Long Intergenic Noncoding RNA OIN1 Promotes Ovarian Cancer Growth by Modulating Apoptosis-Related Gene Expression
Abstract
:1. Introduction
2. Results
2.1. OIN1 Is Highly Expressed in Ovarian Cancer Tissues and Cells
2.2. OIN1 Promotes Proliferation and Suppresses Apoptosis of Ovarian Cancer Cells
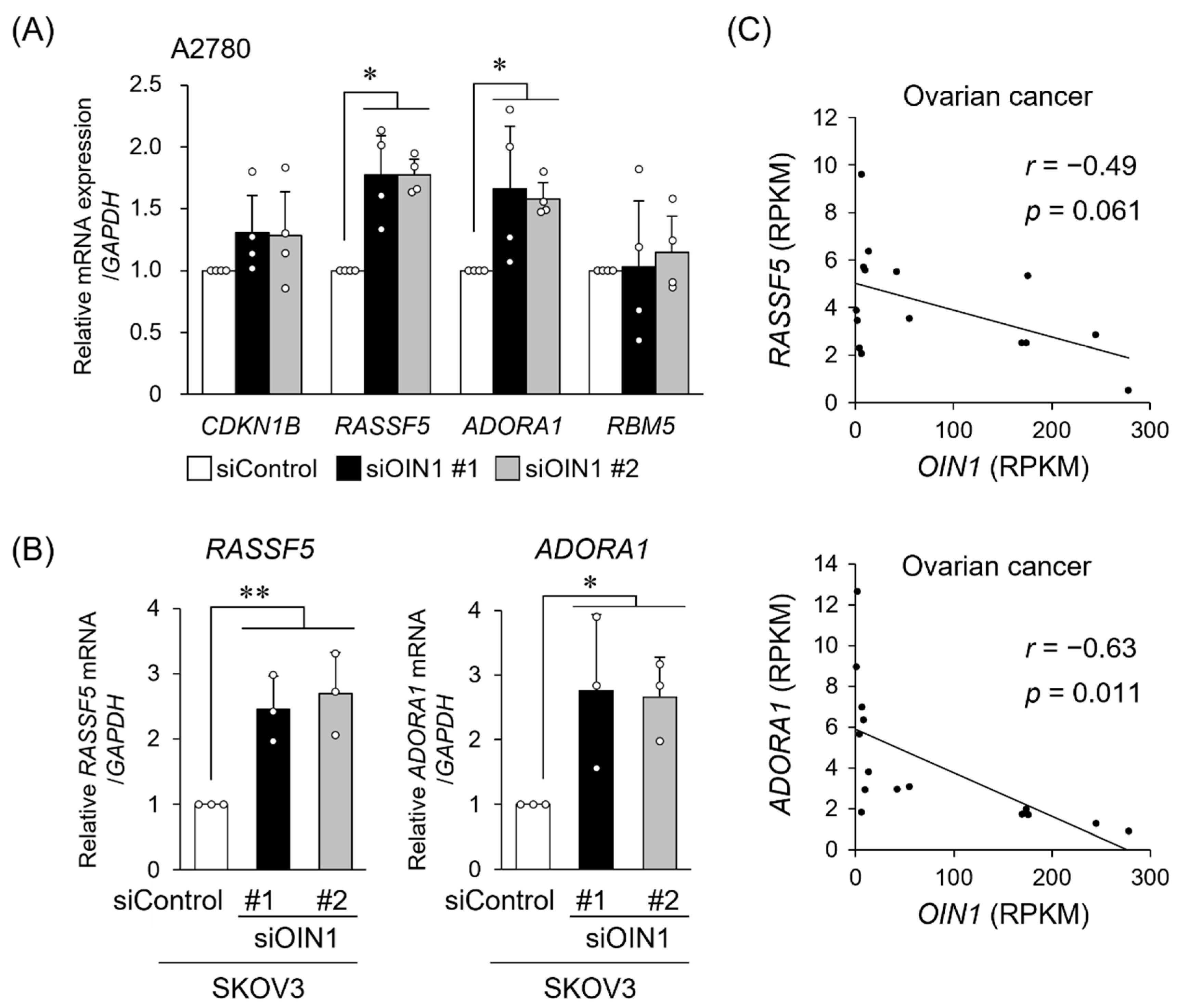
2.3. OIN1 Modulates the Expression of Apoptosis- or Cell Proliferation-Related Genes
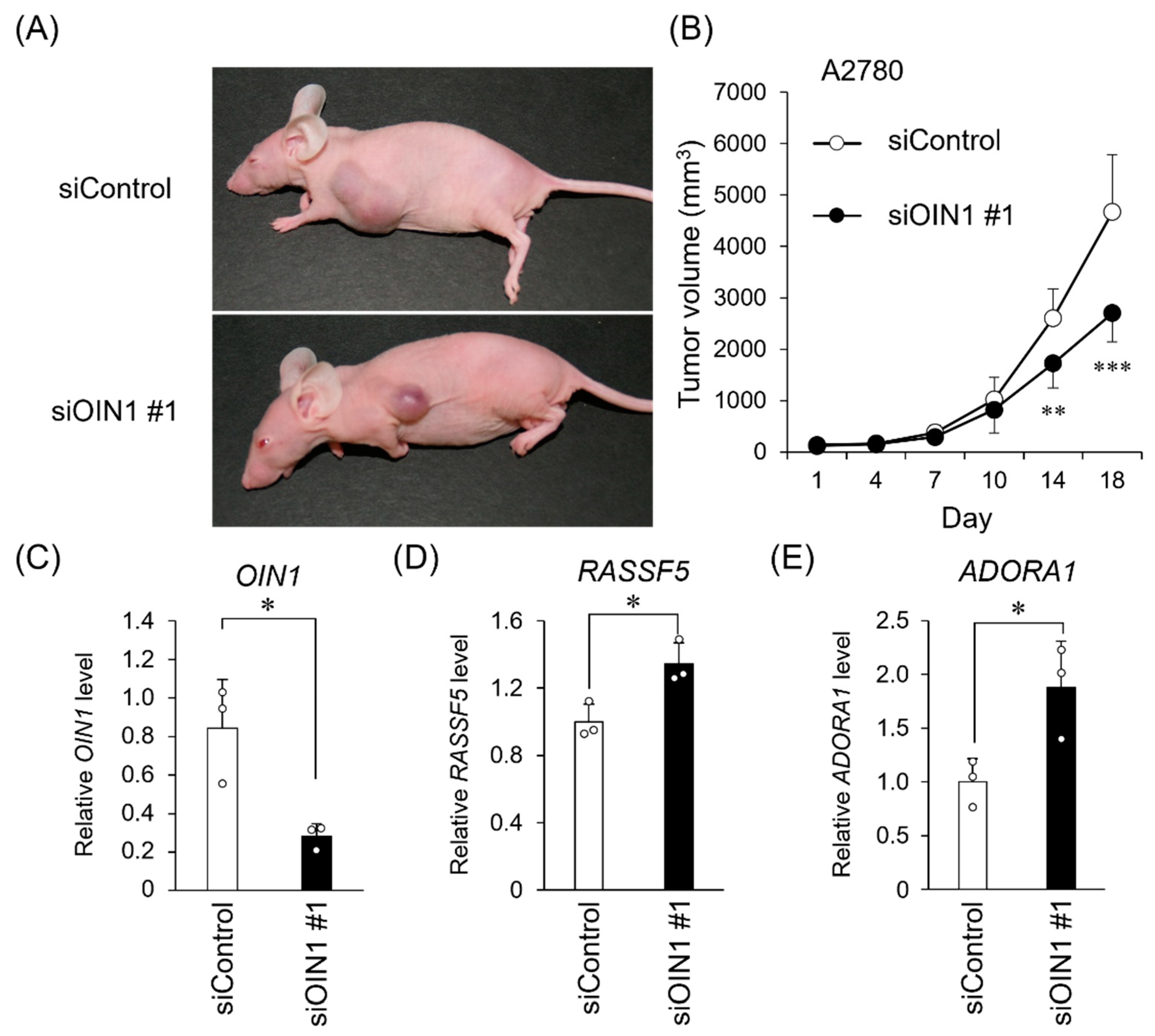
2.4. OIN1 Silencing Suppresses In Vivo Tumor Growth of Ovarian Cancer Cells
3. Discussion
4. Materials and Methods
4.1. RNA-Seq Analysis of Clinical Specimens from Normal and Ovarian Cancer Tissues
4.2. Human Ovarian Cancer Cell Culture
4.3. siRNA and Plasmid Transfection
4.4. RNA Extraction and qRT-PCR
4.5. Cell Proliferation Assay (DNA Assay)
4.6. Apoptosis Assay
4.7. Western Blotting
4.8. In Vivo Tumor Formation and siRNA Treatment
4.9. Statistical Analysis
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Momenimovahed, Z.; Tiznobaik, A.; Taheri, S.; Salehiniya, H. Ovarian cancer in the world: Epidemiology and risk factors. Int. J. Women’s Health 2019, 11, 287–299. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coburn, S.B.; Bray, F.; Sherman, M.E.; Trabert, B. International patterns and trends in ovarian cancer incidence, overall and by histologic subtype. Int. J. Cancer 2017, 140, 2451–2460. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Torre, L.A.; Trabert, B.; DeSantis, C.E.; Miller, K.D.; Samimi, G.; Runowicz, C.D.; Gaudet, M.M.; Jemal, A.; Siegel, R.L. Ovarian cancer statistics, 2018. CA Cancer J. Clin. 2018, 68, 284–296. [Google Scholar] [CrossRef]
- Kashi, K.; Henderson, L.; Bonetti, A.; Carninci, P. Discovery and functional analysis of lncRNAs: Methodologies to investigate an uncharacterized transcriptome. Biochim. Biophys. Acta 2016, 1859, 3–15. [Google Scholar] [CrossRef] [Green Version]
- Anastasiadou, E.; Messina, E.; Sanavia, T.; Labruna, V.; Ceccarelli, S.; Megiorni, F.; Gerini, G.; Pontecorvi, P.; Camero, S.; Perniola, G.; et al. Calcineurin gamma catalytic subunit PPP3CC inhibition by miR-200c-3p affects apoptosis in epithelial ovarian cancer. Genes 2021, 12, 1400. [Google Scholar] [CrossRef]
- Alshamrani, A.A. Roles of microRNAs in ovarian cancer tumorigenesis: Two decades later, what have we learned. Front. Oncol. 2020, 10, 1084. [Google Scholar] [CrossRef]
- Takayama, K.I.; Inoue, S. The emerging role of noncoding RNA in prostate cancer progression and its implication on diagnosis and treatment. Brief. Funct. Genom. 2016, 15, 257–265. [Google Scholar] [CrossRef] [Green Version]
- Misawa, A.; Takayama, K.I.; Inoue, S. Long non-coding RNAs and prostate cancer. Cancer Sci. 2017, 108, 2107–2114. [Google Scholar] [CrossRef]
- Mitobe, Y.; Takayama, K.I.; Horie-Inoue, K.; Inoue, S. Prostate cancer-associated lncRNAs. Cancer Lett. 2018, 418, 159–166. [Google Scholar] [CrossRef]
- Arun, G.; Diermeier, S.D.; Spector, D.L. Therapeutic targeting of long non-coding RNAs in cancer. Trends Mol. Med. 2018, 24, 257–277. [Google Scholar] [CrossRef] [PubMed]
- Takeiwa, T.; Ikeda, K.; Mitobe, Y.; Horie-Inoue, K.; Inoue, S. Long noncoding RNAs involved in the endocrine therapy resistance of breast cancer. Cancers 2020, 12, 1424. [Google Scholar] [CrossRef]
- Wang, J.Y.; Lu, A.Q.; Chen, L.J. LncRNAs in ovarian cancer. Clin. Chim. Acta 2019, 490, 17–27. [Google Scholar] [CrossRef] [PubMed]
- Zheng, J.; Guo, J.; Zhang, H.; Cao, B.; Xu, G.; Zhang, Z.; Tong, J. Four prognosis-associated lncRNAs serve as biomarkers in ovarian cancer. Front. Genet. 2021, 12, 672674. [Google Scholar] [CrossRef] [PubMed]
- Takeiwa, T.; Ikeda, K.; Horie-Inoue, K.; Inoue, S. Mechanisms of apoptosis-related long non-coding RNAs in ovarian cancer. Front. Cell Dev. Biol. 2021, 9, 641963. [Google Scholar] [CrossRef] [PubMed]
- Nagasawa, S.; Ikeda, K.; Horie-Inoue, K.; Sato, S.; Itakura, A.; Takeda, S.; Hasegawa, K.; Inoue, S. Systematic identification of characteristic genes of ovarian clear cell carcinoma compared with high-grade serous carcinoma based on RNA-sequencing. Int. J. Mol. Sci. 2019, 20, 4330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nagasawa, S.; Ikeda, K.; Horie-Inoue, K.; Sato, S.; Takeda, S.; Hasegawa, K.; Inoue, S. Identification of novel mutations of ovarian cancer-related genes from RNA-sequencing data for Japanese epithelial ovarian cancer patients. Endocr. J. 2020, 67, 219–229. [Google Scholar] [CrossRef] [Green Version]
- Ransohoff, J.D.; Wei, Y.; Khavari, P.A. The functions and unique features of long intergenic non-coding RNA. Nat. Rev. Mol. Cell Biol. 2018, 19, 143–157. [Google Scholar] [CrossRef]
- Xie, C.; Yuan, J.; Li, H.; Li, M.; Zhao, G.; Bu, D.; Zhu, W.; Wu, W.; Chen, R.; Zhao, Y. NONCODEv4: Exploring the world of long non-coding RNA genes. Nucleic Acids Res. 2014, 42, D98–D103. [Google Scholar] [CrossRef] [Green Version]
- Gao, Y.; Meng, H.; Liu, S.; Hu, J.; Zhang, Y.; Jiao, T.; Liu, Y.; Ou, J.; Wang, D.; Yao, L.; et al. LncRNA-HOST2 regulates cell biological behaviors in epithelial ovarian cancer through a mechanism involving microRNA let-7b. Hum. Mol. Genet. 2015, 24, 841–852. [Google Scholar] [CrossRef] [Green Version]
- Wang, F.; Zhou, J.; Xie, X.; Hu, J.; Chen, L.; Hu, Q.; Guo, H.; Yu, C. Involvement of SRPK1 in cisplatin resistance related to long non-coding RNA UCA1 in human ovarian cancer cells. Neoplasma 2015, 62, 432–438. [Google Scholar] [CrossRef] [Green Version]
- Li, Z.; Niu, H.; Qin, Q.; Yang, S.; Wang, Q.; Yu, C.; Wei, Z.; Jin, Z.; Wang, X.; Yang, A.; et al. lncRNA UCA1 mediates resistance to cisplatin by regulating the miR-143/FOSL2-signaling pathway in ovarian cancer. Mol. Ther. Nucleic Acids 2019, 17, 92–101. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, X.; Spindler, T.J.; de Souza Fonseca, M.A.; Corona, R.I.; Seo, J.H.; Dezem, F.S.; Li, L.; Lee, J.M.; Long, H.W.; Sellers, T.A.; et al. Super-enhancer-associated LncRNA UCA1 interacts directly with AMOT to activate YAP target genes in epithelial ovarian cancer. iScience 2019, 17, 242–255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.; Liu, Y.; Cai, Y.; Han, P.; Wang, R.; Cao, L.; He, S. Downregulation of LINC00958 inhibits proliferation, invasion and migration, and promotes apoptosis of colorectal cancer cells by targeting miR-3619-5p. Oncol. Rep. 2020, 44, 1574–1582. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Domcke, S.; Sinha, R.; Levine, D.A.; Sander, C.; Schultz, N. Evaluating cell lines as tumour models by comparison of genomic profiles. Nat. Commun. 2013, 4, 2126. [Google Scholar] [CrossRef]
- Beaufort, C.M.; Helmijr, J.C.; Piskorz, A.M.; Hoogstraat, M.; Ruigrok-Ritstier, K.; Besselink, N.; Murtaza, M.; van IJcken, W.F.; Heine, A.A.; Smid, M.; et al. Ovarian cancer cell line panel (OCCP): Clinical importance of in vitro morphological subtypes. PLoS ONE 2014, 9, e103988. [Google Scholar] [CrossRef]
- Stordal, B.; Timms, K.; Farrelly, A.; Gallagher, D.; Busschots, S.; Renaud, M.; Thery, J.; Williams, D.; Potter, J.; Tran, T.; et al. BRCA1/2 mutation analysis in 41 ovarian cell lines reveals only one functionally deleterious BRCA1 mutation. Mol. Oncol. 2013, 7, 567–579. [Google Scholar] [CrossRef] [Green Version]
- Liao, T.J.; Tsai, C.J.; Jang, H.; Fushman, D.; Nussinov, R. RASSF5: An MST activator and tumor suppressor in vivo but opposite in vitro. Curr. Opin. Struct. Biol. 2016, 41, 217–224. [Google Scholar] [CrossRef]
- Zhou, X.H.; Yang, C.Q.; Zhang, C.L.; Gao, Y.; Yuan, H.B.; Wang, C. RASSF5 inhibits growth and invasion and induces apoptosis in osteosarcoma cells through activation of MST1/LATS1 signaling. Oncol. Rep. 2014, 32, 1505–1512. [Google Scholar] [CrossRef] [Green Version]
- Aoyama, Y.; Avruch, J.; Zhang, X.F. Nore1 inhibits tumor cell growth independent of Ras or the MST1/2 kinases. Oncogene 2004, 23, 3426–3433. [Google Scholar] [CrossRef] [Green Version]
- Park, J.; Kang, S.I.; Lee, S.Y.; Zhang, X.F.; Kim, M.S.; Beers, L.F.; Lim, D.S.; Avruch, J.; Kim, H.S.; Lee, S.B. Tumor suppressor ras association domain family 5 (RASSF5/NORE1) mediates death receptor ligand-induced apoptosis. J. Biol. Chem. 2010, 285, 35029–35038. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Elmetwali, T.; Salman, A.; Palmer, D.H. NORE1A induction by membrane-bound CD40L (mCD40L) contributes to CD40L-induced cell death and G1 growth arrest in p21-mediated mechanism. Cell Death Dis. 2016, 7, e2146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.T.; Yu, C.; Xu, Y.; Liu, S.B.; Fan, H.Y.; Pan, W.W. TET1 inhibits cell proliferation by inducing RASSF5 expression. Oncotarget 2017, 8, 86395–86409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kazemi, M.H.; Raoofi Mohseni, S.; Hojjat-Farsangi, M.; Anvari, E.; Ghalamfarsa, G.; Mohammadi, H.; Jadidi-Niaragh, F. Adenosine and adenosine receptors in the immunopathogenesis and treatment of cancer. J. Cell Physiol. 2018, 233, 2032–2057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Tong, L.; Chu, X.; Deng, F.; Tang, J.; Tang, Y.; Dai, Y. The adenosine A1 receptor antagonist DPCPX inhibits tumor progression via the ERK/JNK pathway in renal cell carcinoma. Cell. Physiol. Biochem. 2017, 43, 733–742. [Google Scholar] [CrossRef]
- Sureechatchaiyan, P.; Hamacher, A.; Brockmann, N.; Stork, B.; Kassack, M.U. Adenosine enhances cisplatin sensitivity in human ovarian cancer cells. Purinergic Signal. 2018, 14, 395–408. [Google Scholar] [CrossRef] [PubMed]
- Yu, H.; Xu, Y.; Zhang, D.; Liu, G. Long noncoding RNA LUCAT1 promotes malignancy of ovarian cancer through regulation of miR-612/HOXA13 pathway. Biochem. Biophys. Res. Commun. 2018, 503, 2095–2100. [Google Scholar] [CrossRef] [PubMed]
- Gu, L.; Li, Q.; Liu, H.; Lu, X.; Zhu, M. Long noncoding RNA TUG1 promotes autophagy-associated paclitaxel resistance by sponging miR-29b-3p in ovarian cancer cells. Onco Targets Ther. 2020, 13, 2007–2019. [Google Scholar] [CrossRef] [Green Version]
- Deng, B.; Xu, W.; Wang, Z.; Liu, C.; Lin, P.; Li, B.; Huang, Q.; Yang, J.; Zhou, H.; Qu, L. An LTR retrotransposon-derived lncRNA interacts with RNF169 to promote homologous recombination. EMBO Rep. 2019, 20, e47650. [Google Scholar] [CrossRef] [PubMed]
- Zapatka, M.; Borozan, I.; Brewer, D.S.; Iskar, M.; Grundhoff, A.; Alawi, M.; Desai, N.; Sültmann, H.; Moch, H.; Cooper, C.S.; et al. The landscape of viral associations in human cancers. Nat. Genet. 2020, 52, 320–330. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ishiguro, T.; Sato, A.; Ohata, H.; Ikarashi, Y.; Takahashi, R.U.; Ochiya, T.; Yoshida, M.; Tsuda, H.; Onda, T.; Kato, T.; et al. Establishment and characterization of an in vitro model of ovarian cancer stem-like cells with an enhanced proliferative capacity. Cancer Res. 2016, 76, 150–160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Namekawa, T.; Ikeda, K.; Horie-Inoue, K.; Inoue, S. Application of prostate cancer models for preclinical study: Advantages and limitations of cell lines, patient-derived xenografts, and three-dimensional culture of patient-derived cells. Cells 2019, 8, 74. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maru, Y.; Hippo, Y. Current status of patient-derived ovarian cancer models. Cells 2019, 8, 505. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shiba, S.; Ikeda, K.; Suzuki, T.; Shintani, D.; Okamoto, K.; Horie-Inoue, K.; Hasegawa, K.; Inoue, S. Hormonal regulation of patient-derived endometrial cancer stem-like cells generated by three-dimensional culture. Endocrinology 2019, 160, 1895–1906. [Google Scholar] [CrossRef]
- Kamada, S.; Namekawa, T.; Ikeda, K.; Suzuki, T.; Kagawa, M.; Takeshita, H.; Yano, A.; Okamoto, K.; Ichikawa, T.; Horie-Inoue, K.; et al. Functional inhibition of cancer stemness-related protein DPP4 rescues tyrosine kinase inhibitor resistance in renal cell carcinoma. Oncogene 2021, 40, 3899–3913. [Google Scholar] [CrossRef]
- Mitobe, Y.; Ikeda, K.; Sato, W.; Kodama, Y.; Naito, M.; Gotoh, N.; Miyata, K.; Kataoka, K.; Sasaki, H.; Horie-Inoue, K.; et al. Proliferation-associated long noncoding RNA, TMPO-AS1, is a potential therapeutic target for triple-negative breast cancer. Cancer Sci. 2020, 111, 2440–2450. [Google Scholar] [CrossRef]
- Sato, W.; Ikeda, K.; Urano, T.; Abe, Y.; Nakasato, N.; Horie-Inoue, K.; Takeda, S.; Inoue, S. Efp promotes in vitro and in vivo growth of endometrial cancer cells along with the activation of nuclear factor-κB signaling. PLoS ONE 2018, 13, e0208351. [Google Scholar] [CrossRef] [Green Version]
- Mitobe, Y.; Ikeda, K.; Suzuki, T.; Takagi, K.; Kawabata, H.; Horie-Inoue, K.; Inoue, S. ESR1-stabilizing long noncoding RNA TMPO-AS1 promotes hormone-refractory breast cancer progression. Mol. Cell. Biol. 2019, 39, e00261-19. [Google Scholar] [CrossRef]
- Ueyama, K.; Ikeda, K.; Sato, W.; Nakasato, N.; Horie-Inoue, K.; Takeda, S.; Inoue, S. Knockdown of Efp by DNA-modified small interfering RNA inhibits breast cancer cell proliferation and in vivo tumor growth. Cancer Gene Ther. 2010, 17, 624–632. [Google Scholar] [CrossRef]


| NONCODE ID | Chr | Start | End | Strand | Alias | Normal Ovary | Ovarian Cancer | Fold Change b | q-Value | ||
|---|---|---|---|---|---|---|---|---|---|---|---|
| Mean RPKM | SEM RPKM | Mean RPKM | SEM RPKM | ||||||||
| NONHSAT013448 | 10 | 54,785,023 | 54,789,855 | - | OIN1 | 3.0 | 2.6 | 79.2 | 24.8 | 26.1 | 5.6 × 10−3 |
| NONHSAT099419 | 4 | 182,443,812 | 182,444,154 | + | 3.2 | 2.7 | 40.4 | 5.5 | 12.7 | 2.9 × 10−4 | |
| NONHSAT017219 | 11 | 287,304 | 288,298 | + | 0.6 | 0.2 | 38.6 | 18.3 | 62.9 | 1.0 × 10−2 | |
| NONHSAT027397 | 12 | 26,383,751 | 26,472,653 | - | 0.5 | 0.2 | 27.4 | 11.7 | 51.5 | 1.6 × 10−3 | |
| NONHSAT015316 | 10 | 85,926,985 | 85,931,832 | - | CERNA2/HOST2 | 0.8 | 0.7 | 17.2 | 5.9 | 20.2 | 7.4 × 10−4 |
| NONHSAT032437 | 13 | 23,477,401 | 23,493,348 | + | 0.3 | 0.1 | 9.2 | 3.0 | 31.0 | 1.5 × 10−4 | |
| NONHSAT080725 | 20 | 60,880,487 | 60,881,452 | - | 0.5 | 0.2 | 8.8 | 3.8 | 19.2 | 1.3 × 10−3 | |
| NONHSAT122583 | 7 | 104,581,509 | 104,602,507 | + | 0.3 | 0.2 | 8.2 | 3.7 | 27.0 | 3.0 × 10−2 | |
| NONHSAT061517 | 19 | 15,939,789 | 15,947,064 | + | UCA1 | 0.6 | 0.5 | 6.7 | 2.0 | 11.3 | 8.1 × 10−3 |
| NONHSAT018088 | 11 | 13,002,033 | 13,005,839 | - | LINC00958 | 0.6 | 0.5 | 5.7 | 0.9 | 10.3 | 2.1 × 10−2 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Takeiwa, T.; Mitobe, Y.; Ikeda, K.; Hasegawa, K.; Horie, K.; Inoue, S. Long Intergenic Noncoding RNA OIN1 Promotes Ovarian Cancer Growth by Modulating Apoptosis-Related Gene Expression. Int. J. Mol. Sci. 2021, 22, 11242. https://doi.org/10.3390/ijms222011242
Takeiwa T, Mitobe Y, Ikeda K, Hasegawa K, Horie K, Inoue S. Long Intergenic Noncoding RNA OIN1 Promotes Ovarian Cancer Growth by Modulating Apoptosis-Related Gene Expression. International Journal of Molecular Sciences. 2021; 22(20):11242. https://doi.org/10.3390/ijms222011242
Chicago/Turabian StyleTakeiwa, Toshihiko, Yuichi Mitobe, Kazuhiro Ikeda, Kosei Hasegawa, Kuniko Horie, and Satoshi Inoue. 2021. "Long Intergenic Noncoding RNA OIN1 Promotes Ovarian Cancer Growth by Modulating Apoptosis-Related Gene Expression" International Journal of Molecular Sciences 22, no. 20: 11242. https://doi.org/10.3390/ijms222011242
APA StyleTakeiwa, T., Mitobe, Y., Ikeda, K., Hasegawa, K., Horie, K., & Inoue, S. (2021). Long Intergenic Noncoding RNA OIN1 Promotes Ovarian Cancer Growth by Modulating Apoptosis-Related Gene Expression. International Journal of Molecular Sciences, 22(20), 11242. https://doi.org/10.3390/ijms222011242







