Updates and Perspectives on Aquaporin-2 and Water Balance Disorders
Abstract
:1. Introduction
2. Upon Vasopressin Stimulation AQP2 Increases Water Reabsorption and Urine Concentration
3. Phosphorylation Process of AQP2
4. The Role of Calcium in the Regulation of AQP2
5. The Role of the Cytoskeleton in AQP2 Trafficking
6. AQP2 Recycling and Endocytosis
7. The Molecular Mechanism Driving AQP2 Movement
8. The Water Channel Activity of Individual AQP2 Proteins
9. The Role of AQP2 in Fluctuating Osmolality
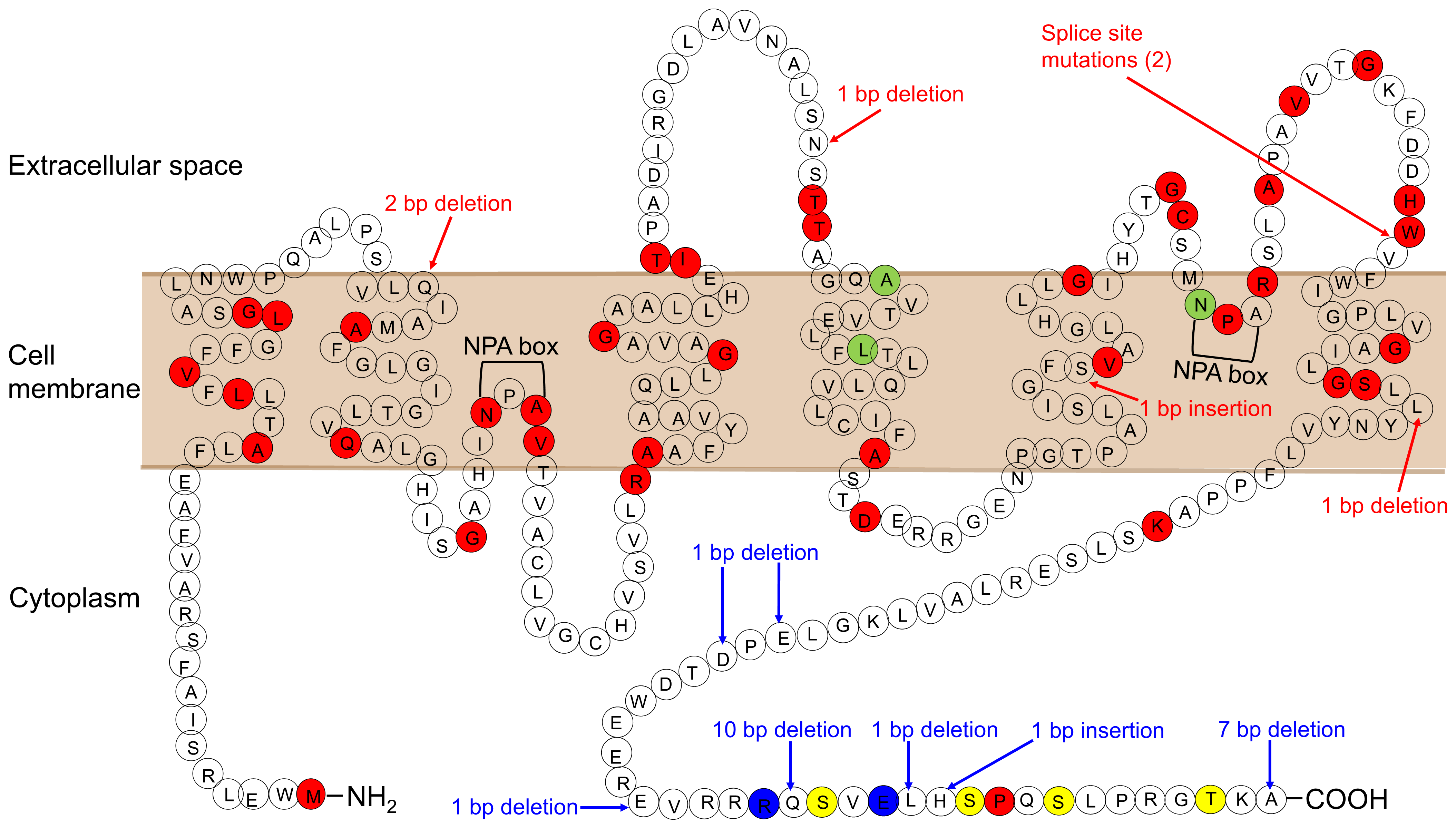
10. NDI
11. Congenital NDI
12. Acquired NDI
13. Water Retention by AQP2 Dysregulation
14. Development of Therapeutics for NDI by Targeting AQP2 Regulation
15. Vasopressin V2 Antagonists as Current Treatments for Water Retention, and Future Strategies for Pure Aquaretics by Direct AQP2 Inhibition
16. Conclusions
Author Contributions
Funding
Conflicts of Interest
References
- Noda, Y.; Sasaki, S. Regulation of water balance: Urine concentration and dilution. In Schrier’s Diseases of the Kidney, 9th ed.; Coffman, T.M., Falk., R.J., Molitoris, B.A., Neilson, E.G., Schrier, R.W., Eds.; Lippincott Williams & Wilkins: Philadelphia, PA, USA, 2012; pp. 132–158. [Google Scholar]
- Fushimi, K.; Uchida, S.; Hara, Y.; Hirata, Y.; Marumo, F.; Sasaki, S. Cloning and expression of apical membrane water channel of rat kidney collecting tubule. Nature 1993, 361, 549–552. [Google Scholar] [CrossRef] [PubMed]
- Agre, P.; Sasaki, S.; Chrispeels, M.J. Aquaporins: A family of water channel proteins. Am. J. Physiol. 1993, 265, F461. [Google Scholar] [CrossRef]
- Sasaki, S.; Fushimi, K.; Saito, H.; Saito, F.; Uchida, S.; Ishibashi, K.; Kuwahara, M.; Ikeuchi, T.; Inui, K.; Nakajima, K. Cloning, characterization, and chromosomal mapping of human aquaporin of collecting duct. J. Clin. Investig. 1994, 93, 1250–1256. [Google Scholar] [CrossRef]
- Noda, Y.; Sohara, E.; Ohta, E.; Sasaki, S. Aquaporins in kidney pathophysiology. Nat. Rev. Nephrol. 2010, 6, 168–178. [Google Scholar] [CrossRef] [PubMed]
- Noda, Y. Dynamic regulation and dysregulation of the water channel aquaporin-2: A common cause of and promising therapeutic target for water balance disorders. Clin. Exp. Nephrol. 2014, 18, 558–570. [Google Scholar] [CrossRef] [PubMed]
- Matsuzaki, T.; Yaguchi, T.; Shimizu, K.; Kita, A.; Ishibashi, K.; Takata, K. The distribution and function of aquaporins in the kidney: Resolved and unresolved questions. Anat. Sci. Int. 2017, 92, 187–199. [Google Scholar] [CrossRef]
- Noda, Y.; Sasaki, S. Regulation of aquaporin-2 trafficking and its binding protein complex. Biochim. Biophys. Acta 2006, 1758, 1117–1125. [Google Scholar] [CrossRef] [Green Version]
- Lolait, S.J.; O’Carroll, A.M.; McBride, O.W.; Konig, M.; Morel, A.; Brownstein, M.J. Cloning and characterization of a vasopressin V2 receptor and possible link to nephrogenic diabetes insipidus. Nature 1992, 357, 336–339. [Google Scholar] [CrossRef]
- Birnbaumer, M.; Seibold, A.; Gilbert, S.; Ishido, M.; Barberis, C.; Antaramian, A.; Brabet, P.; Rosenthal, W. Molecular cloning of the receptor for human antidiuretic hormone. Nature 1992, 357, 333–335. [Google Scholar] [CrossRef]
- Sands, J.M.; Nonoguchi, H.; Knepper, M.A. Vasopressin effects on urea and H2O transport in inner medullary collecting duct subsegments. Am. J. Physiol. 1987, 253, F823–F832. [Google Scholar] [CrossRef]
- Pohl, M.; Shan, Q.; Petsch, T.; Styp-Rekowska, B.; Matthey, P.; Bleich, M.; Bachmann, S.; Theilig, F. Short-term functional adaptation of aquaporin-1 surface expression in the proximal tubule, a component of glomerulotubular balance. J. Am. Soc. Nephrol. 2015, 26, 1269–1278. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ciappelloni, S.; Bouchet, D.; Dubourdieu, N.; Boué-Grabot, E.; Kellermayer, B.; Manso, C.; Marignier, R.; Oliet, S.H.R.; Tourdias, T.; Groc, L. Aquaporin-4 Surface Trafficking Regulates Astrocytic Process Motility and Synaptic Activity in Health and Autoimmune Disease. Cell Rep. 2019, 27, 3860–3872. [Google Scholar] [CrossRef] [Green Version]
- Kitchen, P.; Salman, M.M.; Halsey, A.M.; Clarke-Bland, C.; MacDonald, J.A.; Ishida, H.; Vogel, H.J.; Almutiri, S.; Logan, A.; Kreida, S.; et al. Targeting Aquaporin-4 Subcellular Localization to Treat Central Nervous System Edema. Cell 2020, 181, 784–799. [Google Scholar] [CrossRef]
- Chivasso, C.; Hagströmer, C.J.; Rose, K.L.; Lhotellerie, F.; Leblanc, L.; Wang, Z.; Moscato, S.; Chevalier, C.; Zindy, E.; Martin, M.; et al. Ezrin Is a Novel Protein Partner of Aquaporin-5 in Human Salivary Glands and Shows Altered Expression and Cellular Localization in Sjögren’s Syndrome. Int. J. Mol. Sci. 2021, 22, 9213. [Google Scholar] [CrossRef]
- Kikuchi, H.; Jung, H.J.; Raghuram, V.; Leo, K.T.; Park, E.; Yang, C.R.; Chou, C.L.; Chen, L.; Knepper, M.A. Bayesian identification of candidate transcription factors for the regulation of Aqp2 gene expression. Am. J. Physiol. Ren. Physiol. 2021, 321, F389–F401. [Google Scholar] [CrossRef] [PubMed]
- Saito, T.; Higashiyama, M.; Nagasaka, S.; Sasaki, S.; Saito, T.; Ishikawa, S.E. Role of aquaporin-2 gene expression in hyponatremic rats with chronic vasopressin-induced antidiuresis. Kidney Int. 2001, 60, 1266–1276. [Google Scholar] [CrossRef] [Green Version]
- Isobe, K.; Jung, H.J.; Yang, C.R.; Claxton, J.; Sandoval, P.; Burg, M.B.; Raghuram, V.; Knepper, M.A. Systems-level identification of PKA-dependent signaling in epithelial cells. Proc. Natl. Acad. Sci. USA 2017, 114, E8875–E8884. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yui, N.; Ando, F.; Sasaki, S.; Uchida, S. Ser-261 phospho-regulation is involved in pS256 and pS269-mediated aquaporin-2 apical translocation. Biochem. Biophys. Res. Commun. 2017, 490, 1039–1044. [Google Scholar] [CrossRef]
- Sakai, M.; Yamamoto, K.; Mizumura, H.; Matsumoto, T.; Tanaka, Y.; Noda, Y.; Ishibashi, K.; Yamamoto, T.; Sasaki, S. Phosphorylation profile of human AQP2 in urinary exosomes by LC-MS/MS phosphoproteomic analysis. Clin. Exp. Nephrol. 2020, 24, 762–769. [Google Scholar] [CrossRef]
- Procino, G.; Carmosino, M.; Marin, O.; Brunati, A.M.; Contri, A.; Pinna, L.A.; Mannucci, R.; Nielsen, S.; Kwon, T.H.; Svelto, M.; et al. Ser-256 phosphorylation dynamics of aquaporin 2 during maturation from the endoplasmic reticulum to the vesicular compartment in renal cells. FASEB J. 2003, 17, 1886–1888. [Google Scholar] [CrossRef] [Green Version]
- Van Balkom, B.W.; Savelkoul, P.J.; Markovich, D.; Hofman, E.; Nielsen, S.; van der Sluijs, P.; Deen, P.M. The role of putative phosphorylation sites in the targeting and shuttling of the aquaporin-2 water channel. J. Biol. Chem. 2002, 277, 41473–41479. [Google Scholar] [CrossRef] [Green Version]
- Bouley, R.; Breton, S.; Sun, T.; McLaughlin, M.; Nsumu, N.N.; Lin, H.Y.; Ausiello, D.A.; Brown, D. Nitric oxide and atrial natriuretic factor stimulate cGMP-dependent membrane insertion of aquaporin 2 in renal epithelial cells. J. Clin. Investig. 2000, 106, 1115–1126. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouley, R.; Pastor-Soler, N.; Cohen, O.; McLaughlin, M.; Breton, S.; Brown, D. Stimulation of AQP2 membrane insertion in renal epithelial cells in vitro and in vivo by the cGMP phosphodiesterase inhibitor sildenafil citrate (Viagra). Am. J. Physiol. Ren. Physiol. 2005, 288, F1103–F1112. [Google Scholar] [CrossRef] [Green Version]
- Cheung, P.W.; Terlouw, A.; Janssen, S.A.; Brown, D.; Bouley, R. Inhibition of non-receptor tyrosine kinase Src induces phosphoserine 256-independent aquaporin-2 membrane accumulation. J. Physiol. 2019, 597, 1627–1642. [Google Scholar] [CrossRef] [PubMed]
- Balasubramanian, L.; Sham, J.S.; Yip, K.P. Calcium signaling in vasopressin-induced aquaporin-2 trafficking. Pflug. Arch. 2008, 456, 747–754. [Google Scholar] [CrossRef]
- Li, S.Z.; McDill, B.W.; Kovach, P.A.; Ding, L.; Go, W.Y.; Ho, S.N.; Chen, F. Calcineurin-NFATc signaling pathway regulates AQP2 expression in response to calcium signals and osmotic stress. Am. J. Physiol. Cell Physiol. 2007, 292, C1606–C1616. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Veeman, M.; Axelrod, J.; Moon, R. A second canon: Functions and mechanisms of beta-catenin-independent Wnt signaling. Dev. Cell 2003, 5, 367–377. [Google Scholar] [CrossRef] [Green Version]
- Ando, F.; Sohara, E.; Morimoto, T.; Yui, N.; Nomura, N.; Kikuchi, E.; Takahashi, D.; Mori, T.; Vandewalle, A.; Rai, T.; et al. Wnt5a induces renal AQP2 expression by activating calcineurin signalling pathway. Nat. Commun. 2016, 7, 13636. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sylvain, N.J.; Salman, M.M.; Pushie, M.J.; Hou, H.; Meher, V.; Herlo, R.; Peeling, L.; Kelly, M.E. The effects of trifluoperazine on brain edema, aquaporin-4 expression and metabolic markers during the acute phase of stroke using photothrombotic mouse model. Biochim. Biophys. Acta Biomembr. 2021, 1863, 183573. [Google Scholar] [CrossRef] [PubMed]
- Salman, M.M.; Kitchen, P.; Iliff, J.J.; Bill, R.M. Aquaporin 4 and glymphatic flow have central roles in brain fluid homeostasis. Nat. Rev. Neurosci. 2021, 22, 650–651. [Google Scholar] [CrossRef]
- Salman, M.M.; Kitchen, P.; Halsey, A.; Wang, M.X.; Tornroth-Horsefield, S.; Conner, A.C.; Badaut, J.; Iliff, J.J.; Bill, R.M. Emerging roles for dynamic aquaporin-4 subcellular relocalization in CNS water homeostasis. Brain 2021, awab311. [Google Scholar] [CrossRef] [PubMed]
- Sands, J.M.; Flores, F.; Kato, A.; Baum, M.A.; Brown, E.M.; Ward, D.T.; Hebert, S.C.; Harris, H.W. Vasopressin-elicited water and urea permeabilities are altered in IMCD in hypercalcemic rats. Am. J. Physiol. 1998, 274, F978–F985. [Google Scholar] [CrossRef] [PubMed]
- Procino, G.; Carmosino, M.; Tamma, G.; Gouraud, S.; Laera, A.; Riccardi, D.; Svelto, M.; Valenti, G. Extracellular calcium antagonizes forskolin-induced aquaporin 2 trafficking in collecting duct cells. Kidney Int. 2004, 66, 2245–2255. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranieri, M.; Tamma, G.; Di Mise, A.; Russo, A.; Centrone, M.; Svelto, M.; Calamita, G.; Valenti, G. Negative feedback from CaSR signaling to aquaporin-2 sensitizes vasopressin to extracellular Ca2. J. Cell Sci. 2015, 128, 2350–2360. [Google Scholar] [CrossRef] [Green Version]
- Ranieri, M. Renal Ca2+ and Water handling in response to calcium sensing receptor signaling: Physiopathological aspects and role of CaSR-regulated microRNAs. Int. J. Mol. Sci. 2019, 20, 5341. [Google Scholar] [CrossRef] [Green Version]
- Noda, Y.; Sasaki, S. The role of actin remodeling in the trafficking of intracellular vesicles, transporters, and channels: Focusing on aquaporin-2. Pflug. Arch. 2008, 456, 737–745. [Google Scholar] [CrossRef]
- Noda, Y.; Sasaki, S. Actin-binding channels. Prog. Brain Res. 2008, 170, 551–557. [Google Scholar]
- Tamma, G.; Wiesner, B.; Furkert, J.; Hahm, D.; Oksche, A.; Schaefer, M.; Valenti, G.; Rosenthal, W.; Klussmann, E. The prostaglandin E2 analogue sulprostone antagonizes vasopressin-induced antidiuresis through activation of Rho. J. Cell Sci. 2003, 116, 3285–3294. [Google Scholar] [CrossRef] [Green Version]
- Tajika, Y.; Matsuzaki, T.; Suzuki, T.; Ablimit, A.; Aoki, T.; Hagiwara, H.; Kuwahara, M.; Sasaki, S.; Takata, K. Differential regulation of AQP2 trafficking in endosomes by microtubules and actin filaments. Histochem. Cell Biol. 2005, 124, 1–12. [Google Scholar] [CrossRef]
- Tamma, G.; Carmosino, M.; Svelto, M.; Valenti, G. Bradykinin signaling counteracts cAMP-elicited aquaporin 2 translocation in renal cells. J. Am. Soc. Nephrol. 2005, 16, 2881–2889. [Google Scholar] [CrossRef] [Green Version]
- Noda, Y.; Horikawa, S.; Furukawa, T.; Hirai, K.; Katayama, Y.; Asai, T.; Kuwahara, M.; Katagiri, K.; Kinashi, T.; Hattori, M.; et al. Aquaporin-2 trafficking is regulated by PDZ-domain containing protein SPA-1. FEBS Lett. 2004, 568, 139–145. [Google Scholar] [CrossRef] [Green Version]
- Noda, Y.; Sasaki, S. Molecular mechanisms and drug development in aquaporin water channel diseases: Molecular mechanism of water channel aquaporin-2 trafficking. J. Pharmacol. Sci. 2004, 96, 249–254. [Google Scholar] [CrossRef] [Green Version]
- Harazaki, M.; Kawai, Y.; Su, L.; Hamazaki, Y.; Nakahata, T.; Minato, N.; Hattori, M. Specific recruitment of SPA-1 to the immunological synapse: Involvement of actin-bundling protein actinin. Immunol. Lett. 2004, 92, 221–226. [Google Scholar] [CrossRef] [Green Version]
- Kometani, K.; Aoki, M.; Kawamata, S.; Shinozuka, Y.; Era, T.; Taniwaki, M.; Hattori, M.; Minato, N. Role of SPA-1 in phenotypes of chronic myelogenous leukemia induced by BCR-ABL-expressing hematopoietic progenitors in a mouse model. Cancer Res. 2006, 66, 9967–9976. [Google Scholar] [CrossRef] [Green Version]
- Kuwahara, M.; Iwai, K.; Ooeda, T.; Igarashi, T.; Ogawa, E.; Katsushima, Y.; Shinbo, I.; Uchida, S.; Terada, Y.; Arthus, M.F.; et al. Three families with autosomal dominant nephrogenic diabetes insipidus caused by aquaporin-2 mutations in the C-terminus. Am. J. Hum. Genet. 2001, 69, 738–748. [Google Scholar] [CrossRef] [Green Version]
- Kuwahara, M.; Asai, T.; Terada, Y.; Sasaki, S. The C-terminal tail of aquaporin-2 determines apical trafficking. Kidney Int. 2005, 68, 1999–2009. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noda, Y.; Horikawa, S.; Katayama, Y.; Sasaki, S. Identification of a multiprotein “motor” complex binding to water channel aquaporin-2. Biochem. Biophys. Res. Commun. 2005, 330, 1041–1047. [Google Scholar] [CrossRef] [PubMed]
- Nedvetsky, P.I.; Stefan, E.; Frische, S.; Santamaria, K.; Wiesner, B.; Valenti, G.; Hammer, J.A., 3rd; Nielsen, S.; Goldenring, J.R.; Rosenthal, W.; et al. A Role of myosin Vb and Rab11-FIP2 in the aquaporin-2 shuttle. Traffic 2007, 8, 110–123. [Google Scholar] [CrossRef]
- Chou, C.L.; Christensen, B.M.; Frische, S.; Vorum, H.; Desai, R.A.; Hoffert, J.D.; de Lanerolle, P.; Nielsen, S.; Knepper, M.A. Non-muscle myosin II and myosin light chain kinase are downstream targets for vasopressin signaling in the renal collecting duct. J. Biol. Chem. 2004, 279, 49026–49035. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Isobe, K.; Raghuram, V.; Krishnan, L.; Chou, C.L.; Yang, C.R.; Knepper, M.A. CRISPR-Cas9/phosphoproteomics identifies multiple noncanonical targets of myosin light chain kinase. Am. J. Physiol. Ren. Physiol. 2020, 318, F600–F616. [Google Scholar] [CrossRef] [PubMed]
- Liu, C.S.; Cheung, P.W.; Dinesh, A.; Baylor, N.; Paunescu, T.C.; Nair, A.V.; Bouley, R.; Brown, D. Actin-related protein 2/3 complex plays a critical role in the aquaporin-2 exocytotic pathway. Am. J. Physiol. Ren. Physiol. 2021, 321, F179–F194. [Google Scholar] [CrossRef] [PubMed]
- Holst, M.R.; Gammelgaard, L.; Aaron, J.; Login, F.H.; Rajkumar, S.; Hahn, U.; Nejsum, L.N. Regulated exocytosis: Renal Aquaporin-2 3D Vesicular Network Organization and Association with F-actin. Am. J. Physiol. Cell Physiol. 2021. [Google Scholar] [CrossRef] [PubMed]
- Brown, D.; Orci, L. Vasopressin stimulates formation of coated pits in rat kidney collecting ducts. Nature 1983, 302, 253–255. [Google Scholar] [CrossRef] [PubMed]
- Sun, T.X.; van Hoek, A.; Huang, Y.; Bouley, R.; McLaughlin, M.; Brown, D. Aquaporin-2 localization in clathrin-coated pits: Inhibition of endocytosis by dominant-negative dynamin. Am. J. Physiol. 2002, 282, F998–F1011. [Google Scholar] [CrossRef] [Green Version]
- Lu, H.; Sun, T.X.; Bouley, R.; Blackburn, K.; McLaughlin, M.; Brown, D. Inhibition of endocytosis causes phosphorylation (S256)-independent plasma membrane accumulation of AQP2. Am. J. Physiol. 2004, 286, F233–F243. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, H.A.; Sun, T.X.; Matsuzaki, T.; Yi, X.H.; Eswara, J.; Bouley, R.; McKee, M.; Brown, D. Heat shock protein 70 interacts with aquaporin-2 (AQP2) and regulates its trafficking. J. Biol. Chem. 2007, 282, 28721–28732. [Google Scholar] [CrossRef] [Green Version]
- Kamsteeg, E.J.; Hendriks, G.; Boone, M.; Konings, I.B.; Oorschot, V.; van der Sluijs, P.; Klumperman, J.; Deen, P.M. Short-chain ubiquitination mediates the regulated endocytosis of the aquaporin-2 water channel. Proc. Natl. Acad. Sci. USA 2006, 103, 18344–18349. [Google Scholar] [CrossRef] [Green Version]
- Wu, Q.; Moeller, H.B.; Stevens, D.A.; Sanchez-Hodge, R.; Childers, G.; Kortenoeven, M.L.A.; Cheng, L.; Rosenbaek, L.L.; Rubel, C.; Patterson, C.; et al. CHIP Regulates Aquaporin-2 Quality Control and Body Water Homeostasis. J. Am. Soc. Nephrol. 2018, 29, 936–948. [Google Scholar] [CrossRef]
- Noda, Y.; Horikawa, S.; Katayama, Y.; Sasaki, S. Water channel aquaporin-2 directly binds to actin. Biochem. Biophys. Res. Commun. 2004, 322, 740. [Google Scholar] [CrossRef]
- Noda, Y.; Sasaki, S. Trafficking mechanism of water channel aquaporin-2. Biol. Cell. 2005, 97, 885–892. [Google Scholar] [CrossRef]
- Noda, Y.; Horikawa, S.; Kanda, E.; Yamashita, M.; Meng, H.; Eto, K.; Li, Y.; Kuwahara, M.; Hirai, K.; Pack, C.; et al. Reciprocal interaction with G-actin and tropomyosin is essential for aquaporin-2 trafficking. J. Cell Biol. 2008, 182, 587–601. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kuwahara, M.; Fushimi, K.; Terada, Y.; Bai, L.; Marumo, F.; Sasaki, S. cAMP-dependent phosphorylation stimulates water permeability of aquaporin-collecting duct water channel protein expressed in Xenopus oocytes. J. Biol. Chem. 1995, 270, 10384–10387. [Google Scholar] [CrossRef] [Green Version]
- Moeller, H.B.; MacAulay, N.; Knepper, M.A.; Fenton, R.A. Role of multiple phosphorylation sites in the COOH-terminal tail of aquaporin-2 for water transport: Evidence against channel gating. Am. J. Physiol Ren. Physiol. 2009, 296, F649–F657. [Google Scholar] [CrossRef] [Green Version]
- Eto, K.; Noda, Y.; Horikawa, S.; Uchida, S.; Sasaki, S. Phosphorylation of aquaporin-2 regulates its water permeability. J. Biol. Chem. 2010, 285, 40777–40784. [Google Scholar] [CrossRef] [Green Version]
- Kasono, K.; Saito, T.; Saito, T.; Tamemoto, H.; Yanagidate, C.; Uchida, S.; Kawakami, M.; Sasaki, S.; Ishikawa, S.E. Hypertonicity regulates the aquaporin-2 promoter independently of arginine vasopressin. Nephrol. Dial. Transplant. 2005, 20, 509–515. [Google Scholar] [CrossRef] [Green Version]
- Saito, T.; Saito, T.; Kasono, K.; Tamemoto, H.; Kawakami, M.; Sasaki, S.; Ishikawa, S.E. Hypotonicity reduces the activity of murine aquaporin-2 promoter induced by dibutyryl cAMP. Exp. Physiol. 2008, 93, 1147–1156. [Google Scholar] [CrossRef] [PubMed]
- Hasler, U.; Nunes, P.; Bouley, R.; Lu, H.A.; Matsuzaki, T.; Brown, D. Acute hypertonicity alters aquaporin-2 trafficking and induces a MAP kinase-dependent accumulation at the plasma membrane of renal epithelial cells. J. Biol. Chem. 2008, 283, 26643–26661. [Google Scholar] [PubMed] [Green Version]
- Van Balkom, B.W.; van Raak, M.; Breton, S.; Pastor-Soler, N.; Bouley, R.; van der Sluijs, P.; Brown, D.; Deen, P.M. Hypertonicity is involved in redirecting the aquaporin-2 water channel into the basolateral, instead of the apical, plasma membrane of renal epithelial cells. J. Biol. Chem. 2003, 278, 1101–1107. [Google Scholar]
- Okada, Y.; Maeno, E.; Shimizu, T.; Dezaki, K.; Wang, J.; Morishima, S. Receptor-mediated control of regulatory volume decrease (RVD) and apoptotic volume decrease (AVD). J. Physiol. 2001, 532, 3–16. [Google Scholar] [CrossRef]
- Tamma, G.; Procino, G.; Strafino, A.; Bononi, E.; Meyer, G.; Paulmichl, M.; Formoso, V.; Svelto, M.; Valenti, G. Hypotonicity induces aquaporin-2 internalization and cytosol-to-membrane translocation of ICln in renal cells. Endocrinology 2007, 148, 1118–1130. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.H.; Eto, K.; Horikawa, S.; Uchida, S.; Sasaki, S.; Li, X.J.; Noda, Y. Aquaporin-2 regulates cell volume recovery via tropomyosin. Int. J. Biochem. Cell Biol. 2009, 41, 2466–2476. [Google Scholar] [CrossRef] [PubMed]
- Savelkoul, P.J.; de Mattia, F.; Li, Y.; Kamsteeg, E.J.; Konings, I.B.; van der Sluijs, P.; Deen, P.M. p.R254Q mutation in the aquaporin-2 water channel causing dominant nephrogenic diabetes insipidus is due to a lack of arginine vasopressin-induced phosphorylation. Hum. Mutat. 2009, 30, E891–E903. [Google Scholar] [CrossRef]
- De Mattia, F.; Savelkoul, P.J.; Kamsteeg, E.J.; Konings, I.B.; van der Sluijs, P.; Mallmann, R.; Oksche, A.; Deen, P.M. Lack of arginine vasopressin-induced phosphorylation of aquaporin-2 mutant AQP2-R254L explains dominant nephrogenic diabetes insipidus. J. Am. Soc. Nephrol. 2005, 16, 2872–2880. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kamsteeg, E.J.; Savelkoul, P.J.; Hendriks, G.; Konings, I.B.; Nivillac, N.M.; Lagendijk, A.K.; van der Sluijs, P.; Deen, P.M. Missorting of the Aquaporin-2 mutant E258K to multivesicular bodies/lysosomes in dominant NDI is associated with its monoubiquitination and increased phosphorylation by PKC but is due to the loss of E258. Pflug. Arch. 2008, 455, 1041–1054. [Google Scholar] [CrossRef] [PubMed]
- Asai, T.; Kuwahara, M.; Kurihara, H.; Sakai, T.; Terada, Y.; Marumo, F.; Sasaki, S. Pathogenesis of nephrogenic diabetes insipidus by aquaporin-2 C-terminus mutations. Kidney Int. 2003, 64, 2–10. [Google Scholar]
- Sohara, E.; Rai, T.; Yang, S.S.; Uchida, K.; Nitta, K.; Horita, S.; Ohno, M.; Harada, A.; Sasaki, S.; Uchida, S. Pathogenesis and treatment of autosomal-dominant nephrogenic diabetes insipidus caused by an aquaporin 2 mutation. Proc. Natl. Acad. Sci. USA 2006, 103, 14217–14222. [Google Scholar] [CrossRef] [Green Version]
- Grünfeld, J.P.; Rossier, B.C. Lithium nephrotoxicity revisited. Nat. Rev. Nephrol. 2009, 5, 270–276. [Google Scholar] [CrossRef]
- Rao, R.; Zhang, M.Z.; Zhao, M.; Cai, H.; Harris, R.C.; Breyer, M.D.; Hao, C.M. Lithium treatment inhibits renal GSK-3 activity and promotes cyclooxygenase 2-dependent polyuria. Am. J. Physiol. Ren. Physiol. 2005, 288, F642–F649. [Google Scholar] [CrossRef] [Green Version]
- Rao, R.; Patel, S.; Hao, C.; Woodgett, J.; Harris, R. GSK3beta mediates renal response to vasopressin by modulating adenylate cyclase activity. J. Am. Soc. Nephrol. 2010, 21, 428–437. [Google Scholar] [CrossRef] [Green Version]
- Christensen, B.M.; Marples, D.; Kim, Y.H.; Wang, W.; Frøkiaer, J.; Nielsen, S. Changes in cellular composition of kidney collecting duct cells in rats with lithium-induced NDI. Am. J. Physiol. Cell Physiol. 2004, 286, C952–C964. [Google Scholar] [CrossRef]
- Khositseth, S.; Uawithya, P.; Somparn, P.; Charngkaew, K.; Thippamom, N.; Hoffert, J.D.; Saeed, F.; Michael Payne, D.; Chen, S.H.; Fenton, R.A.; et al. Autophagic degradation of aquaporin-2 is an early event in hypokalemia-induced nephrogenic diabetes insipidus. Sci. Rep. 2015, 5, 18311. [Google Scholar] [CrossRef] [Green Version]
- Khositseth, S.; Charngkaew, K.; Boonkrai, C.; Somparn, P.; Uawithya, P.; Chomanee, N.; Payne, D.M.; Fenton, R.A.; Pisitkun, T. Hypercalcemia induces targeted autophagic degradation of aquaporin-2 at the onset of nephrogenic diabetes insipidus. Kidney Int. 2017, 91, 1070–1087. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Iervolino, A.; Prosperi, F.; de La Motte, L.R.; Petrillo, F.; Spagnuolo, M.; D’Acierno, M.; Siccardi, S.; Perna, A.F.; Christensen, B.M.; Frische, S.; et al. Potassium depletion induces cellular conversion in the outer medullary collecting duct altering Notch signaling pathway. Sci. Rep. 2020, 10, 5708. [Google Scholar] [CrossRef] [PubMed]
- Somparn, P.; Boonkrai, C.; Charngkaew, K.; Chomanee, N.; Hodge, K.G.; Fenton, R.A.; Pisitkun, T.; Khositseth, S. Bilateral ureteral obstruction is rapidly accompanied by ER stress and activation of autophagic degradation of IMCD proteins, including AQP2. Am. J. Physiol. Ren. Physiol. 2020, 318, F135–F147. [Google Scholar] [CrossRef] [PubMed]
- Schrier, R.W. Vasopressin and aquaporin 2 in clinical disorders of water homeostasis. Semin. Nephrol. 2008, 28, 289–296. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gheorghiade, M.; Gattis, W.A.; O’Connor, C.M.; Adams, K.F.; Elkayam, U., Jr.; Barbagelata, A.; Ghali, J.K.; Benza, R.L.; McGrew, F.A.; Klapholz, M.; et al. Effects of tolvaptan, a vasopressin antagonist, in patients hospitalized with worsening heart failure: A randomized controlled trial. JAMA 2004, 291, 1963–1971. [Google Scholar] [CrossRef] [Green Version]
- Schrier, R.W.; Gross, P.; Gheorghiade, M.; Berl, T.; Verbalis, J.G.; Czerwiec, F.S.; Orlandi, C.; SALT Investigators. Tolvaptan, a selective oral vasopressin V2-receptor antagonist, for hyponatremia. N. Engl. J. Med. 2006, 355, 2099–2112. [Google Scholar] [CrossRef] [Green Version]
- Abraham, W.T.; Shamshirsaz, A.A.; McFann, K.; Oren, R.M.; Schrier, R.W. Aquaretic effect of lixivaptan, an oral non-peptide, selective V2 receptor vasopressin antagonist, in the New York Heart Association functional class II and III chronic heart failure patients. J. Am. Coll. Cardiol. 2006, 47, 1615–1621. [Google Scholar] [CrossRef] [Green Version]
- Gheorghiade, M.; Konstam, M.A.; Burnett, J.C., Jr.; Grinfeld, L.; Maggioni, A.P.; Swedberg, K.; Udelson, J.E.; Zannad, F.; Cook, T.; Ouyang, J.; et al. Short-term clinical effects of tolvaptan, an oral vasopressin antagonist, in patients hospitalized for heart failure: The EVEREST clinical status trials. JAMA 2007, 297, 1332–1343. [Google Scholar] [CrossRef] [Green Version]
- Asahina, Y.; Izumi, N.; Enomoto, N.; Sasaki, S.; Fushimi, K.; Marumo, F.; Sato, C. Increased gene expression of water channel in cirrhotic rat kidneys. Hepatology 1995, 21, 169–173. [Google Scholar] [CrossRef]
- Fukui, H. Do vasopressin V2 receptor antagonists benefit cirrhotics with refractory ascites? World J. Gastroenterol. 2015, 21, 11584–11596. [Google Scholar] [CrossRef]
- Ishikawa, S.E.; Saito, T.; Saito, T.; Kasono, K.; Funayama, H. Pathophysiological role of aquaporin-2 in impaired water excretion. Prog. Brain Res. 2008, 170, 581–588. [Google Scholar]
- Saito, T.; Ishikawa, S.; Abe, K.; Kamoi, K.; Yamada, K.; Shimizu, K.; Saruta, T.; Yoshida, S. Acute aquaresis by the nonpeptide arginine vasopressin (AVP) antagonist OPC-31260 improves hyponatremia in patients with syndrome of inappropriate secretion of antidiuretic hormone (SIADH). J. Clin. Endocrinol. Metab. 1997, 82, 1054–1057. [Google Scholar] [CrossRef]
- Kazama, I.; Hatano, R.; Michimata, M.; Suzuki, K.; Arata, T.; Suzuki, M.; Miyama, N.; Sato, A.; Satomi, S.; Ejima, Y.; et al. BSC1 inhibition complements effects of vasopressin V2 receptor antagonist on hyponatremia in SIADH rats. Kidney Int. 2005, 67, 1855–1867. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kanno, K.; Sasaki, S.; Hirata, Y.; Ishikawa, S.; Fushimi, K.; Nakanishi, S.; Bichet, D.G.; Marumo, F. Urinary excretion of aquaporin-2 in patients with diabetes insipidus. N. Engl. J. Med. 1995, 332, 1540–1545. [Google Scholar] [CrossRef] [PubMed]
- Sasaki, S.; Ohmoto, Y.; Mori, T.; Iwata, F.; Muraguchi, M. Daily variance of urinary excretion of AQP2 determined by sandwich ELISA method. Clin. Exp. Nephrol. 2012, 16, 406–410. [Google Scholar] [CrossRef] [PubMed]
- Pisitkun, T.; Shen, R.F.; Knepper, M.A. Identification and proteomic profiling of exosomes in human urine. Proc. Natl. Acad. Sci. USA 2004, 101, 1338–13373. [Google Scholar] [CrossRef] [Green Version]
- Martin, P.Y.; Abraham, W.T.; Lieming, X.; Olson, B.R.; Oren, R.M.; Ohara, M.; Schrier, R.W. Selective V2-receptor vasopressin antagonism decreases urinary aquaporin-2 excretion in patients with chronic heart failure. J. Am. Soc. Nephrol. 1999, 10, 2165–2170. [Google Scholar] [CrossRef]
- Ivarsen, P.; Frøkiaer, J.; Aagaard, N.K.; Hansen, E.F.; Bendtsen, F.; Nielsen, S.; Vilstrup, H. Increased urinary excretion of aquaporin 2 in patients with liver cirrhosis. Gut 2003, 52, 1194–1199. [Google Scholar] [CrossRef]
- Nakanishi, H.; Kurosaki, M.; Hosokawa, T.; Takahashi, Y.; Itakura, J.; Suzuki, S.; Yasui, Y.; Tamaki, N.; Nakakuki, N.; Takada, H.; et al. Urinary excretion of the water channel aquaporin 2 correlated with the pharmacological effect of tolvaptan in cirrhotic patients with ascites. J. Gastroenterol. 2016, 51, 620–627. [Google Scholar] [CrossRef]
- Ishikawa, S.E.; Saito, T.; Fukagawa, A.; Higashiyama, M.; Nakamura, T.; Kusaka, I.; Nagasaka, S.; Honda, K.; Saito, T. Close association of urinary excretion of aquaporin-2 with appropriate and inappropriate arginine vasopressin-dependent antidiuresis in hyponatremia in elderly subjects. J. Clin. Endocrinol. Metab. 2001, 86, 1665–1671. [Google Scholar]
- Imamura, T.; Kinugawa, K.; Fujino, T.; Inaba, T.; Maki, H.; Hatano, M.; Yao, A.; Komuro, I. Increased urine aquaporin-2 relative to plasma arginine vasopressin is a novel marker of response to tolvaptan in patients with decompensated heart failure. Circ. J. 2014, 78, 2240–2249. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bouley, R.; Lu, H.A.; Nunes, P.; Da Silva, N.; McLaughlin, M.; Chen, Y.; Brown, D. Calcitonin has a vasopressin-like effect on aquaporin-2 trafficking and urinary concentration. J. Am. Soc. Nephrol. 2011, 22, 59–72. [Google Scholar] [CrossRef] [Green Version]
- Procino, G.; Milano, S.; Carmosino, M.; Barbieri, C.; Nicoletti, M.C.; Li, J.H.; Wess, J.; Svelto, M. Combination of secretin and fluvastatin ameliorates the polyuria associated with X-linked nephrogenic diabetes insipidus in mice. Kidney Int. 2014, 86, 127–138. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gao, M.; Cao, R.; Du, S.; Jia, X.; Zheng, S.; Huang, S.; Han, Q.; Liu, J.; Zhang, X.; Miao, Y.; et al. Disruption of prostaglandin E2 receptor EP4 impairs urinary concentration via decreasing aquaporin 2 in renal collecting ducts. Proc. Natl. Acad. Sci. USA 2015, 112, 8397–8402. [Google Scholar] [CrossRef] [Green Version]
- Li, J.H.; Chou, C.L.; Li, B.; Gavrilova, O.; Eisner, C.; Schnermann, J.; Anderson, S.A.; Deng, C.X.; Knepper, M.A.; Wess, J. A selective EP4 PGE2 receptor agonist alleviates disease in a new mouse model of X-linked nephrogenic diabetes insipidus. J. Clin. Investig. 2009, 119, 3115–3126. [Google Scholar] [CrossRef] [Green Version]
- Olesen, E.T.; Rützler, M.R.; Moeller, H.B.; Praetorius, H.A.; Fenton, R.A. Vasopressin-independent targeting of aquaporin-2 by selective E-prostanoid receptor agonists alleviates nephrogenic diabetes insipidus. Proc. Natl. Acad. Sci. USA 2011, 108, 12949–12954. [Google Scholar] [CrossRef] [Green Version]
- Olesen, E.T.; Moeller, H.B.; Assentoft, M.; MacAulay, N.; Fenton, R.A. The vasopressin type 2 receptor and prostaglandin receptors EP2 and EP4 can increase aquaporin-2 plasma membrane targeting through a cAMP-independent pathway. Am. J. Physiol. Renal Physiol. 2016, 311, F935–F944. [Google Scholar] [CrossRef] [Green Version]
- Hinrichs, G.R.; Mortensen, L.A.; Bistrup, C.; Dieperink, H.H.; Jensen, B.L. Treatment of Nephrogenic Diabetes Insipidus Patients with cGMP-Stimulating Drugs Does Not Mitigate Polyuria or Increase Urinary Concentrating Ability. Kidney Int. Rep. 2020, 5, 1319–1325. [Google Scholar] [CrossRef]
- Suga, H.; Nagasaki, H.; Kondo, T.A.; Okajima, Y.; Suzuki, C.; Ozaki, N.; Arima, H.; Yamamoto, T.; Ozaki, N.; Akai, M.; et al. Novel treatment for lithiuminduced nephrogenic diabetes insipidus rat model using the Sendai-virus vector carrying aquaporin 2 gene. Endocrinology 2008, 149, 5803–5810. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Y.; Peti-Peterdi, J.; Müller, C.E.; Carlson, N.G.; Baqi, Y.; Strasburg, D.L.; Heiney, K.M.; Villanueva, K.; Kohan, D.E.; Kishore, B.K. P2Y12 Receptor Localizes in the Renal Collecting Duct and Its Blockade Augments Arginine Vasopressin Action and Alleviates Nephrogenic Diabetes Insipidus. J. Am. Soc. Nephrol. 2015, 26, 2978–2987. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Zhang, Y.; Bouley, R.; Chen, Y.; Matsuzaki, T.; Nunes, P.; Hasler, U.; Brown, D.; Lu, H.A. Simvastatin enhances aquaporin-2 surface expression and urinary concentration in vasopressin-deficient Brattleboro rats through modulation of Rho GTPase. Am. J. Physiol. Ren. Physiol. 2011, 301, F309–F318. [Google Scholar] [CrossRef] [Green Version]
- Procino, G.; Portincasa, P.; Mastrofrancesco, L.; Castorani, L.; Bonfrate, L.; Addabbo, F.; Carmosino, M.; Di Ciaula, A.; Svelto, M. Simvastatin increases AQP2 urinary excretion in hypercholesterolemic patients: A pleiotropic effect of interest for patients with impaired AQP2 trafficking. Clin. Pharmacol. Ther. 2016, 99, 528–537. [Google Scholar] [CrossRef] [PubMed]
- Bech, A.P.; Wetzels, J.F.M.; Nijenhuis, T. Effects of sildenafil, metformin, and simvastatin on ADH-independent urine concentration in healthy volunteers. Physiol. Rep. 2018, 6, e13665. [Google Scholar] [CrossRef]
- Fotso Soh, J.; Beaulieu, S.; Trepiccione, F.; Linnaranta, O.; Torres-Platas, G.; Platt, R.W.; Renaud, S.; Su, C.L.; Mucsi, I.; D’Apolito, L.; et al. A double-blind, randomized, placebo-controlled pilot trial of atorvastatin for nephrogenic diabetes insipidus in lithium users. Bipolar Disord. 2021, 23, 66–75. [Google Scholar] [CrossRef] [PubMed]
- Efe, O.; Klein, J.D.; LaRocque, L.M.; Ren, H.; Sands, J.M. Metformin improves urine concentration in rodents with nephrogenic diabetes insipidus. JCI Insight 2016, 1, e88409. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kim, J.E.; Jung, H.J.; Lee, Y.J.; Kwon, T.H. Vasopressin-regulated miRNAs and AQP2-targeting miRNAs in kidney collecting duct cells. Am. J. Physiol. Ren. Physiol. 2015, 308, F749–F764. [Google Scholar] [CrossRef] [Green Version]
- Ranieri, M.; Zahedi, K.; Tamma, G.; Centrone, M.; Di Mise, A.; Soleimani, M.; Valenti, G. CaSR signaling down-regulates AQP2 expression via a novel microRNA pathway in pendrin and NaCl cotransporter knockout mice. FASEB J. 2018, 32, 2148–2159. [Google Scholar] [CrossRef] [Green Version]
- Petrillo, F.; Iervolino, A.; Angrisano, T.; Jelen, S.; Costanzo, V.; D’Acierno, M.; Cheng, L.; Wu, Q.; Guerriero, I.; Mazzarella, M.C.; et al. Dysregulation of Principal Cell miRNAs Facilitates Epigenetic Regulation of AQP2 and Results in Nephrogenic Diabetes Insipidus. J. Am. Soc. Nephrol. 2021, 32, 1339–1354. [Google Scholar] [CrossRef] [PubMed]
- Bogum, J.; Faust, D.; Zühlke, K.; Eichhorst, J.; Moutty, M.C.; Furkert, J.; Eldahshan, A.; Neuenschwander, M.; von Kries, J.P.; Wiesner, B.; et al. Small-molecule screening identifies modulators of aquaporin-2 trafficking. J. Am. Soc. Nephrol. 2013, 24, 744–758. [Google Scholar] [CrossRef] [Green Version]
- Vukićević, T.; Hinze, C.; Baltzer, S.; Himmerkus, N.; Quintanova, C.; Zühlke, K.; Compton, F.; Ahlborn, R.; Dema, A.; Eichhorst, J.; et al. Fluconazole increases osmotic water transport in renal collecting duct through effects on aquaporin-2 trafficking. J. Am. Soc. Nephrol 2019, 30, 795–810. [Google Scholar] [CrossRef] [PubMed]
- Ando, F.; Mori, S.; Yui, N.; Morimoto, T.; Nomura, N.; Sohara, E.; Rai, T.; Sasaki, S.; Kondo, Y.; Kagechika, H.; et al. AKAPs-PKA disruptors increase AQP2 activity independently of vasopressin in a model of nephrogenic diabetes insipidus. Nat. Commun. 2018, 9, 1411. [Google Scholar] [CrossRef] [PubMed]
- Konstam, M.A.; Gheorghiade, M.; Burnett, J.C., Jr.; Grinfeld, L.; Maggioni, A.P.; Swedberg, K.; Udelson, J.E.; Zannad, F.; Cook, T.; Ouyang, J.; et al. Effects of oral tolvaptan in patients hospitalized for worsening heart failure: The EVEREST outcome Trial. JAMA 2007, 297, 1319–1331. [Google Scholar] [CrossRef]
- Berl, T.; Quittnat-Pelletier, F.; Verbalis, J.G.; Schrier, R.W.; Bichet, D.G.; Ouyang, J.; Czerwiec, F.S.; SALTWATER Investigators. Oral Tolvaptan is safe and effective in chronic hyponatremia. J. Am. Soc. Nephrol. 2010, 21, 705–712. [Google Scholar] [CrossRef] [Green Version]
- Soupart, A.; Gross, P.; Legros, J.J.; Alföldi, S.; Annane, D.; Heshmati, H.M.; Decaux, G. Successful long-term treatment of hyponatremia in syndrome of inappropriate antidiuretic hormone secretion with satavaptan (SR121463B), an orally active nonpeptide vasopressin V2-receptor antagonist. Clin. J. Am. Soc. Nephrol. 2006, 1, 1154–1160. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mondritzki, T.; Mai, T.A.; Vogel, J.; Pook, E.; Wasnaire, P.; Schmeck, C.; Hüser, J.; Dinh, W.; Truebel, H.; Kolkhof, P. Cardiac output improvement by pecavaptan: A novel dual-acting vasopressin V1a/V2 receptor antagonist in experimental heart failure. Eur. J. Heart. Fail. 2021, 23, 743–750. [Google Scholar] [CrossRef]
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Noda, Y.; Sasaki, S. Updates and Perspectives on Aquaporin-2 and Water Balance Disorders. Int. J. Mol. Sci. 2021, 22, 12950. https://doi.org/10.3390/ijms222312950
Noda Y, Sasaki S. Updates and Perspectives on Aquaporin-2 and Water Balance Disorders. International Journal of Molecular Sciences. 2021; 22(23):12950. https://doi.org/10.3390/ijms222312950
Chicago/Turabian StyleNoda, Yumi, and Sei Sasaki. 2021. "Updates and Perspectives on Aquaporin-2 and Water Balance Disorders" International Journal of Molecular Sciences 22, no. 23: 12950. https://doi.org/10.3390/ijms222312950
APA StyleNoda, Y., & Sasaki, S. (2021). Updates and Perspectives on Aquaporin-2 and Water Balance Disorders. International Journal of Molecular Sciences, 22(23), 12950. https://doi.org/10.3390/ijms222312950





