. Expert Opin. Drug Saf. 2017, 16, 493–500. [Google Scholar] [CrossRef]
- Grom, A.A.; Horne, A.; De Benedetti, F. Macrophage activation syndrome in the era of biologic therapy. Nat. Rev. Rheumatol. 2016, 12, 259–268. [Google Scholar] [CrossRef] [PubMed]
- Sarilumab. LiverTox: Clinical and Research Information on Drug-Induced Liver Injury [Internet]: Bethesda (MD), National Institute of Diabetes and Digestive and Kidney Diseases. 2012. Available online: https://pubmed.ncbi.nlm.nih.gov/31643297/ (accessed on 11 May 2021).
- Lamb, Y.N.; Deeks, E.D. Sarilumab: A Review in Moderate to Severe Rheumatoid Arthritis. Drugs 2018, 78, 929–940. [Google Scholar] [CrossRef]
- Siltuximab. Drugs and Lactation Database (LactMed) [Internet]: Bethesda (MD), National Library of Medicine (US). 2006. Available online: https://pubmed.ncbi.nlm.nih.gov/29999789/ (accessed on 19 April 2021).
- Sitenga, J.; Aird, G.; Ahmed, A.; Silberstein, P.T. Impact of siltuximab on patient-related outcomes in multicentric Castleman’s disease. Patient Relat. Outcome Meas. 2018, 9, 35–41. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ferrario, A.; Merli, M.; Basilico, C.; Maffioli, M.; Passamonti, F. Siltuximab and hematologic malignancies. A focus in non Hodgkin lymphoma. Expert Opin. Investig. Drugs. 2017, 26, 367–373. [Google Scholar] [CrossRef]
- Yao, X.; Huang, J.; Zhong, H.; Shen, N.; Faggioni, R.; Fung, M.; Yao, Y. Targeting interleukin-6 in inflammatory autoimmune diseases and cancers. Pharmacol. Ther. 2014, 141, 125–139. [Google Scholar] [CrossRef] [PubMed]
- Iznardo, H.; Puig, L. Exploring the Role of IL-36 Cytokines as a New Target in Psoriatic Disease. Int. J. Mol. Sci. 2021, 22, 4344. [Google Scholar] [CrossRef] [PubMed]
- Malcova, H.; Strizova, Z.; Milota, T.; Striz, I.; Sediva, A.; Cebecauerova, D.; Horvath, R. IL-1 Inhibitors in the Treatment of Monogenic Periodic Fever Syndromes: From the Past to the Future Perspectives. Front. Immunol. 2021, 11, 619257. [Google Scholar] [CrossRef]
- Mahmudpour, M.; Roozbeh, J.; Keshavarz, M.; Farrokhi, S.; Nabipour, I. COVID-19 cytokine storm: The anger of inflammation. Cytokine 2020, 133, 155151. [Google Scholar] [CrossRef] [PubMed]
- Giamarellos-Bourboulis, E.J.; Netea, M.G.; Rovina, N.; Akinosoglou, K.; Antoni-adou, A.; Antonakos, N.; Damoraki, G.; Gkavogianni, T.; Adami, M.E.; Katsaounou, P.; et al. Complex Immune Dysregulation in COVID-19 Patients with Severe Respiratory Failure. Cell Host Microbe. 2020, 27, 992–1000.e3. [Google Scholar] [CrossRef]
- Ferrara, J.L.; Abhyankar, S.; Gilliland, D.G. Cytokine storm of graft-versus-host disease: A critical effector role for interleukin-1. Transpl. Proc. 1993, 25, 1216–1217. [Google Scholar]
- Han, P.; Hodge, G. Intracellular cytokine production and cytokine receptor interaction of cord mononuclear cells: Relevance to cord blood transplantation. Br. J. Haematol. 1999, 107, 450–457. [Google Scholar] [CrossRef]
- Cohen, S.B.; Wang, X.N.; Dickinson, A. Can cord blood cells support the cytokine storm in GvHD? Cytokine Growth Factor Rev. 2000, 11, 185–197. [Google Scholar] [CrossRef]
- Hill, G.R.; Ferrara, J.L. The primacy of the gastrointestinal tract as a target organ of acute graft-versus-host disease: Rationale for the use of cytokine shields in allogeneic bone marrow transplantation. Blood 2000, 95, 2754–2759. [Google Scholar] [CrossRef] [PubMed]
- Wong, S.S.; Yuen, K.Y. Avian influenza virus infections in humans. Chest 2006, 129, 156–168. [Google Scholar] [CrossRef] [PubMed]
- Theron, M.; Huang, K.J.; Chen, Y.W.; Liu, C.C.; Lei, H.Y. A probable role for IFN-gamma in the development of a lung immunopathology in SARS. Cytokine 2005, 32, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Salomon, R.; Hoffmann, E.; Webster, R.G. Inhibition of the cytokine response does not protect against lethal H5N1 influenza infection. Proc. Natl. Acad. Sci. USA 2007, 104, 12479–12481. [Google Scholar] [CrossRef] [Green Version]
- López-Reyes, A.; Martinez-Armenta, C.; Espinosa-Velázquez, R.; Vázquez-Cárdenas, P.; Cruz-Ramos, M.; Palacios-Gonzalez, B.; Gomez-Quiroz, L.E.; Martínez-Nava, G.A. NLRP3 Inflammasome: The Stormy Link Between Obesity and COVID-19. Front. Immunol. 2020, 11, 570251. [Google Scholar] [CrossRef]
- Zhang, Y.; Sun, H.; Pei, R.; Mao, B.; Zhao, Z.; Li, H.; Lin, Y.; Lu, K. The SARS-CoV-2 protein ORF3a inhibits fusion of autophagosomes with lysosomes. Cell Discov. 2021, 7, 31. [Google Scholar] [CrossRef] [PubMed]
- Miao, G.; Zhao, H.; Li, Y.; Ji, M.; Chen, Y.; Shi, Y.; Bi, Y.; Wang, P.; Zhang, H. ORF3a of the COVID-19 virus SARS-CoV-2 blocks HOPS complex-mediated assembly of the SNARE complex required for autolysosome formation. Dev Cell. 2021, 56, 427–442.e5. [Google Scholar] [CrossRef]
- Bianchi, M.; Borsetti, A.; Ciccozzi, M.; Pascarella, S. SARS-Cov-2 ORF3a: Mutability and function. Int. J. Biol. Macromol. 2021, 170, 820–826. [Google Scholar] [CrossRef]
- Yount, B.; Roberts, R.S.; Sims, A.C.; Deming, D.; Frieman, M.B.; Sparks, J.; Denison, M.R.; Davis, N.; Baric, R.S. Severe acute respiratory syndrome coronavirus group-specific open reading frames encode nonessential functions for replication in cell cultures and mice. J. Virol. 2005, 79, 14909–14922. [Google Scholar] [CrossRef] [Green Version]
- Van den Berg, D.F.; Te Velde, A.A. Severe COVID-19: NLRP3 Inflammasome Dysregulated. Front. Immunol. 2020, 11, 1580. [Google Scholar] [CrossRef]
- Quagliariello, V.; Bonelli, A.; Caronna, A.; Lombari, M.C.; Conforti, G.; Libutti, M.; Iaffaioli, R.V.; Berretta, M.; Botti, G.; Maurea, N. SARS-CoV-2 infection: NLRP3 inflammasome as plausible target to prevent cardiopulmonary complications? Eur. Rev. Med. Pharmacol. Sci. 2020, 24, 9169–9171. [Google Scholar]
- Paul, O.; Tao, J.Q.; Litzky, L.; Feldman, M.; Montone, K.; Rajapakse, C.; Bermudez, C.; Chatterjee, S. Vascular Inflammation in Lungs of Patients with Fatal Coronavirus Disease 2019 (COVID-19) Infection: Possible role for the NLRP3 inflammasome. medRxiv 2021, 22. [Google Scholar] [CrossRef]
- Courjon, J.; Dufies, O.; Robert, A.; Bailly, L.; Torre, C.; Chirio, D.; Contenti, J.; Vitale, S.; Loubatier, C.; Doye, A.; et al. Heterogeneous NLRP3 inflammasome signature in circulating myeloid cells as a biomarker of COVID-19 severity. Blood Adv. 2021, 5, 1523–1534. [Google Scholar] [CrossRef]
- He, Y.; Zeng, M.Y.; Yang, D.; Motro, B.; Núñez, G. NEK7 is an essential mediator of NLRP3 activation downstream of potassium efflux. Nature 2016, 530, 354–357. [Google Scholar] [CrossRef] [Green Version]
- Shi, H.; Wang, Y.; Li, X.; Zhan, X.; Tang, M.; Fina, M.; Su, L.; Pratt, D.; Bu, C.H.; Hildebrand, S.; et al. NLRP3 activation and mitosis are mutually exclusive events coordinated by NEK7, a new inflammasome component. Nat. Immunol. 2016, 17, 250–258. [Google Scholar] [CrossRef] [PubMed]
- Kaewborisuth, C.; Koonpaew, S.; Srisutthisamphan, K.; Viriyakitkosol, R.; Jaru-Ampornpan, P.; Jongkaewwattana, A. PEDV ORF3 Independently Regulates IκB Kinase β-Mediated NF-κB and IFN-β Promoter Activities. Pathogens 2020, 9, 376. [Google Scholar] [CrossRef]
- Dea, S.; Gagnon, C.A.; Mardassi, H.; Pirzadeh, B.; Rogan, D. Current knowledge on the structural proteins of porcine reproductive and respiratory syndrome (PRRS) virus: Comparison of the North American and European isolates. Arch. Virol. 2000, 145, 659–688. [Google Scholar] [CrossRef] [PubMed]
- Siu, K.L.; Yuen, K.S.; Castaño-Rodriguez, C.; Ye, Z.W.; Yeung, M.L.; Fung, S.Y.; Yuan, S.; Chan, C.P.; Yuen, K.Y.; Enjuanes, L.; et al. Severe acute respiratory syndrome coronavirus ORF3a protein activates the NLRP3 inflammasome by promoting TRAF3-dependent ubiquitination of ASC. FASEB J. 2019, 33, 8865–8877. [Google Scholar] [CrossRef] [PubMed]
- Pacheco, P.A.F.; Faria, R.X. The potential involvement of P2X7 receptor in COVID-19 pathogenesis: A new therapeutic target? Scand. J. Immunol. 2021, 93, e12960. [Google Scholar] [CrossRef]
- Di Virgilio, F.; Tang, Y.; Sarti, A.C.; Rossato, M. A rationale for targeting the P2X7 receptor in Coronavirus disease. Br. J. Pharmacol. 2020, 177, 4990–4994. [Google Scholar] [CrossRef]
- Clark, I.A. The advent of the cytokine storm. Immunol Cell Biol. 2007, 85, 271–273. [Google Scholar] [CrossRef]
- Ghosh, K.; Shetty, S. Blood coagulation in falciparum malaria—A review. Parasitol. Res. 2008, 102, 571–576. [Google Scholar] [CrossRef]
- Schulert, G.S.; Grom, A.A. Pathogenesis of macrophage activation syndrome and potential for cytokine—Directed therapies. Annu. Rev. Med. 2015, 66, 145–159. [Google Scholar] [CrossRef] [Green Version]
- Hu, B.; Huang, S.; Yin, L. The cytokine storm and COVID-19. J. Med. Virol. 2021, 93, 250–256. [Google Scholar] [CrossRef] [PubMed]
- Soy, M.; Keser, G.; Atagündüz, P.; Tabak, F.; Atagündüz, I.; Kayhan, S. Cytokine storm in COVID-19: Pathogenesis and overview of anti-inflammatory agents used in treatment. Clin. Rheumatol. 2020, 39, 2085–2094. [Google Scholar] [CrossRef]
- Jamilloux, Y.; Henry, T.; Belot, A.; Viel, S.; Fauter, M.; El Jammal, T.; Walzer, T.; François, B.; Sève, P. Should we stimulate or suppress immune responses in COVID-19? Cytokine and anti-cytokine inter-ventions. Autoimmun. Rev. 2020, 19, 102567. [Google Scholar] [CrossRef] [PubMed]
- Zhang, C.; Wu, Z.; Li, J.W.; Zhao, H.; Wang, G.Q. Cytokine release syndrome in severe COVID-19: Interleukin-6 receptor antagonist tocilizumab may be the key to reduce mortality. Int. J. Antimicrob. Agents 2020, 55, 105954. [Google Scholar] [CrossRef] [PubMed]
- Somers, E.C.; Eschenauer, G.A.; Troost, J.P.; Golob, J.L.; Gandhi, T.N.; Wang, L.; Zhou, N.; Petty, L.A.; Baang, J.H.; Dillman, N.O.; et al. Tocilizumab for Treatment of Mechanically Ventilated Patients With COVID-19. Clin. Infect. Dis. 2021, 73, e445–e454. [Google Scholar] [CrossRef]
- Leaf, D.E.; Gupta, S.; Wang, W. Tocilizumab in Covid-19. N. Engl. J. Med. 2021, 384, 86–87. [Google Scholar]
- Landi, L.; Ravaglia, C.; Russo, E.; Cataleta, P.; Fusari, M.; Boschi, A.; Giannarelli, D.; Facondini, F.; Valentini, I.; Panzini, I.; et al. Blockage of interleukin-1beta with canakinumab in patients with Covid-19. Sci. Rep. 2020, 10, 21775. [Google Scholar] [CrossRef]
- Huet, T.; Beaussier, H.; Voisin, O.; Jouveshomme, S.; Dauriat, G.; Lazareth, I.; Sacco, E.; Naccache, J.M.; Bézie, Y.; Laplanche, S.; et al. Anakinra for severe forms of COVID-19: A cohort study. Lancet Rheumatol. 2020, 2, e393–e400. [Google Scholar] [CrossRef]
- Cavalli, G.; De Luca, G.; Campochiaro, C.; Della-Torre, E.; Ripa, M.; Canetti, D.; Oltolini, C.; Castiglioni, B.; Tassan Din, C.; Boffini, N.; et al. Interleukin-1 blockade with high-dose anakinra in patients with COVID-19, acute respiratory distress syndrome, and hyperinflammation: A retrospective cohort study. Lancet Rheumatol. 2020, 2, e325–e331. [Google Scholar] [CrossRef]
- Mehta, P.; Fajgenbaum, D.C. Is severe COVID-19 a cytokine storm syndrome: A hyperinflammatory debate. Curr. Opin. Rheumatol. 2021, 33, 419–430. [Google Scholar] [CrossRef]
- Slifka, M.K.; Whitton, J.L. Clinical implications of dysregulated cytokine production. J. Mol. Med. 2000, 78, 74–80. [Google Scholar] [CrossRef]
- Bekkering, S.; Domínguez-Andrés, J.; Joosten, L.A.B.; Riksen, N.P.; Netea, M.G. Trained Immunity: Reprogramming Innate Immunity in Health and Disease. Annu. Rev. Immunol. 2021, 39, 667–693. [Google Scholar] [CrossRef] [PubMed]
- Arneth, B. Trained innate immunity. Immunol. Res. 2021, 69, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Palmieri, B.; Vadala, M.; Palmieri, L. Immune memory: An evolutionary perspective. Hum. Vaccin. Immunother. 2021, 17, 1604–1606. [Google Scholar] [CrossRef] [PubMed]
- Drummer, C.; Saaoud, F.; Shao, Y.; Sun, Y.; Xu, K.; Lu, Y.; Ni, D.; Atar, D.; Jiang, X.; Wang, H.; et al. Trained Immunity and Reactivity of Macrophages and Endothelial Cells. Arterioscler. Thromb. Vasc. Biol. 2021, 41, 1032–1046. [Google Scholar] [CrossRef] [PubMed]
- Van der Meer, J.W.; Joosten, L.A.; Riksen, N.; Netea, M.G. Trained immunity: A smart way to enhance innate immune defence. Mol. Immunol. 2015, 68, 40–44. [Google Scholar] [CrossRef] [PubMed]
- Cunningham, K.T.; Mills, K.H.G. Trained Innate Immunity in Hematopoietic Stem Cell and Solid Organ Transplantation. Transplantation 2021, 105, 1666–1676. [Google Scholar] [CrossRef]
- Heng, Y.; Zhang, X.; Borggrewe, M.; van Weering, H.R.J.; Brummer, M.L.; Nijboer, T.W.; Joosten, L.A.B.; Netea, M.G.; Boddeke, E.W.G.M.; Laman, J.D.; et al. Systemic administration of beta-glucan induces immune training in microglia. J. Neuroinflamm. 2021, 18, 57. [Google Scholar] [CrossRef] [PubMed]
- Sohrabi, Y.; Dos Santos, J.C.; Dorenkamp, M.; Findeisen, H.; Godfrey, R.; Netea, M.G.; Joosten, L.A. Trained immunity as a novel approach against COVID-19 with a focus on Bacillus Calmette-Guerin vaccine: Mechanisms, challenges and perspectives. Clin. Transl. Immunol. 2020, 9, e1228. [Google Scholar] [CrossRef]
- Covián, C.; Ríos, M.; Berríos-Rojas, R.V.; Bueno, S.M.; Kalergis, A.M. Induction of Trained Immunity by Recombinant Vaccines. Front. Immunol. 2021, 11, 611946. [Google Scholar] [CrossRef]
- Moorlag, S.J.C.F.M.; Rodriguez-Rosales, Y.A.; Gillard, J.; Fanucchi, S.; Theunissen, K.; Novakovic, B.; de Bont, C.M.; Negishi, Y.; Fok, E.T.; Kalafati, L.; et al. BCG Vaccination Induces Long-Term Functional Reprogramming of Human Neutrophils. Cell Rep. 2020, 33, 108387. [Google Scholar] [CrossRef]
- Luo, Y.; Chen, X.; O’Donnell, M.A. Role of Th1 and Th2 cytokines in BCG-induced IFN-gamma production: Cytokine promotion and simula-tion of BCG effect. Cytokine 2003, 21, 17–26. [Google Scholar] [CrossRef]
- Caron, J.; Ridgley, L.A.; Bodman-Smith, M. How to Train Your Dragon: Harnessing Gamma Delta T Cells Antiviral Functions and Trained Immunity in a Pandemic Era. Front. Immunol. 2021, 12, 666983. [Google Scholar] [CrossRef]
- Zhong, C.; Yang, X.; Feng, Y.; Yu, J. Trained Immunity: An Underlying Driver of Inflammatory Atherosclerosis. Front. Immunol. 2020, 11, 284. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wendeln, A.C.; Degenhardt, K.; Kaurani, L.; Gertig, M.; Ulas, T.; Jain, G.; Wagner, J.; Häsler, L.M.; Wild, K.; Skodras, A.; et al. Innate immune memory in the brain shapes neurological disease hallmarks. Nature 2018, 556, 332–338. [Google Scholar] [CrossRef] [PubMed]
- Ennerfelt, H.E.; Lukens, J.R. The role of innate immunity in Alzheimer’s disease. Immunol. Rev. 2020, 297, 225–246. [Google Scholar] [CrossRef]
- Multhoff, G.; Molls, M.; Radons, J. Chronic inflammation in cancer development. Front. Immunol. 2012, 2, 98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dimitriou, F.; Hogan, S.; Menzies, A.M.; Dummer, R.; Long, G.V. Interleukin-6 blockade for prophylaxis and management of immune-related adverse events in cancer immunotherapy. Eur. J. Cancer. 2021, 157, 214–224. [Google Scholar] [CrossRef] [PubMed]
- Balkwill, F. TNF-alpha in promotion and progression of cancer. Cancer Metastasis Rev. 2006, 25, 409–416. [Google Scholar] [CrossRef]
- Montfort, A.; Colacios, C.; Levade, T.; Andrieu-Abadie, N.; Meyer, N.; Ségui, B. The TNF Paradox in Cancer Progression and Immunotherapy. Front. Immunol. 2019, 10, 1818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Peckham, D.; Scambler, T.; Savic, S.; McDermott, M.F. The burgeoning field of innate immune-mediated disease and autoinflammation. J. Pathol. 2017, 241, 123–139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Devulapalli, C.S. COVIder in childD-19 is milren possibly due to cross-immunity. Acta Paediatr. 2020, 109, 2422. [Google Scholar] [CrossRef]
- Han, X.; Li, X.; Xiao, Y.; Yang, R.; Wang, Y.; Wei, X. Distinct Characteristics of COVID-19 Infection in Children. Front. Pediatr. 2021, 9, 619738. [Google Scholar] [CrossRef]
- Falahi, S.; Abdoli, A.; Kenarkoohi, A. Claims and reasons about mild COVID-19 in children. New Microbes New Infect. 2021, 41, 100864. [Google Scholar] [CrossRef]
- Akbarpour, M.; Sharifi, L.; Safdarian, A.R.; Farhangnia, P.; Borjkhani, M.; Rezaei, N. Potential Antiviral Immune Response Against COVID-19: Lessons Learned from SARS-CoV. Adv. Exp. Med. Biol. 2021, 1318, 149–167. [Google Scholar]
- Amato, M.; Werba, J.P.; Frigerio, B.; Coggi, D.; Sansaro, D.; Ravani, A.; Ferrante, P.; Veglia, F.; Tremoli, E.; Baldassarre, D. Relationship between Influenza Vaccination Coverage Rate and COVID-19 Outbreak: An Italian Ecological Study. Vaccines 2020, 8, 535. [Google Scholar] [CrossRef]
- Candelli, M.; Pignataro, G.; Torelli, E.; Gullì, A.; Nista, E.C.; Petrucci, M.; Saviano, A.; Marchesini, D.; Covino, M.; Ojetti, V.; et al. Effect of influenza vaccine on COVID-19 mortality: A retrospective study. Intern. Emerg. Med. 2021, 20, 1–7. [Google Scholar]
- Jones, M.E.; Kohn, A.H.; Pourali, S.P.; Rajkumar, J.R.; Gutierrez, Y.; Yim, R.M.; Armstrong, A.W. The Use of Biologics During the COVID-19 Pandemic. Dermatol Clin. 2021, 39, 545–553. [Google Scholar] [CrossRef]
- Montesu, M.A.; Biondi, G.; Sotgiu, G.; Sucato, F.; Satta, R. Biologic drugs during COVID-19 outbreak. Int. J. Dermatol. 2020, 59, 1293. [Google Scholar] [CrossRef]
- Della-Torre, E.; Lanzillotta, M.; Campochiaro, C.; Cavalli, G.; De Luca, G.; Tomelleri, A.; Boffini, N.; De Lorenzo, R.; Ruggeri, A.; Rovere-Querini, P.; et al. Respiratory Impairment Predicts Response to IL-1 and IL-6 Blockade in COVID-19 Patients With Severe Pneumonia and Hyper Inflammation. Front. Immunol. 2021, 12, 675678. [Google Scholar] [CrossRef]
- Rabaan, A.A.; Al-Ahmed, S.H.; Muhammad, J.; Khan, A.; Sule, A.A.; Tirupathi, R.; Mutair, A.A.; Alhumaid, S.; Al-Omari, A.; Dhawan, M.; et al. Role of Inflammatory Cytokines in COVID-19 Patients: A Review on Molecular Mechanisms, Immune Functions, Immunopathology and Immunomodulatory Drugs to Counter Cytokine Storm. Vaccines 2021, 9, 436. [Google Scholar] [CrossRef]
- Maslennikov, R.; Ivashkin, V.; Vasilieva, E.; Chipurik, M.; Semikova, P.; Semenets, V.; Russkova, T.; Levshina, A.; Grigoriadis, D.; Magomedov, S.; et al. Tofacitinib reduces mortality in coronavirus disease 2019 Tofacitinib in COVID-19. Pulm. Pharmacol. Ther. 2021, 69, 102039. [Google Scholar] [CrossRef]
- Welzel, T.; Samba, S.D.; Klein, R.; van den Anker, J.N. Kuemmerle-Deschner JB. COVID-19 in Autoinflammatory Diseases with Immunosuppressive Treatment. J. Clin. Med. 2021, 10, 605. [Google Scholar] [CrossRef] [PubMed]
- Peng, J.; Fu, M.; Mei, H.; Zheng, H.; Liang, G.; She, X.; Wang, Q.; Liu, W. Efficacy and secondary infection risk of tocilizumab, sarilumab and anakinra in COVID-19 patients: A systematic review and meta-analysis. Rev. Med. Virol. 2021, e2295. [Google Scholar] [CrossRef]
- Kyriazopoulou, E.; Poulakou, G.; Milionis, H.; Metallidis, S.; Adamis, G.; Tsiakos, K.; Fragkou, A.; Rapti, A.; Damoulari, C.; Fantoni, M.; et al. Early treatment of COVID-19 with anakinra guided by soluble urokinase plasminogen receptor plasma levels: A double-blind, randomized controlled phase 3 trial. Nat. Med. 2021, 27, 1–9. [Google Scholar] [CrossRef]
| Cytokine Target | Principal Cell Source Cytokine | Principal Cellular Targets and Biologic Effects | Biological Drug | Structure | Disease | |
|---|---|---|---|---|---|---|
| TNF-α | Macrophages, T cells | Endothelial cells. Neutrophils and inflammation activation. Induces apoptosis in many cell types. | TNF inhibitors [69] | ADALIMUMAB [70] | Human Monoclonal Antibody | - plaque psoriasis [71] - psoriatic arthritis [72] - rheumatoid arthritis [73] - hidradenitis suppurativa [74] - non-infectious uveitis [75] - polyarticular juvenile idiopathic arthritis [76] - moderate-to-severe Crohn’s disease and ulcerative colitis [77] |
| GOLIMUMAB [78] | Human Monoclonal Antibody | -moderate to severe ulcerative colitis [79] - rheumatoid arthritis, psoriatic arthritis/spondyloarthritis [80] | ||||
| ETANERCEPT [81] | TNF- α receptor-Fc fusion | - plaque psoriasis [82] - psoriasis [83] - juvenile idiopathic arthritis [84] - psoriatic arthritis [85] - rheumatoid arthritis [86] | ||||
| INFLIXIMAB [87] | Chimeric monoclonal antibody | - idiopathic inflammatory myopathies [88] - moderate-to-severe Crohn’s disease and ulcerative colitis [77] - Behçet’s disease [89] - rheumatoid arthritis [90] - ankylosing spondylitis [91] - psoriasis [92] | ||||
| CERTOLIZUMAB-pegol [93] | Mouse Monoclonal Antibody (Fab’ fragment) | - Crohn’s Disease [94] - rheumatoid arthritis [95] - psoriasis [96] | ||||
| IL-1 β | Macrophages, endothelial cells, epithelial cells | Endothelial cells, hypothalamus, liver | IL-1 β inhibitors [97] | ANAKINRA [98] | Interleukin-1 receptor antagonist | - Still’s disease [99] - rheumatoid arthritis [100] - cryopyrin-associated periodic syndrome [101] - autoinflammatory disorders [102,103] |
| CANAKINUMAB [104] | Human Monoclonal Antibody | -autoinflammatory disease [105] - hyperimmunoglobulin D syndrome [106] - Familial Mediterranean Fever [107] - juvenile idiopathic arthritis [108] | ||||
| RILONACEPT [109] | Interleukin-1 receptor antagonist | - chronic inflammatory disorders [110] - cryopyrin-associated periodic syndromes [111] - recurrent pericarditis [112] - gout [113] | ||||
| IL-6 | Macrophages, endothelial cells, T cells | Liver, B cells | IL-6 inhibitors [114] | TOCILIZUMAB [115] | IL-6 receptor monoclonal antibodies | - rheumatoid arthritis [116] - giant cell arteritis [117] - Still’s disease [118] - juvenile idiopathic arthritis [119] - Macrophage activation syndrome [120] |
| SARILUMAB [121] | IL-6 receptor monoclonal antibodies | - moderate-to-severe Rheumatoid Arthritis [122] | ||||
| SILTUXIMAB [123] | IL-6 monoclonal antibodies | - Castleman’s disease [124] - non-Hodgkin lymphoma [125] - autoimmune diseases [126] | ||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Marcuzzi, A.; Melloni, E.; Zauli, G.; Romani, A.; Secchiero, P.; Maximova, N.; Rimondi, E. Autoinflammatory Diseases and Cytokine Storms—Imbalances of Innate and Adaptative Immunity. Int. J. Mol. Sci. 2021, 22, 11241. https://doi.org/10.3390/ijms222011241
Marcuzzi A, Melloni E, Zauli G, Romani A, Secchiero P, Maximova N, Rimondi E. Autoinflammatory Diseases and Cytokine Storms—Imbalances of Innate and Adaptative Immunity. International Journal of Molecular Sciences. 2021; 22(20):11241. https://doi.org/10.3390/ijms222011241
Chicago/Turabian StyleMarcuzzi, Annalisa, Elisabetta Melloni, Giorgio Zauli, Arianna Romani, Paola Secchiero, Natalia Maximova, and Erika Rimondi. 2021. "Autoinflammatory Diseases and Cytokine Storms—Imbalances of Innate and Adaptative Immunity" International Journal of Molecular Sciences 22, no. 20: 11241. https://doi.org/10.3390/ijms222011241
APA StyleMarcuzzi, A., Melloni, E., Zauli, G., Romani, A., Secchiero, P., Maximova, N., & Rimondi, E. (2021). Autoinflammatory Diseases and Cytokine Storms—Imbalances of Innate and Adaptative Immunity. International Journal of Molecular Sciences, 22(20), 11241. https://doi.org/10.3390/ijms222011241











