Macrophages Modulate the Function of MSC- and iPSC-Derived Fibroblasts in the Presence of Polyethylene Particles
Abstract
:1. Introduction
2. Results
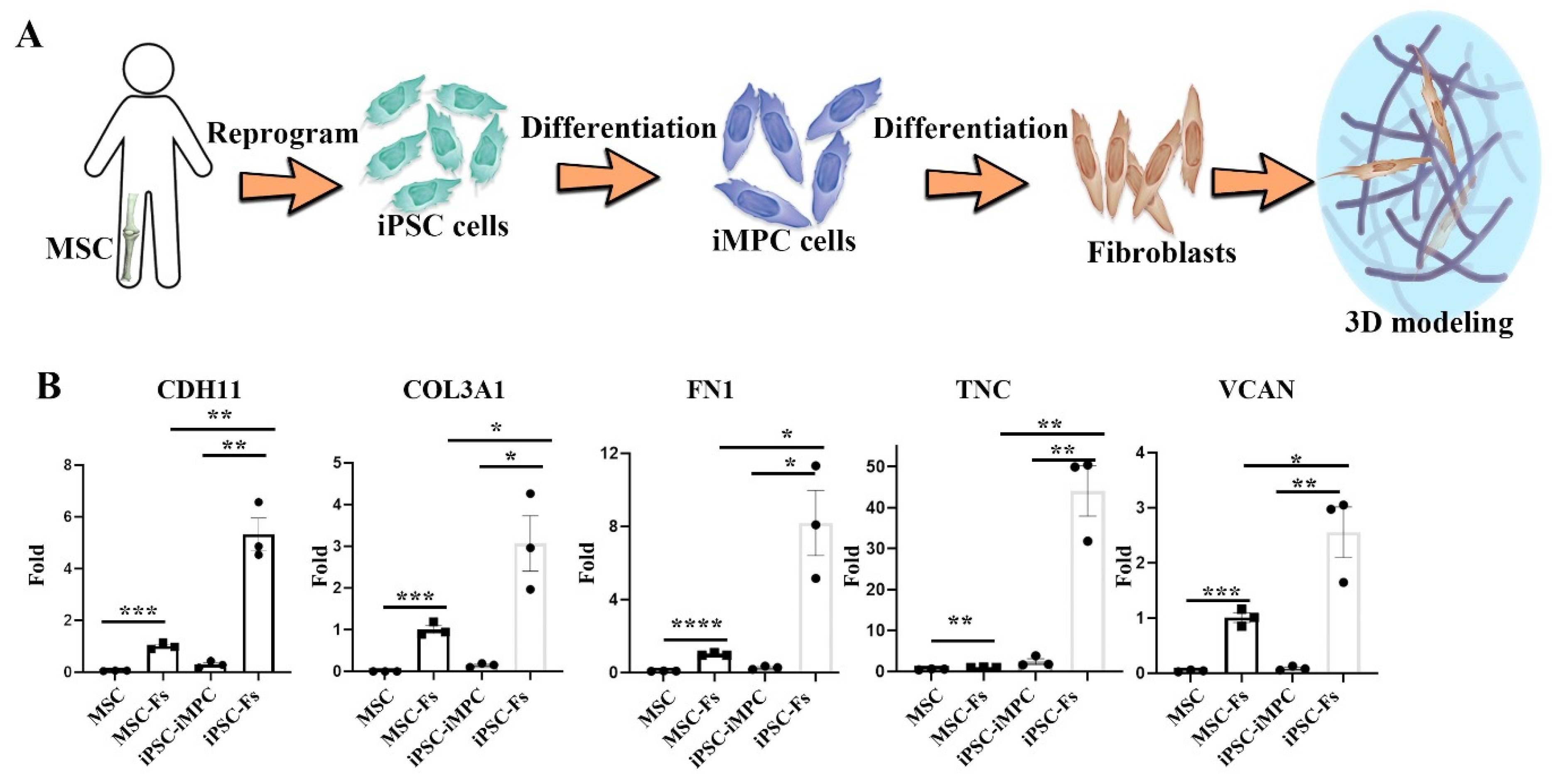
2.1. Polyethylene Particles Induce the Expression of Functional Fibroblast Markers
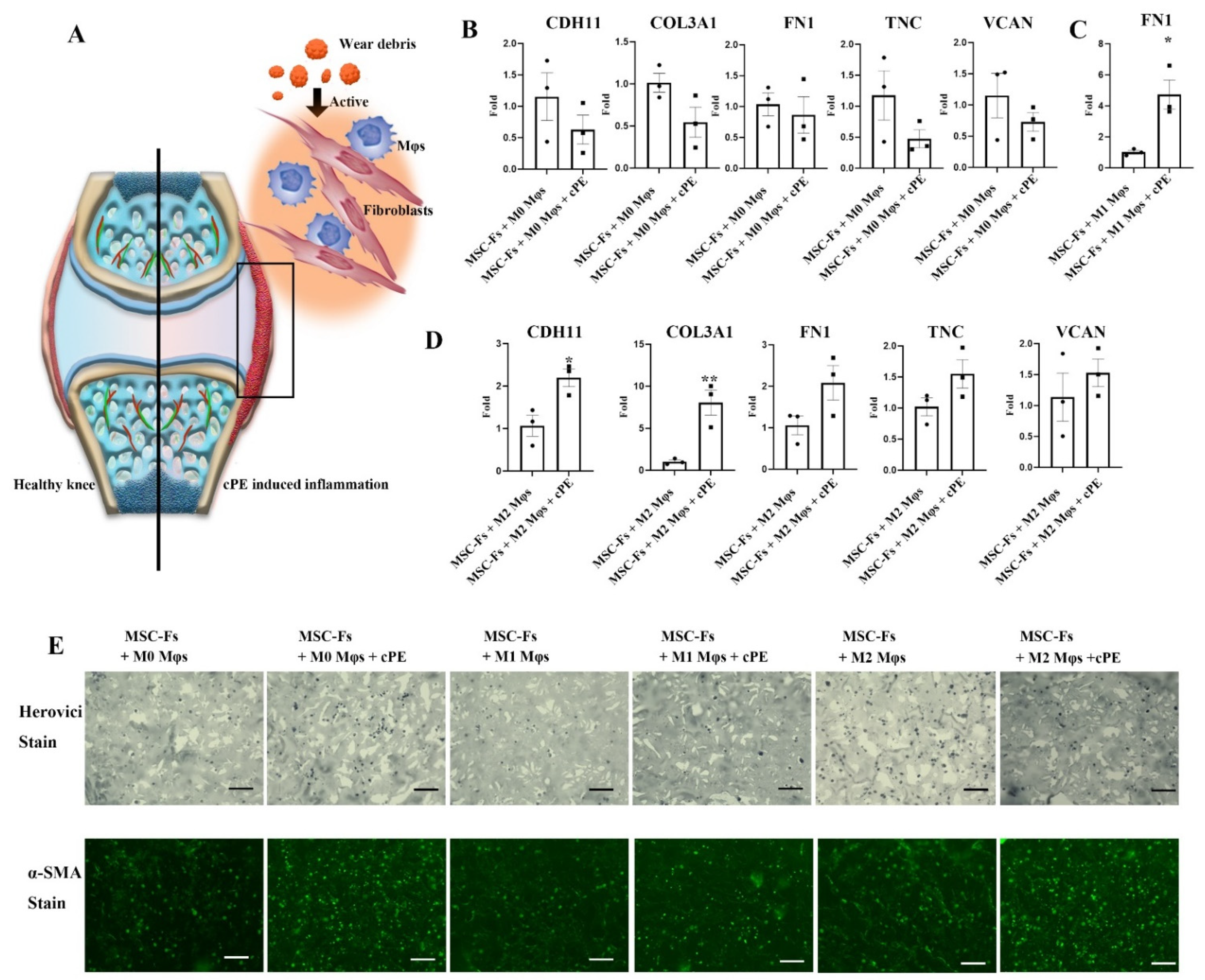
2.2. Mφ Accelerate Fibrosis in the Presence of Polyethylene Particles
2.3. Cell Source-Specific Model for Polyethylene Particle Disease
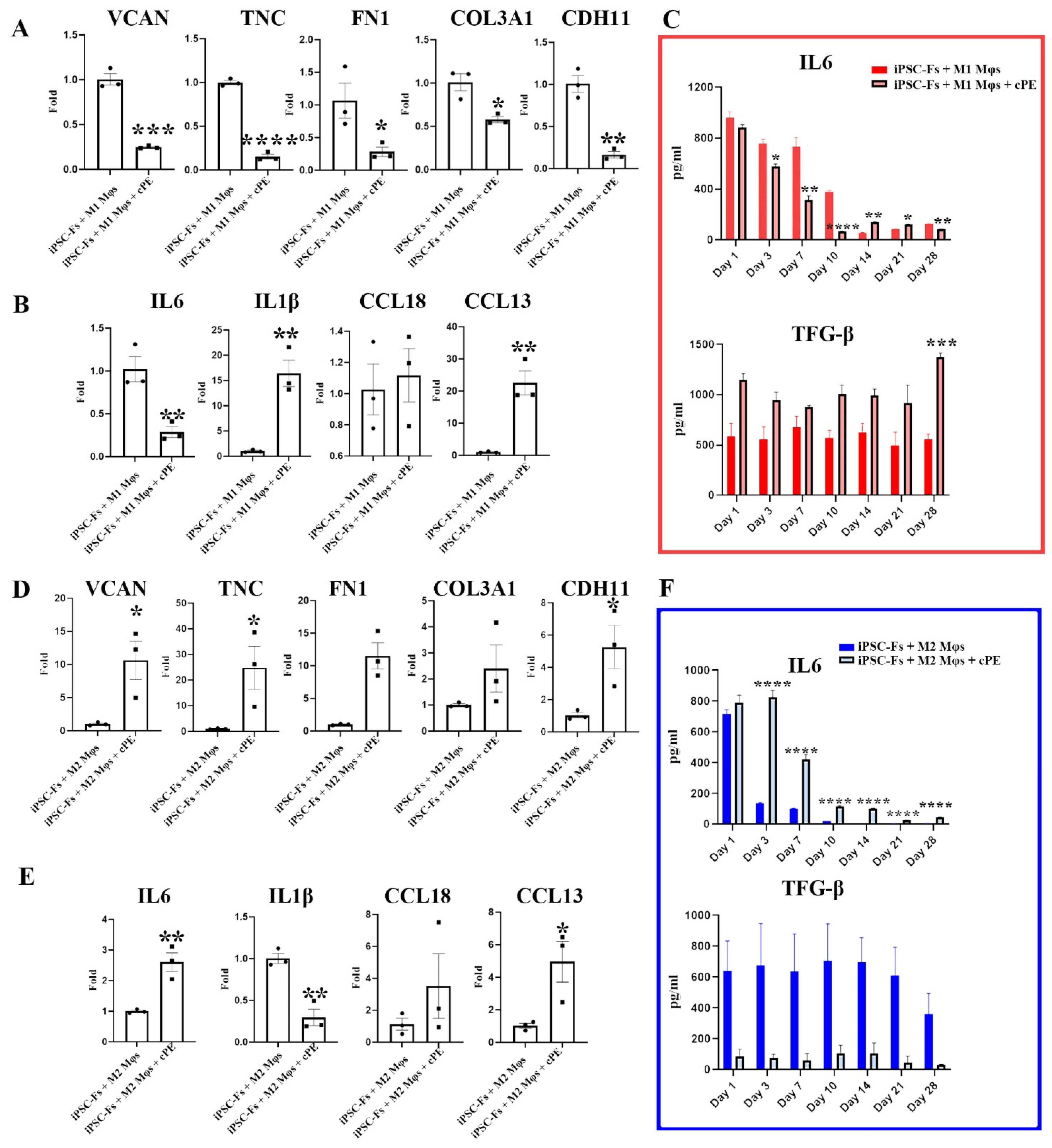
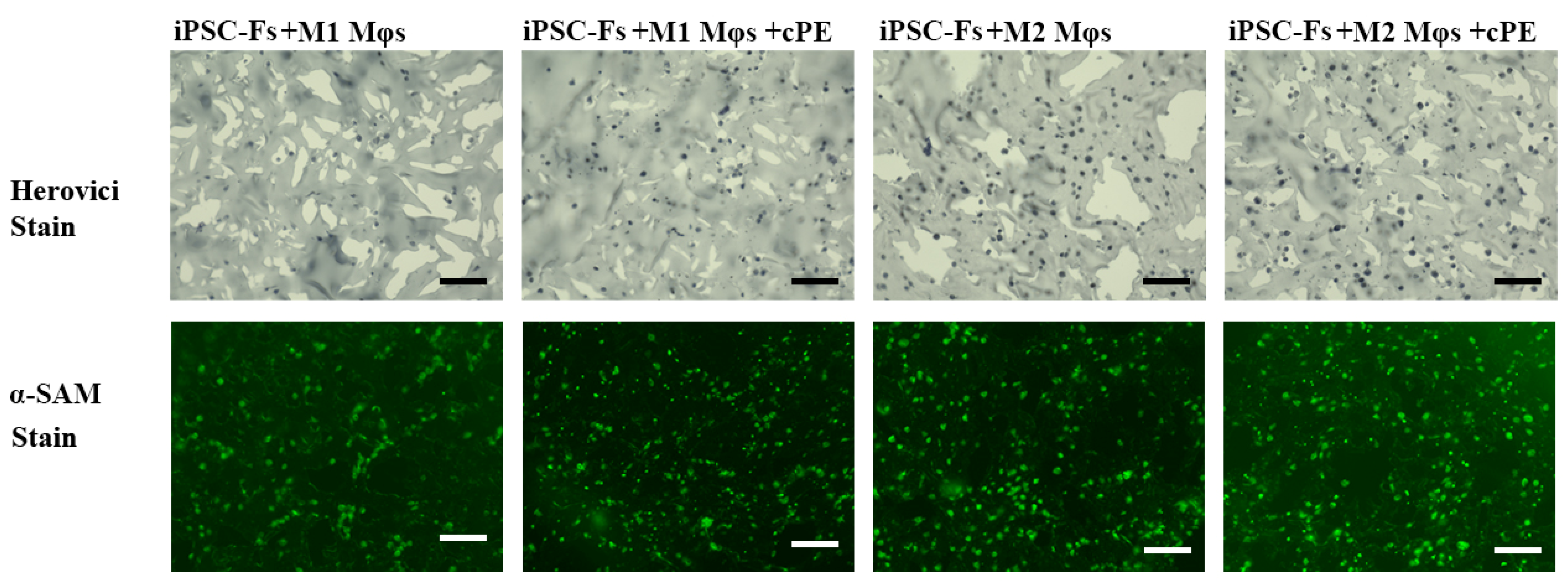
2.4. M1 Mφs Inhibit Fibrosis in iPSC-Fs
3. Discussion
4. Materials and Methods
4.1. Cell Culture and Differentiation
4.2. iPSC Culture and Differentiation
4.3. Bioreactor Setup
4.4. qPCR Analysis
4.5. Enzyme-Linked Immunosorbent Assay (ELISA)
4.6. Histology and Imaging
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
References
- Abdul, N.; Dixon, D.; Walker, A.; Horabin, J.; Smith, N.; Weir, D.J.; Brewster, N.T.; Deehan, D.J.; Mann, D.A.; Borthwick, L.A. Fibrosis is a common outcome following total knee arthroplasty. Sci. Rep. 2015, 5, 16469. [Google Scholar] [CrossRef] [Green Version]
- Bonnans, C.; Chou, J.; Werb, Z. Remodelling the extracellular matrix in development and disease. Nat. Rev. Mol. Cell Biol. 2014, 15, 786–801. [Google Scholar] [CrossRef]
- Remst, D.F.G.; Blaney Davidson, E.N.; van der Kraan, P.M. Unravelling osteoarthritis-related synovial fibrosis: A step closer to solving joint stiffness. Rheumatology 2015, 54, 1954–1963. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hodgeson, S.; O’Brien, S.; Simkin, J.; Plakotaris, E.; McCarthy, C.; Dasa, V.; Marrero, L. Differences in synovial fibrosis relative to range of motion in knee osteoarthritis patients. J. Orthop. Res. 2021, 1–11. [Google Scholar] [CrossRef] [PubMed]
- Thakur, R.R.; Ast, M.P.; McGraw, M.; Bostrom, M.P.; Rodriguez, J.A.; Parks, M.L. Severe persistent synovitis after cobalt-chromium total knee arthroplasty requiring revision. Orthopedics 2013, 36, 520–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bonnet, C.S.; Walsh, D.A. Osteoarthritis, angiogenesis and inflammation. Rheumatology 2005, 44, 7–16. [Google Scholar] [CrossRef]
- Li, N.; Gao, J.; Mi, L.; Zhang, G.; Zhang, L.; Zhang, N.; Huo, R.; Hu, J.; Xu, K. Synovial membrane mesenchymal stem cells: Past life, current situation, and application in bone and joint diseases. Stem Cell Res. Ther. 2020, 11, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Meng, X.; Du, Z.; Wang, Y. Characteristics of wear particles and wear behavior of retrieved PEEK-on-HXLPE total knee implants: A preliminary study. RSC Adv. 2018, 8, 30330–30339. [Google Scholar] [CrossRef] [Green Version]
- Revell, P.A. The combined role of wear particles, macrophages and lymphocytes in the loosening of total joint prostheses. J. R. Soc. Interface 2008, 5, 1263–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, P.; Dai, Z.; Zhang, Y.S.; Liu, H.; Niu, W.; Li, K.; Wang, L.; Hu, Y.; Xie, J. Macrophage inhibits the osteogenesis of fibroblasts in ultrahigh molecular weight polyethylene (UHMWPE) wear particle-induced osteolysis. J. Orthop. Surg. Res. 2019, 14, 1–7. [Google Scholar] [CrossRef] [Green Version]
- Wynn, T.A.; Vannella, K.M. Macrophages in Tissue Repair, Regeneration, and Fibrosis. Immunity 2016, 44, 450–462. [Google Scholar] [CrossRef] [Green Version]
- Denu, R.A.; Nemcek, S.; Bloom, D.D.; Goodrich, A.D.; Kim, J.; Mosher, D.F.; Hematti, P. Fibroblasts and Mesenchymal Stromal/Stem Cells Are Phenotypically Indistinguishable. Acta Haematol. 2016, 136, 85–97. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Imere, A.; Ligorio, C.; O’Brien, M.; Wong, J.K.F.; Domingos, M.; Cartmell, S.H. Engineering a cell-hydrogel-fibre composite to mimic the structure and function of the tendon synovial sheath. Acta Biomater. 2021, 119, 140–154. [Google Scholar] [CrossRef]
- Wight, T.N.; Kang, I.; Evanko, S.P.; Harten, I.A.; Chang, M.Y.; Pearce, O.M.T.; Allen, C.E.; Frevert, C.W. Versican—A Critical Extracellular Matrix Regulator of Immunity and Inflammation. Front. Immunol. 2020, 11, 512. [Google Scholar] [CrossRef] [Green Version]
- Ortiz-Vitali, J.L.; Darabi, R. iPSCs as a Platform for Disease Modeling, Drug Screening, and Personalized Therapy in Muscular Dystrophies. Cells 2019, 8, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, Y.; Sallam, K.; Schwartz, P.J.; Wu, J.C. Patient-Specific iPSC-Based Disease Model for Pathogenesis Studies and Clinical Pharmacotherapy. Physiol. Behav. 2016, 176, 139–148. [Google Scholar] [CrossRef]
- Sharma, A.; Sances, S.; Workman, M.J.; Svendsen, C.N. Multi-lineage Human iPSC-Derived Platforms for Disease Modeling and Drug Discovery. Cell Stem Cell 2020, 26, 309–329. [Google Scholar] [CrossRef]
- Maglaviceanu, A.; Wu, B.; Kapoor, M. Fibroblast-like synoviocytes: Role in synovial fibrosis associated with osteoarthritis. Wound Repair Regen. 2021, 29, 642–649. [Google Scholar] [CrossRef]
- Gao, Q.; Rhee, C.; Maruyama, M.; Li, Z.; Shen, H.; Zhang, N.; Utsunomiya, T.; Huang, E.E.; Yao, Z.; Bunnell, B.A.; et al. The effects of macrophage phenotype on osteogenic differentiation of mscs in the presence of polyethylene particles. Biomedicines 2021, 9, 499. [Google Scholar] [CrossRef]
- Lin, T.; Kohno, Y.; Huang, J.F.; Romero-Lopez, M.; Pajarinen, J.; Maruyama, M.; Nathan, K.; Yao, Z.; Goodman, S.B. NFκB sensing IL-4 secreting mesenchymal stem cells mitigate the proinflammatory response of macrophages exposed to polyethylene wear particles. J. Biomed. Mater. Res.-Part A 2018, 106, 2744–2752. [Google Scholar] [CrossRef] [PubMed]
- Karsdal, M.A.; Daniels, S.J.; Holm Nielsen, S.; Bager, C.; Rasmussen, D.G.K.; Loomba, R.; Surabattula, R.; Villesen, I.F.; Luo, Y.; Shevell, D.; et al. Collagen biology and non-invasive biomarkers of liver fibrosis. Liver Int. 2020, 40, 736–750. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hinz, B.; Phan, S.H.; Thannickal, V.J.; Galli, A.; Bochaton-Piallat, M.L.; Gabbiani, G. The myofibroblast: One function, multiple origins. Am. J. Pathol. 2007, 170, 1807–1816. [Google Scholar] [CrossRef] [PubMed]
- Holm Nielsen, S.; Willumsen, N.; Leeming, D.J.; Daniels, S.J.; Brix, S.; Karsdal, M.A.; Genovese, F.; Nielsen, M.J. Serological Assessment of Activated Fibroblasts by alpha-Smooth Muscle Actin (α-SMA): A Noninvasive Biomarker of Activated Fibroblasts in Lung Disorders. Transl. Oncol. 2019, 12, 368–374. [Google Scholar] [CrossRef]
- Shinde, A.V.; Humeres, C.; Frangogiannis, N.G. The role of α-smooth muscle actin in fibroblast-mediated matrix contraction and remodeling. Biochim. Biophys. Acta-Mol. Basis Dis. 2017, 1863, 298–309. [Google Scholar] [CrossRef]
- Diederichs, S.; Tuan, R.S. Functional comparison of human-induced pluripotent stem cell-derived mesenchymal cells and bone marrow-derived mesenchymal stromal cells from the same donor. Stem Cells Dev. 2014, 23, 1594–1610. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Z.; Li, Z.; Li, E.N.; Li, X.; Del Duke, C.J.; Shen, H.; Hao, T.; O’Donnell, B.; Bunnell, B.A.; Goodman, S.B.; et al. Osteochondral Tissue Chip Derived From iPSCs: Modeling OA Pathologies and Testing Drugs. Front. Bioeng. Biotechnol. 2019, 7, 411. [Google Scholar] [CrossRef]
- Singh, P.; Carraher, C.; Schwarzbauer, J.E. Assembly of fibronectin extracellular matrix. Annu. Rev. Cell Dev. Biol. 2010, 26, 397–419. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Brösicke, N.; Faissner, A. Role of tenascins in the ECM of gliomas. Cell Adhes. Migr. 2015, 9, 131–140. [Google Scholar] [CrossRef] [Green Version]
- Rutnam, Z.J.; Wight, T.N.; Yang, B.B. MiRNAs regulate expression and function of extracellular matrix molecules. Matrix Biol. 2013, 32, 74–85. [Google Scholar] [CrossRef] [Green Version]
- Bertrand, J.; Awiszus, F.; Lohmann, C.H.; Delfosse, D.; Mai, V.; Harnisch, K. Ceramic prosthesis surfaces induce an inflammatory cell response and fibrotic tissue changes. Bone Jt. J. 2018, 100B, 882–890. [Google Scholar] [CrossRef]
- Gu, Q.; Yang, H.; Shi, Q. Macrophages and bone inflammation. J. Orthop. Transl. 2017, 10, 86–93. [Google Scholar] [CrossRef] [PubMed]
- Kurowska-Stolarska, M.; Alivernini, S. Synovial tissue macrophages: Friend or foe? RMD Open 2017, 3, e000527. [Google Scholar] [CrossRef] [PubMed]
- Menarim, B.C.; Gillis, K.H.; Oliver, A.; Ngo, Y.; Werre, S.R.; Barrett, S.H.; Rodgerson, D.H.; Dahlgren, L.A. Macrophage Activation in the Synovium of Healthy and Osteoarthritic Equine Joints. Front. Vet. Sci. 2020, 7, 568756. [Google Scholar] [CrossRef]
- Oishi, Y.; Manabe, I. Macrophages in inflammation, repair and regeneration. Int. Immunol. 2018, 30, 511–528. [Google Scholar] [CrossRef] [PubMed]
- Hesketh, M.; Sahin, K.B.; West, Z.E.; Murray, R.Z. Macrophage phenotypes regulate scar formation and chronic wound healing. Int. J. Mol. Sci. 2017, 18, 1545. [Google Scholar] [CrossRef] [Green Version]
- Davidson, S.; Coles, M.; Thomas, T.; Kollias, G.; Ludewig, B.; Turley, S.; Brenner, M.; Buckley, C.D. Fibroblasts as immune regulators in infection, inflammation and cancer. Nat. Rev. Immunol. 2021, 21, 704–717. [Google Scholar] [CrossRef]
- Hou, J.; Shi, J.; Chen, L.; Lv, Z.; Chen, X.; Cao, H.; Xiang, Z.; Han, X. M2 macrophages promote myofibroblast differentiation of LR-MSCs and are associated with pulmonary fibrogenesis 11 Medical and Health Sciences 1102 Cardiorespiratory Medicine and Haematology. Cell Commun. Signal. 2018, 16, 89. [Google Scholar] [CrossRef] [Green Version]
- Ng-Blichfeldt, J.P.; de Jong, T.; Kortekaas, R.K.; Wu, X.; Lindner, M.; Guryev, V.; Hiemstra, P.S.; Stolk, J.; Königshoff, M.; Gosens, R. Tgf-β activation impairs fibroblast ability to support adult lung epithelial progenitor cell organoid formation. Am. J. Physiol.-Lung Cell. Mol. Physiol. 2019, 317, L14–L28. [Google Scholar] [CrossRef]
- Lin, H.; Cheng, A.W.-M.; Alexander, P.G.; Beck, A.M.; Tuan, R.S. Cartilage tissue engineering application of injectable gelatin hydrogel with in situ visible-light-activated gelation capability in both air and aqueous solution. Tissue Eng. Part A 2014, 20, 2402–2411. [Google Scholar] [CrossRef] [Green Version]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gao, Q.; Li, Z.; Rhee, C.; Xiang, S.; Maruyama, M.; Huang, E.E.; Yao, Z.; Bunnell, B.A.; Tuan, R.S.; Lin, H.; et al. Macrophages Modulate the Function of MSC- and iPSC-Derived Fibroblasts in the Presence of Polyethylene Particles. Int. J. Mol. Sci. 2021, 22, 12837. https://doi.org/10.3390/ijms222312837
Gao Q, Li Z, Rhee C, Xiang S, Maruyama M, Huang EE, Yao Z, Bunnell BA, Tuan RS, Lin H, et al. Macrophages Modulate the Function of MSC- and iPSC-Derived Fibroblasts in the Presence of Polyethylene Particles. International Journal of Molecular Sciences. 2021; 22(23):12837. https://doi.org/10.3390/ijms222312837
Chicago/Turabian StyleGao, Qi, Zhong Li, Claire Rhee, Shiqi Xiang, Masahiro Maruyama, Elijah Ejun Huang, Zhenyu Yao, Bruce A. Bunnell, Rocky S. Tuan, Hang Lin, and et al. 2021. "Macrophages Modulate the Function of MSC- and iPSC-Derived Fibroblasts in the Presence of Polyethylene Particles" International Journal of Molecular Sciences 22, no. 23: 12837. https://doi.org/10.3390/ijms222312837
APA StyleGao, Q., Li, Z., Rhee, C., Xiang, S., Maruyama, M., Huang, E. E., Yao, Z., Bunnell, B. A., Tuan, R. S., Lin, H., Gold, M. S., & Goodman, S. B. (2021). Macrophages Modulate the Function of MSC- and iPSC-Derived Fibroblasts in the Presence of Polyethylene Particles. International Journal of Molecular Sciences, 22(23), 12837. https://doi.org/10.3390/ijms222312837









