Practical Considerations of Dissolved Oxygen Levels for Platelet Function under Hypoxia
Abstract
:1. Introduction
2. Results
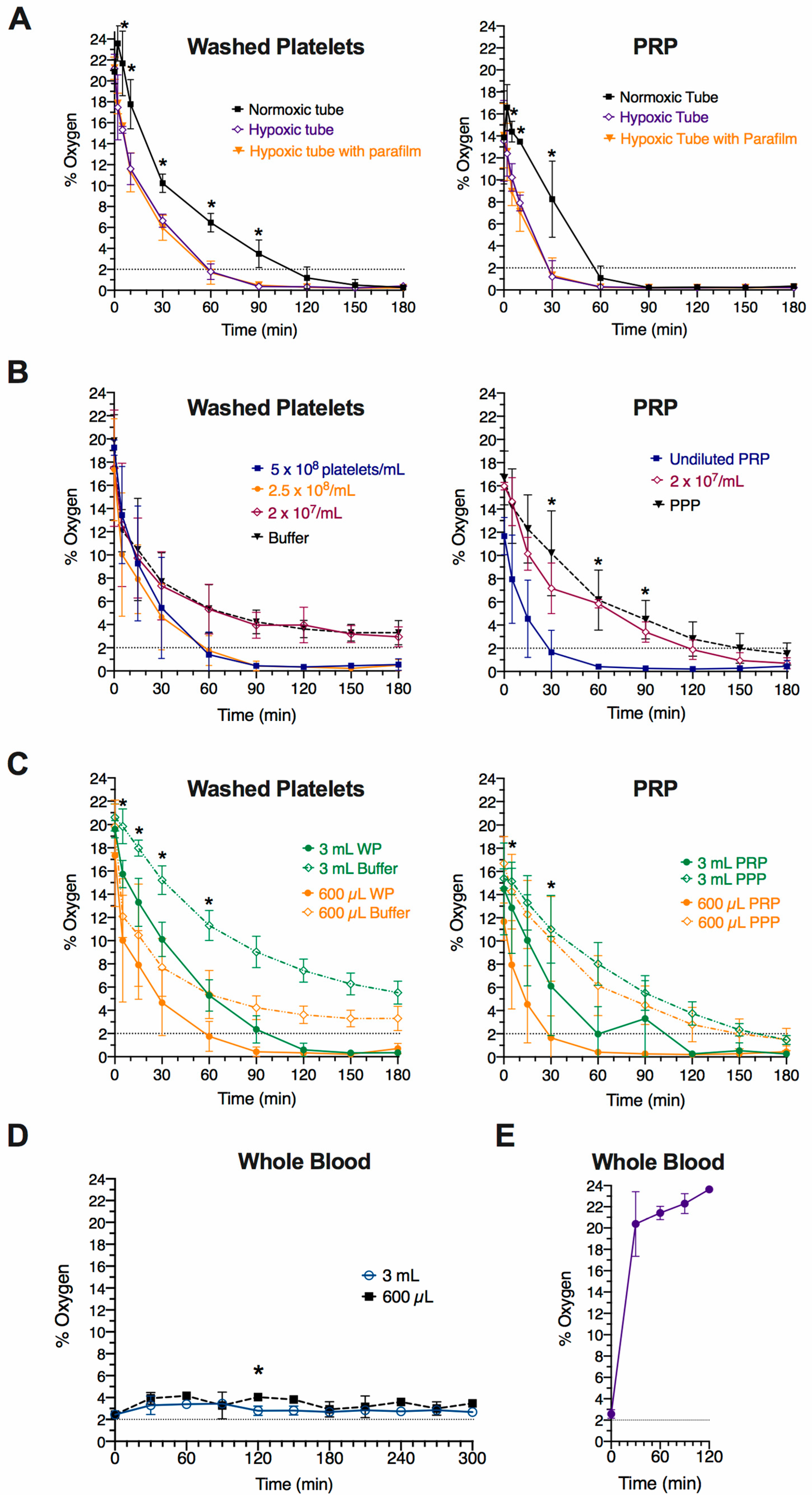
2.1. Effect of Tubes on Oxygen Level Changes in Platelet Suspensions Exposed to 2% Oxygen
2.2. Effect of Platelet Concentration and Volume on Oxygen Level Changes in Platelet Suspensions Expose to 2% Oxygen
2.3. Changes in Oxygen Levels on Whole Blood Samples Exposed to 2% Oxygen
2.4. Changes in Oxygen Levels in Platelet Suspensions Kept at Atmospheric Conditions
2.5. Re-Oxygenation of Hypoxia-Treated Platelet Suspensions upon Exposure to Atmospheric Conditions
2.6. Platelet Spreading on Fibrinogen at Atmospheric and 2% Oxygen
3. Discussion
4. Materials and Methods
4.1. Reagents
4.2. Human Samples and Ethical Considerations
4.3. Platelet Preparation and Hypoxia Incubation
4.4. Measurement of Dissolved Oxygen in Cell Suspensions
4.5. Platelet Spreading Assay
4.6. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Rocke, A.; Paterson, G.; Barber, M.; Jackson, A.; Main, S.; Stannett, C.; Schnopp, M.; Baillie, J.; Horne, E.; Moores, C.; et al. Thromboelastometry and platelet function during acclimatization to high altitude. Thromb. Haemost. 2018, 118, 63–71. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gupta, N.; Ashraf, M. Exposure to high altitude: A risk factor for venous thromboembolism? Semin. Thromb. Hemost. 2012, 38, 156–163. [Google Scholar] [CrossRef]
- Jha, S.K.; Anand, A.C.; Sharma, V.; Kumar, N.; Adya, C.M. Stroke at high altitude: Indian experience. High Alt. Med. Biol. 2002, 3, 21–27. [Google Scholar] [CrossRef] [PubMed]
- Anand, A.C.; Jha, S.K.; Saha, A.; Sharma, V.; Adya, C.M. Thrombosis as a complication of extended stay at high altitude. Natl. Med. J. India 2001, 4, 197–201. [Google Scholar]
- Tyagi, T.; Ahmad, S.; Gupta, N.; Sahu, A.; Ahmad, Y.; Nair, V.; Chatterjee, T.; Bajaj, N.; Sengupta, S.; Ganju, L.; et al. Altered expression of platelet proteins and calpain activity mediate hypoxia-induced prothrombotic phenotype. Blood 2014, 123, 1250–1260. [Google Scholar] [CrossRef] [Green Version]
- Shang, C.; Wuren, T.; Ga, Q.; Bai, Z.; Guo, L.; Eustes, A.S.; McComas, K.N.; Rondina, M.T.; Ge, R. The human platelet transcriptome and proteome is altered and pro-thrombotic functional responses are increased during prolonged hypoxia exposure at high altitude. Platelets 2019, 31, 33–42. [Google Scholar] [CrossRef] [PubMed]
- Paterson, G.; Young, J.; Willson, J.; Graham, C.; Dru, R.; Lee, E.; Torpey, G.; Walmsley, S.; Chan, M.; Warner, T.; et al. hypoxia modulates platelet purinergic signalling pathways. Thromb. Haemost. 2019, 120, 253–261. [Google Scholar] [CrossRef]
- Oga, T.; Chin, K.; Tabuchi, A.; Kawato, M.; Morimoto, T.; Takahashi, K.; Handa, T.; Takahashi, K.; Taniguchi, R.; Kondo, H.; et al. Effects of obstructive sleep apnea with intermittent hypoxia on platelet aggregability. J. Atheroscler. Thromb. 2010, 16, 862–869. [Google Scholar] [CrossRef] [Green Version]
- Sánchez-de-la-Torre, M.; Campos-Rodriguez, F.; Barbé, F. Obstructive sleep apnoea and cardiovascular disease. Lancet Respir. Med. 2013, 1, 61–72. [Google Scholar] [CrossRef]
- Marin, J.M.; Carrizo, S.J.; Vicente, E.; Agusti, A.G. Long-term cardiovascular outcomes in men with obstructive sleep apnoea-hypopnoea with or without treatment with continuous positive airway pressure: An observational study. Lancet 2005, 365, 1046–1053. [Google Scholar] [CrossRef]
- Wedzicha, J.A.; Syndercombe-Court, D.; Tan, K.C. Increased platelet aggregate formation in patients with chronic airflow obstruction and hypoxaemia. Thorax 1991, 46, 504. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Curkendall, S.M.; de Luise, C.; Jones, J.K.; Lanes, S.; Stang, M.R.; Goehring, E.; She, D. Cardiovascular disease in patients with chronic obstructive pulmonary disease, saskatchewan canada cardiovascular disease in COPD Patients. Ann. Epidemiol. 2006, 16, 63–70. [Google Scholar] [CrossRef] [PubMed]
- Feary, J.R.; Rodrigues, L.C.; Smith, C.J.; Hubbard, R.B.; Gibson, J.E. Prevalence of major comorbidities in subjects with COPD and incidence of myocardial infarction and stroke: A comprehensive analysis using data from primary care. Thorax 2010, 65, 956. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Delaney, C.; Davizon-Castillo, P.; Allawzi, A.; Posey, J.; Gandjeva, A.; Neeves, K.; Tuder, R.M.; Paola, J.D.; Stenmark, K.R.; Nozik, E.S. Platelet activation contributes to hypoxia-induced inflammation. Am. J. Physiol. Lung C 2021, 320, L413–L421. [Google Scholar] [CrossRef]
- Cameron, S.J.; Mix, D.S.; Ture, S.K.; Schmidt, R.A.; Mohan, A.; Pariser, D.; Stoner, M.C.; Shah, P.; Chen, L.; Zhang, H.; et al. Hypoxia and ischemia promote a maladaptive platelet phenotype. Arterioscler. Thromb. Vasc. Biol. 2018, 38, 1594–1606. [Google Scholar] [CrossRef]
- Kiouptsi, K.; Gambaryan, S.; Walter, E.; Walter, U.; Jurk, K.; Reinhardt, C. Hypoxia impairs agonist-induced integrin αiibβ3 activation and platelet aggregation. Sci. Rep. 2017, 7, 7621. [Google Scholar] [CrossRef]
- Chaurasia, S.N.; Kushwaha, G.; Kulkarni, P.P.; Mallick, R.L.; Latheef, N.A.; Mishra, J.K.; Dash, D. Platelet HIF-2α promotes thrombogenicity through PAI-1 synthesis and extracellular vesicle release. Haematologica 2019, 104, 2482–2492. [Google Scholar] [CrossRef]
- Zhang, K.; Zhao, T.; Huang, X.; He, Y.; Zhou, Y.; Wu, L.; Wu, K.; Fan, M.; Zhu, L. Dissolved oxygen concentration in the medium during cell culture: Defects and improvements. Cell Biol. Int. 2016, 40, 354–360. [Google Scholar] [CrossRef]
- Place, T.L.; Domann, F.E.; Case, A.J. Limitations of oxygen delivery to cells in culture: An underappreciated problem in basic and translational research. Free Radic. Biol. Med. 2017, 113, 311–322. [Google Scholar] [CrossRef] [PubMed]
- Allen, C.B.; Schneider, B.K.; White, C.W. Limitations to oxygen diffusion and equilibration in in vitro cell exposure systems in hyperoxia and hypoxia. Am. J. Physiol. Lung C 2001, 281, L1021–L1027. [Google Scholar] [CrossRef] [PubMed]
- Newby, D.; Marks, L.; Lyall, F. Dissolved oxygen concentration in culture medium: Assumptions and pitfalls. Placenta 2005, 26, 353–357. [Google Scholar] [CrossRef] [PubMed]
- Ellsworth, M.L.; Forrester, T.; Ellis, C.G.; Dietrich, H.H. The erythrocyte as a regulator of vascular tone. Am. J. Physiol. 1995, 6, H2155–H2161. [Google Scholar] [CrossRef]
- Dietrich, H.H.; Ellsworth, M.L.; Sprague, R.S.; Dacey, R.G. Red blood cell regulation of microvascular tone through adenosine triphosphate. Am. J. Physiol. Heart C 2000, 278, H1294–H1298. [Google Scholar] [CrossRef] [PubMed]
- Klatt, C.; Krüger, I.; Zey, S.; Krott, K.-J.; Spelleken, M.; Gowert, N.S.; Oberhuber, A.; Pfaff, L.; Lückstädt, W.; Jurk, K.; et al. Platelet-RBC interaction mediated by FasL-FasR induces procoagulant activity important for thrombosis. J. Clin. Investig. 2018, 128, 3906–3925. [Google Scholar] [CrossRef] [PubMed]


Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Kusanto, B.; Gordon, A.; Naylor-Adamson, L.; Atkinson, L.; Coupland, C.; Booth, Z.; Ahmed, Y.; Pires, I.M.; Stasiuk, G.J.; Sturmey, R.; et al. Practical Considerations of Dissolved Oxygen Levels for Platelet Function under Hypoxia. Int. J. Mol. Sci. 2021, 22, 13223. https://doi.org/10.3390/ijms222413223
Kusanto B, Gordon A, Naylor-Adamson L, Atkinson L, Coupland C, Booth Z, Ahmed Y, Pires IM, Stasiuk GJ, Sturmey R, et al. Practical Considerations of Dissolved Oxygen Levels for Platelet Function under Hypoxia. International Journal of Molecular Sciences. 2021; 22(24):13223. https://doi.org/10.3390/ijms222413223
Chicago/Turabian StyleKusanto, Branden, Andrew Gordon, Leigh Naylor-Adamson, Lloyd Atkinson, Charlie Coupland, Zoe Booth, Yusra Ahmed, Isabel M. Pires, Graeme J. Stasiuk, Roger Sturmey, and et al. 2021. "Practical Considerations of Dissolved Oxygen Levels for Platelet Function under Hypoxia" International Journal of Molecular Sciences 22, no. 24: 13223. https://doi.org/10.3390/ijms222413223
APA StyleKusanto, B., Gordon, A., Naylor-Adamson, L., Atkinson, L., Coupland, C., Booth, Z., Ahmed, Y., Pires, I. M., Stasiuk, G. J., Sturmey, R., Calaminus, S. D. J., & Arman, M. (2021). Practical Considerations of Dissolved Oxygen Levels for Platelet Function under Hypoxia. International Journal of Molecular Sciences, 22(24), 13223. https://doi.org/10.3390/ijms222413223





