Exogenous Application of Low-Concentration Sugar Enhances Brassinosteroid Signaling for Skotomorphogenesis by Promoting BIN2 Degradation
Abstract
:1. Introduction
2. Results
2.1. BR Biosynthesis Is Required for Sucrose Promotion of Skotomorphogenesis
2.2. The Roles of TOR Kinase and BZR1 Protein in Skotomorphogenesis
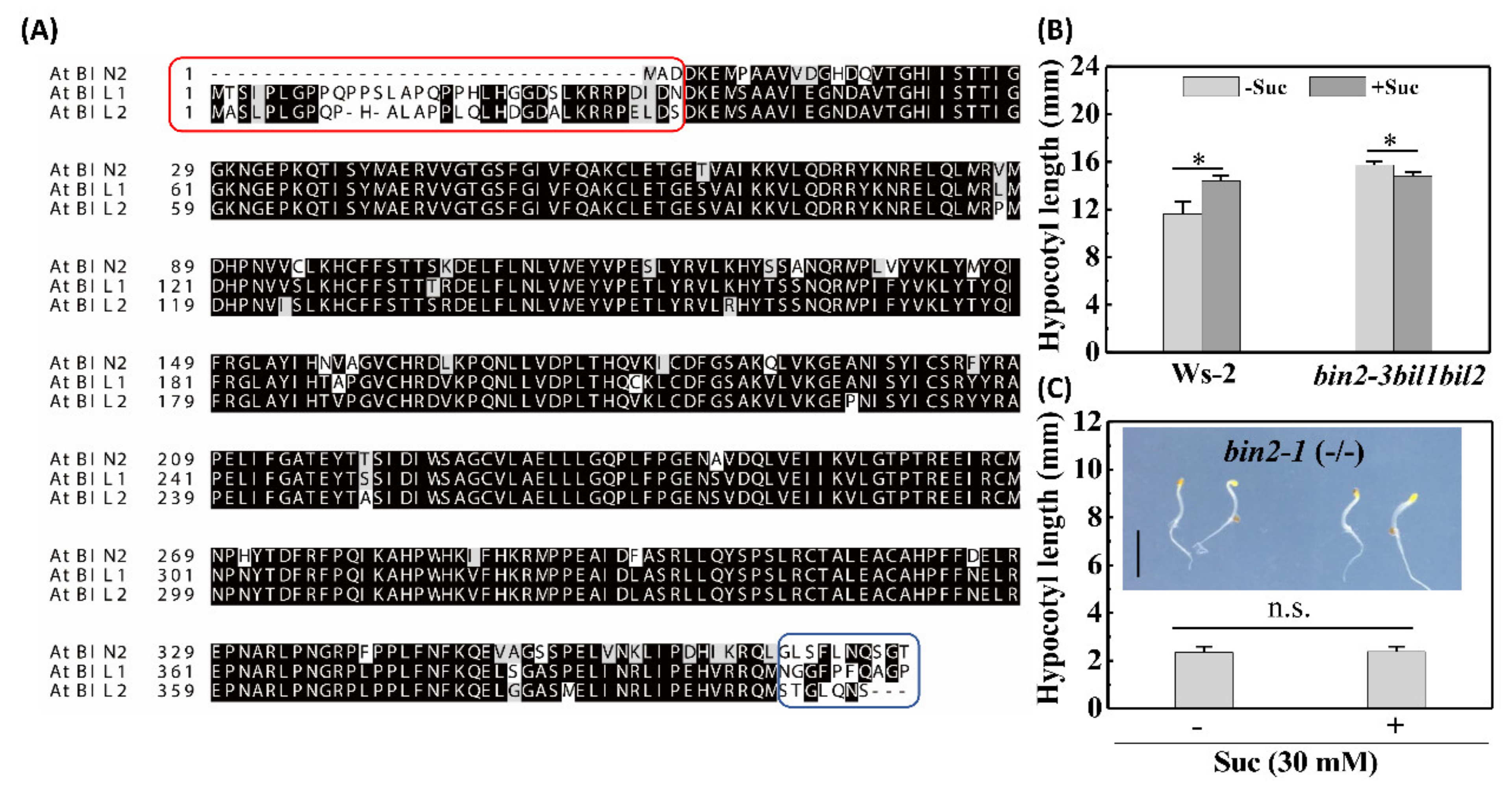
2.3. BIN2 and Its Homologs Negatively Regulate Etiolated Seedling Growth (Skotomorphogenesis)
2.4. Sucrose–TOR Signaling Reduces the Abundance of BIN2
2.5. S6K2 Downstream of TOR Interacts with and Phosphorylates BIN2 to Induce Skotomorphogenesis
2.6. Effects of Sucrose on Crystalline Cellulose Biosynthesis
3. Discussion
3.1. BZR1 Partially Functions in Sugar-Mediated Skotomorphogenesis
3.2. Sucrose-Mediated BIN2 Degradation Promotes Skotomorphogenesis
3.3. Putative Mechanisms of Sucrose-Induced BIN2 Inactivation in Darkness
3.4. Sucrose Enhances Cellulose Synthesis for Skotomorphogenesis
4. Materials and Methods
4.1. Plant Material and Growth Conditions
4.2. Hypocotyl Growth Measurements
4.3. Protein Extraction and Western Blot Assays
4.4. RNA Isolation and Quantitative Real-Time PCR Analysis
4.5. Split-Luciferase Complementation Assay
4.6. Measurements of Crystalline Cellulose Content
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Wingler, A. Transitioning to the next phase: The role of sugar signaling throughout the plant life cycle. Plant Physiol. 2018, 176, 1075–1084. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Shi, L.; Li, L.; Fu, L.; Liu, Y.; Xiong, Y.; Sheen, J. Integration of nutrient, energy, light, and hormone signalling via TOR in plants. J. Exp. Bot. 2019, 70, 2227–2238. [Google Scholar] [CrossRef] [PubMed]
- Yoon, J.; Cho, L.H.; Tun, W.; Jeon, J.S.; An, G. Sucrose signaling in higher plants. Plant Sci. 2020, 302, 110703. [Google Scholar] [CrossRef] [PubMed]
- Cho, Y.H.; Yoo, S.D.; Sheen, J. Regulatory functions of nuclear hexokinase1 complex in glucose signaling. Cell 2006, 127, 579–589. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Barbier, F.F.; Cao, D.; Fichtner, F.; Weiste, C.; Perez-Garcia, M.D.; Caradeuc, M.; Gourrierec, J.M.; Sakr, M.; Beveridge, C.A. HEXOKINASE1 signalling promotes shoot branching and interacts with cytokinin and strigolactone pathways. New Phytol. 2021, 231, 1088–1104. [Google Scholar] [CrossRef]
- Cho, Y.H.; Hong, J.W.; Kim, E.C.; Yoo, S.D. Regulatory functions of SnRK1 in stress-responsive gene expression and in plant growth and development. Plant Physiol. 2012, 158, 1955–1964. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Margalha, L.; Confraria, A.; Baena-González, E. SnRK1 and TOR: Modulating growth–defense trade-offs in plant stress responses. J. Exp. Bot. 2019, 70, 2261–2274. [Google Scholar] [CrossRef] [PubMed]
- Wang, B.; Zhao, X.; Zhao, Y.; Shanklin, J.; Zhao, Q.; Liu, C.J. Arabidopsis SnRK1 negatively regulates phenylpropanoid metabolism via Kelch domain-containing F-box proteins. New Phytol. 2021, 229, 3345–3359. [Google Scholar] [CrossRef]
- Díaz-Granados, V.H.; López-López, J.M.; Flores-Sánchez, J.; Olguin-Alor, R.; Bedoya-López, A.; Dinkova, T.D.; Salazar-Díaz, K.; Vázquez-Santana, S.; Vázquez-Ramos, J.M.; Lara-Núñez, A. Glucose modulates proliferation in root apical meristems via TOR in maize during germination. Plant Physiol. Biochem. 2020, 155, 126–135. [Google Scholar] [CrossRef] [PubMed]
- O’Leary, B.M.; Oh, G.G.K.; Lee, C.P.; Millar, A.H. Metabolite regulatory interactions control plant respiratory metabolism via Target of Rapamycin (TOR) kinase activation. Plant Cell 2020, 32, 666–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fu, L.; Liu, Y.; Qin, G.; Wu, P.; Zi, H.; Xu, Z.; Zhao, X.; Wang, Y.; Li, Y.; Yang, S.; et al. The TOR-EIN2 axis mediates nuclear signalling to modulate plant growth. Nature 2021, 591, 288–292. [Google Scholar] [CrossRef] [PubMed]
- Chiou, T.J.; Bush, D.R. Sucrose is a signal molecule in assimilate partitioning. Proc. Natl. Acad. Sci. USA 1998, 95, 4784–4788. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ruan, Y.L. Sucrose metabolism: Gateway to diverse carbon use and sugar signaling. Ann. Rev. Plant Biol. 2014, 65, 33–67. [Google Scholar] [CrossRef] [PubMed]
- Li, L.; Sheen, J. Dynamic and diverse sugar signaling. Curr. Opin. Plant Biol. 2016, 33, 116–125. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, J.; Wang, H.; Ren, L.; Chen, S.; Chen, F.; Jiang, J. CmFTL2 is involved in the photoperiod-and sucrose-mediated control of flowering time in chrysanthemum. Hortic. Res. 2017, 4, 17001. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Camañes, G.; Cerezo, M.; Primo-Millo, E.; Gojon, A.; García-Agustín, P. Ammonium transport and CitAMT1 expression are regulated by light and sucrose in Citrus plants. J. Exp. Bot. 2007, 58, 2811–2825. [Google Scholar] [CrossRef] [PubMed]
- Li, D.; Zhang, X.; Xu, Y.; Li, L.; Aghdam, M.S.; Luo, Z. Effect of exogenous sucrose on anthocyanin synthesis in postharvest strawberry fruit. Food Chem. 2019, 289, 112–120. [Google Scholar] [CrossRef] [PubMed]
- Eckstein, A.; Zięba, P.; Gabryś, H. Sugar and light effects on the condition of the photosynthetic apparatus of Arabidopsis thaliana cultured in vitro. J. Plant Growth Regul. 2012, 31, 90–101. [Google Scholar] [CrossRef] [Green Version]
- Li, J.; Nam, K.H.; Vafeados, D.; Chory, J. BIN2, a new brassinosteroid-insensitive locus in Arabidopsis. Plant Physiol. 2001, 127, 14–22. [Google Scholar] [CrossRef] [Green Version]
- He, J.X.; Gendron, J.M.; Yang, Y.; Li, J.; Wang, Z.Y. The GSK3-like kinase BIN2 phosphorylates and destabilizes BZR1, a positive regulator of the brassinosteroid signaling pathway in Arabidopsis. Proc. Natl. Acad. Sci. USA 2002, 99, 10185–10190. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tang, W.; Yuan, M.; Wang, R.; Yang, Y.; Wang, C.; Oses-Prieto, J.A.; Kim, T.W.; Zhou, H.W.; Deng, Z.; Gampala, S.S.; et al. PP2A activates brassinosteroid-responsive gene expression and plant growth by dephosphorylating BZR1. Nat. Cell Biol. 2011, 13, 124–131. [Google Scholar] [CrossRef] [PubMed]
- Wang, Z.Y.; Bai, M.Y.; Oh, E.; Zhu, J.Y. Brassinosteroid signaling network and regulation of photomorphogenesis. Annu. Rev. Genet. 2012, 46, 701–724. [Google Scholar] [CrossRef] [PubMed]
- Schlüter, U.; Köpke, D.; Altmann, T.; Müssig, C. Analysis of carbohydrate metabolism of CPD antisense plants and the brassinosteroid-deficient cbb1 mutant. Plant Cell Environ. 2002, 25, 783–791. [Google Scholar] [CrossRef]
- Gupta, A.; Singh, M.; Laxmi, A. Multiple interactions between glucose and brassinosteroid signal transduction pathways in Arabidopsis are uncovered by whole-genome transcriptional profiling. Plant Physiol. 2015, 168, 1091–1105. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitterlich, M.; Krügel, U.; Boldt-Burisch, K.; Franken, P.; Kühn, C. The sucrose transporter SlSUT2 from tomato interacts with brassinosteroid functioning and affects arbuscular mycorrhiza formation. Plant J. 2014, 78, 877–889. [Google Scholar] [CrossRef] [PubMed]
- Hansch, F.; Jaspar, H.; von Sivers, L.; Bitterlich, M.; Franken, P.; Kühn, C. Brassinosteroids and sucrose transport in mycorrhizal tomato plants. Plant Signal. Behav. 2020, 15, 1714292. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Josse, E.M.; Halliday, K.J. Skotomorphogenesis: The dark side of light signalling. Curr. Biol. 2008, 18, R1144–R1146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Y.; Liu, Z.; Wang, J.; Chen, Y.; Bi, Y.; He, J. Brassinosteroid is required for sugar promotion of hypocotyl elongation in Arabidopsis in darkness. Planta 2015, 242, 881–893. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Z.; Zhu, J.Y.; Roh, J.; Marchive, C.; Kim, S.K.; Meyer, C.; Sun, Y.; Wang, W.; Wang, Z.Y. TOR signaling promotes accumulation of BZR1 to balance growth with carbon availability in Arabidopsis. Curr. Biol. 2016, 26, 1854–1860. [Google Scholar] [CrossRef] [PubMed]
- Yan, Z.; Zhao, J.; Peng, P.; Chihara, R.K.; Li, J. BIN2 functions redundantly with other Arabidopsis GSK3-like kinases to regulate brassinosteroid signaling. Plant Physiol. 2009, 150, 710–721. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Xiong, F.; Zhang, R.; Meng, Z.; Deng, K.; Que, Y.; Zhuo, F.; Feng, L.; Guo, S.; Datla, R.; Ren, M. Brassinosteriod Insensitive 2 (BIN2) acts as a downstream effector of the Target of Rapamycin (TOR) signaling pathway to regulate photoautotrophic growth in Arabidopsis. New Phytol. 2017, 213, 233–249. [Google Scholar] [CrossRef] [Green Version]
- Rayapuram, N.; Bigeard, J.; Alhoraibi, H.; Bonhomme, L.; Hesse, A.M.; Vinh, J.; Hirt, H.; Pflieger, D. Quantitative Phosphoproteomic Analysis Reveals Shared and Specific Targets of Arabidopsis Mitogen-Activated Protein Kinases (MAPKs) MPK3, MPK4, and MPK6. Mol Cell Proteom. 2018, 17, 61–80. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, H.; Chen, S. Plant silicon-cell wall complexes: Identification, model of covalent bond formation and biofunction. Plant Physiol. Biochem. 2020, 155, 13–19. [Google Scholar] [CrossRef]
- Zhang, Y.; Liu, Z.; Wang, L.; Zheng, S.; Xie, J.; Bi, Y. Sucrose-induced hypocotyl elongation of Arabidopsis seedlings in darkness depends on the presence of gibberellins. J. Plant Physiol. 2010, 167, 1130–1136. [Google Scholar] [CrossRef] [PubMed]
- Alabadí, D.; Gil, J.; Blázquez, M.A.; García-Martínez, J.L. Gibberellins repress photomorphogenesis in darkness. Plant Physiol. 2004, 134, 1050–1057. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Feng, S.; Martinez, C.; Gusmaroli, G.; Wang, Y.; Zhou, J.; Wang, F.; Chen, L.; Yu, L.; Iglesias-Pedraz, J.M.; Kircher, S.; et al. Coordinated regulation of Arabidopsis thaliana development by light and gibberellins. Nature 2008, 451, 475–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Liu, Z.; Zhang, Y.; Liu, R.; Hao, H.; Wang, Z.; Bi, Y. Phytochrome interacting factors (PIFs) are essential regulators for sucrose-induced hypocotyl elongation in Arabidopsis. J. Plant Physiol. 2011, 168, 1771–1779. [Google Scholar] [CrossRef] [PubMed]
- Oh, E.; Zhu, J.Y.; Wang, Z.Y. Interaction between BZR1 and PIF4 integrates brassinosteroid and environmental responses. Nat. Cell Biol. 2012, 14, 802–809. [Google Scholar] [CrossRef] [Green Version]
- Bouquin, T.; Meier, C.; Foster, R.; Nielsen, M.E.; Mundy, J. Control of specific gene expression by gibberellin and brassinosteroid. Plant Physiol. 2001, 127, 450–458. [Google Scholar] [CrossRef] [PubMed]
- Hao, Y.; Wang, H.; Qiao, S.; Leng, L.; Wang, X. Histone deacetylase HDA6 enhances brassinosteroid signaling by inhibiting the BIN2 kinase. Proc. Natl. Acad. Sci. USA 2016, 113, 10418–10423. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Song, S.; Wang, H.; Sun, M.; Tang, J.; Zheng, B.; Wang, X.; Tan, Y.W. Reactive oxygen species-mediated BIN 2 activity revealed by single-molecule analysis. New Phytol. 2019, 223, 692–704. [Google Scholar] [CrossRef]
- Peng, Y.; Chen, L.; Li, S.; Zhang, Y.; Xu, R.; Liu, Z.; Liu, W.; Kong, J.; Huang, X.; Wang, Y.; et al. BRI1 and BAK1 interact with G proteins and regulate sugar-responsive growth and development in Arabidopsis. Nat. Commun. 2018, 9, 1522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Sun, Y.; Jiang, X.; Wang, W.; Wang, Z.Y. Sugar inhibits brassinosteroid signaling by enhancing BIN2 phosphorylation of BZR1. PLoS Genet. 2021, 17, e1009540. [Google Scholar] [CrossRef] [PubMed]
- Xiong, Y.; McCormack, M.; Li, L.; Hall, Q.; Xiang, C.; Sheen, J. Glucose–TOR signalling reprograms the transcriptome and activates meristems. Nature 2013, 496, 181–186. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheng, H.; Ma, J.; Pu, J.; Wang, L. Cell wall-bound silicon optimizes ammonium uptake and metabolism in rice cells. Ann. Bot. 2018, 122, 303–313. [Google Scholar] [CrossRef] [PubMed]
- Wang, L.; Hart, B.E.; Khan, G.A.; Cruz, E.R.; Persson, S.; Wallace, I.S. Associations between phytohormones and cellulose biosynthesis in land plants. Ann. Bot. 2020, 126, 807–824. [Google Scholar] [CrossRef] [PubMed]
- Sun, Y.; Veerabomma, S.; Abdel-Mageed, H.A.; Fokar, M.; Asami, T.; Yoshida, S.; Allen, R.D. Brassinosteroid regulates fiber development on cultured cotton ovules. Plant Cell Physiol. 2005, 46, 1384–1391. [Google Scholar] [CrossRef]
- Xie, L.; Yang, C.; Wang, X. Brassinosteroids can regulate cellulose biosynthesis by controlling the expression of CESA genes in Arabidopsis. J. Exp. Bot. 2011, 62, 4495–4506. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Park, C.H.; Kim, T.W.; Son, S.H.; Hwang, J.Y.; Lee, S.C.; Chang, S.C.; Kim, S.H.; Kim, S.K.; Kim, S.K. Brassinosteroids control AtEXPA5 gene expression in Arabidopsis thaliana. Phytochemistry 2010, 71, 380–387. [Google Scholar] [CrossRef] [PubMed]
- Sánchez-Rodríguez, C.; Ketelaar, K.; Schneider, R.; Villalobos, J.A.; Somerville, C.R.; Persson, S.; Wallace, I.S. BRASSINOSTEROID INSENSITIVE2 negatively regulates cellulose synthesis in Arabidopsis by phosphorylating cellulose synthase 1. Proc. Natl. Acad. Sci. USA 2017, 114, 3533–3538. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ivakov, A.; Flis, A.; Apelt, F.; Fünfgeld, M.; Scherer, U.; Stitt, M.; Kragler, F.; Vissenberg, K.; Persson, S.; Suslov, D. Cellulose synthesis and cell expansion are regulated by different mechanisms in growing Arabidopsis hypocotyls. Plant Cell 2017, 29, 1305–1315. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, J.; Nagpal, P.; Vitart, V.; McMorris, T.C.; Chory, J. A role for brassinosteroids in light-dependent development of Arabidopsis. Science 1996, 272, 398–401. [Google Scholar] [CrossRef]
- Wang, Z.Y.; Nakano, T.; Gendron, J.; He, J.; Chen, M.; Vafeados, D.; Yang, Y.; Fujioka, S.; Yoshida, S.; Asami, T.; et al. Nuclear-localized BZR1 mediates brassinosteroid-induced growth and feedback suppression of brassinosteroid biosynthesis. Dev. Cell 2002, 2, 505–513. [Google Scholar] [CrossRef] [Green Version]
- Updegraff, D.M. Semimicro determination of cellulose inbiological materials. Anal. Biochem. 1969, 32, 420–424. [Google Scholar] [CrossRef]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Sheng, H.; Zhang, S.; Wei, Y.; Chen, S. Exogenous Application of Low-Concentration Sugar Enhances Brassinosteroid Signaling for Skotomorphogenesis by Promoting BIN2 Degradation. Int. J. Mol. Sci. 2021, 22, 13588. https://doi.org/10.3390/ijms222413588
Sheng H, Zhang S, Wei Y, Chen S. Exogenous Application of Low-Concentration Sugar Enhances Brassinosteroid Signaling for Skotomorphogenesis by Promoting BIN2 Degradation. International Journal of Molecular Sciences. 2021; 22(24):13588. https://doi.org/10.3390/ijms222413588
Chicago/Turabian StyleSheng, Huachun, Shuangxi Zhang, Yanping Wei, and Shaolin Chen. 2021. "Exogenous Application of Low-Concentration Sugar Enhances Brassinosteroid Signaling for Skotomorphogenesis by Promoting BIN2 Degradation" International Journal of Molecular Sciences 22, no. 24: 13588. https://doi.org/10.3390/ijms222413588
APA StyleSheng, H., Zhang, S., Wei, Y., & Chen, S. (2021). Exogenous Application of Low-Concentration Sugar Enhances Brassinosteroid Signaling for Skotomorphogenesis by Promoting BIN2 Degradation. International Journal of Molecular Sciences, 22(24), 13588. https://doi.org/10.3390/ijms222413588






