Transcriptional Responses and GCMS Analysis for the Biosynthesis of Pyrethrins and Volatile Terpenes in Tanacetum coccineum
Abstract
:1. Introduction
2. Results
2.1. Transcriptome Sequencing and de novo Assembly of T. coccineum
2.2. Functional Annotation of Unigenes
2.3. Differentially Expressed Gene (DEG) Analysis
2.4. GO and KEGG Enrichment Analysis of DEGs
2.5. DEGs Involved in the Terpenoid Biosynthesis Pathway
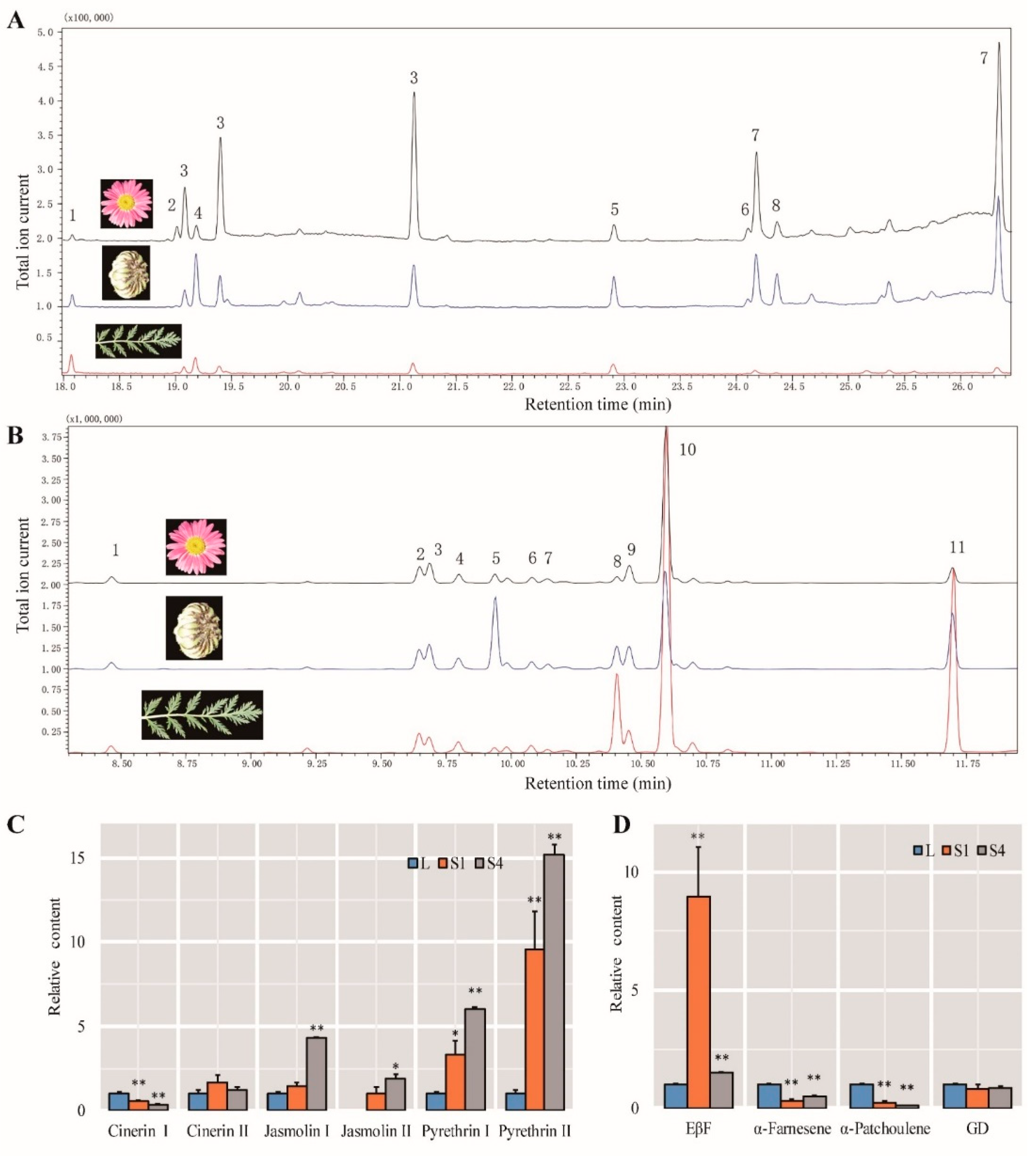
2.6. DEGs Involved in Pyrethrin Biosynthetic Pathways
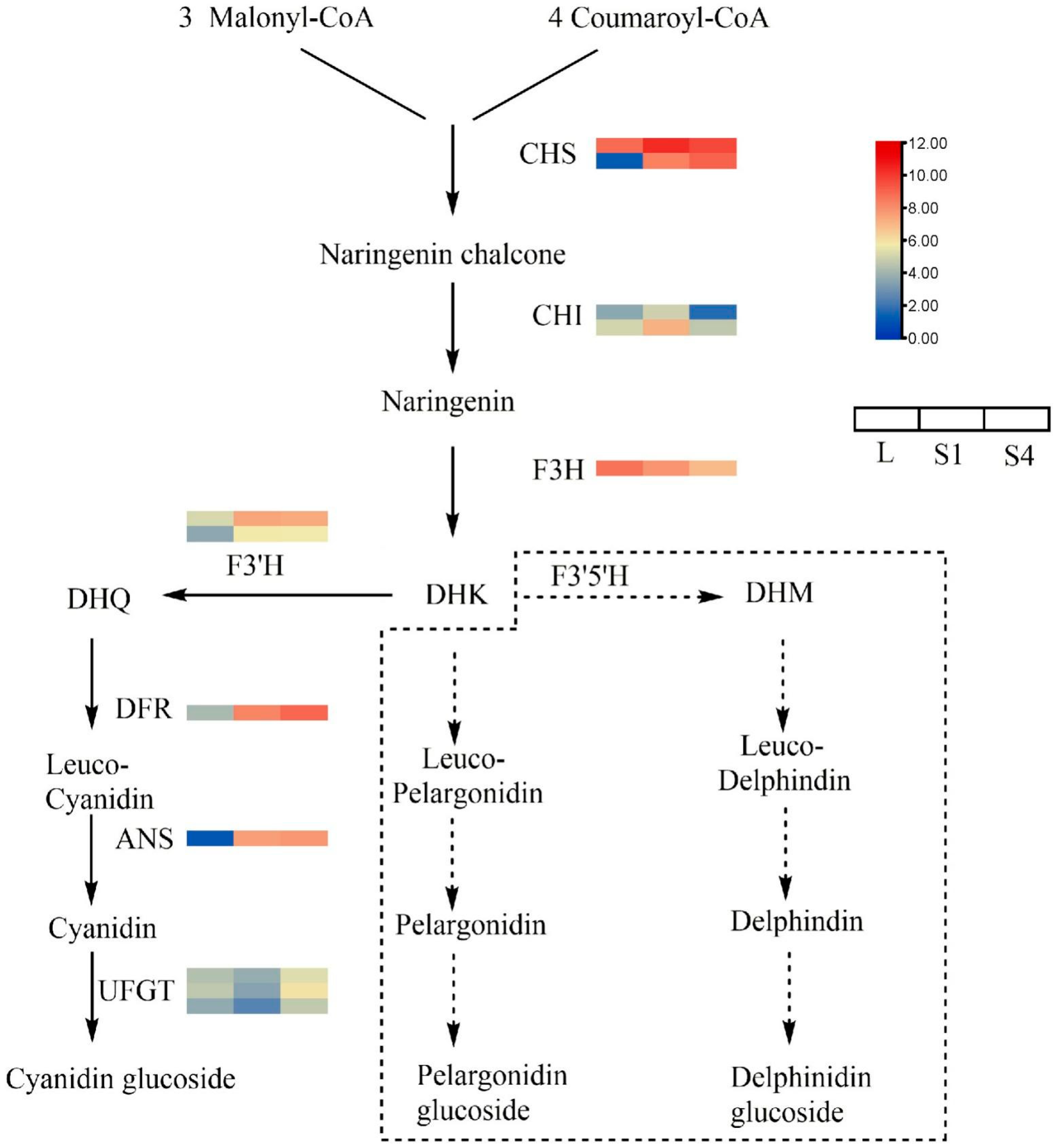
2.7. DEGs Involved in Anthocyanin Biosynthetic Pathways
2.8. qRT-PCR Validation of DEGs
2.9. Identification of Compounds
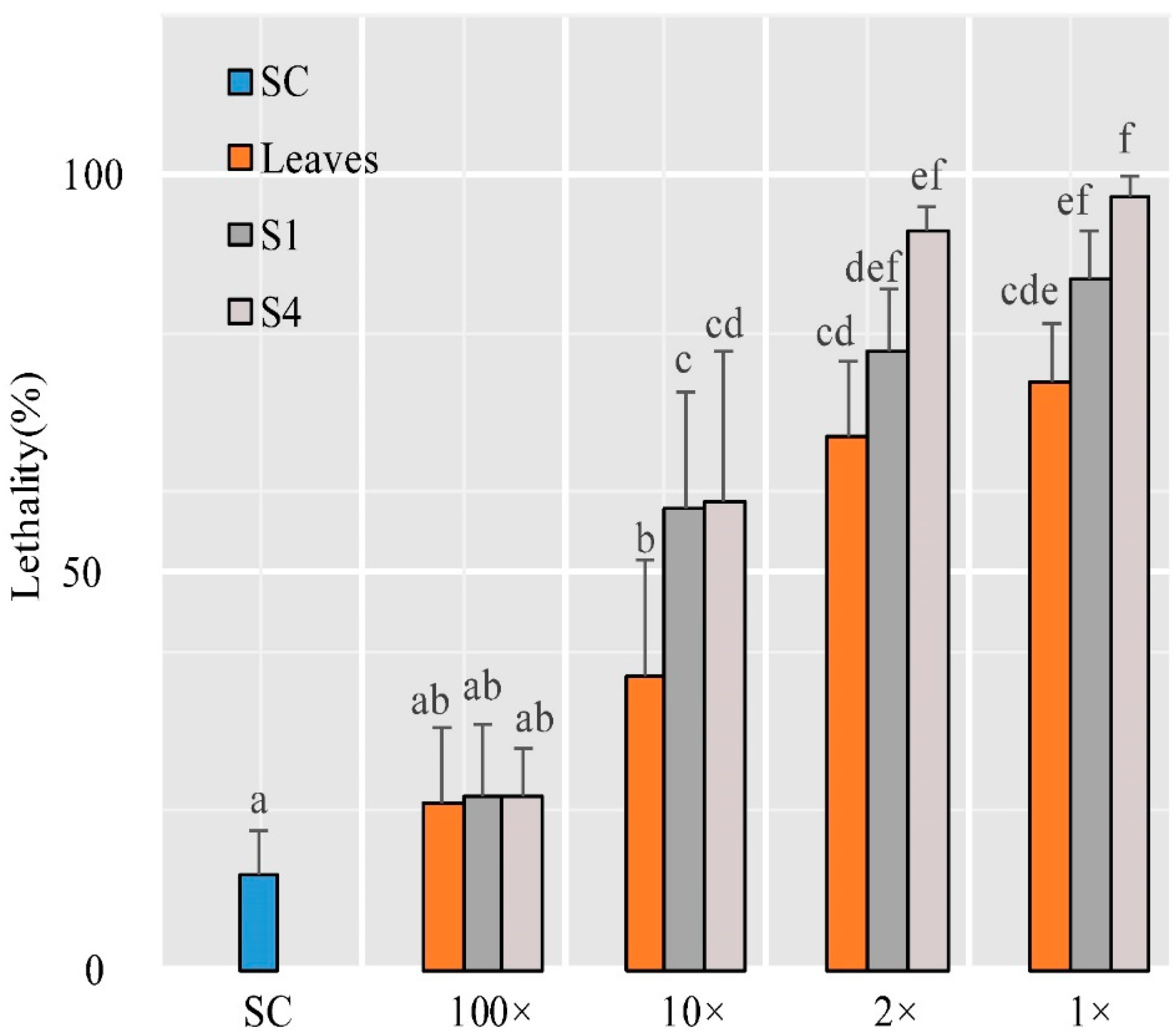
2.10. Insect Test
3. Discussion
4. Materials and Methods
4.1. Plant and Insect Materials
4.2. RNA Isolation, cDNA Library Construction, and RNA Sequencing
4.3. De novo Assembly and Clustering
4.4. Sequence Annotation and Functional Characterization
4.5. Differential Expression Analysis
4.6. Quantitative Real-Time PCR (qRT-PCR) Analysis
4.7. Gas Chromatography/Mass Spectrometry Analysis
4.8. Insect Test
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Salamci, E.; Kordali, S.; Kotan, R.; Cakir, A.; Kaya, Y. Chemical compositions, antimicrobial and herbicidal effects of essential oils isolated from Turkish Tanacetum aucheranum and Tanacetum chiliophyllum var. chiliophyllum. Biochem. Syst. Ecol. 2007, 35, 569–581. [Google Scholar] [CrossRef]
- Baczek, K.B.; Kosakowska, O.; Przybyl, J.; Pioro-Jabrucka, E.; Costa, R.; Mondello, L.; Gniewosz, M.; Synowiec, A.; Weglarz, Z. Antibacterial and antioxidant activity of essential oils and extracts from costmary (Tanacetum balsamita L.) and tansy (Tanacetum vulgare L.). Ind. Crop. Prod. 2017, 102, 154–163. [Google Scholar] [CrossRef]
- Mossa, A.H.; Mohafrash, S.M.M.; Chandrasekaran, N. Safety of natural insecticides: Toxic effects on experimental animals. BioMed Res. Int. 2018, 2018, 4308054. [Google Scholar] [CrossRef] [Green Version]
- Lybrand, D.B.; Xu, H.; Last, R.L.; Pichersky, E. How plants synthesize pyrethrins: Safe and biodegradable insecticides. Trends Plant Sci. 2020, 25, 1240–1251. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Zhou, F.; Pichersky, E. Jasmone hydroxylase, a key enzyme in the synthesis of the alcohol moiety of pyrethrin insecticides. Plant Physiol. 2018, 177, 1498–1509. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, W.; Lybrand, D.B.; Zhou, F.; Last, R.L.; Pichersky, E. Pyrethrin biosynthesis: The cytochrome P450 oxidoreductase CYP82Q3 converts jasmolone to pyrethrolone. Plant Physiol. 2019, 181, 934–944. [Google Scholar] [CrossRef] [Green Version]
- Rivera, S.B.; Swedlund, B.D.; King, G.J.; Bell, R.N.; Hussey, C.E.; Shattuck-Eidens, D.M.; Wrobel, W.M.; Peiser, G.D.; Poulter, C.D. Chrysanthemyl diphosphate synthase: Isolation of the gene and characterization of the recombinant non-head-to-tail monoterpene synthase from Chrysanthemum cinerariaefolium. Proc. Natl. Acad. Sci. USA 2001, 98, 4373–4378. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hu, H.; Li, J.; Delatte, T.; Vervoort, J.; Gao, L.; Verstappen, F.; Xiong, W.; Gan, J.; Jongsma, M.A.; Wang, C. Modification of chrysanthemum odour and taste with chrysanthemol synthase induces strong dual resistance against cotton aphids. Plant Biotechnol. J. 2018, 16, 1434–1445. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Thulasiram, H.V.; Erickson, H.K.; Poulter, C.D. Chimeras of two isoprenoid synthases catalyze all four coupling reactions in isoprenoid biosynthesis. Science 2007, 316, 73–76. [Google Scholar] [CrossRef] [PubMed]
- Xu, H.; Moghe, G.D.; Wiegert-Rininger, K.; Schilmiller, A.L.; Barry, C.S.; Last, R.L.; Pichersky, E. Coexpression analysis identifies two oxidoreductases involved in the biosynthesis of the monoterpene acid moiety of natural pyrethrin insecticides in Tanacetum cinerariifolium. Plant Physiol. 2018, 176, 524–537. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xu, H.; Li, W.; Schilmiller, A.L.; van Eekelen, H.; de Vos, R.C.H.; Jongsma, M.A.; Pichersky, E. Pyrethric acid of natural pyrethrin insecticide: Complete pathway elucidation and reconstitution in Nicotiana benthamiana. New Phytol. 2019, 223, 751–765. [Google Scholar] [CrossRef] [PubMed]
- Kikuta, Y.; Ueda, H.; Takahashi, M.; Mitsumori, T.; Yamada, G.; Sakamori, K.; Takeda, K.; Furutani, S.; Nakayama, K.; Katsuda, Y.; et al. Identification and characterization of a GDSL lipase-like protein that catalyzes the ester-forming reaction for pyrethrin biosynthesis in Tanacetum cinerariifolium—A new target for plant protection. Plant J. 2012, 71, 183–193. [Google Scholar] [CrossRef] [PubMed]
- Katsuda, Y. Development of and future prospects for pyrethroid chemistry. Pestic. Sci. 1999, 55, 775–782. [Google Scholar] [CrossRef]
- Bhat, B.M.R.C. Scanning electron microscopic study of oil glands in pyrethrum flowers. Pyrethrum. Post 1975, 15, 11–15. [Google Scholar]
- Ramirez, A.M. Pyrethrum Secondary Metabolism: Biosynthesis, Localization and Ecology of Defense Compounds; Wageningen University: Wageningen, The Netherlands, 2013. [Google Scholar]
- Vosteen, I.; Weisser, W.W.; Kunert, G. Is there any evidence that aphid alarm pheromones work as prey and host finding kairomones for natural enemies? Ecol. Entomol. 2016, 41, 1–12. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Hu, H.; Mao, J.; Yu, L.; Stoopen, G.; Wang, M.; Mumm, R.; de Ruijter, N.C.A.; Dicke, M.; Jongsma, M.A.; et al. Defense of pyrethrum flowers: Repelling herbivores and recruiting carnivores by producing aphid alarm pheromone. New Phytol. 2019, 223, 1607–1620. [Google Scholar] [CrossRef]
- Almohamad, R.; Verheggen, F.J.; Francis, F.; Haubruge, E. Predatory hoverflies select their oviposition site according to aphid host plant and aphid species. Entomol. Exp. Appl. 2007, 125, 13–21. [Google Scholar] [CrossRef] [Green Version]
- Liu, F.; Wang, Q.; Xu, P.; Andreazza, F.; Valbon, W.R.; Bandason, E.; Chen, M.; Yan, R.; Feng, B.; Smith, L.B.; et al. A dual-target molecular mechanism of pyrethrum repellency against mosquitoes. Nat. Commun. 2021, 12, 2553. [Google Scholar] [CrossRef] [PubMed]
- Nieuwenhuizen, N.J.; Green, S.; Atkinson, R.G. Floral sesquiterpenes and their synthesis in dioecious kiwifruit. Plant Signal. Behav. 2010, 5, 61–63. [Google Scholar] [CrossRef] [Green Version]
- Brewer, J.G. Incompatibility relationship in pyrethrum (Chrysanthemum cinerariaefolium vis). Euphytica 1974, 23, 45–47. [Google Scholar] [CrossRef]
- Raguso, R.A. Wake up and smell the roses: The ecology and evolution of floral scent. Annu. Rev. Ecol. Evol. Syst. 2008, 39, 549–569. [Google Scholar] [CrossRef]
- Willmer, P. Pollination and Floral Ecology; Princeton University Press: Princeton, NJ, USA, 2011. [Google Scholar]
- Li, J.; Hu, H.; Chen, Y.; Xie, J.; Li, J.; Zeng, T.; Wang, M.; Luo, J.; Zheng, R.; Jongsma, M.A.; et al. Tissue specificity of (E)-beta-farnesene and germacrene D accumulation in pyrethrum flowers. Phytochemistry 2021, 187, 112768. [Google Scholar] [CrossRef] [PubMed]
- Contant, R.B. Pyrethrum: Chrysanthemum spp. (Compositae); Evolution of Crop Plants; Simmonds, N.W., Ed.; Longman: London, UK; New York, NY, USA, 1976. [Google Scholar]
- Pavela, R. History, presence and perspective of using plant extracts as commercial botanical insecticides and farm products for protection against insects–a review. Plant Prot. Sci. 2016, 52, 229–241. [Google Scholar]
- Dolinsek, J.A.; Kovac, M.; Zel, J.; Camloh, M. Pyrethrum (Tanacetum cinerariifolium) from the northern adriatic as a potential source of natural insecticide. Ann. Ser. Hist. Nat. 2007, 17, 39–46. [Google Scholar]
- Kobayashi, T.; Tabuchi, T. Anatomical and histochemical characteristics of leaves and flowers of Tanacetum cinerariifolium and Tanacetum coccineum. Acta Hortic. 2017, 1169, 157–164. [Google Scholar] [CrossRef]
- Zeng, T.; Li, J.W.; Wang, C.Y.; He, J.F. The complete chloroplast genome of Tanacetum coccineum. Mitochondrial DNA B 2020, 5, 2233–2234. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Xia, X.L.; Jiang, J.F.; Chen, S.M.; Chen, F.D.; Lv, G.S. Salicylic acid-induced changes in physiological parameters and genes of the flavonoid biosynthesis pathway in Artemisia vulgaris and Dendranthema nankingense during aphid feeding. Genet. Mol. Res. 2016, 15, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Lim, S.H.; Park, B.; Kim, D.H.; Park, S.; Yang, J.H.; Jung, J.A.; Lee, J.; Lee, J.Y. Cloning and functional characterization of dihydroflavonol 4-reductase gene involved in anthocyanin biosynthesis of Chrysanthemum. Int. J. Mol. Sci. 2020, 21, 7960. [Google Scholar] [CrossRef]
- Puangkrit, T.; Narumi-Kawasaki, T.; Takamura, T.; Fukai, S. Isolation and analysis of the key enzyme genes in the flavonoid biosynthesis pathway in Chrysanthemum. Acta Hortic. 2018, 1208, 53–59. [Google Scholar] [CrossRef]
- Ramirez, A.M.; Stoopen, G.; Menzel, T.R.; Gols, R.; Bouwmeester, H.J.; Dicke, M.; Jongsma, M.A. Bidirectional secretions from glandular trichomes of pyrethrum enable immunization of seedlings. Plant Cell 2012, 24, 4252–4265. [Google Scholar] [CrossRef] [Green Version]
- Matsuda, K.; Kikuta, Y.; Haba, A.; Nakayama, K.; Katsuda, Y.; Hatanaka, A.; Komai, K. Biosynthesis of pyrethrin I in seedlings of Chrysanthemum cinerariaefolium. Phytochemistry 2005, 66, 1529–1535. [Google Scholar] [CrossRef] [PubMed]
- Umlauf, D.; Zapp, J.; Becker, H.; Adam, K.P. Biosynthesis of the irregular monoterpene artemisia ketone, the sesquiterpene germacrene D and other isoprenoids in Tanacetum vulgare L. (Asteraceae). Phytochemistry 2004, 65, 2463–2470. [Google Scholar] [CrossRef] [PubMed]
- Lv, Z.; Zhang, F.; Pan, Q.; Fu, X.; Jiang, W.; Shen, Q.; Yan, T.; Shi, P.; Lu, X.; Sun, X.; et al. Branch pathway blocking in Artemisia annua is a useful method for obtaining high yield artemisinin. Plant Cell Physiol. 2016, 57, 588–602. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jacobsen, D.J.; Raguso, R.A. Lingering effects of herbivory and plant defenses on pollinators. Curr. Biol. 2018, 28, R1164–R1169. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Turlings, T.C.J.; Erb, M. Tritrophic interactions mediated by herbivore-induced plant volatiles: Mechanisms, ecological relevance, and application potential. Annual review of entomology. Annu. Rev. Entomol. 2018, 63, 433–452. [Google Scholar] [CrossRef] [PubMed]
- Wester, P.; Lunau, K. Plant-pollinator communication. In How Plants Communicate with Their Biotic Environment; Becard, G., Ed.; Academic Press Ltd.-Elsevier Science Ltd.: London, UK, 2017; Volume 82, pp. 225–257. [Google Scholar]
- Chen, S.F.; Zhou, Y.Q.; Chen, Y.R.; Gu, J. Fastp: An ultra-fast all-in-one FASTQ preprocessor. Bioinformatics 2018, 34, 884–890. [Google Scholar] [CrossRef]
- Grabherr, M.G.; Haas, B.J.; Yassour, M.; Levin, J.Z.; Thompson, D.A.; Amit, I.; Adiconis, X.; Fan, L.; Raychowdhury, R.; Zeng, Q.; et al. Full-length transcriptome assembly from RNA-Seq data without a reference genome. Nat. Biotechnol. 2011, 29, 644–652. [Google Scholar] [CrossRef] [Green Version]
- Simão, F.A.; Waterhouse, R.M.; Ioannidis, P.; Kriventseva, E.V. BUSCO: Assessing genome assembly and annotation completeness with single-copy orthologs. Bioinformatics 2015, 19, 3210–3212. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Langmead, B.; Salzberg, S.L. Fast gapped-read alignment with Bowtie 2. Nat. Methods 2012, 9, 357–359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, B.; Dewey, C.N. RSEM: Accurate transcript quantification from RNA-Seq data with or without a reference genome. BMC Bioinform. 2011, 12, 16. [Google Scholar] [CrossRef] [Green Version]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; Van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Anders, S.; Huber, W. Differential expression analysis for sequence count data. Genome Biol. 2010, 11, R106. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yu, G.; Wang, L.G.; Han, Y.; He, Q.Y. ClusterProfiler: An R package for comparing biological themes among gene clusters. OMICS 2012, 16, 284–287. [Google Scholar] [CrossRef] [PubMed]
- Chen, C.; Chen, H.; Zhang, Y.; Thomas, H.R.; Frank, M.H.; He, Y.; Xia, R. TBtools: An integrative toolkit developed for interactive analyses of big biological data. Mol. Plant. 2020, 13, 1194–1202. [Google Scholar] [CrossRef] [PubMed]
- Livak, K.J.; Schmittgen, T.D. Analysis of relative gene expression data using real-time quantitative PCR and the 2−ΔΔCT method. Methods 2001, 408, 402–408. [Google Scholar] [CrossRef] [PubMed]
- Li, J.; Jongsma, M.A.; Wang, C.Y. Comparative analysis of pyrethrin content improvement by mass selection, family selection and polycross in pyrethrum [Tanacetum cinerariifolium (Trevir.) Sch.Bip.] populations. Ind. Crop. Prod. 2014, 53, 268–273. [Google Scholar] [CrossRef]





Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zeng, T.; Li, J.-W.; Zhou, L.; Xu, Z.-Z.; Li, J.-J.; Hu, H.; Luo, J.; Zheng, R.-R.; Wang, Y.-Y.; Wang, C.-Y. Transcriptional Responses and GCMS Analysis for the Biosynthesis of Pyrethrins and Volatile Terpenes in Tanacetum coccineum. Int. J. Mol. Sci. 2021, 22, 13005. https://doi.org/10.3390/ijms222313005
Zeng T, Li J-W, Zhou L, Xu Z-Z, Li J-J, Hu H, Luo J, Zheng R-R, Wang Y-Y, Wang C-Y. Transcriptional Responses and GCMS Analysis for the Biosynthesis of Pyrethrins and Volatile Terpenes in Tanacetum coccineum. International Journal of Molecular Sciences. 2021; 22(23):13005. https://doi.org/10.3390/ijms222313005
Chicago/Turabian StyleZeng, Tuo, Jia-Wen Li, Li Zhou, Zhi-Zhuo Xu, Jin-Jin Li, Hao Hu, Jing Luo, Ri-Ru Zheng, Yuan-Yuan Wang, and Cai-Yun Wang. 2021. "Transcriptional Responses and GCMS Analysis for the Biosynthesis of Pyrethrins and Volatile Terpenes in Tanacetum coccineum" International Journal of Molecular Sciences 22, no. 23: 13005. https://doi.org/10.3390/ijms222313005
APA StyleZeng, T., Li, J.-W., Zhou, L., Xu, Z.-Z., Li, J.-J., Hu, H., Luo, J., Zheng, R.-R., Wang, Y.-Y., & Wang, C.-Y. (2021). Transcriptional Responses and GCMS Analysis for the Biosynthesis of Pyrethrins and Volatile Terpenes in Tanacetum coccineum. International Journal of Molecular Sciences, 22(23), 13005. https://doi.org/10.3390/ijms222313005






