Signaling at Physical Barriers during Pollen–Pistil Interactions
Abstract
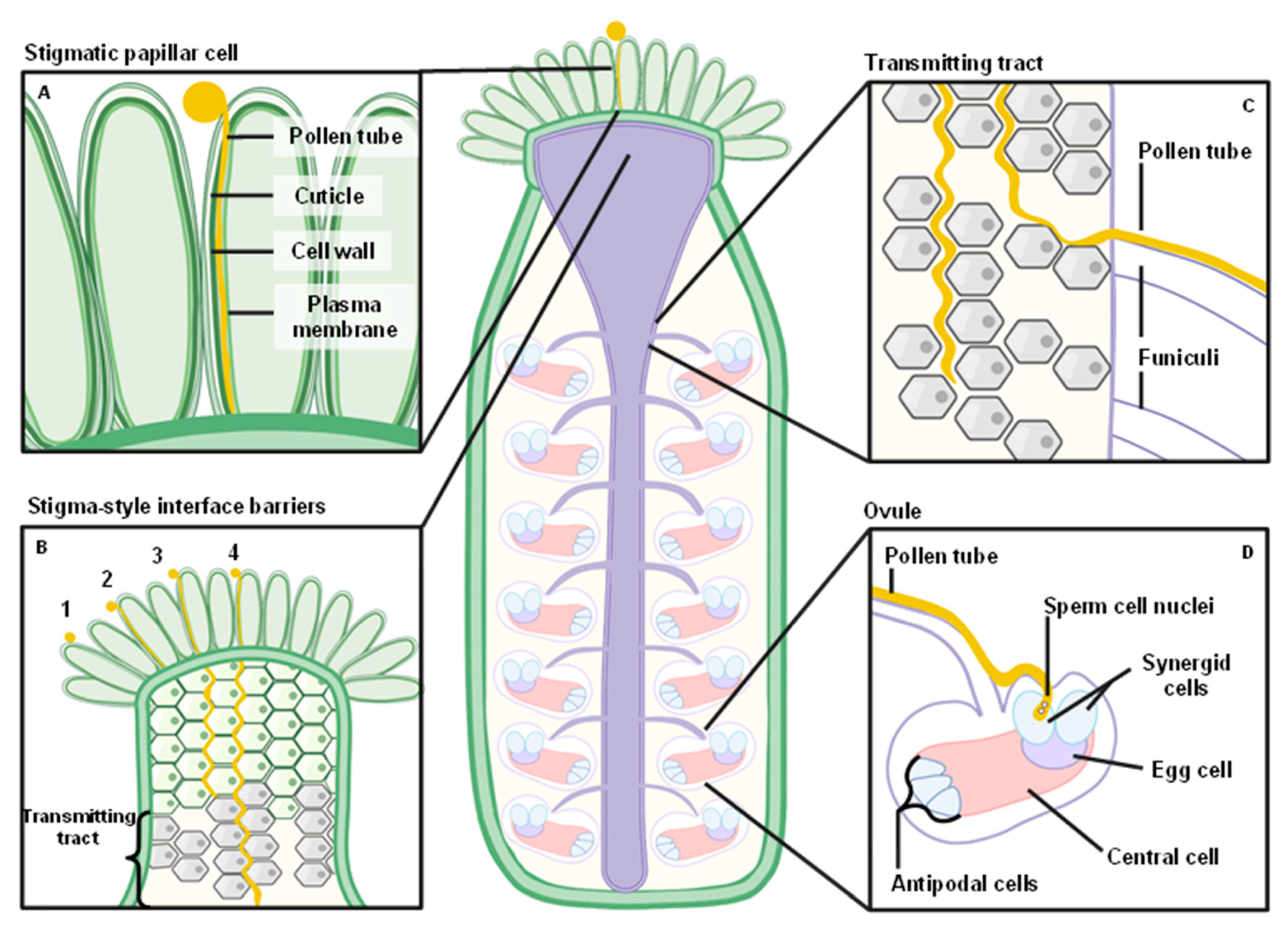
:1. Introduction
2. Pollen Tube Growth and Cell Wall Composition
3. Pollen Tube Behavior during Encounters with Inanimate Barriers
4. Physical Interactions with the Stigmatic Papillae
5. Penetrating through the Stigma-Style Interface
6. Growth in the Transmitting Tract
7. Synergid Cell Penetration and Sperm Cell Discharge
8. Sperm Cell Fusion
9. Conclusions and Future Perspectives
Funding
Conflicts of Interest
References
- Johnson, M.A.; Harper, J.F.; Palanivelu, R. A Fruitful Journey: Pollen Tube Navigation from Germination to Fertilization. Annu. Rev. Plant Biol. 2019, 70, 809–837. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Higashiyama, T.; Yang, W. Gametophytic Pollen Tube Guidance: Attractant Peptides, Gametic Controls, and Receptors. Plant Physiol. 2017, 173, 112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dresselhaus, T.; Sprunck, S.; Wessel, G.M. Fertilization Mechanisms in Flowering Plants. Curr. Biol. 2016, 26, R125–R139. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nezhad, A.S.; Geitmann, A. The cellular mechanics of an invasive lifestyle. J. Exp. Bot. 2013, 64, 4709–4728. [Google Scholar] [CrossRef] [Green Version]
- Chae, K.; Lord, E.M. Pollen tube growth and guidance: Roles of small, secreted proteins. Ann. Bot. 2011, 108, 627–636. [Google Scholar] [CrossRef] [Green Version]
- Krichevsky, A.; Kozlovsky, S.V.; Tian, G.W.; Chen, M.H.; Zaltsman, A.; Citovsky, V. How pollen tubes grow. Dev. Biol. 2007, 303, 405–420. [Google Scholar] [CrossRef] [Green Version]
- Burton, R.A.; Gidley, M.J.; Fincher, G.B. Heterogeneity in the chemistry, structure and function of plant cell walls. Nat. Chem. Biol. 2010, 6, 724–732. [Google Scholar] [CrossRef]
- Somerville, C.; Bauer, S.; Brininstool, G.; Facette, M.; Hamann, T.; Milne, J.; Osborne, E.; Paredez, A.; Persson, S.; Raab, T.; et al. REVIEW Toward a systems approach to understanding plant cell walls. Science 2004, 306, 2206–2211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mollet, J.C.; Leroux, C.; Dardelle, F.; Lehner, A. Cell wall composition, biosynthesis and remodeling during pollen tube growth. Plants 2013, 2, 107. [Google Scholar] [CrossRef] [Green Version]
- Williams, J.H.; Edwards, J.A.; Ramsey, A.J. Economy, efficiency, and the evolution of pollen tube growth rates. Am. J. Bot. 2016, 103, 471–483. [Google Scholar] [CrossRef] [Green Version]
- Dardelle, F.; Lehner, A.; Ramdani, Y.; Bardor, M.; Lerouge, P.; Driouich, A.; Mollet, J.-C. Biochemical and Immunocytological Characterizations of Arabidopsis Pollen Tube Cell Wall. Plant Physiol. 2010, 153, 1563–1576. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parre, E.; Geitmann, A. Pectin and the role of the physical properties of the cell wall in pollen tube growth of Solanum chacoense. Planta 2005, 220, 582–592. [Google Scholar] [CrossRef] [PubMed]
- Sterling, J.D.; Atmodjo, M.A.; Inwood, S.E.; Kolli, V.S.K.; Quigley, H.F.; Hahn, M.G.; Mohnen, D. Functional identification of an Arabidopsis pectin biosynthetic homogalacturonan galacturonosyltransferase. Proc. Natl. Acad. Sci. USA 2006, 103, 5236–5241. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atmodjo, M.A.; Sakuragi, Y.; Zhu, X.; Burrell, A.J.; Mohanty, S.S.; Atwood, J.A.; Orlando, R.; Scheller, H.V.; Mohnen, D. Galacturonosyltransferase (GAUT)1 and GAUT7 are the core of a plant cell wall pectin biosynthetic homogalacturonan:galacturonosyltransferase complex. Proc. Natl. Acad. Sci. USA 2011, 108, 20225–20230. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, L.; Wang, W.; Wang, Y.Q.; Liu, Y.Y.; Wang, J.X.; Zhang, X.Q.; Ye, D.; Chen, L.Q. Arabidopsis galacturonosyltransferase (GAUT) 13 and GAUT14 have redundant functions in pollen tube growth. Mol. Plant 2013, 6, 1131–1148. [Google Scholar] [CrossRef] [Green Version]
- Caffall, K.H.; Mohnen, D. The structure, function, and biosynthesis of plant cell wall pectic polysaccharides. Carbohydr. Res. 2009, 344, 1879–1900. [Google Scholar] [CrossRef]
- Atmodjo, M.A.; Hao, Z.; Mohnen, D. Evolving views of pectin biosynthesis. Annu. Rev. Plant Biol. 2013, 64, 747–779. [Google Scholar] [CrossRef] [Green Version]
- Stonebloom, S.; Ebert, B.; Xiong, G.; Pattathil, S.; Birdseye, D.; Lao, J.; Pauly, M.; Hahn, M.G.; Heazlewood, J.L.; Scheller, H.V. A DUF-246 family glycosyltransferase-like gene affects male fertility and the biosynthesis of pectic arabinogalactans. BMC Plant Biol. 2016, 16, 90. [Google Scholar] [CrossRef] [Green Version]
- O’Neill, M.A.; Ishii, T.; Albersheim, P.; Darvill, A.G. RHAMNOGALACTURONAN II: Structure and Function of a Borate Cross-Linked Cell Wall Pectic Polysaccharide. Annu. Rev. Plant Biol. 2004, 55, 109–139. [Google Scholar] [CrossRef] [Green Version]
- Routray, P.; Li, T.; Yamasaki, A.; Yoshinari, A.; Takano, J.; Choi, W.G.; Sams, C.E.; Roberts, D.M. Nodulin Intrinsic Protein 7;1 Is a Tapetal Boric Acid Channel Involved in Pollen Cell Wall Formation. Plant Physiol. 2018, 178, 1269. [Google Scholar] [CrossRef] [Green Version]
- Iwai, H.; Hokura, A.; Oishi, M.; Chida, H.; Ishii, T.; Sakai, S.; Satoh, S. The gene responsible for borate cross-linking of pectin Rhamnogalacturonan-II is required for plant reproductive tissue development and fertilization. Proc. Natl. Acad. Sci. USA 2006, 103, 16592–16597. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delmas, F.; Séveno, M.; Northey, J.G.B.; Hernould, M.; Lerouge, P.; McCourt, P.; Chevalier, C. The synthesis of the rhamnogalacturonan II component 3-deoxy-D-manno-2-octulosonic acid (Kdo) is required for pollen tube growth and elongation. J. Exp. Bot. 2008, 59, 2639–2647. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kobayashi, M.; Kouzu, N.; Inami, A.; Toyooka, K.; Konishi, Y.; Matsuoka, K.; Matoh, T. Characterization of Arabidopsis CTP:3-Deoxy-d-manno-2-Octulosonate Cytidylyltransferase (CMP-KDO synthetase), the Enzyme that Activates KDO During Rhamnogalacturonan II Biosynthesis. Plant Cell Physiol. 2011, 52, 1832–1843. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Dumont, M.; Lehner, A.; Bouton, S.; Kiefer-Meyer, M.C.; Voxeur, A.; Pelloux, J.; Lerouge, P.; Mollet, J.-C. The cell wall pectic polymer rhamnogalacturonan-II is required for proper pollen tube elongation: Implications of a putative sialyltransferase-like protein. Ann. Bot. 2014, 114, 1177. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Ebert, B.; Zhang, B.; Liu, H.; Zhang, Y.; Zeng, W.; Rautengarten, C.; Li, H.; Chen, X.; Bacic, A.; et al. UDP-Api/UDP-Xyl synthases affect plant development by controlling the content of UDP-Api to regulate the RG-II-borate complex. Plant J. 2020, 104, 252–267. [Google Scholar] [CrossRef] [PubMed]
- Liu, X.-L.; Liu, L.; Niu, Q.-K.; Xia, C.; Yang, K.-Z.; Li, R.; Chen, L.-Q.; Zhang, X.-Q.; Zhou, Y.; Ye, D. MALE GAMETOPHYTE DEFECTIVE 4 encodes a rhamnogalacturonan II xylosyltransferase and is important for growth of pollen tubes and roots in Arabidopsis. Plant J. 2011, 65, 647–660. [Google Scholar] [CrossRef]
- Mizuta, Y.; Kurihara, D.; Higashiyama, T. Two-photon imaging with longer wavelength excitation in intact Arabidopsis tissues. Protoplasma 2015, 252, 1231–1240. [Google Scholar] [CrossRef]
- Noble, J.A.; Palanivelu, R. Workflow to characterize mutants with reproductive defects. In Methods in Molecular Biology; Humana Press Inc.: Clifton, NJ, USA, 2020; Volume 2160, pp. 109–128. [Google Scholar]
- Nezhad, A.S.; Naghavi, M.; Packirisamy, M.; Bhat, R.; Geitmann, A. Quantification of cellular penetrative forces using lab-on-a-chip technology and finite element modeling. Proc. Natl. Acad. Sci. USA 2013, 110, 8093–8098. [Google Scholar] [CrossRef] [Green Version]
- Yanagisawa, N.; Sugimoto, N.; Arata, H.; Higashiyama, T.; Sato, Y. Capability of tip-growing plant cells to penetrate into extremely narrow gaps. Sci. Rep. 2017, 7, 1403. [Google Scholar] [CrossRef] [Green Version]
- Edlund, A.F.; Swanson, R.; Preuss, D. Pollen and stigma structure and function: The role of diversity in pollination. Plant Cell 2004, 16, S84–S97. [Google Scholar] [CrossRef] [Green Version]
- Ndinyanka Fabrice, T.; Kaech, A.; Barmettler, G.; Eichenberger, C.; Knox, J.P.; Grossniklaus, U.; Ringli, C. Efficient preparation of Arabidopsis pollen tubes for ultrastructural analysis using chemical and cryo-fixation. BMC Plant Biol. 2017, 17, 176. [Google Scholar] [CrossRef] [Green Version]
- Chapman, L.A.; Goring, D.R. Pollen-pistil interactions regulating successful fertilization in the Brassicaceae. J. Exp. Bot. 2010, 61, 1987–1999. [Google Scholar] [CrossRef] [Green Version]
- Palanivelu, R.; Tsukamoto, T. Pathfinding in angiosperm reproduction: Pollen tube guidance by pistils ensures successful double fertilization. Wiley Interdiscip. Rev. Dev. Biol. 2012, 1, 96–113. [Google Scholar] [CrossRef] [PubMed]
- Riglet, L.; Rozier, F.; Kodera, C.; Bovio, S.; Sechet, J.; Fobis-Loisy, I.; Gaude, T. KATANIN-dependent mechanical properties of the stigmatic cell wall mediate the pollen tube path in arabidopsis. eLife 2020, 9, e57282. [Google Scholar] [CrossRef]
- Rozier, F.; Riglet, L.; Kodera, C.; Bayle, V.; Durand, E.; Schnabel, J.; Gaude, T.; Fobis-Loisy, I. Live-cell imaging of early events following pollen perception in self-incompatible Arabidopsis Thaliana. J. Exp. Bot. 2020, 71, 2513–2526. [Google Scholar] [CrossRef] [PubMed]
- Takahashi, K.; Shimada, T.; Kondo, M.; Tamai, A.; Mori, M.; Nishimura, M.; Hara-Nishimura, I. Ectopic expression of an esterase, which is a candidate for the unidentified plant cutinase, causes cuticular defects in arabidopsis thaliana. Plant Cell Physiol. 2010, 51, 123–131. [Google Scholar] [CrossRef] [PubMed]
- Paredez, A.R.; Somerville, C.; Ehrhardt, D. Visualization of Cellulose Synthase with Microtubules. Science 2006, 312, 1491–1495. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qin, Y.; Leydon, A.R.; Manziello, A.; Pandey, R.; Mount, D.; Denic, S.; Vasic, B.; Johnson, M.A.; Palanivelu, R. Penetration of the stigma and style elicits a novel transcriptome in pollen tubes, pointing to genes critical for growth in a pistil. PLoS Genet. 2009, 5, e1000621. [Google Scholar] [CrossRef] [Green Version]
- Leydon, A.R.; Weinreb, C.; Venable, E.; Reinders, A.; Ward, J.M.; Johnson, M.A. The molecular dialog between flowering plant reproductive partners defined by snp-informed rna-sequencing. Plant Cell 2017, 29, 984–1006. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Osaka, M.; Matsuda, T.; Sakazono, S.; Masuko-Suzuki, H.; Maeda, S.; Sewaki, M.; Sone, M.; Takahashi, H.; Nakazono, M.; Iwano, M.; et al. Cell type-specific transcriptome of brassicaceae stigmatic papilla cells from a combination of laser microdissection and RNA sequencing. Plant Cell Physiol. 2013, 54, 1894–1906. [Google Scholar] [CrossRef] [Green Version]
- Wang, Y.; Zhang, W.Z.; Song, L.F.; Zou, J.J.; Su, Z.; Wu, W.H. Transcriptome analyses show changes in gene expression to accompany pollen germination and tube growth in arabidopsis. Plant Physiol. 2008, 148, 1201–1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wan, L.; Essuman, K.; Anderson, R.G.; Sasaki, Y.; Monteiro, F.; Chung, E.H.; Nishimura, E.O.; DiAntonio, A.; Milbrandt, J.; Dangl, J.L.; et al. TIR domains of plant immune receptors are NAD+-cleaving enzymes that promote cell death. Science 2019, 365, 799–803. [Google Scholar] [CrossRef] [PubMed]
- Marchal, C.; Delorme-Hinoux, V.; Bariat, L.; Siala, W.; Belin, C.; Saez-Vasquez, J.; Riondet, C.; Reichheld, J.P. NTR/NRX Define a New Thioredoxin System in the Nucleus of Arabidopsis thaliana Cells. Mol. Plant 2014, 7, 30–44. [Google Scholar] [CrossRef] [Green Version]
- Cruz-Ramírez, A.; López-Bucio, J.; Ramírez-Pimentel, G.; Zurita-Silva, A.; Sánchez-Calderon, L.; Ramírez-Chávez, E.; González-Ortega, E.; Herrera-Estrella, L. The xipotl mutant of arabidopsis reveals a critical role for phospholipid metabolism in root system development and epidermal cell integrity. Plant Cell 2004, 16, 2020–2034. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, D.K.; Jones, D.M.; Lau, J.B.R.; Cruz, E.R.; Brown, E.; Harper, J.F.; Wallacea, I.S. A putative protein O-fucosyltransferase facilitates pollen tube penetration through the stigma–style interface. Plant Physiol. 2018, 176, 2804–2818. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Weigand, C.; Harper, J. Decapitation crosses to test pollen fertility mutations for defects in stigma-style penetration. In Methods in Molecular Biology; Humana Press Inc.: Clifton, NJ, USA, 2020; Volume 2160, pp. 29–40. [Google Scholar]
- Okajima, T.; Xu, A.; Irvine, K.D. Modulation of Notch-Ligand Binding by Protein O-Fucosyltransferase 1 and Fringe. J. Biol. Chem. 2003, 278, 42340–42345. [Google Scholar] [CrossRef] [Green Version]
- Sasamura, T.; Sasaki, N.; Miyashita, F.; Nakao, S.; Ishikawa, H.O.; Ito, M.; Kitagawa, M.; Harigaya, K.; Spana, E.; Bilder, D.; et al. Neurotic, a novel maternal neurogenic gene, encodes an O-fucosyltransferase that is essential for Notch-Delta interactions. Development 2003, 130, 4785–4795. [Google Scholar] [CrossRef] [Green Version]
- Shi, S.; Stanley, P. Protein O-fucosyltransferase 1 is an essential component of Notch signaling pathways. Proc. Natl. Acad. Sci. USA 2003, 100, 5234–5239. [Google Scholar] [CrossRef] [Green Version]
- Rampal, R.; Li, A.S.Y.; Moloney, D.J.; Georgiou, S.A.; Luther, K.B.; Nita-Lazar, A.; Haltiwanger, R.S. Lunatic fringe, manic fringe, and radical fringe recognize similar specificity determinants in O-fucosylated epidermal growth factor-like repeats. J. Biol. Chem. 2005, 280, 42454–42463. [Google Scholar] [CrossRef] [Green Version]
- Stahl, M.; Uemura, K.; Ge, C.; Shi, S.; Tashima, Y.; Stanley, P. Roles of Pofut1 and O-fucose in mammalian Notch signaling. J. Biol. Chem. 2008, 283, 13638–13651. [Google Scholar] [CrossRef] [Green Version]
- Luca, V.C.; Kim, B.C.; Ge, C.; Kakuda, S.; Wu, D.; Roein-Peikar, M.; Haltiwanger, R.S.; Zhu, C.; Ha, T.; Garcia, K.C. Notch-Jagged complex structure implicates a catch bond in tuning ligand sensitivity. Science 2017, 355, 1320. [Google Scholar] [CrossRef] [Green Version]
- Smith, D.K.; Harper, J.F.; Wallace, I.S. A potential role for protein O-fucosylation during pollen-pistil interactions. Plant Signal. Behav. 2018, 13, e1467687. [Google Scholar] [CrossRef] [PubMed]
- Takenaka, Y.; Kato, K.; Ogawa-Ohnishi, M.; Tsuruhama, K.; Kajiura, H.; Yagyu, K.; Takeda, A.; Takeda, Y.; Kunieda, T.; Hara-Nishimura, I.; et al. Pectin RG-I rhamnosyltransferases represent a novel plant-specific glycosyltransferase family. Nat. Plants 2018, 4, 669–676. [Google Scholar] [CrossRef] [PubMed]
- Wachananawat, B.; Kuroha, T.; Takenaka, Y.; Kajiura, H.; Naramoto, S.; Yokoyama, R.; Ishizaki, K.; Nishitani, K.; Ishimizu, T. Diversity of Pectin Rhamnogalacturonan I Rhamnosyltransferases in Glycosyltransferase Family 106. Front. Plant Sci. 2020, 11, 997. [Google Scholar] [CrossRef] [PubMed]
- Hao, L.; Liu, J.; Zhong, S.; Gu, H.; Qu, L.J. AtVPS41-mediated endocytic pathway is essential for pollen tube-stigma interaction in Arabidopsis. Proc. Natl. Acad. Sci. USA 2016, 113, 6307–6312. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, L.; Yang, S.-L.; Xie, L.-F.; Puah, C.S.; Zhang, X.-Q.; Yang, W.-C.; Sundaresan, V.; Ye, D. VANGUARD1 Encodes a Pectin Methylesterase That Enhances Pollen Tube Growth in the Arabidopsis Style and Transmitting Tract. Plant Cell 2005, 17, 584–596. [Google Scholar] [CrossRef] [Green Version]
- Boisson-Dernier, A.; Roy, S.; Kritsas, K.; Grobei, M.A.; Jaciubek, M.; Schroeder, J.I.; Grossniklaus, U. Disruption of the pollen-expressed FERONIA homologs ANXUR1 and ANXUR2 triggers pollen tube discharge. Development 2009, 136, 3279. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.; Bergonci, T.; Zhao, Y.; Zou, Y.; Du, S.; Liu, M.-C.; Luo, X.; Ruan, H.; García-Valencia, L.E.; Zhong, S.; et al. Arabidopsis pollen tube integrity and sperm release are regulated by RALF-mediated signaling HHS Public Access. Science 2017, 358, 1596–1600. [Google Scholar] [CrossRef] [Green Version]
- Zhu, L.; Chu, L.C.; Liang, Y.; Zhang, X.Q.; Chen, L.Q.; Ye, D. The arabidopsis CrRLK1L protein kinases BUPS1 and BUPS2 are required for normal growth of pollen tubes in the pistil. Plant J. 2018, 95, 474–486. [Google Scholar] [CrossRef] [Green Version]
- Ge, Z.; Zhao, Y.; Liu, M.C.; Zhou, L.Z.; Wang, L.; Zhong, S.; Hou, S.; Jiang, J.; Liu, T.; Huang, Q.; et al. LLG2/3 Are Co-receptors in BUPS/ANX-RALF Signaling to Regulate Arabidopsis Pollen Tube Integrity. Curr. Biol. 2019, 29, 3256–3265.e5. [Google Scholar] [CrossRef]
- Feng, H.; Liu, C.; Fu, R.; Zhang, M.; Li, H.; Shen, L.; Wei, Q.; Sun, X.; Xu, L.; Ni, B.; et al. LORELEI-LIKE GPI-ANCHORED PROTEINS 2/3 Regulate Pollen Tube Growth as Chaperones and Coreceptors for ANXUR/BUPS Receptor Kinases in Arabidopsis. Mol. Plant 2019, 12, 1612–1623. [Google Scholar] [CrossRef]
- Haruta, M.; Sabat, G.; Stecker, K.; Minkoff, B.B.; Sussman, M.R. A Peptide Hormone and Its Receptor Protein Kinase Regulate Plant Cell Expansion. Science 2014, 343, 408. [Google Scholar] [CrossRef] [Green Version]
- Hwang, J.-U.; Gu, Y.; Lee, Y.-J.; Yang, Z. Oscillatory ROP GTPase Activation Leads the Oscillatory Polarized Growth of Pollen Tubes. Mol. Biol. Cell 2005, 16, 5385. [Google Scholar] [CrossRef]
- Zhou, X.; Lu, J.; Zhang, Y.; Guo, J.; Lin, W.; Van Norman, J.M.; Qin, Y.; Zhu, X.; Yang, Z. Membrane receptor-mediated mechano-transduction maintains cell integrity during pollen tube growth within the pistil. Dev. Cell 2021, 56, 1030–1042.e6. [Google Scholar] [CrossRef]
- Wu, H.M.; Wong, E.; Ogdahl, J.; Cheung, A.Y. A pollen tube growth-promoting arabinogalactan protein from Nicotiana alata is similar to the tobacco TTS protein. Plant J. 2000, 22, 165–176. [Google Scholar] [CrossRef] [PubMed]
- Cheung, A.Y.; Wang, H.; Wu, H. ming A floral transmitting tissue-specific glycoprotein attracts pollen tubes and stimulates their growth. Cell 1995, 82, 383–393. [Google Scholar] [CrossRef] [Green Version]
- Costa, M.; Nobre, M.S.; Becker, J.D.; Masiero, S.; Amorim, M.I.; Pereira, L.G.; Coimbra, S. Expression-based and co-localization detection of arabinogalactan protein 6 and arabinogalactan protein 11 interactors in Arabidopsis pollen and pollen tubes. BMC Plant Biol. 2013, 13, 7. [Google Scholar] [CrossRef] [Green Version]
- Palanivelu, R.; Brass, L.; Edlund, A.F.; Preuss, D. Pollen Tube Growth and Guidance Is Regulated by POP2, an Arabidopsis Gene that Controls GABA Levels. Cell 2003, 114, 47–59. [Google Scholar] [CrossRef] [Green Version]
- Yu, G.-H.; Zou, J.; Feng, J.; Peng, X.-B.; Wu, J.-Y.; Wu, Y.-L.; Palanivelu, R.; Sun, M.-X. Exogenous γ-aminobutyric acid (GABA) affects pollen tube growth via modulating putative Ca2+-permeable membrane channels and is coupled to negative regulation on glutamate decarboxylase. J. Exp. Bot. 2014, 65, 3235. [Google Scholar] [CrossRef]
- Okuda, S.; Tsutsui, H.; Shiina, K.; Sprunck, S.; Takeuchi, H.; Yui, R.; Kasahara, R.D.; Hamamura, Y.; Mizukami, A.; Susaki, D.; et al. Defensin-like polypeptide LUREs are pollen tube attractants secreted from synergid cells. Nature 2009, 458, 357–361. [Google Scholar] [CrossRef] [PubMed]
- Takeuchi, H.; Higashiyama, T. A Species-Specific Cluster of Defensin-Like Genes Encodes Diffusible Pollen Tube Attractants in Arabidopsis. PLoS Biol. 2012, 10, e1001449. [Google Scholar] [CrossRef] [Green Version]
- Takeuchi, H.; Higashiyama, T. Tip-localized receptors control pollen tube growth and LURE sensing in Arabidopsis. Nature 2016, 531, 245–248. [Google Scholar] [CrossRef] [PubMed]
- Mizukami, A.G.; Inatsugi, R.; Jiao, J.; Kotake, T.; Kuwata, K.; Ootani, K.; Okuda, S.; Sankaranarayanan, S.; Sato, Y.; Maruyama, D.; et al. The AMOR Arabinogalactan Sugar Chain Induces Pollen-Tube Competency to Respond to Ovular Guidance. Curr. Biol. 2016, 26, 1091–1097. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rotman, N.; Rozier, F.; Boavida, L.; Dumas, C.; Berger, F.; Faure, J.E. Female control of male gamete delivery during fertilization in Arabidopsis thaliana. Curr. Biol. 2003, 13, 432–436. [Google Scholar] [CrossRef] [Green Version]
- Liu, X.; Castro, C.; Wang, Y.; Noble, J.; Ponvert, N.; Bundy, M.; Hoel, C.; Shpak, E.; Palanivelu, R. The Role of LORELEI in Pollen Tube Reception at the Interface of the Synergid Cell and Pollen Tube Requires the Modified Eight-Cysteine Motif and the Receptor-Like Kinase FERONIA. Plant Cell 2016, 28, 1035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Galindo-Trigo, S.; Blanco-Touriñán, N.; DeFalco, T.A.; Wells, E.S.; Gray, J.E.; Zipfel, C.; Smith, L.M. CrRLK1L receptor-like kinases HERK1 and ANJEA are female determinants of pollen tube reception. EMBO Rep. 2020, 21, e48466. [Google Scholar] [CrossRef]
- Kessler, S.A.; Shimosato-Asano, H.; Keinath, N.F.; Wuest, S.E.; Ingram, G.; Panstruga, R.; Grossniklaus, U. Conserved molecular components for pollen tube reception and fungal invasion. Science 2010, 330, 968–971. [Google Scholar] [CrossRef]
- Duan, Q.; Kita, D.; Johnson, E.A.; Aggarwal, M.; Gates, L.; Wu, H.-M.; Cheung, A.Y. Reactive oxygen species mediate pollen tube rupture to release sperm for fertilization in Arabidopsis. Nat. Commun. 2014, 5, 3129. [Google Scholar] [CrossRef]
- Duan, Q.; Liu, M.-C.J.; Kita, D.; Jordan, S.S.; Yeh, F.-L.J.; Yvon, R.; Carpenter, H.; Federico, A.N.; Garcia-Valencia, L.E.; Eyles, S.J.; et al. FERONIA controls pectin- and nitric oxide-mediated male–female interaction. Nature 2020, 579, 561–566. [Google Scholar] [CrossRef]
- Leydon, A.R.; Beale, K.M.; Woroniecka, K.; Castner, E.; Chen, J.; Horgan, C.; Palanivelu, R.; Johnson, M.A. Three MYB transcription factors control pollen tube differentiation required for sperm release. Curr. Biol. 2013, 23, 1209–1214. [Google Scholar] [CrossRef] [Green Version]
- Hamamura, Y.; Saito, C.; Awai, C.; Kurihara, D.; Miyawaki, A.; Nakagawa, T.; Kanaoka, M.M.; Sasaki, N.; Nakano, A.; Berger, F.; et al. Live-Cell Imaging Reveals the Dynamics of Two Sperm Cells during Double Fertilization in Arabidopsis thaliana. Curr. Biol. 2011, 21, 497–502. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sprunck, S.; Rademacher, S.; Vogler, F.; Gheyselinck, J.; Grossniklaus, U.; Dresselhaus, T. Egg cell—Secreted EC1 triggers sperm cell activation during double fertilization. Science 2012, 338, 1093–1097. [Google Scholar] [CrossRef] [PubMed]
- Mori, T.; Igawa, T.; Tamiya, G.; Miyagishima, S.Y.; Berger, F. Gamete Attachment Requires GEX2 for Successful Fertilization in Arabidopsis. Curr. Biol. 2014, 24, 170–175. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cyprys, P.; Lindemeier, M.; Sprunck, S. Gamete fusion is facilitated by two sperm cell-expressed DUF679 membrane proteins. Nat. Plants 2019, 5, 253–257. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Yu, Y.; Liang, Y.; Anderson, C.T.; Cao, J. A Profusion of Molecular Scissors for Pectins: Classification, Expression, and Functions of Plant Polygalacturonases. Front. Plant Sci. 2018, 9, 1208. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grashoff, C.; Hoffman, B.D.; Brenner, M.D.; Zhou, R.; Parsons, M.; Yang, M.T.; McLean, M.A.; Sligar, S.G.; Chen, C.S.; Ha, T.; et al. Measuring mechanical tension across vinculin reveals regulation of focal adhesion dynamics. Nature 2010, 466, 263. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bechtel, T.J.; Reyes-Robles, T.; Fadeyi, O.O.; Oslund, R.C. Strategies for monitoring cell–cell interactions. Nat. Chem. Biol. 2021, 17, 641–652. [Google Scholar] [CrossRef]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Robichaux, K.J.; Wallace, I.S. Signaling at Physical Barriers during Pollen–Pistil Interactions. Int. J. Mol. Sci. 2021, 22, 12230. https://doi.org/10.3390/ijms222212230
Robichaux KJ, Wallace IS. Signaling at Physical Barriers during Pollen–Pistil Interactions. International Journal of Molecular Sciences. 2021; 22(22):12230. https://doi.org/10.3390/ijms222212230
Chicago/Turabian StyleRobichaux, Kayleigh J., and Ian S. Wallace. 2021. "Signaling at Physical Barriers during Pollen–Pistil Interactions" International Journal of Molecular Sciences 22, no. 22: 12230. https://doi.org/10.3390/ijms222212230
APA StyleRobichaux, K. J., & Wallace, I. S. (2021). Signaling at Physical Barriers during Pollen–Pistil Interactions. International Journal of Molecular Sciences, 22(22), 12230. https://doi.org/10.3390/ijms222212230





