Revisiting the Role of Ser982 Phosphorylation in Stoichiometry Shift of the Electrogenic Na+/qHCO3− Cotransporter NBCe1
Abstract
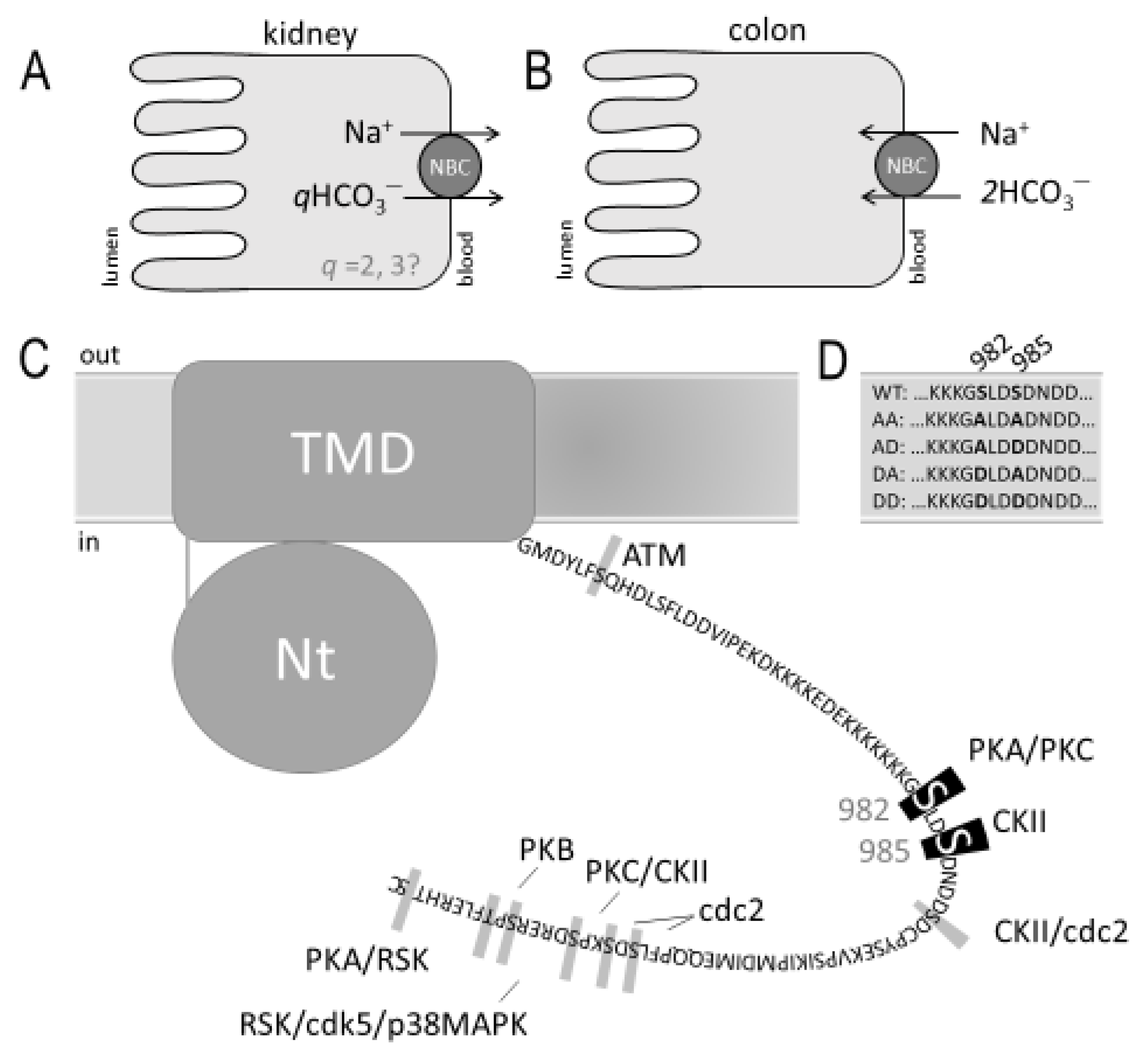
:1. Introduction
2. Results
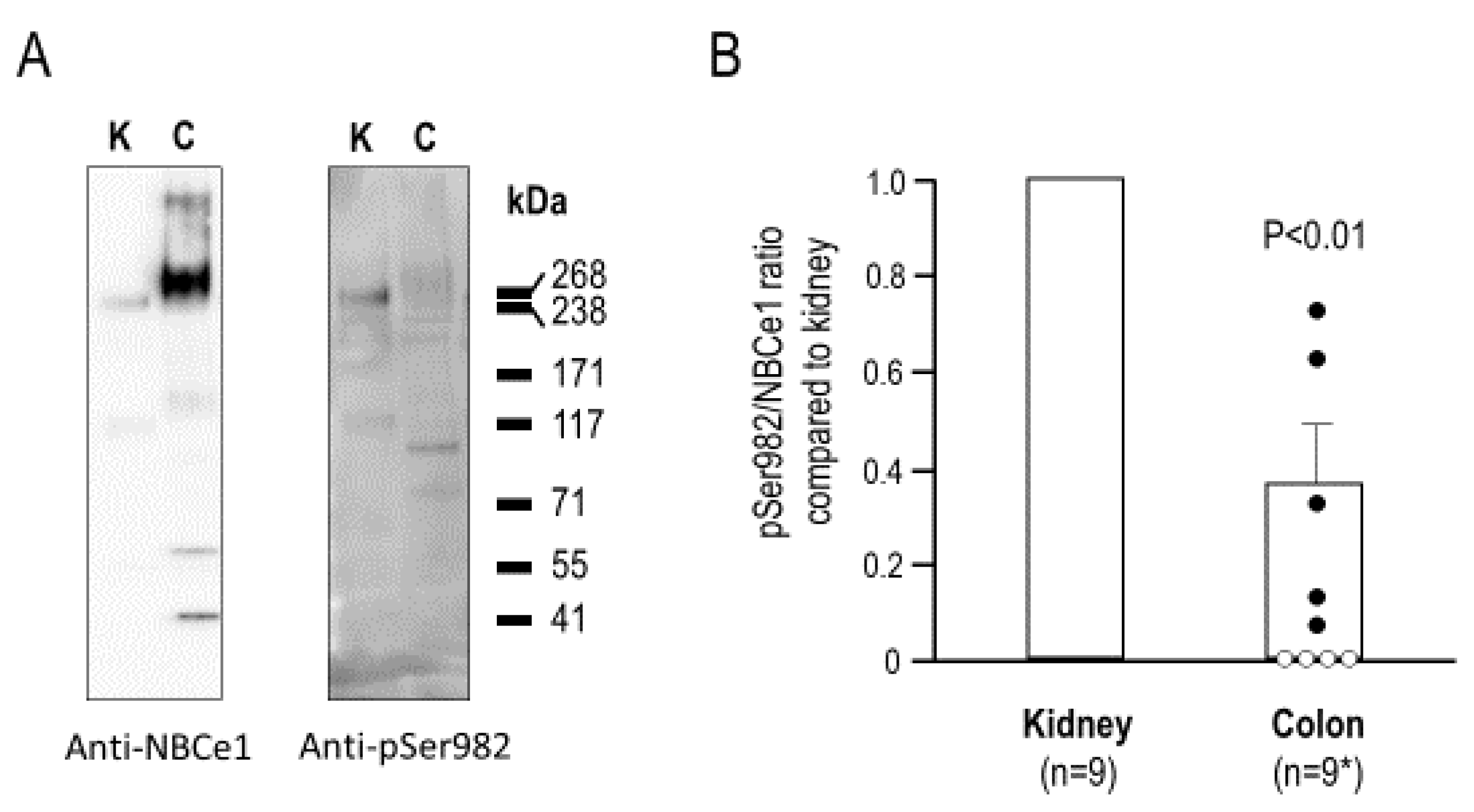
2.1. Validating the Anti-pSer982 Antibody
2.2. The Relative Phosphorylation Status of NBCe1/Ser982 in Murine Kidney versus Colon
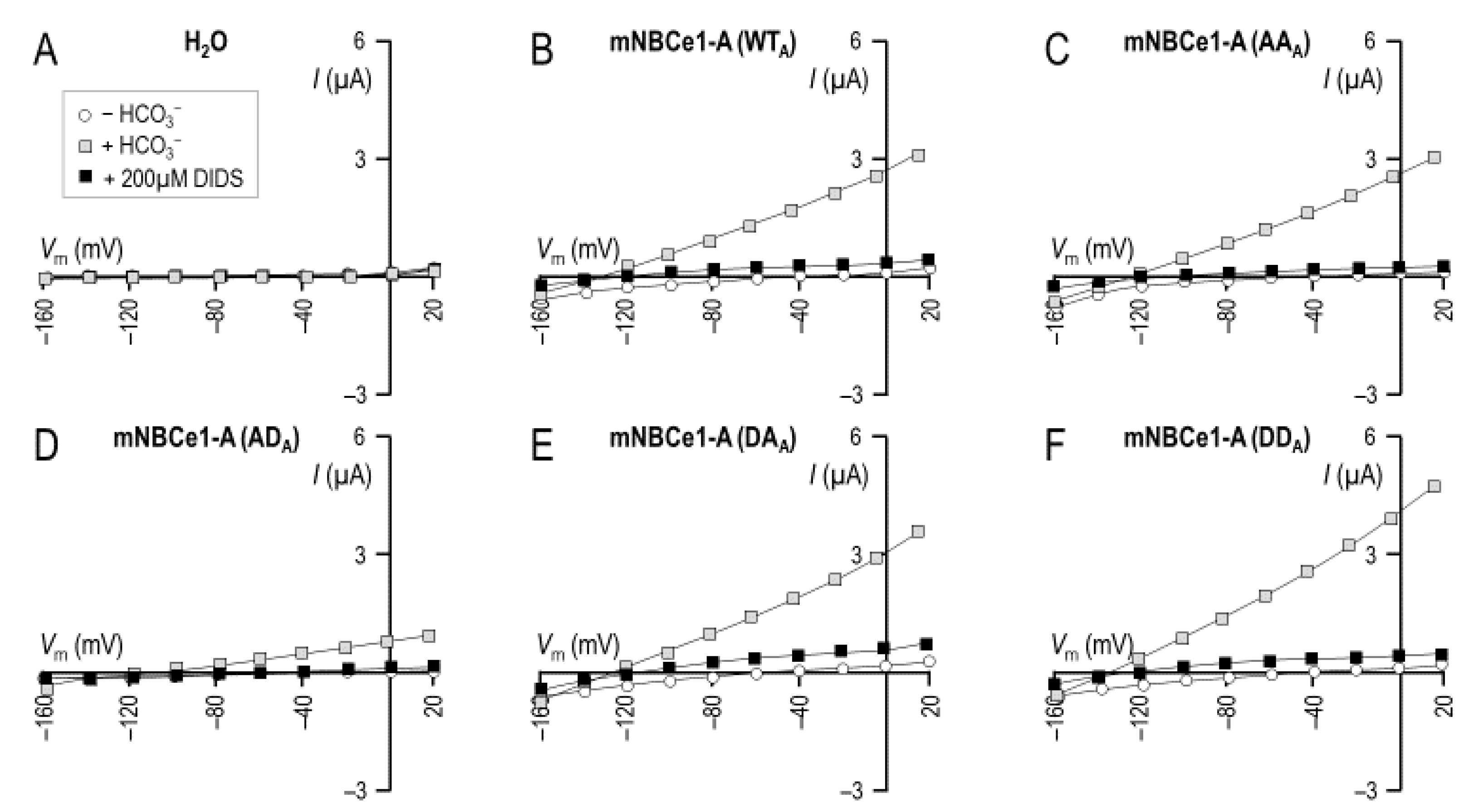
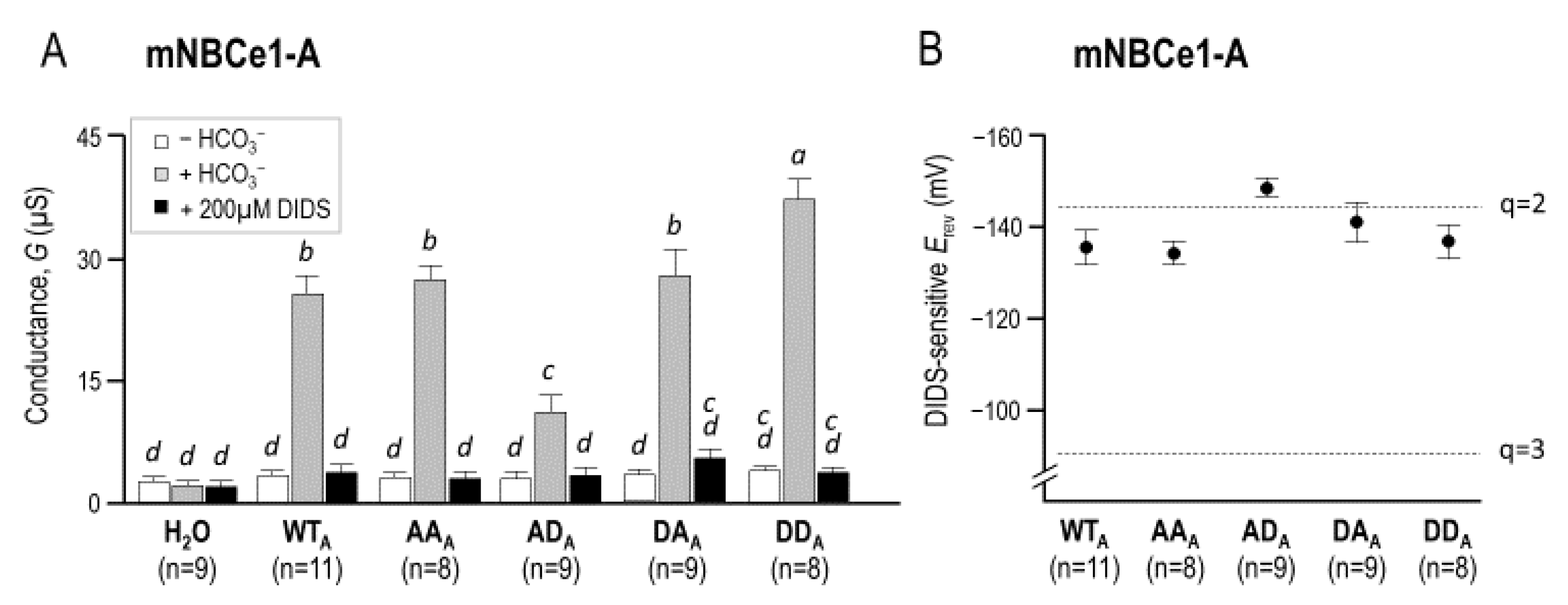
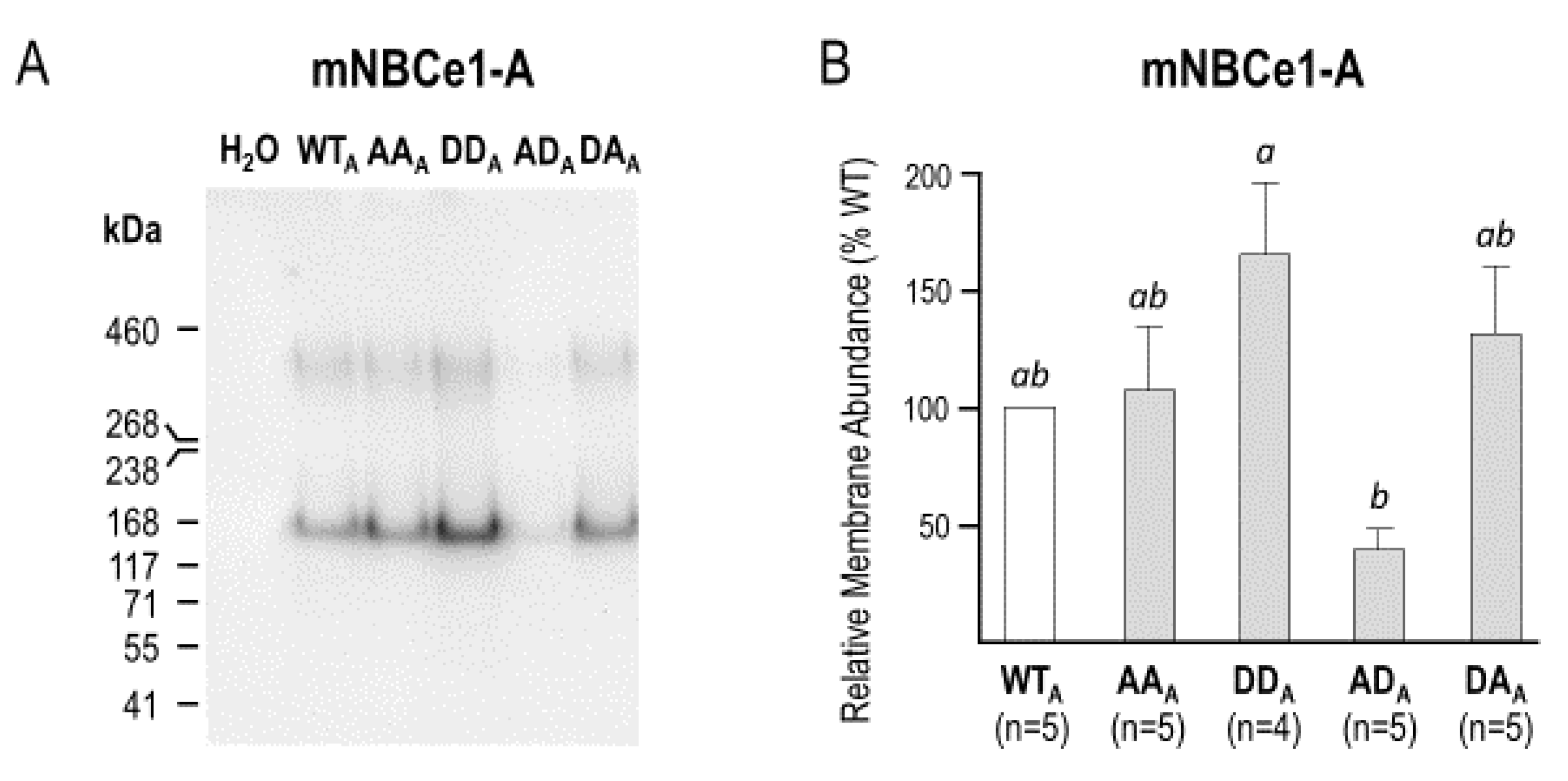
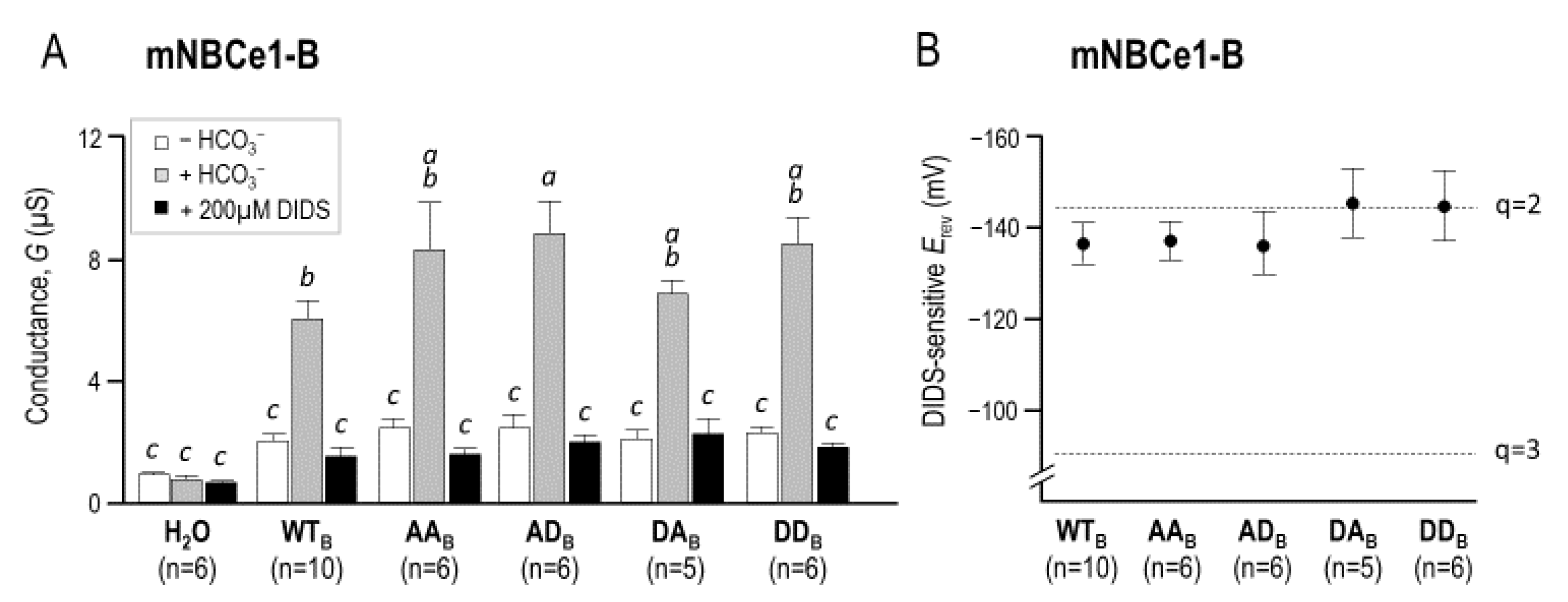
2.3. The Electrophysiological Properties of Double (de)Phosphomimetic Mutants of NBCe1-A and NBCe1-B in Xenopus Oocytes
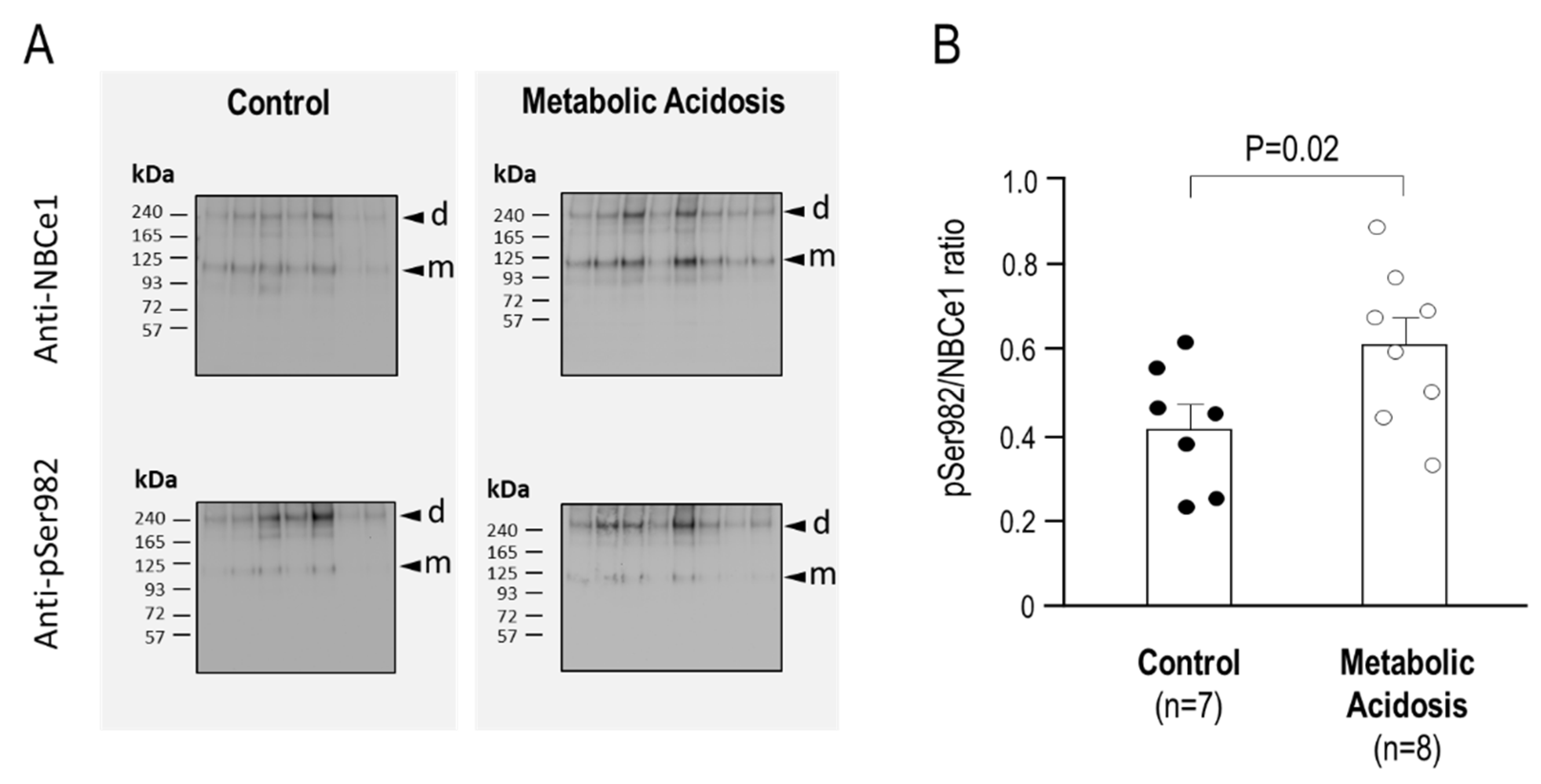
2.4. The Relative Phosphorylation Status of NBCe1/Ser982 in Murine Kidneys after 24 h Metabolic Acidosis
3. Discussion
4. Materials and Methods
4.1. cDNA Clones and Mutagenesis
4.2. cRNA Preparation
4.3. Oocyte Preparation
4.4. Electrophysiology
4.5. Oocyte Membrane Protein Preparation
4.6. Mouse Studies and Institutional Approval
4.7. Mouse Kidney and Colon Membrane Protein Preparation
4.8. Generation of the Anti-pSer982 Antibody
4.9. Protein Electrophoresis and Western Blotting
4.10. Data Analysis and Statistics
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Parker, M.D.; Boron, W.F. The Divergence, Actions, Roles, and Relatives of Sodium-Coupled Bicarbonate Transporters. Physiol. Rev. 2013, 93, 803–959. [Google Scholar] [CrossRef] [Green Version]
- Romero, M.F.; Hediger, M.A.; Boulpaep, E.L.; Boron, W.F. Expression Cloning and Characterization of a Renal Electrogenic Na+/HCO3− Cotransporter. Nature 1997, 387, 409–413. [Google Scholar] [CrossRef]
- Liu, Y.; Xu, J.-Y.; Wang, D.-K.; Wang, L.; Chen, L.-M. Cloning and Identification of Two Novel NBCe1 Splice Variants from Mouse Reproductive Tract Tissues: A Comparative Study of NCBT Genes. Genomics 2011, 98, 112–119. [Google Scholar] [CrossRef] [Green Version]
- McAlear, S.D.; Liu, X.; Williams, J.B.; McNicholas-Bevensee, C.M.; Bevensee, M.O. Electrogenic Na/HCO3 Cotransporter (NBCe1) Variants Expressed in Xenopus Oocytes: Functional Comparison and Roles of the Amino and Carboxy Termini. J. Gen. Physiol. 2006, 127, 639–658. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giebisch, G.; Windhager, E. Transport of Acids and Bases. In Medical Physiology. A Cellular and Molecular Approach; Boron, W.F., Boulpaep, E.L., Eds.; Elsevier Saunders: Philadelphia, PA, USA, 2012; pp. 851–865. [Google Scholar]
- Shirakabe, K.; Priori, G.; Yamada, H.; Ando, H.; Horita, S.; Fujita, T.; Fujimoto, I.; Mizutani, A.; Seki, G.; Mikoshiba, K. IRBIT, an Inositol 1,4,5-Trisphosphate Receptor-Binding Protein, Specifically Binds to and Activates Pancreas-Type Na+/HCO3− Cotransporter 1 (PNBC1). Proc. Natl. Acad. Sci. USA 2006, 103, 9542–9547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lee, S.-K.; Boron, W.F.; Parker, M.D. Relief of Autoinhibition of the Electrogenic Na-HCO3 Cotransporter NBCe1-B: Role of IRBIT vs.Amino-Terminal Truncation. Am. J. Physiol. Cell Physiol. 2012, 302, C518–C526. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, H.; Riederer, B.; Stieger, N.; Boron, W.F.; Shull, G.E.; Manns, M.P.; Seidler, U.E.; Bachmann, O. Secretagogue Stimulation Enhances NBCe1 (Electrogenic Na+/HCO3− Cotransporter) Surface Expression in Murine Colonic Crypts. Am. J. Physiol. Gastrointest. Liver Physiol. 2009, 297, G1223–G1231. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shao, X.M.; Kao, L.; Kurtz, I. A Novel Delta Current Method for Transport Stoichiometry Estimation. BMC Biophys. 2014, 7, 14. [Google Scholar] [CrossRef] [Green Version]
- Heyer, M.; Müller-Berger, S.; Romero, M.F.; Boron, W.F.; Frömter, E. Stoichiometry of the Rat Kidney Na+-HCO3− Cotransporter Expressed in Xenopus Laevis Oocytes. Pflug. Arch. 1999, 438, 322–329. [Google Scholar] [CrossRef]
- Yoshitomi, K.; Frömter, E. How Big Is the Electrochemical Potential Difference of Na+ across Rat Renal Proximal Tubular Cell Membranes in Vivo? Pflug. Arch. 1985, 405 (Suppl. S1), S121–S126. [Google Scholar] [CrossRef]
- Gross, E.; Hawkins, K.; Abuladze, N.; Pushkin, A.; Cotton, C.U.; Hopfer, U.; Kurtz, I. The Stoichiometry of the Electrogenic Sodium Bicarbonate Cotransporter NBC1 Is Cell-Type Dependent. J. Physiol. 2001, 531, 597–603. [Google Scholar] [CrossRef]
- Vidyasagar, S.; Rajendran, V.M.; Binder, H.J. Three Distinct Mechanisms of HCO3− Secretion in Rat Distal Colon. Am. J. Physiol. Cell Physiol. 2004, 287, C612–C621. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feric, M.; Zhao, B.; Hoffert, J.; Pisitkun, T.; Knepper, M. Renal Cortical Membrane Phosphoproteome Database (RCMPD). Available online: http://dir.nhlbi.nih.gov/papers/lkem/rcmpd/ (accessed on 24 February 2021).
- Gross, E.; Hawkins, K.; Pushkin, A.; Sassani, P.; Dukkipati, R.; Abuladze, N.; Hopfer, U.; Kurtz, I. Phosphorylation of Ser(982) in the Sodium Bicarbonate Cotransporter KNBC1 Shifts the HCO3−: Na+ Stoichiometry from 3:1 to 2:1 in Murine Proximal Tubule Cells. J. Physiol. 2001, 537, 659–665. [Google Scholar] [CrossRef] [PubMed]
- Feric, M.; Zhao, B.; Hoffert, J.D.; Pisitkun, T.; Knepper, M.A. Large-Scale Phosphoproteomic Analysis of Membrane Proteins in Renal Proximal and Distal Tubule. Am. J. Physiol. Cell Physiol. 2011, 300, C755–C770. [Google Scholar] [CrossRef]
- Toye, A.M.; Parker, M.D.; Daly, C.M.; Lu, J.; Virkki, L.V.; Pelletier, M.F.; Boron, W.F. The Human NBCe1-A Mutant R881C, Associated with Proximal Renal Tubular Acidosis, Retains Function but Is Mistargeted in Polarized Renal Epithelia. Am. J. Physiol. Cell Physiol. 2006, 291, C788–C801. [Google Scholar] [CrossRef] [PubMed]
- Millar, I.D.; Brown, P.D. NBCe2 Exhibits a 3 HCO3−:1 Na+ Stoichiometry in Mouse Choroid Plexus Epithelial Cells. Biochem. Biophys. Res. Commun. 2008, 373, 550–554. [Google Scholar] [CrossRef]
- Theparambil, S.M.; Naoshin, Z.; Thyssen, A.; Deitmer, J.W. Reversed Electrogenic Sodium Bicarbonate Cotransporter1 Is the Major Acid Loader during Recovery from Cytosolic Alkalosis in Mouse Cortical Astrocytes. J. Physiol. 2015, 593, 3533–3547. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Theparambil, S.M.; Hosford, P.S.; Ruminot, I.; Kopach, O.; Reynolds, J.R.; Sandoval, P.Y.; Rusakov, D.A.; Barros, L.F.; Gourine, A.V. Astrocytes Regulate Brain Extracellular PH via a Neuronal Activity-Dependent Bicarbonate Shuttle. Nat. Commun. 2020, 11, 5073. [Google Scholar] [CrossRef] [PubMed]
- Gross, E.; Pushkin, A.; Abuladze, N.; Fedotoff, O.; Kurtz, I. Regulation of the Sodium Bicarbonate Cotransporter KNBC1 Function: Role of Asp(986), Asp(988) and KNBC1-Carbonic Anhydrase II Binding. J. Physiol. 2002, 544, 679–685. [Google Scholar] [CrossRef] [PubMed]
- Seki, G.; Coppola, S.; Frömter, E. The Na+-HCO3− Cotransporter Operates with a Coupling Ratio of 2 HCO3− to 1 Na+ in Isolated Rabbit Renal Proximal Tubule. Pflug. Arch. 1993, 425, 409–416. [Google Scholar] [CrossRef]
- Akiba, T.; Rocco, V.K.; Warnock, D.G. Parallel Adaptation of the Rabbit Renal Cortical Sodium/Proton Antiporter and Sodium/Bicarbonate Cotransporter in Metabolic Acidosis and Alkalosis. J. Clin. Investig. 1987, 80, 308–315. [Google Scholar] [CrossRef] [PubMed]
- Preisig, P.A.; Alpern, R.J. Chronic Metabolic Acidosis Causes an Adaptation in the Apical Membrane Na/H Antiporter and Basolateral Membrane Na(HCO3)3 Symporter in the Rat Proximal Convoluted Tubule. J. Clin. Investig. 1988, 82, 1445–1453. [Google Scholar] [CrossRef]
- Zhu, Q.; Shao, X.M.; Kao, L.; Azimov, R.; Weinstein, A.M.; Newman, D.; Liu, W.; Kurtz, I. Missense Mutation T485S Alters NBCe1-A Electrogenicity Causing Proximal Renal Tubular Acidosis. Am. J. Physiol. Cell Physiol. 2013, 305, C392–C405. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, F.Y.; Cogan, M.G. Role of Protein Kinase C in Proximal Bicarbonate Absorption and Angiotensin Signaling. Am. J. Physiol. 1990, 258, F927–F933. [Google Scholar] [CrossRef] [PubMed]
- Skelton, L.A.; Boron, W.F.; Zhou, Y. Acid-Base Transport by the Renal Proximal Tubule. J. Nephrol. 2010, 23 (Suppl. S16), S4–S18. [Google Scholar] [PubMed]
- Bachmann, O.; Rossmann, H.; Berger, U.V.; Colledge, W.H.; Ratcliff, R.; Evans, M.J.; Gregor, M.; Seidler, U. CAMP-Mediated Regulation of Murine Intestinal/Pancreatic Na+/HCO3− Cotransporter Subtype PNBC1. Am. J. Physiol. Gastrointest. Liver Physiol. 2003, 284, G37–G45. [Google Scholar] [CrossRef] [Green Version]
- Bachmann, O.; Reichelt, D.; Tuo, B.; Manns, M.P.; Seidler, U. Carbachol Increases Na+-HCO3− Cotransport Activity in Murine Colonic Crypts in a M3-, Ca2+/Calmodulin-, and PKC-Dependent Manner. Am. J. Physiol. Gastrointest. Liver Physiol. 2006, 291, G650–G657. [Google Scholar] [CrossRef]
- Myers, E.J.; Marshall, A.; Parker, M.D. Normal Intrinsic Na/HCO3 Cotransport Actiity but Altered Plasma Membrane Abundance of Phosphomimetic NBCe1-A Mutants. J. Am. Soc. Nephrol. 2016, 27, 650A. [Google Scholar]
- Sergeev, M.; Godin, A.G.; Kao, L.; Abuladze, N.; Wiseman, P.W.; Kurtz, I. Determination of Membrane Protein Transporter Oligomerization in Native Tissue Using Spatial Fluorescence Intensity Fluctuation Analysis. PLoS ONE 2012, 7, e36215. [Google Scholar] [CrossRef] [Green Version]
- Chang, M.-H.; Chen, A.-P.; Romero, M.F. NBCe1A Dimer Assemble Visualized by Bimolecular Fluorescence Complementation. Am. J. Physiol. Ren. Physiol. 2014, 306, F672–F680. [Google Scholar] [CrossRef] [PubMed]
- Khakipoor, S.; Giannaki, M.; Theparambil, S.M.; Zecha, J.; Küster, B.; Heermann, S.; Deitmer, J.W.; Roussa, E. Functional Expression of Electrogenic Sodium Bicarbonate Cotransporter 1 (NBCe1) in Mouse Cortical Astrocytes Is Dependent on S255–257 and Regulated by MTOR. Glia 2019, 67, 2264–2278. [Google Scholar] [CrossRef] [Green Version]
- Vachel, L.; Shcheynikov, N.; Yamazaki, O.; Fremder, M.; Ohana, E.; Son, A.; Shin, D.M.; Yamazaki-Nakazawa, A.; Yang, C.-R.; Knepper, M.A.; et al. Modulation of Cl- Signaling and Ion Transport by Recruitment of Kinases and Phosphatases Mediated by the Regulatory Protein IRBIT. Sci. Signal 2018, 11. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, L.-M.; Kelly, M.L.; Rojas, J.D.; Parker, M.D.; Gill, H.S.; Davis, B.A.; Boron, W.F. Use of a New Polyclonal Antibody to Study the Distribution and Glycosylation of the Sodium-Coupled Bicarbonate Transporter NCBE in Rodent Brain. Neuroscience 2008, 151, 374–385. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Quade, B.N.; Marshall, A.; Parker, M.D. PH Dependence of the Slc4a11-Mediated H+ Conductance Is Influenced by Intracellular Lysine Residues and Modified by Disease-Linked Mutations. Am. J. Physiol. Cell Physiol. 2020, 319, C359–C370. [Google Scholar] [CrossRef]
- Nowik, M.; Kampik, N.B.; Mihailova, M.; Eladari, D.; Wagner, C.A. Induction of Metabolic Acidosis with Ammonium Chloride (NH4Cl) in Mice and Rats--Species Differences and Technical Considerations. Cell Physiol. Biochem. 2010, 26, 1059–1072. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salerno, E.E.; Patel, S.P.; Marshall, A.; Marshall, J.; Alsufayan, T.; Mballo, C.S.A.; Quade, B.N.; Parker, M.D. Extrarenal Signs of Proximal Renal Tubular Acidosis Persist in Nonacidemic Nbce1b/c-Null Mice. J. Am. Soc. Nephrol. 2019, 30, 979–989. [Google Scholar] [CrossRef]
- Bevensee, M.O.; Schmitt, B.M.; Choi, I.; Romero, M.F.; Boron, W.F. An Electrogenic Na+-HCO3− Cotransporter (NBC) with a Novel COOH-Terminus, Cloned from Rat Brain. Am. J. Physiol. Cell Physiol. 2000, 278, C1200–C1211. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Parker, M.D.; Qin, X.; Williamson, R.C.; Toye, A.M.; Boron, W.F. HCO3−-Independent Conductance with a Mutant Na+/HCO3− Cotransporter (SLC4A4) in a Case of Proximal Renal Tubular Acidosis with Hypokalaemic Paralysis. J. Physiol. 2012, 590, 2009–2034. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [Green Version]


| Mutant | Title 2 |
|---|---|
| AA | FWD: 5′-GAAGAAAGGAGCTTTGGATGCCGACAATGACGATTCTG-3′ RVS: 5′-CAGAATCGTCATTGTCGGCATCCAAAGCTCCTTTCTTC-3′ |
| AD | FWD: 5′-GAAGAAAGGAGCTTTGGATGACGACAATGACGATTCTG-3′ RVS: 5′-CAGAATCGTCATTGTCGTCATCCAAAGCTCCTTTCTTC-3′ |
| DA | FWD: 5′-GAAGAAAGGAGATTTGGATGCCGACAATGACGATTCTG-3′ RVS: 5′-CAGAATCGTCATTGTCGGCATCCAAATCTCCTTTCTTC-3′ |
| DD | FWD: 5′-GAAGAAAGGAGATTTGGATGACGACAATGACGATTCTG-3′ RVS: 5′- CAGAATCGTCATTGTCGTCATCCAAATCTCCTTTCTTC-3′ |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Alsufayan, T.A.; Myers, E.J.; Quade, B.N.; Brady, C.T.; Marshall, A.; Haque, N.; Duffey, M.E.; Parker, M.D. Revisiting the Role of Ser982 Phosphorylation in Stoichiometry Shift of the Electrogenic Na+/qHCO3− Cotransporter NBCe1. Int. J. Mol. Sci. 2021, 22, 12817. https://doi.org/10.3390/ijms222312817
Alsufayan TA, Myers EJ, Quade BN, Brady CT, Marshall A, Haque N, Duffey ME, Parker MD. Revisiting the Role of Ser982 Phosphorylation in Stoichiometry Shift of the Electrogenic Na+/qHCO3− Cotransporter NBCe1. International Journal of Molecular Sciences. 2021; 22(23):12817. https://doi.org/10.3390/ijms222312817
Chicago/Turabian StyleAlsufayan, Thamer A., Evan J. Myers, Bianca N. Quade, Clayton T. Brady, Aniko Marshall, Nayem Haque, Michael E. Duffey, and Mark D. Parker. 2021. "Revisiting the Role of Ser982 Phosphorylation in Stoichiometry Shift of the Electrogenic Na+/qHCO3− Cotransporter NBCe1" International Journal of Molecular Sciences 22, no. 23: 12817. https://doi.org/10.3390/ijms222312817
APA StyleAlsufayan, T. A., Myers, E. J., Quade, B. N., Brady, C. T., Marshall, A., Haque, N., Duffey, M. E., & Parker, M. D. (2021). Revisiting the Role of Ser982 Phosphorylation in Stoichiometry Shift of the Electrogenic Na+/qHCO3− Cotransporter NBCe1. International Journal of Molecular Sciences, 22(23), 12817. https://doi.org/10.3390/ijms222312817







