The ECM: To Scaffold, or Not to Scaffold, That Is the Question
Abstract
:1. Introduction
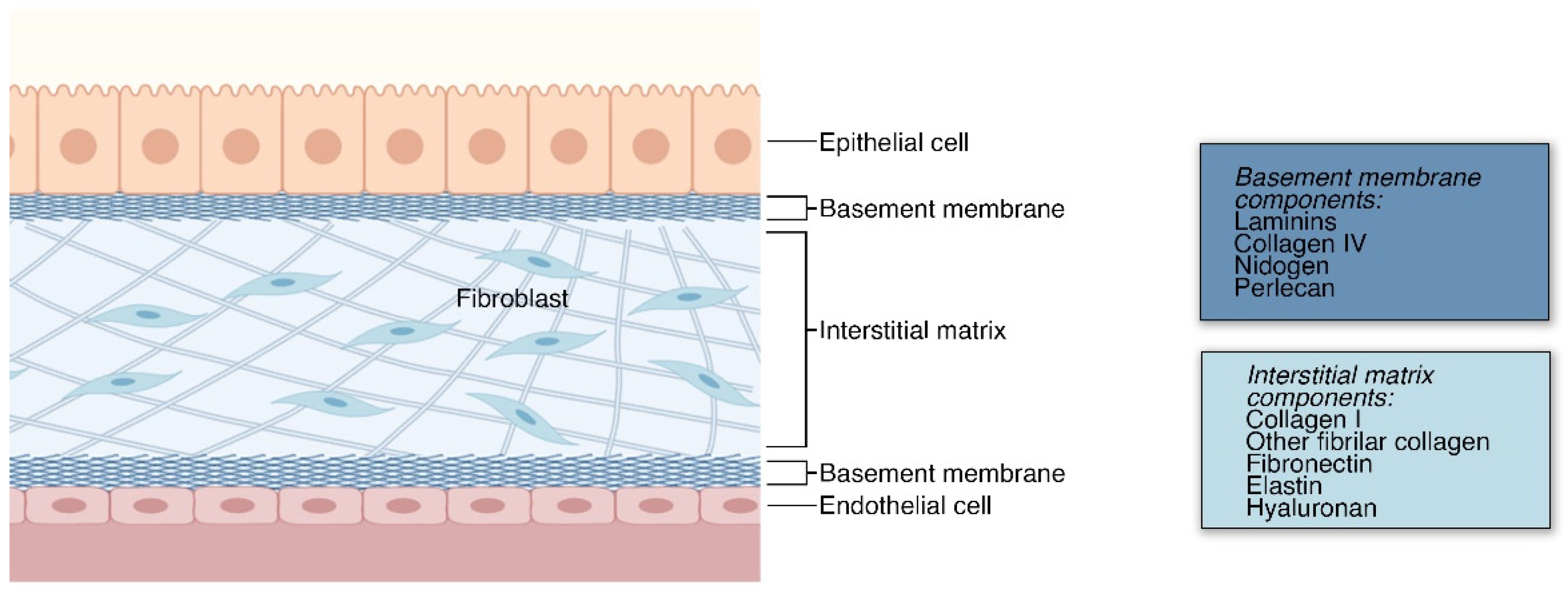
2. The Extracellular Matrix
2.1. ECM in Structural Support and Anchorage
2.2. ECM in Receptor Signaling

2.2.1. ECM in Morphogenesis
2.2.2. ECM in Proliferation
2.2.3. ECM in Cell Survival and Cell Death
2.3. Synthetic Materials as ECM Biomimetic
| Backbone | Structure | Citation |
|---|---|---|
| PEG | 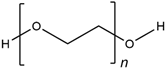 | Wake, et al., 1996 [118] Hern and Hubbell, 1998 [119] Namba, et al., 2009 [120] |
| PVA | 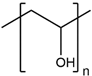 | Wake, et al., 1995 [121] Annabi, et al., 2013 [109] |
| PHEMA |  | Flynn, et al., 2003 [122] Annabi, et al., 2013 [109] |
| PAMAM | 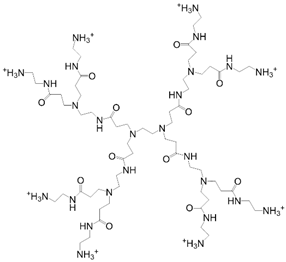 | Kawase, et al., 1999 [123] Kim and Kino-oka, 2014 [117] |
| Dextran | 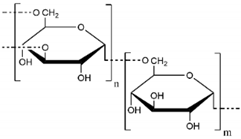 | Chupa, et al., 2000 [124] Möller, et al., 2007 [125] Liu, et al., 2021 [116] |
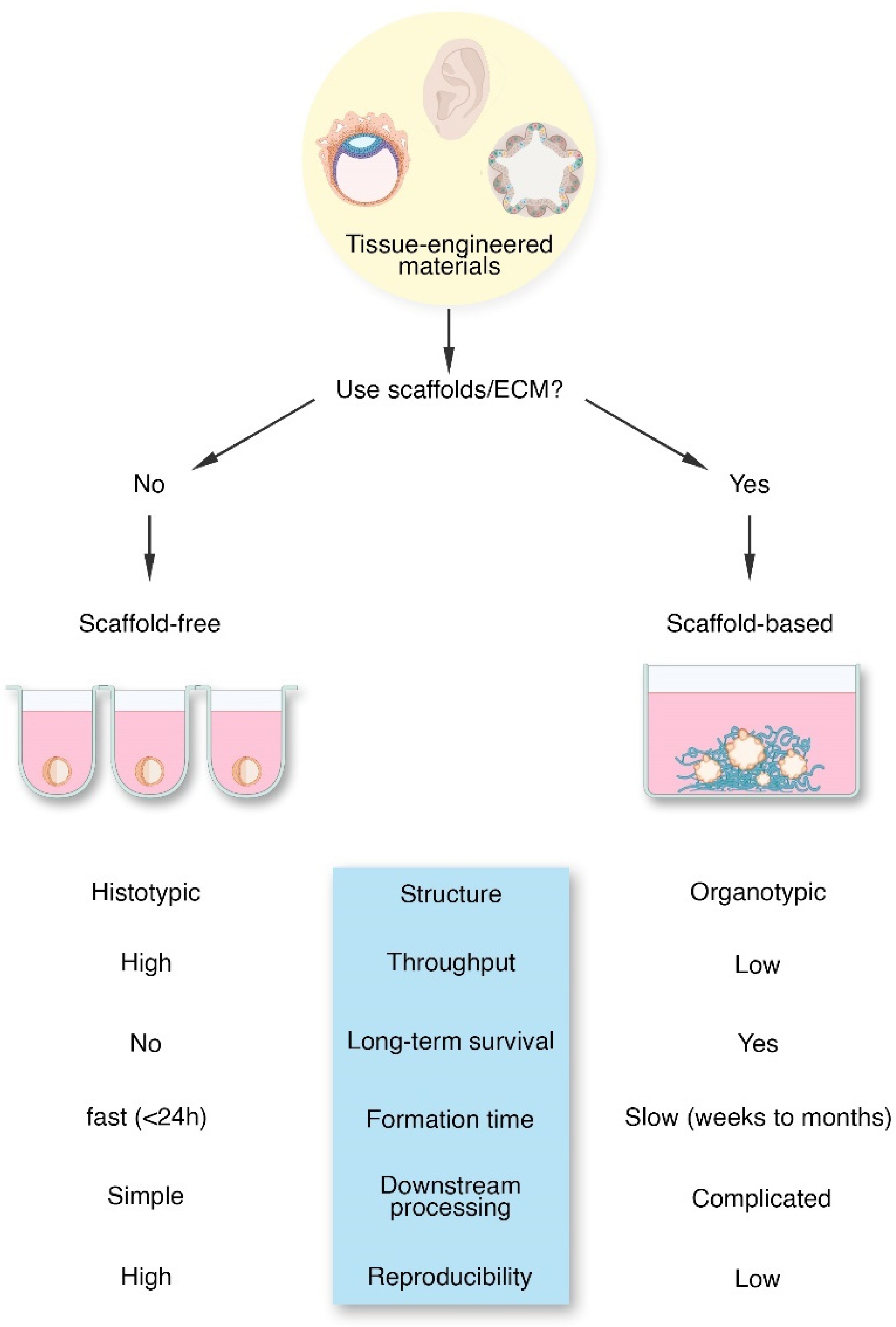
3. The Tissue Engineering Dichotomy
3.1. Scaffold-Free Tissue Engineering
3.2. Scaffold-Based Tissue Engineering
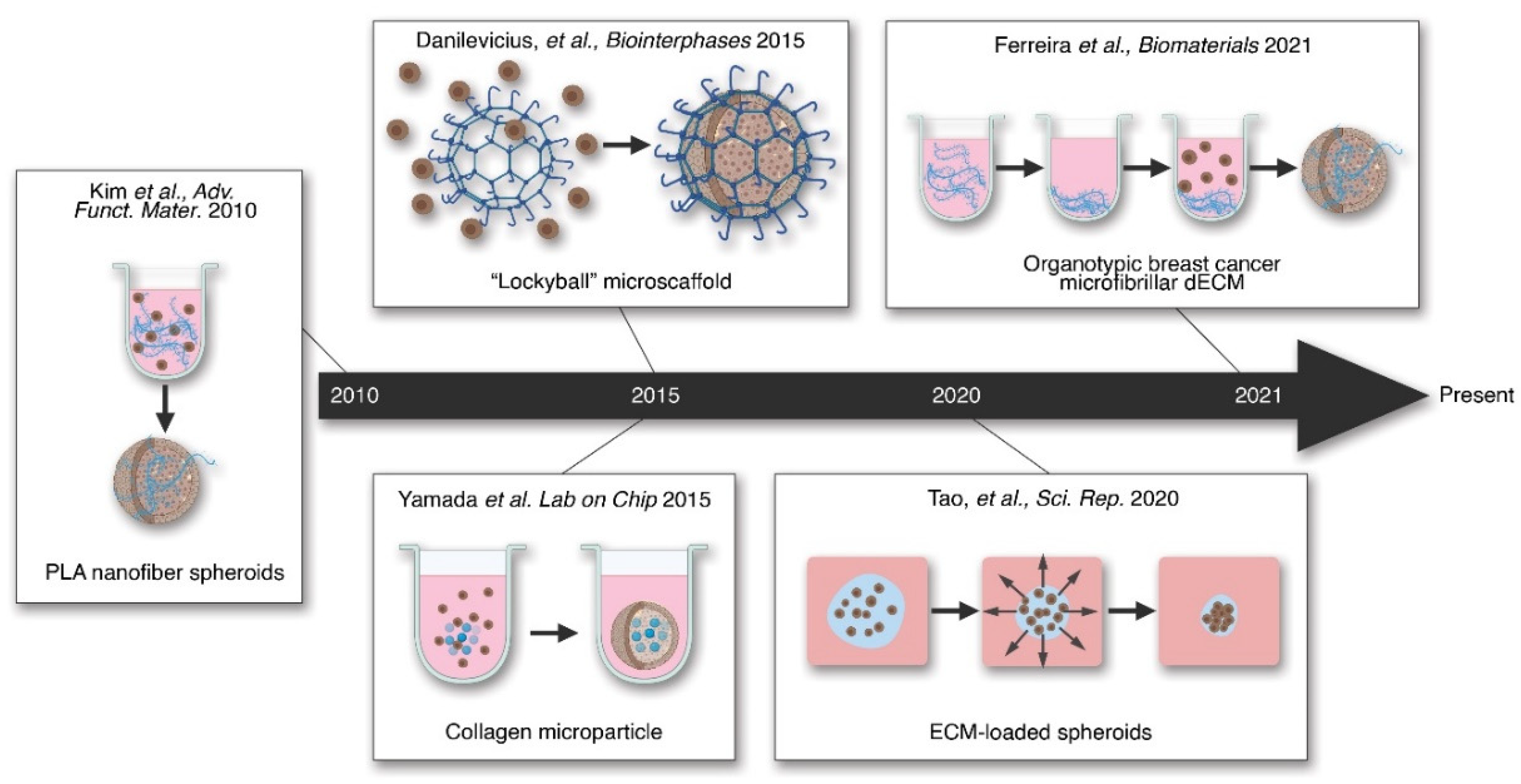
4. Hybrid Tissue Engineering and Use of ECM
5. Conclusions and Future Outlook
6. Glossary
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Bell, C.C.; Hendriks, D.; Moro, S.M.L.; Ellis, E.; Walsh, J.; Renblom, A.; Puigvert, L.F.; Dankers, A.C.A.; Jacobs, F.; Snoeys, J.; et al. Characterization of primary human hepatocyte spheroids as a model system for drug-induced liver injury, liver function and disease. Sci. Rep. 2016, 6, 25187. [Google Scholar] [CrossRef] [Green Version]
- Younesi, M.; Goldberg, V.M.; Akkus, O. A micro-architecturally biomimetic collagen template for mesenchymal condensation based cartilage regeneration. Acta Biomater. 2016, 30, 212–221. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caplan, A.I. Tissue Engineering: Then, Now, and the Future. Tissue Eng. Part A 2019, 25, 515–517. [Google Scholar] [CrossRef] [PubMed]
- Lentine, K.L.; Schnitzler, M.A. The Economic Impact of Addressing the Organ Shortage with Clinically High-Risk Allografts. Mol. Med. 2011, 108, 275–279. [Google Scholar]
- Kondo, J.; Inoue, M. Application of Cancer Organoid Model for Drug Screening and Personalized Therapy. Cells 2019, 8, 470. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Driehuis, E.; Kolders, S.; Spelier, S.; Lõhmussaar, K.; Willems, S.M.; Devriese, L.A.; De Bree, R.; De Ruiter, E.J.; Korving, J.; Begthel, H.; et al. Oral Mucosal Organoids as a Potential Platform for Personalized Cancer Therapy. Cancer Discov. 2019, 9, 852–871. [Google Scholar] [CrossRef]
- Yuki, K.; Cheng, N.; Nakano, M.; Kuo, C.J. Organoid Models of Tumor Immunology. Trends Immunol. 2020, 41, 652–664. [Google Scholar] [CrossRef]
- Ovsianikov, A.; Khademhosseini, A.; Mironov, V. The Synergy of Scaffold-Based and Scaffold-Free Tissue Engineering Strategies. Trends Biotechnol. 2018, 36, 348–357. [Google Scholar] [CrossRef]
- Kuo, H.-J.; Maslen, C.L.; Keene, D.R.; Glanville, R.W. Type VI Collagen Anchors Endothelial Basement Membranes by Interacting with Type IV Collagen. J. Biol. Chem. 1997, 272, 26522–26529. [Google Scholar] [CrossRef] [Green Version]
- Wang, S.; Sekiguchi, R.; Daley, W.P.; Yamada, K.M. Patterned cell and matrix dynamics in branching morphogenesis. J. Cell Biol. 2017, 216, 559–570. [Google Scholar] [CrossRef] [Green Version]
- Hynes, R.O. The extracellular matrix: Not just pretty fibrils. Science 2009, 326, 1216–1219. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hastings, J.F.; Skhinas, J.N.; Fey, D.; Croucher, D.R.; Cox, T.R. The extracellular matrix as a key regulator of intracellular signalling networks. Br. J. Pharmacol. 2019, 176, 82–92. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frantz, C.; Stewart, K.M.; Weaver, V.M. The extracellular matrix at a glance. J. Cell Sci. 2010, 123 Pt 24, 4195–4200. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Paulsson, M. Basement membrane proteins: Structure, assembly, and cellular interactions. Crit. Rev. Biochem. Mol. Biol. 1992, 27, 93–127. [Google Scholar] [PubMed]
- HintonJr, R.B.; Lincoln, J.; Deutsch, G.H.; Osinska, H.; Manning, P.B.; Benson, D.W.; Yutzey, K.E. Extracellular Matrix Remodeling and Organization in Developing and Diseased Aortic Valves. Circ. Res. 2006, 98, 1431–1438. [Google Scholar] [CrossRef] [Green Version]
- Loganathan, R.; Rongish, B.J.; Smith, C.; Filla, M.B.; Czirok, A.; Bénazéraf, B.; Little, C.D. Extracellular matrix motion and early morphogenesis. Development 2016, 143, 2056–2065. [Google Scholar] [CrossRef] [Green Version]
- Aleksandrova, A.; Czirok, A.; Kosa, E.; Galkin, O.; Cheuvront, T.J.; Rongish, B.J. The endoderm and myocardium join forces to drive early heart tube assembly. Dev. Biol. 2015, 404, 40–54. [Google Scholar] [CrossRef] [Green Version]
- Kruegel, J.; Miosge, N. Basement membrane components are key players in specialized extracellular matrices. Cell. Mol. Life Sci. 2010, 67, 2879–2895. [Google Scholar] [CrossRef] [Green Version]
- Duan, P.; Chen, S.; Zeng, Y.; Xu, H.; Liu, Y. Osteopontin Upregulates Col IV Expression by Repressing miR-29a in Human Retinal Capillary Endothelial Cells. Mol. Ther. Nucleic Acids 2020, 20, 242–251. [Google Scholar] [CrossRef]
- Chung, H.J.; Uitto, J. Type VII Collagen: The Anchoring Fibril Protein at Fault in Dystrophic Epidermolysis Bullosa. Dermatol. Clin. 2010, 28, 93–105. [Google Scholar] [CrossRef] [Green Version]
- Pöschl, E.; Schlötzer-Schrehardt, U.; Brachvogel, B.; Saito, K.; Ninomiya, Y.; Mayer, U. Collagen IV is essential for basement membrane stability but dispensable for initiation of its assembly during early development. Development 2004, 131, 1619–1628. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Timpl, R.; Aumailley, M. Biochemistry of basement membranes. Adv. Nephrol. Necker Hosp. 1989, 18, 59–76. [Google Scholar] [PubMed]
- Kuhn, K. Basement membrane (type IV) collagen. Matrix Biol. 1995, 14, 439–445. [Google Scholar] [CrossRef]
- Boudko, S.P.; Danylevych, N.; Hudson, B.G.; Pedchenko, V.K. Basement membrane collagen IV: Isolation of functional domains. Methods Cell Biol. 2018, 143, 171–185. [Google Scholar] [CrossRef] [PubMed]
- Aumailley, M.; Timpl, R. Attachment of cells to basement membrane collagen type IV. J. Cell Biol. 1986, 103, 1569–1575. [Google Scholar] [CrossRef] [Green Version]
- Xu, H.; Pumiglia, K.; LaFlamme, S.E. Laminin-511 and α6 integrins regulate the expression of CXCR4 to promote endothelial morphogenesis. J. Cell Sci. 2020, 133, 6595. [Google Scholar] [CrossRef] [PubMed]
- Koper, A.; Schenck, A.; Prokop, A. Analysis of Adhesion Molecules and Basement Membrane Contributions to Synaptic Adhesion at the Drosophila Embryonic NMJ. PLoS ONE 2012, 7, e36339. [Google Scholar] [CrossRef] [Green Version]
- Sekiguchi, R.; Yamada, K.M. Basement Membranes in Development and Disease. Curr. Top. Dev. Biol. 2018, 130, 143–191. [Google Scholar] [CrossRef]
- Ida-Yonemochi, H.; Ohshiro, K.; Swelam, W.; Metwaly, H.; Saku, T. Perlecan, a Basement Membrane-type Heparan Sulfate Proteoglycan, in the Enamel Organ: Its Intraepithelial Localization in the Stellate Reticulum. J. Histochem. Cytochem. 2005, 53, 763–772. [Google Scholar] [CrossRef] [Green Version]
- Egeblad, M.; Rasch, M.G.; Weaver, V.M. Dynamic interplay between the collagen scaffold and tumor evolution. Curr. Opin. Cell Biol. 2010, 22, 697–706. [Google Scholar] [CrossRef] [Green Version]
- Jarvelainen, H.; Sainio, A.; Koulu, M.; Wight, T.N.; Penttinen, R. Extracellular matrix molecules: Potential targets in pharmacotherapy. Pharmacol. Rev. 2009, 61, 198–223. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fan, D.; Creemers, E.; Kassiri, Z. Matrix as an Interstitial Transport System. Circ. Res. 2014, 114, 889–902. [Google Scholar] [CrossRef] [Green Version]
- Netti, P.A.; Berk, D.A.; Swartz, M.A.; Grodzinsky, A.J.; Jain, R.K. Role of extracellular matrix assembly in interstitial transport in solid tumors. Cancer Res. 2000, 60, 2497–2503. [Google Scholar] [PubMed]
- Wong, K.M.; Horton, K.J.; Coveler, A.L.; Hingorani, S.; Harris, W.P. Targeting the Tumor Stroma: The Biology and Clinical Development of Pegylated Recombinant Human Hyaluronidase (PEGPH20). Curr. Oncol. Rep. 2017, 19, 47. [Google Scholar] [CrossRef] [PubMed]
- Mow, V.C.; Wang, C.C.; Hung, C.T. The extracellular matrix, interstitial fluid and ions as a mechanical signal transducer in articular cartilage. Osteoarthr. Cartil. 1999, 7, 41–58. [Google Scholar] [CrossRef] [Green Version]
- Mackie, A.R.; Klyachko, E.; Thorne, T.; Schultz, K.M.; Millay, M.; Ito, A.; Kamide, C.E.; Liu, T.; Gupta, R.; Sahoo, S.; et al. Sonic Hedgehog–Modified Human CD34+ Cells Preserve Cardiac Function After Acute Myocardial Infarction. Circ. Res. 2012, 111, 312–321. [Google Scholar] [CrossRef] [PubMed]
- Arroyo, J.; Chevillet, J.; Kroh, E.M.; Ruf, I.K.; Pritchard, C.C.; Gibson, D.F.; Mitchell, P.; Bennett, C.; Pogosova-Agadjanyan, E.L.; Stirewalt, D.L.; et al. Argonaute2 complexes carry a population of circulating microRNAs independent of vesicles in human plasma. Proc. Natl. Acad. Sci. USA 2011, 108, 5003–5008. [Google Scholar] [CrossRef] [Green Version]
- Noguchi, S.; Saito, A.; Nagase, T. YAP/TAZ Signaling as a Molecular Link between Fibrosis and Cancer. Int. J. Mol. Sci. 2018, 19, 3674. [Google Scholar] [CrossRef] [Green Version]
- Burgy, O.; Königshoff, M. The WNT signaling pathways in wound healing and fibrosis. Matrix Biol. 2018, 68–69, 67–80. [Google Scholar] [CrossRef]
- Meng, X.M.; Nikolic-Paterson, D.J.; Lan, H.Y. TGF-beta: The master regulator of fibrosis. Nat. Rev. Nephrol. 2016, 12, 325–338. [Google Scholar] [CrossRef]
- Wynn, T.A.; Ramalingam, T.R. Mechanisms of fibrosis: Therapeutic translation for fibrotic disease. Nat. Med. 2012, 18, 1028–1040. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Piersma, B.; Bank, R.A.; Boersema, M. Signaling in Fibrosis: TGF-beta, WNT, and YAP/TAZ Converge. Front. Med. 2015, 2, 59. [Google Scholar] [CrossRef] [PubMed]
- Maller, O.; Drain, A.P.; Barrett, A.S.; Borgquist, S.; Ruffell, B.; Zakharevich, I.; Pham, T.T.; Gruosso, T.; Kuasne, H.; Lakins, J.N.; et al. Tumour-associated macrophages drive stromal cell-dependent collagen crosslinking and stiffening to promote breast cancer aggression. Nat. Mater. 2021, 20, 548–559. [Google Scholar] [CrossRef]
- Xu, J.; Chen, L.-J.; Yu, J.; Wang, H.-J.; Zhang, F.; Liu, Q.; Wu, J. Involvement of Advanced Glycation End Products in the Pathogenesis of Diabetic Retinopathy. Cell. Physiol. Biochem. 2018, 48, 705–717. [Google Scholar] [CrossRef]
- Mason, R.M.; Wahab, N.A. Extracellular Matrix Metabolism in Diabetic Nephropathy. J. Am. Soc. Nephrol. 2003, 14, 1358–1373. [Google Scholar] [CrossRef] [Green Version]
- Davies, K.E.; Nowak, K.J. Molecular mechanisms of muscular dystrophies: Old and new players. Nat. Rev. Mol. Cell Biol. 2006, 7, 762–773. [Google Scholar] [CrossRef] [PubMed]
- Mohassel, P.; Foley, A.R.; Bönnemann, C.G. Extracellular matrix-driven congenital muscular dystrophies. Matrix Biol. 2018, 71–72, 188–204. [Google Scholar] [CrossRef] [PubMed]
- Van Ry, P.M.; Fontelonga, T.M.; Barraza-Flores, P.; Sarathy, A.; Nunes, A.M.; Burkin, D.J. ECM-Related Myopathies and Muscular Dystrophies: Pros and Cons of Protein Therapies. Compr. Physiol. 2017, 7, 1519–1536. [Google Scholar] [CrossRef]
- Beenakker, J.-W.M.; Ashcroft, B.A.; Lindeman, J.H.; Oosterkamp, T.H. Mechanical Properties of the Extracellular Matrix of the Aorta Studied by Enzymatic Treatments. Biophys. J. 2012, 102, 1731–1737. [Google Scholar] [CrossRef] [Green Version]
- Black, L.; Allen, P.G.; Morris, S.M.; Stone, P.J.; Suki, B. Mechanical and Failure Properties of Extracellular Matrix Sheets as a Function of Structural Protein Composition. Biophys. J. 2008, 94, 1916–1929. [Google Scholar] [CrossRef] [Green Version]
- Badylak, S.; Brown, B.N.; Gilbert, T.W. Tissue Engineering with Decellularized Tissues. In Biomaterials Science; Elsevier BV: Cambridge, MA, USA, 2013; pp. 1316–1331. [Google Scholar]
- Badylak, S.F.; Freytes, D.O.; Gilbert, T.W. Extracellular matrix as a biological scaffold material: Structure and function. Acta Biomater. 2009, 5, 1–13. [Google Scholar] [CrossRef]
- Liu, Y.; Ballarini, R.; Eppell, S.J. Tension tests on mammalian collagen fibrils. Interface Focus 2016, 6, 20150080. [Google Scholar] [CrossRef] [PubMed]
- Yue, B. Biology of the extracellular matrix: An overview. J. Glaucoma 2014, 23 (Suppl. S18), S20–S23. [Google Scholar] [CrossRef] [PubMed]
- Guthold, M.; Liu, W.; Sparks, E.A.; Jawerth, L.M.; Peng, L.; Falvo, M.; Superfine, R.; Hantgan, R.R.; Lord, S.T. A Comparison of the Mechanical and Structural Properties of Fibrin Fibers with Other Protein Fibers. Cell Biophys. 2007, 49, 165–181. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bax, D.V.; Smalley, H.E.; Farndale, R.W.; Best, S.M.; Cameron, R.E. Cellular response to collagen-elastin composite materials. Acta Biomater. 2019, 86, 158–170. [Google Scholar] [CrossRef]
- Henke, E.; Nandigama, R.; Ergün, S. Extracellular Matrix in the Tumor Microenvironment and Its Impact on Cancer Therapy. Front. Mol. Biosci. 2020, 6, 160. [Google Scholar] [CrossRef] [Green Version]
- Domogatskaya, A.; Rodin, S.; Tryggvason, K. Functional Diversity of Laminins. Annu. Rev. Cell Dev. Biol. 2012, 28, 523–553. [Google Scholar] [CrossRef]
- Kim, S.-H.; Turnbull, J.; Guimond, S. Extracellular matrix and cell signalling: The dynamic cooperation of integrin, proteoglycan and growth factor receptor. J. Endocrinol. 2011, 209, 139–151. [Google Scholar] [CrossRef] [Green Version]
- Barczyk, M.; Carracedo, S.; Gullberg, D. Integrins. Cell Tissue Res. 2010, 339, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Alam, N.; Goel, H.L.; Zarif, M.J.; Butterfield, J.E.; Perkins, H.M.; Sansoucy, B.G.; Sawyer, T.K.; Languino, L.R. The integrin—growth factor receptor duet. J. Cell. Physiol. 2007, 213, 649–653. [Google Scholar] [CrossRef]
- Mezu-Ndubuisi, O.J.; Maheshwari, A. The role of integrins in inflammation and angiogenesis. Pediatr. Res. 2021, 89, 1619–1626. [Google Scholar] [CrossRef] [PubMed]
- Hynes, R.O. Integrins: Versatility, modulation, and signaling in cell adhesion. Cell 1992, 69, 11–25. [Google Scholar] [CrossRef]
- Keely, P.J. Mechanisms by Which the Extracellular Matrix and Integrin Signaling Act to Regulate the Switch Between Tumor Suppression and Tumor Promotion. J. Mammary Gland. Biol. Neoplasia 2011, 16, 205–219. [Google Scholar] [CrossRef] [PubMed]
- Wehrle-Haller, B.; Imhof, B. Integrin-dependent pathologies. J. Pathol. 2003, 200, 481–487. [Google Scholar] [CrossRef] [PubMed]
- Cooper, J.; Giancotti, F.G. Integrin Signaling in Cancer: Mechanotransduction, Stemness, Epithelial Plasticity, and Therapeutic Resistance. Cancer Cell 2019, 35, 347–367. [Google Scholar] [CrossRef]
- Ellert-Miklaszewska, A.; Poleszak, K.; Pasierbińska, M.; Kaminska, B. Integrin Signaling in Glioma Pathogenesis: From Biology to Therapy. Int. J. Mol. Sci. 2020, 21, 888. [Google Scholar] [CrossRef] [Green Version]
- Frisch, S.M.; Ruoslahti, E. Integrins and anoikis. Curr. Opin. Cell Biol. 1997, 9, 701–706. [Google Scholar] [CrossRef]
- Lee, J.L.; Streuli, C.H. Integrins and epithelial cell polarity. J. Cell Sci. 2014, 127, 3217–3225. [Google Scholar] [CrossRef] [Green Version]
- Yamada, K.M.; Even-Ram, S. Integrin regulation of growth factor receptors. Nat. Cell Biol. 2002, 4, E75–E76. [Google Scholar] [CrossRef]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Lu, P.; Takai, K.; Weaver, V.M.; Werb, Z. Extracellular Matrix Degradation and Remodeling in Development and Disease. Cold Spring Harb. Perspect. Biol. 2011, 3, a005058. [Google Scholar] [CrossRef]
- Gaggar, A.; Jackson, P.L.; Noerager, B.D.; O’Reilly, P.J.; McQuaid, D.B.; Rowe, S.M.; Clancy, J.P.; Blalock, J.E. A Novel Proteolytic Cascade Generates an Extracellular Matrix-Derived Chemoattractant in Chronic Neutrophilic Inflammation. J. Immunol. 2008, 180, 5662–5669. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haun, F.; Neumann, S.; Peintner, L.; Wieland, K.; Habicht, J.; Schwan, C.; Østevold, K.; Koczorowska, M.M.; Biniossek, M.; Kist, M.; et al. Identification of a novel anoikis signalling pathway using the fungal virulence factor gliotoxin. Nat. Commun. 2018, 9, 3524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Watanabe, K.; Ueno, M.; Kamiya, D.; Nishiyama, A.; Matsumura, M.; Wataya, T.; Takahashi, J.B.; Nishikawa, S.; Nishikawa, S.-I.; Muguruma, K.; et al. A ROCK inhibitor permits survival of dissociated human embryonic stem cells. Nat. Biotechnol. 2007, 25, 681–686. [Google Scholar] [CrossRef] [PubMed]
- Backer, M.V.; Backer, J.M. Imaging Key Biomarkers of Tumor Angiogenesis. Theranostics 2012, 2, 502–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parkin, A.; Man, J.; Timpson, P.; Pajic, M. Targeting the complexity of Src signalling in the tumour microenvironment of pancreatic cancer: From mechanism to therapy. FEBS J. 2019, 286, 3510–3539. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinman, H.K.; Philp, D.; Hoffman, M.P. Role of the extracellular matrix in morphogenesis. Curr. Opin. Biotechnol. 2003, 14, 526–532. [Google Scholar] [CrossRef]
- Xu, C.; Inokuma, M.S.; Denham, J.; Golds, K.; Kundu, P.; Gold, J.D.; Carpenter, M.K. Feeder-free growth of undifferentiated human embryonic stem cells. Nat. Biotechnol. 2001, 19, 971–974. [Google Scholar] [CrossRef]
- Berdichevsky, F.; Alford, D.; D’Souza, B.; Taylor-Papadimitriou, J. Branching morphogenesis of human mammary epithelial cells in collagen gels. J. Cell Sci. 1994, 107, 3557–3568. [Google Scholar] [CrossRef]
- Stahl, S.; Weitzman, S.; Jones, J. The role of laminin-5 and its receptors in mammary epithelial cell branching morphogenesis. J. Cell Sci. 1997, 110, 55–63. [Google Scholar] [CrossRef]
- Rozario, T.; DeSimone, D.W. The extracellular matrix in development and morphogenesis: A dynamic view. Dev. Biol. 2010, 341, 126–140. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garreta, E.; Prado, P.; Tarantino, C.; Oria, R.; Fanlo, L.; Martí, E.; Zalvidea, D.; Trepat, X.; Roca-Cusachs, P.; Gavaldà-Navarro, A.; et al. Fine tuning the extracellular environment accelerates the derivation of kidney organoids from human pluripotent stem cells. Nat. Mater. 2019, 18, 397–405. [Google Scholar] [CrossRef]
- Park, J.S.; Chu, J.S.; Tsou, A.D.; Diop, R.; Tang, Z.; Wang, A.; Li, S. The effect of matrix stiffness on the differentiation of mesenchymal stem cells in response to TGF-beta. Biomaterials 2011, 32, 3921–3930. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Neve, A.; Cantatore, F.P.; Maruotti, N.; Corrado, A.; Ribatti, D. Extracellular Matrix Modulates Angiogenesis in Physiological and Pathological Conditions. BioMed Res. Int. 2014, 2014, 756078. [Google Scholar] [CrossRef] [Green Version]
- Brooke, B.S.; Karnik, S.K.; Li, D.Y. Extracellular matrix in vascular morphogenesis and disease: Structure versus signal. Trends Cell Biol. 2003, 13, 51–56. [Google Scholar] [CrossRef]
- Li, D.Y.; Brooke, B.; Davis, E.C.; Mecham, R.P.; Sorensen, L.K.; Boak, B.B.; Eichwald, E.; Keating, M.T. Elastin is an essential determinant of arterial morphogenesis. Nat. Cell Biol. 1998, 393, 276–280. [Google Scholar] [CrossRef]
- Karnik, S.K.; Brooke, B.S.; Bayes-Genis, A.; Sorensen, L.; Wythe, J.; Schwartz, R.S.; Keating, M.T.; Li, D.Y. A critical role for elastin signaling in vascular morphogenesis and disease. Development 2003, 130, 411–423. [Google Scholar] [CrossRef] [Green Version]
- Ambesi, A.; McKeown-Longo, P.J. Conformational remodeling of the fibronectin matrix selectively regulates VEGF signaling. J. Cell Sci. 2014, 127, 3805–3816. [Google Scholar] [CrossRef] [Green Version]
- Bunton, T.E.; Biery, N.J.; Myers, L.; Gayraud, B.; Ramirez, F.; Dietz, H.C. Phenotypic Alteration of Vascular Smooth Muscle Cells Precedes Elastolysis in a Mouse Model of Marfan Syndrome. Circ. Res. 2001, 88, 37–43. [Google Scholar] [CrossRef] [Green Version]
- Yamamoto, M.; Yamamoto, K.; Noumura, T. Type I Collagen Promotes Modulation of Cultured Rabbit Arterial Smooth Muscle Cells from a Contractile to a Synthetic Phenotype. Exp. Cell Res. 1993, 204, 121–129. [Google Scholar] [CrossRef]
- Paoli, P.; Giannoni, E.; Chiarugi, P. Anoikis molecular pathways and its role in cancer progression. Biochim. Biophys. Acta (BBA) Bioenerg. 2013, 1833, 3481–3498. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gospodarowicz, D.; Delgado, D.; Vlodavsky, I. Permissive effect of the extracellular matrix on cell proliferation in vitro. Proc. Natl. Acad. Sci. USA 1980, 77, 4094–4098. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Miyamoto, S.; Teramoto, H.; Gutkind, J.S.; Yamada, K. Integrins can collaborate with growth factors for phosphorylation of receptor tyrosine kinases and MAP kinase activation: Roles of integrin aggregation and occupancy of receptors. J. Cell Biol. 1996, 135, 1633–1642. [Google Scholar] [CrossRef]
- Maik-Rachline, G.; Hacohen-Lev-Ran, A.; Seger, R. Nuclear ERK: Mechanism of Translocation, Substrates, and Role in Cancer. Int. J. Mol. Sci. 2019, 20, 1194. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Impey, S.; Obrietan, K.; Wong, S.T.; Poser, S.; Yano, S.; Wayman, G.; Deloulme, J.C.; Chan, G.; Storm, D.R. Cross Talk between ERK and PKA Is Required for Ca2+ Stimulation of CREB-Dependent Transcription and ERK Nuclear Translocation. Neuron 1998, 21, 869–883. [Google Scholar] [CrossRef] [Green Version]
- Powers, C.J.; McLeskey, S.W.; Wellstein, A. Fibroblast growth factors, their receptors and signaling. Endocr. Relat. Cancer 2000, 7, 165–197. [Google Scholar] [CrossRef] [Green Version]
- Meredith, J.E.; Fazeli, B.; Schwartz, M. The extracellular matrix as a cell survival factor. Mol. Biol. Cell 1993, 4, 953–961. [Google Scholar] [CrossRef] [Green Version]
- Grossmann, J.; Walther, K.; Artinger, M.; Kiessling, S.; Schölmerich, J. Apoptotic signaling during initiation of detachment-induced apoptosis (“anoikis”) of primary human intestinal epithelial cells. Cell Growth Differ. Mol. Biol. J. Am. Assoc. Cancer Res. 2001, 12, 147–155. [Google Scholar]
- Coleman, M.; Olson, M. Rho GTPase signalling pathways in the morphological changes associated with apoptosis. Cell Death Differ. 2002, 9, 493–504. [Google Scholar] [CrossRef] [Green Version]
- Schafer, Z.T.; Grassian, A.R.; Song, L.; Jiang, Z.; Gerhart-Hines, Z.; Irie, H.Y.; Gao, S.; Puigserver, P.; Brugge, J.S. Antioxidant and oncogene rescue of metabolic defects caused by loss of matrix attachment. Nat. Cell Biol. 2009, 461, 109–113. [Google Scholar] [CrossRef] [Green Version]
- Orkin, R.W.; Gehron, P.; McGoodwin, E.B.; Martin, G.R.; Valentine, T.; Swarm, R. A murine tumor producing a matrix of basement membrane. J. Exp. Med. 1977, 145, 204–220. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kleinman, H.K.; Martin, G.R. Matrigel: Basement membrane matrix with biological activity. Semin. Cancer Biol. 2005, 15, 378–386. [Google Scholar] [CrossRef] [PubMed]
- Baatout, S.; CheŢa, N. Matrigel: A useful tool to study endothelial differentiation. Rom. J. Intern. Med. 1996, 34, 263–269. [Google Scholar] [PubMed]
- Abe, Y.; Tada, A.; Isoyama, J.; Nagayama, S.; Yao, R.; Adachi, J.; Tomonaga, T. Improved phosphoproteomic analysis for phosphosignaling and active-kinome profiling in Matrigel-embedded spheroids and patient-derived organoids. Sci. Rep. 2018, 8, 11401. [Google Scholar] [CrossRef]
- Gjorevski, N.; Sachs, N.; Manfrin, A.; Giger, S.; Bragina, M.E.; Ordóñez-Morán, P.; Clevers, N.S.H.; Lutolf, M.P. Designer matrices for intestinal stem cell and organoid culture. Nat. Cell Biol. 2016, 539, 560–564. [Google Scholar] [CrossRef] [PubMed]
- Aisenbrey, E.A.; Murphy, W.L. Synthetic alternatives to Matrigel. Nat. Rev. Mater. 2020, 5, 539–551. [Google Scholar] [CrossRef]
- Wang, W.; Narain, R.; Zeng, H. Chapter 10—Hydrogels. In Polymer Science and Nanotechnology; Narain, R., Ed.; Elsevier: Amsterdam, The Netherlands, 2020; pp. 203–244. [Google Scholar]
- Annabi, N.; Tamayol, A.; Uquillas, J.A.; Akbari, M.; Bertassoni, L.E.; Cha, C.; Camci-Unal, G.; Dokmeci, M.R.; Peppas, N.A.; Khademhosseini, A. 25th Anniversary Article: Rational Design and Applications of Hydrogels in Regenerative Medicine. Adv. Mater. 2014, 26, 85–124. [Google Scholar] [CrossRef]
- Engler, A.; Sen, S.; Sweeney, H.L.; Discher, D.E. Matrix Elasticity Directs Stem Cell Lineage Specification. Cell 2006, 126, 677–689. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Mooney, D.J. Designing hydrogels for controlled drug delivery. Nat. Rev. Mater. 2016, 1, 16071. [Google Scholar] [CrossRef]
- D’Souza, S.E.; Ginsberg, M.H.; Plow, E.F. Arginyl-glycyl-aspartic acid (RGD): A cell adhesion motif. Trends Biochem. Sci. 1991, 16, 246–250. [Google Scholar] [CrossRef]
- Kyburz, K.A.; Anseth, K.S. Synthetic Mimics of the Extracellular Matrix: How Simple is Complex Enough? Ann. Biomed. Eng. 2015, 43, 489–500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gallagher, L.B.; Dolan, E.B.; O’Sullivan, J.; Levey, R.; Cavanagh, B.L.; Kovarova, L.; Pravda, M.; Velebny, V.; Farrell, T.; O’Brien, F.J.; et al. Pre-culture of mesenchymal stem cells within RGD-modified hyaluronic acid hydrogel improves their resilience to ischaemic conditions. Acta Biomater. 2020, 107, 78–90. [Google Scholar] [CrossRef] [PubMed]
- García, A.; Reyes, C. Bio-adhesive Surfaces to Promote Osteoblast Differentiation and Bone Formation. J. Dent. Res. 2005, 84, 407–413. [Google Scholar] [CrossRef]
- Liu, J.; Long, H.; Zeuschner, D.; Räder, A.F.B.; Polacheck, W.J.; Kessler, H.; Sorokin, L.; Trappmann, B. Synthetic extracellular matrices with tailored adhesiveness and degradability support lumen formation during angiogenic sprouting. Nat. Commun. 2021, 12, 1–12. [Google Scholar] [CrossRef]
- Kim, M.-H.; Kino-Oka, M. Switching between self-renewal and lineage commitment of human induced pluripotent stem cells via cell–substrate and cell–cell interactions on a dendrimer-immobilized surface. Biomaterials 2014, 35, 5670–5678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wake, M.C.; Gupta, P.K.; Mikos, A.G. Fabrication of pliable biodegradable polymer foams to engineer soft tissues. Cell Transplant. 1996, 5, 465–473. [Google Scholar] [CrossRef] [PubMed]
- Hern, D.L.; Hubbell, J. Incorporation of adhesion peptides into nonadhesive hydrogels useful for tissue resurfacing. J. Biomed. Mater. Res. 1998, 39, 266–276. [Google Scholar] [CrossRef]
- Namba, R.; Cole, A.; Bjugstad, K.; Mahoney, M. Development of porous PEG hydrogels that enable efficient, uniform cell-seeding and permit early neural process extension. Acta Biomater. 2009, 5, 1884–1897. [Google Scholar] [CrossRef]
- Wake, M.C.; Mikos, A.G.; Sarakinos, G.; Vacanti, J.P.; Langer, R. Dynamics of fibrovascular tissue ingrowth in hydrogel foams. Cell Transplant. 1995, 4, 275–279. [Google Scholar] [CrossRef] [Green Version]
- Flynn, L.; Dalton, P.D.; Shoichet, M.S. Fiber templating of poly(2-hydroxyethyl methacrylate) for neural tissue engineering. Biomaterials 2003, 24, 4265–4272. [Google Scholar] [CrossRef]
- Kawase, M.; Kurikawa, N.; Higashiyama, S.; Miura, N.; Shiomi, T.; Ozawa, C.; Mizoguchi, T.; Yagi, K. Effectiveness of polyamidoamine dendrimers modified with tripeptide growth factor, glycyl-l-histidyl-l-lysine, for enhancement of function of hepatoma cells. J. Biosci. Bioeng. 1999, 88, 433–437. [Google Scholar] [CrossRef]
- Chupa, J.M.; Foster, A.M.; Sumner, S.R.; Madihally, S.; Matthew, H.W. Vascular cell responses to polysaccharide materials:: In vitro and in vivo evaluations. Biomaterials 2000, 21, 2315–2322. [Google Scholar] [CrossRef]
- Möller, S.; Weisser, J.; Bischoff, S.; Schnabelrauch, M. Dextran and hyaluronan methacrylate based hydrogels as matrices for soft tissue reconstruction. Biomol. Eng. 2007, 24, 496–504. [Google Scholar] [CrossRef] [PubMed]
- Unal, A.Z.; West, J.L. Synthetic ECM: Bioactive Synthetic Hydrogels for 3D Tissue Engineering. Bioconjug. Chem. 2020, 31, 2253–2271. [Google Scholar] [CrossRef] [PubMed]
- Lutolf, M.P.; Raeber, G.P.; Zisch, A.H.; Tirelli, N.; Hubbell, J.A. Cell-Responsive Synthetic Hydrogels. Adv. Mater. 2003, 15, 888–892. [Google Scholar] [CrossRef]
- Patterson, J.; Hubbell, J. Enhanced proteolytic degradation of molecularly engineered PEG hydrogels in response to MMP-1 and MMP-2. Biomaterials 2010, 31, 7836–7845. [Google Scholar] [CrossRef]
- Ren, X.; Zhao, M.; Lash, B.; Martino, M.M.; Julier, Z. Growth Factor Engineering Strategies for Regenerative Medicine Applications. Front. Bioeng. Biotechnol. 2020, 7, 469. [Google Scholar] [CrossRef] [PubMed]
- Willemen, N.G.; Hassan, S.; Gurian, M.; Li, J.; Allijn, I.E.; Shin, S.R.; Leijten, J. Oxygen-Releasing Biomaterials: Current Challenges and Future Applications. Trends Biotechnol. 2021, 39, 1144–1159. [Google Scholar] [CrossRef]
- Fenton, O.S.; Olafson, K.N.; Pillai, P.S.; Mitchell, M.; Langer, R. Advances in Biomaterials for Drug Delivery. Adv. Mater. 2018, 30, e1705328. [Google Scholar] [CrossRef]
- Matsuda, T.; Kondo, A.; Makino, K.; Akutsu, T. Development of a novel artificial matrix with cell adhesion peptides for cell culture and artificial and hybrid organs. ASAIO Trans. 1989, 35, 677–679. [Google Scholar] [CrossRef]
- Howard, D.; Buttery, L.; Shakesheff, K.M.; Roberts, S.J. Tissue engineering: Strategies, stem cells and scaffolds. J. Anat. 2008, 213, 66–72. [Google Scholar] [CrossRef] [PubMed]
- Marimuthu, M.; Rousset, N.; St-Georges-Robillard, A.; Lateef, M.A.; Ferland, M.; Mes-Masson, A.-M.; Gervais, T. Multi-size spheroid formation using microfluidic funnels. Lab. Chip. 2018, 18, 304–314. [Google Scholar] [CrossRef] [PubMed]
- Laschke, M.W.; Menger, M.D. Life is 3D: Boosting Spheroid Function for Tissue Engineering. Trends Biotechnol. 2017, 35, 133–144. [Google Scholar] [CrossRef] [PubMed]
- Fennema, E.; Rivron, N.; Rouwkema, J.; van Blitterswijk, C.; de Boer, J. Spheroid culture as a tool for creating 3D complex tissues. Trends Biotechnol. 2013, 31, 108–115. [Google Scholar] [CrossRef]
- Mosaad, E.; Chambers, K.F.; Futrega, K.; Clements, J.A.; Doran, M.R. The Microwell-mesh: A high-throughput 3D prostate cancer spheroid and drug-testing platform. Sci. Rep. 2018, 8, 253. [Google Scholar] [CrossRef]
- VeDepo, M.C.; Detamore, M.S.; Hopkins, R.; Converse, G.L. Recellularization of decellularized heart valves: Progress toward the tissue-engineered heart valve. J. Tissue Eng. 2017, 8, 2041731417726327. [Google Scholar] [CrossRef]
- Miyagawa, S.; Domae, K.; Yoshikawa, Y.; Fukushima, S.; Nakamura, T.; Saito, A.; Sakata, Y.; Hamada, S.; Toda, K.; Pak, K.; et al. Phase I Clinical Trial of Autologous Stem Cell–Sheet Transplantation Therapy for Treating Cardiomyopathy. J. Am. Heart Assoc. 2017, 6, 4. [Google Scholar] [CrossRef]
- Tam, W.L.; Mendes, L.F.; Chen, X.; Lesage, R.; Van Hoven, I.; Leysen, E.; Kerckhofs, G.; Bosmans, K.; Chai, Y.C.; Yamashita, A.; et al. Human pluripotent stem cell-derived cartilaginous organoids promote scaffold-free healing of critical size long bone defects. Stem Cell Res. Ther. 2021, 12, 513. [Google Scholar] [CrossRef]
- Glorioso, J.M.; Mao, S.A.; Rodysill, B.; Mounajjed, T.; Kremers, W.K.; Elgilani, F.; Hickey, R.D.; Haugaa, H.; Rose, C.F.; Amiot, B.; et al. Pivotal preclinical trial of the spheroid reservoir bioartificial liver. J. Hepatol. 2015, 63, 388–398. [Google Scholar] [CrossRef] [Green Version]
- Taniguchi, D.; Matsumoto, K.; Tsuchiya, T.; Machino, R.; Takeoka, Y.; Elgalad, A.; Gunge, K.; Takagi, K.; Taura, Y.; Hatachi, G.; et al. Scaffold-free trachea regeneration by tissue engineering with bio-3D printing. Interact. Cardiovasc. Thorac. Surg. 2018, 26, 745–752. [Google Scholar] [CrossRef]
- Han, K.; Pierce, S.E.; Li, A.; Spees, K.; Anderson, G.R.; Seoane, J.A.; Lo, Y.-H.; Dubreuil, M.; Olivas, M.; Kamber, R.A.; et al. CRISPR screens in cancer spheroids identify 3D growth-specific vulnerabilities. Nature 2020, 580, 136–141. [Google Scholar] [CrossRef] [PubMed]
- Djomehri, S.I.; Burman, B.; Gonzalez, M.E.; Takayama, S.; Kleer, C.G. A reproducible scaffold-free 3D organoid model to study neoplastic progression in breast cancer. J. Cell Commun. Signal. 2019, 13, 129–143. [Google Scholar] [CrossRef] [PubMed]
- Leite, S.B.; Roosens, T.; El Taghdouini, A.; Mannaerts, I.; Smout, A.J.; Najimi, M.; Sokal, E.; Noor, F.; Chesne, C.; van Grunsven, L.A. Novel human hepatic organoid model enables testing of drug-induced liver fibrosis in vitro. Biomaterials 2016, 78, 1–10. [Google Scholar] [CrossRef]
- Lee, H.-K.; Sanchez, C.V.; Chen, M.; Morin, P.J.; Wells, J.M.; Hanlon, E.B.; Xia, W. Three Dimensional Human Neuro-Spheroid Model of Alzheimer’s Disease Based on Differentiated Induced Pluripotent Stem Cells. PLoS ONE 2016, 11, e0163072. [Google Scholar] [CrossRef] [Green Version]
- Yan, Y.; Bejoy, J.; Xia, J.; Griffin, K.; Guan, J.; Li, Y. Cell population balance of cardiovascular spheroids derived from human induced pluripotent stem cells. Sci. Rep. 2019, 9, 1–12. [Google Scholar] [CrossRef]
- Rabata, A.; Fedr, R.; Soucek, K.; Hampl, A.; Koledova, Z. 3D Cell Culture Models Demonstrate a Role for FGF and WNT Signaling in Regulation of Lung Epithelial Cell Fate and Morphogenesis. Front. Cell Dev. Biol. 2020, 8, 574. [Google Scholar] [CrossRef]
- Minchinton, A.I.; Tannock, I.F. Drug penetration in solid tumours. Nat. Rev. Cancer 2006, 6, 583–592. [Google Scholar] [CrossRef]
- Sutherland, R.M.; Durand, R. Radiation response of multicell spheroids—An in vitro tumour model. Curr. Top. Radiat. Res. Q. 1976, 11, 87–139. [Google Scholar]
- Sutherland, R.M. Cell and Environment Interactions in Tumor Microregions: The Multicell Spheroid Model. Science 1988, 240, 177–184. [Google Scholar] [CrossRef]
- Zhao, B.; Li, L.; Wang, L.; Wang, C.-Y.; Yu, J.; Guan, K.-L. Cell detachment activates the Hippo pathway via cytoskeleton reorganization to induce anoikis. Genes Dev. 2012, 26, 54–68. [Google Scholar] [CrossRef] [Green Version]
- Nederman, T.; Norling, B.; Glimelius, B.; Carlsson, J.; Brunk, U. Demonstration of an extracellular matrix in multicellular tumor spheroids. Cancer Res. 1984, 44, 3090. [Google Scholar]
- Rescigno, F.; Ceriotti, L.; Meloni, M. Extra Cellular Matrix Deposition and Assembly in Dermis Spheroids. Clin. Cosmet. Investig. Dermatol. 2021, 14, 935–943. [Google Scholar] [CrossRef] [PubMed]
- Shearier, E.; Xing, Q.; Qian, Z.; Zhao, F. Physiologically Low Oxygen Enhances Biomolecule Production and Stemness of Mesenchymal Stem Cell Spheroids. Tissue Eng. Part C Methods 2016, 22, 360–369. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salmenperä, P.; Kankuri, E.; Bizik, J.; Sirén, V.; Virtanen, I.; Takahashi, S.; Leiss, M.; Fässler, R.; Vaheri, A. Formation and activation of fibroblast spheroids depend on fibronectin–integrin interaction. Exp. Cell Res. 2008, 314, 3444–3452. [Google Scholar] [CrossRef] [PubMed]
- Sirén, V.; Salmenperä, P.; Kankuri, E.; Bizik, J.; Sorsa, T.; Tervahartiala, T.; Vaheri, A. Cell-cell contact activation of fibroblasts increases the expression of matrix metalloproteinases. Ann. Med. 2006, 38, 212–220. [Google Scholar] [CrossRef]
- Salmenperä, P.; Karhemo, P.-R.; Räsänen, K.; Laakkonen, P.; Vaheri, A. Fibroblast spheroids as a model to study sustained fibroblast quiescence and their crosstalk with tumor cells. Exp. Cell Res. 2016, 345, 17–24. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vaheri, A.; Enzerink, A.; Räsänen, K.; Salmenperä, P. Nemosis, a novel way of fibroblast activation, in inflammation and cancer. Exp. Cell Res. 2009, 315, 1633–1638. [Google Scholar] [CrossRef]
- Gumbiner, B.M.; Kim, N.G. The Hippo-YAP signaling pathway and contact inhibition of growth. J. Cell Sci. 2014, 127 Pt 4, 709–717. [Google Scholar] [CrossRef] [Green Version]
- Jorgenson, A.J.; Choi, K.M.; Sicard, D.; Smith, K.M.J.; Hiemer, S.E.; Varelas, X.; Tschumperlin, D.J. TAZ activation drives fibroblast spheroid growth, expression of profibrotic paracrine signals, and context-dependent ECM gene expression. Am. J. Physiol. Physiol. 2017, 312, C277–C285. [Google Scholar] [CrossRef]
- Moya, I.M.; Halder, G. Hippo–YAP/TAZ signalling in organ regeneration and regenerative medicine. Nat. Rev. Mol. Cell Biol. 2019, 20, 211–226. [Google Scholar] [CrossRef]
- Rausch, V.; Hansen, C.G. The Hippo Pathway, YAP/TAZ, and the Plasma Membrane. Trends Cell Biol. 2020, 30, 32–48. [Google Scholar] [CrossRef] [PubMed]
- Kim, N.-G.; Gumbiner, B.M. Adhesion to fibronectin regulates Hippo signaling via the FAK–Src–PI3K pathway. J. Cell Biol. 2015, 210, 503–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hofer, M.; Lutolf, M.P. Engineering organoids. Nat. Rev. Mater. 2021, 6, 402–420. [Google Scholar] [CrossRef]
- Nii, T.; Katayama, Y. Biomaterial-Assisted Regenerative Medicine. Int. J. Mol. Sci. 2021, 22, 8657. [Google Scholar] [CrossRef]
- Qu, H.; Fu, H.; Han, Z.; Sun, Y. Biomaterials for bone tissue engineering scaffolds: A review. RSC Adv. 2019, 9, 26252–26262. [Google Scholar] [CrossRef] [Green Version]
- Groeber, F.; Holeiter, M.; Hampel, M.; Hinderer, S.; Schenke-Layland, K. Skin tissue engineering—In vivo and in vitro applications. Adv. Drug Deliv. Rev. 2011, 63, 352–366. [Google Scholar] [CrossRef]
- Nii, T.; Makino, K.; Tabata, Y. Three-Dimensional Culture System of Cancer Cells Combined with Biomaterials for Drug Screening. Cancers 2020, 12, 2754. [Google Scholar] [CrossRef]
- Peña, B.; Laughter, M.; Jett, S.; Rowland, T.J.; Taylor, M.R.G.; Mestroni, L.; Park, D. Injectable Hydrogels for Cardiac Tissue Engineering. Macromol. Biosci. 2018, 18, e1800079. [Google Scholar] [CrossRef]
- Kwee, B.J.; Mooney, D.J. Biomaterials for skeletal muscle tissue engineering. Curr. Opin. Biotechnol. 2017, 47, 16–22. [Google Scholar] [CrossRef]
- Murphy, C.M.; Haugh, M.G.; O’Brien, F.J. The effect of mean pore size on cell attachment, proliferation and migration in collagen–glycosaminoglycan scaffolds for bone tissue engineering. Biomaterials 2010, 31, 461–466. [Google Scholar] [CrossRef]
- Ohata, K.; Ott, H.C. Human-scale lung regeneration based on decellularized matrix scaffolds as a biologic platform. Surg. Today 2020, 50, 633–643. [Google Scholar] [CrossRef] [PubMed]
- Mastrullo, V.; Cathery, W.; Velliou, E.; Madeddu, P.; Campagnolo, P. Angiogenesis in Tissue Engineering: As Nature Intended? Front. Bioeng. Biotechnol. 2020, 8, 188. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mariani, J.; Coppola, G.; Zhang, P.; Abyzov, A.; Provini, L.; Tomasini, L.; Amenduni, M.; Szekely, A.; Palejev, D.; Wilson, M.; et al. FOXG1-Dependent Dysregulation of GABA/Glutamate Neuron Differentiation in Autism Spectrum Disorders. Cell 2015, 162, 375–390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lancaster, M.A.; Renner, M.; Martin, C.-A.; Wenzel, D.; Bicknell, L.S.; Hurles, M.E.; Homfray, T.; Penninger, J.M.; Jackson, A.P.; Knoblich, J.A. Cerebral organoids model human brain development and microcephaly. Nature 2013, 501, 373–379. [Google Scholar] [CrossRef]
- Garcez, P.P.; Loiola, E.C.; Madeiro Da Costa, R.; Higa, L.M.; Trindade, P.; DelVecchio, R.; Nascimento, J.M.; Brindeiro, R.; Tanuri, A.; Rehen, S.K. Zika virus impairs growth in human neurospheres and brain organoids. Science 2016, 352, 816–818. [Google Scholar] [CrossRef] [Green Version]
- Hubert, C.G.; Rivera, M.; Spangler, L.C.; Wu, Q.; Mack, S.C.; Prager, B.C.; Couce, M.; McLendon, R.E.; Sloan, A.E.; Rich, J.N. A Three-Dimensional Organoid Culture System Derived from Human Glioblastomas Recapitulates the Hypoxic Gradients and Cancer Stem Cell Heterogeneity of Tumors Found In Vivo. Cancer Res. 2016, 76, 2465–2477. [Google Scholar] [CrossRef] [Green Version]
- Dotti, I.; Mora-Buch, R.; Ferrer-Picón, E.; Planell, N.; Jung, P.; Masamunt, M.C.; Leal, R.; De Carpi, J.M.; Llach, J.; Ordas, I.; et al. Alterations in the epithelial stem cell compartment could contribute to permanent changes in the mucosa of patients with ulcerative colitis. Gut 2017, 66, 2069–2079. [Google Scholar] [CrossRef] [Green Version]
- Drost, J.; Van Jaarsveld, R.H.; Ponsioen, B.; Zimberlin, C.; Van Boxtel, R.; Buijs, A.; Sachs, N.; Overmeer, R.M.; Offerhaus, G.J.; Begthel, H.; et al. Sequential cancer mutations in cultured human intestinal stem cells. Nature 2015, 521, 43–47. [Google Scholar] [CrossRef]
- Dekkers, J.F.; Berkers, G.; Kruisselbrink, E.; Vonk, A.; de Jonge, H.R.; Janssens, H.M.; Bronsveld, I.; van de Graaf, E.A.; Nieuwenhuis, E.E.S.; Houwen, R.H.J.; et al. Characterizing responses to CFTR-modulating drugs using rectal organoids derived from subjects with cystic fibrosis. Sci. Transl. Med. 2016, 8, 344ra84. [Google Scholar] [CrossRef]
- Yang, H.; Sun, L.; Pang, Y.; Hu, D.; Xu, H.; Mao, S.; Peng, W.; Wang, Y.; Xu, Y.; Zheng, Y.-C.; et al. Three-dimensional bioprinted hepatorganoids prolong survival of mice with liver failure. Gut 2021, 70, 567–574. [Google Scholar] [CrossRef]
- Li, Z.; Qian, Y.; Li, W.; Liu, L.; Yu, L.; Liu, X.; Wu, G.; Wang, Y.; Luo, W.; Fang, F.; et al. Human Lung Adenocarcinoma-Derived Organoid Models for Drug Screening. iScience 2020, 23, 101411. [Google Scholar] [CrossRef] [PubMed]
- Kim, J.-H.; An, G.H.; Kim, J.-Y.; Rasaei, R.; Kim, W.J.; Jin, X.; Woo, D.-H.; Han, C.; Yang, S.-R.; Kim, J.-H.; et al. Human pluripotent stem cell-derived alveolar organoids for modeling pulmonary fibrosis and drug testing. Cell Death Discov. 2021, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Huang, L.; Holtzinger, A.; Jagan, I.; BeGora, M.; Lohse, I.; Ngai, N.; Nostro, C.; Wang, R.; Muthuswamy, L.B.; Crawford, H.C.; et al. Ductal pancreatic cancer modeling and drug screening using human pluripotent stem cell- and patient-derived tumor organoids. Nat. Med. 2015, 21, 1364–1371. [Google Scholar] [CrossRef] [PubMed]
- Rhee, S.; Puetzer, J.L.; Mason, B.N.; Reinhart-King, C.A.; Bonassar, L.J. 3D Bioprinting of Spatially Heterogeneous Collagen Constructs for Cartilage Tissue Engineering. ACS Biomater. Sci. Eng. 2016, 2, 1800–1805. [Google Scholar] [CrossRef] [PubMed]
- Lawlor, K.T.; Vanslambrouck, J.M.; Higgins, J.W.; Chambon, A.; Bishard, K.; Arndt, D.; Er, P.X.; Wilson, S.B.; Howden, S.E.; Tan, K.S.; et al. Cellular extrusion bioprinting improves kidney organoid reproducibility and conformation. Nat. Mater. 2021, 20, 260–271. [Google Scholar] [CrossRef]
- Clevers, H. Modeling Development and Disease with Organoids. Cell 2016, 165, 1586–1597. [Google Scholar] [CrossRef] [Green Version]
- Hughes, C.S.; Postovit, L.M.; Lajoie, G.A. Matrigel: A complex protein mixture required for optimal growth of cell culture. Proteomics 2010, 10, 1886–1890. [Google Scholar] [CrossRef]
- Aamodt, J.M.; Grainger, D.W. Extracellular matrix-based biomaterial scaffolds and the host response. Biomaterials 2016, 86, 68–82. [Google Scholar] [CrossRef] [Green Version]
- Brandenberg, N.; Hoehnel, S.; Kuttler, F.; Homicsko, K.; Ceroni, C.; Ringel, T.; Gjorevski, N.; Schwank, G.; Coukos, G.; Turcatti, G.; et al. High-throughput automated organoid culture via stem-cell aggregation in microcavity arrays. Nat. Biomed. Eng. 2020, 4, 1–12. [Google Scholar] [CrossRef]
- Caliari, S.; Burdick, J.A. A practical guide to hydrogels for cell culture. Nat. Methods 2016, 13, 405–414. [Google Scholar] [CrossRef] [Green Version]
- Ferreira, L.P.; Gaspar, V.M.; Mendes, L.; Duarte, I.F.; Mano, J.F. Organotypic 3D decellularized matrix tumor spheroids for high-throughput drug screening. Biomaterials 2021, 275, 120983. [Google Scholar] [CrossRef]
- Silva, K.R.; Rezende, R.A.; Pereira, F.D.A.S.; Gruber, P.; Stuart, M.P.; Ovsianikov, A.; Brakke, K.; Kasyanovs, V.; Da Silva, J.V.L.; Granjeiro, J.M.; et al. Delivery of Human Adipose Stem Cells Spheroids into Lockyballs. PLoS ONE 2016, 11, e0166073. [Google Scholar] [CrossRef]
- Schuurman, W.; Harimulyo, E.B.; Gawlitta, D.; Woodfield, T.B.F.; Dhert, W.J.A.; van Weeren, P.R.; Malda, J. Three-dimensional assembly of tissue-engineered cartilage constructs results in cartilaginous tissue formation without retainment of zonal characteristics. J. Tissue Eng. Regen. Med. 2016, 10, 315–324. [Google Scholar] [CrossRef] [PubMed]
- Carpenedo, R.L.; Bratt-Leal, A.M.; Marklein, R.A.; Seaman, S.A.; Bowen, N.; McDonald, J.F.; McDevitt, T.C. Homogeneous and organized differentiation within embryoid bodies induced by microsphere-mediated delivery of small molecules. Biomaterials 2009, 30, 2507–2515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, T.G.; Park, S.-H.; Chung, H.J.; Yang, D.-Y.; Park, T.G. Hierarchically Assembled Mesenchymal Stem Cell Spheroids Using Biomimicking Nanofilaments and Microstructured Scaffolds for Vascularized Adipose Tissue Engineering. Adv. Funct. Mater. 2010, 20, 2303–2309. [Google Scholar] [CrossRef]
- Yamada, M.; Hori, A.; Sugaya, S.; Yajima, Y.; Utoh, R.; Yamato, M.; Seki, M. Cell-sized condensed collagen microparticles for preparing microengineered composite spheroids of primary hepatocytes. Lab. Chip. 2015, 15, 3941–3951. [Google Scholar] [CrossRef]
- Tao, F.; Sayo, K.; Sugimoto, K.; Aoki, S.; Kojima, N. Development of a tunable method to generate various three-dimensional microstructures by replenishing macromolecules such as extracellular matrix components and polysaccharides. Sci. Rep. 2020, 10, 1–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeo, G.C.; Weiss, A.S. Soluble matrix protein is a potent modulator of mesenchymal stem cell performance. Proc. Natl. Acad. Sci. USA 2019, 116, 2042–2051. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rothrauff, B.B.; Shimomura, K.; Gottardi, R.; Alexander, P.G.; Tuan, R.S. Anatomical region-dependent enhancement of 3-dimensional chondrogenic differentiation of human mesenchymal stem cells by soluble meniscus extracellular matrix. Acta Biomater. 2017, 49, 140–151. [Google Scholar] [CrossRef] [Green Version]
- Yang, G.; Rothrauff, B.B.; Lin, H.; Gottardi, R.; Alexander, P.G.; Tuan, R.S. Enhancement of tenogenic differentiation of human adipose stem cells by tendon-derived extracellular matrix. Biomaterials 2013, 34, 9295–9306. [Google Scholar] [CrossRef] [Green Version]
- Lecht, S.; Stabler, C.T.; Rylander, A.L.; Chiaverelli, R.; Schulman, E.S.; Marcinkiewicz, C.; Lelkes, P.I. Enhanced reseeding of decellularized rodent lungs with mouse embryonic stem cells. Biomaterials 2014, 35, 3252–3262. [Google Scholar] [CrossRef] [PubMed] [Green Version]
| Peptide | Role/Effect | Polymer | Citation |
|---|---|---|---|
| RGD | Adhesion | PEG | Matsuda, et al., 1989 [132] Hern and Hubbell, 1998 [119] |
| PQ | Adhesion, degradation remodeling | PEG | Lutolf, et al., 2003 [127] |
| Degradation rate variable: GPQGIWG (slow), VPMSMRGG (fast) | Cell migration and Proliferation (with fast construct) | PEG | Patterson and Hubbell, 2010 [128] |
Cleavage linkers:
| Degradable crosslinkers | Dextran | Liu, et al., 2021 [116] |
| Application | Culture Method | Application Method/Results |
|---|---|---|
| Therapeutics | Autologous skeletal muscle tissue sheet | Transplantation to the patient epicardium, improved cardiac disease symptoms [139] |
| Suspension culture of cartilaginous spheroids from human iPSC | Implantation into tibial fractures in nude mice, limited induction of bone remodeling [140] | |
| Suspension culture of primary porcine hepatocyte spheroids | Implantation into extracorporeal device, improved outcomes in a porcine acute liver failure model [141] | |
| Bio-printed trachea from suspension culture of primary rat chondrocyte, mesenchymal stem cells, and lung epithelial cells | Transplantation into rats show vasculogenesis and chondrogenesis [142] | |
| Disease Modeling | Suspension culture of non-small-cell lung cancer spheroids | Genome-wide CRISPR high-throughput drug screen against 3D cancer growth [143] |
| Hanging-drop culture of stable and tumor-derived, murine cells to form mammary spheroids | Studying neoplastic progression in spheroids with high consistency and reproducibility [144] | |
| Suspension spheroid co-culture of HepaRG and primary-derived hepatic stellate cells | Identification of acetaminophen as driver of stellate activation in liver fibrosis [145] | |
| Suspension culture-based differentiation of iPSCs into 3D neuro-spheres from Alzheimer’s disease patients | Drug screen of spheroids showed a patient-specific response to a specific drug [146] | |
| Developmental & Stem cell studies | Suspension culture of human hPSC, differentiated to cardiomyocytes | Characterized the effects of spheroid culture and cell density on cardiomyocyte differentiation [147] |
| “Lungosphere” suspension culture from primary-derived murine lung epithelial cells | Characterization of lung epithelial stem cells, validation of novel assay to separate lung epithelial stem cells [148] |
| Organ Type | Disease Applications |
|---|---|
| Brain | Autism—Patient-derived iPSC developed into neural organoids show overproduction of GABAergic inhibitory neurons [175]. Microcephaly—Patient-derived human iPSC cerebral organoids model microcephaly via loss of the CDK5RAP2 protein [176]. Viral infection—Human neural stem cell organoids infected with Zika virus exhibited decreased size and increased death of brain cells [177]. Cancer—Human glioblastoma-derived organoids maintain tumorigenic potential and heterogeneity [178]. |
| GI tract | Ulcerative colitis—Human epithelial organoid cultures from UC patients exhibited differences from non-UC patient organoids in expression of genes associated with microbial defense, secretion, absorption, and gastric phenotype [179]. Cancer—CRISPR/Cas9 was used to modify 4 commonly mutated colorectal cancer genes in human intestinal stem cells. The organoids produced were xenotransplanted into mice and grew as tumors with features of invasive carcinoma [180]. |
| Cystic Fibrosis—The drug response in primary rectal organoids from CF patients can be used to predict the patient’s specific drug response [181]. | |
| Liver | Liver failure—Human 3D bioprinted hepatorganoids transplanted into Fah-deficient mice rescued liver function and improved survivability of mice [182]. |
| Lung | Cancer—Patient-derived organoids formed from human lung adenocarcinoma cells retained the architecture and gene expression of the tumors they were derived from [183]. Pulmonary fibrosis—Human pluripotent stem cells were used to develop alveolar epithelial organoids of differentiated cells. TGF-β1 treatment induced fibrotic changes [184]. |
| Pancreas | Cancer—Primary human pancreatic adenocarcinoma organoids retain heterogeneity and histoarchitecture of parent tumor as well as physiological changes specific to the patient of origin [185]. |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Valdoz, J.C.; Johnson, B.C.; Jacobs, D.J.; Franks, N.A.; Dodson, E.L.; Sanders, C.; Cribbs, C.G.; Van Ry, P.M. The ECM: To Scaffold, or Not to Scaffold, That Is the Question. Int. J. Mol. Sci. 2021, 22, 12690. https://doi.org/10.3390/ijms222312690
Valdoz JC, Johnson BC, Jacobs DJ, Franks NA, Dodson EL, Sanders C, Cribbs CG, Van Ry PM. The ECM: To Scaffold, or Not to Scaffold, That Is the Question. International Journal of Molecular Sciences. 2021; 22(23):12690. https://doi.org/10.3390/ijms222312690
Chicago/Turabian StyleValdoz, Jonard Corpuz, Benjamin C. Johnson, Dallin J. Jacobs, Nicholas A. Franks, Ethan L. Dodson, Cecilia Sanders, Collin G. Cribbs, and Pam M. Van Ry. 2021. "The ECM: To Scaffold, or Not to Scaffold, That Is the Question" International Journal of Molecular Sciences 22, no. 23: 12690. https://doi.org/10.3390/ijms222312690






