The VirB System Plays a Crucial Role in Brucella Intracellular Infection
Abstract
:1. Introduction
2. Composition of the Brucella VirB Operon
2.1. ATPase
2.2. Core Component VirB6–VirB10
2.3. Surface-Exposed Components VirB2 and VirB5
2.4. Other Components VirB1 and VirB12
3. Brucella Type IV Secretion System
3.1. Formation of T4SS
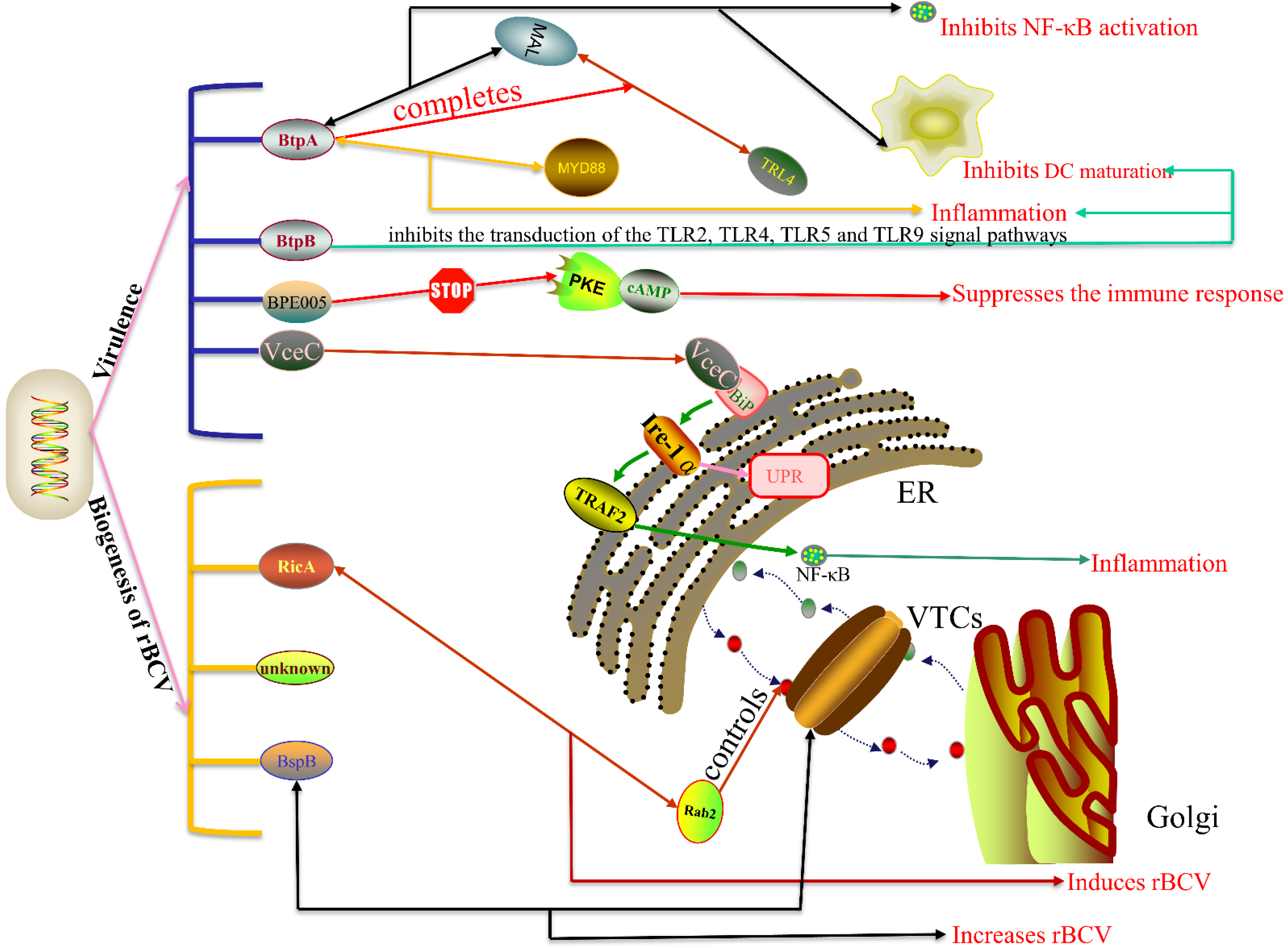
3.2. T4SS Effectors
3.2.1. RicA
3.2.2. VceC and VecA
3.2.3. BtpA and BtpB
3.2.4. BspA, BspB, BspF
3.2.5. Other Effectors
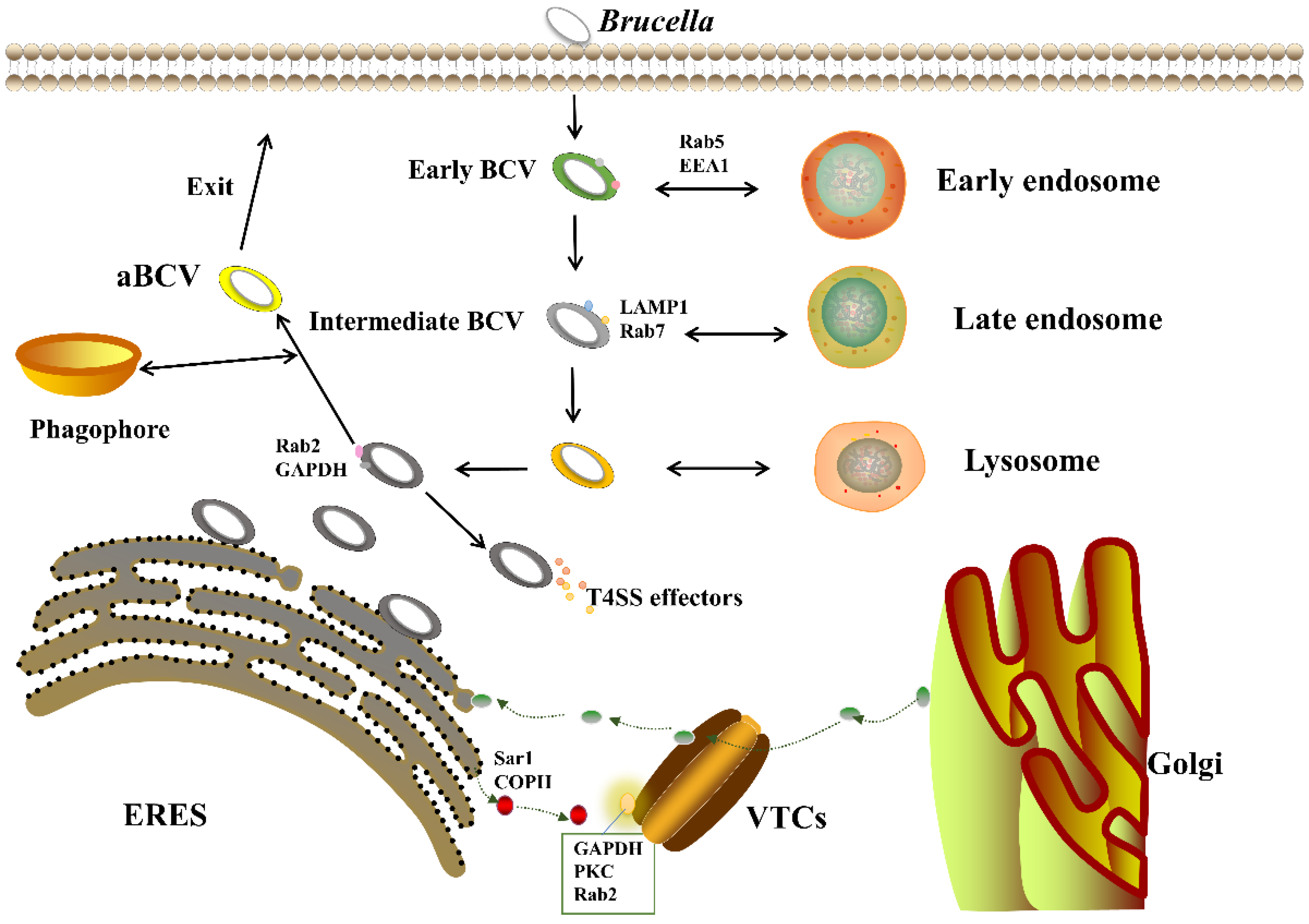
4. Effect of VirB T4SS on the Intracellular Circulation of Brucella
4.1. VirB T4SS Action Stage
4.2. Cellular Pathways Affected by VirB T4SS
4.3. Regulatory Factors of VirB T4SS
5. Conclusions Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Njeru, J.; Wareth, G.; Melzer, F.; Henning, K.; Pletz, M.W.; Heller, R.; Neubauer, H. Systematic review of brucellosis in Kenya: Disease frequency in humans and animals and risk factors for human infection. BMC Public Health 2016, 16, 853. [Google Scholar] [CrossRef] [Green Version]
- Jay, M.; Freddi, L.; Mick, V.; Durand, B.; Girault, G.; Perrot, L.; Taunay, B.; Vuilmet, T.; Azam, D.; Ponsart, C.; et al. Brucella microti-like prevalence in French farms producing frogs. Transbound. Emerg. Dis. 2020, 67, 617–625. [Google Scholar] [CrossRef]
- Perkins, S.D.; Smither, S.J.; Atkins, H.S. Towards a Brucella vaccine for humans. FEMS Microbiol. Rev. 2010, 34, 379–394. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jansen, W.; Demars, A.; Nicaise, C.; Godfroid, J.; de Bolle, X.; Reboul, A.; Al Dahouk, S. Shedding of Brucella melitensis happens through milk macrophages in the murine model of infection. Sci. Rep. 2020, 10, 9421. [Google Scholar] [CrossRef] [PubMed]
- Glowacka, P.; Zakowska, D.; Naylor, K.; Niemcewicz, M.; Bielawska-Drozd, A. Brucella—Virulence Factors, Pathogenesis and Treatment. Pol. J. Microbiol. 2018, 67, 151–161. [Google Scholar] [CrossRef] [Green Version]
- Li, P.; Tian, M.; Hu, H.; Yin, Y.; Guan, X.; Ding, C.; Wang, S.; Yu, S. Lable-free based comparative proteomic analysis of secretory proteins of rough Brucella mutants. J. Proteom. 2019, 195, 66–75. [Google Scholar] [CrossRef]
- Lacerda, T.L.; Salcedo, S.P.; Gorvel, J.P. Brucella T4SS: The VIP pass inside host cells. Curr. Opin. Microbiol. 2013, 16, 45–51. [Google Scholar] [CrossRef]
- Ke, Y.; Wang, Y.; Li, W.; Chen, Z. Type IV secretion system of Brucella spp. and its effectors. Front. Cell. Infect. Microbiol. 2015, 5, 72. [Google Scholar] [PubMed] [Green Version]
- Sieira, R.; Comerci, D.J.; Sanchez, D.O.; Ugalde, R.A. A homologue of an operon required for DNA transfer in Agrobacterium is required in Brucella abortus for virulence and intracellular multiplication. J. Bacteriol. 2000, 182, 4849–4855. [Google Scholar] [CrossRef] [Green Version]
- O’Callaghan, D.; Cazevieille, C.; Allardet-Servent, A.; Boschiroli, M.L.; Bourg, G.; Foulongne, V.; Frutos, P.; Kulakov, Y.; Ramuz, M. A homologue of the Agrobacterium tumefaciens VirB and Bordetella pertussis Ptl type IV secretion systems is essential for intracellular survival of Brucella suis. Mol. Microbiol. 1999, 33, 1210–1220. [Google Scholar] [CrossRef]
- Boschiroli, M.L.; Ouahrani-Bettache, S.; Foulongne, V.; Michaux-Charachon, S.; Bourg, G.; Allardet-Servent, A.; Cazevieille, C.; Liautard, J.P.; Ramuz, M.; O’Callaghan, D. The Brucella suis virB operon is induced intracellularly in macrophages. Proc. Natl. Acad. Sci. USA 2002, 99, 1544–1549. [Google Scholar] [CrossRef] [Green Version]
- Den Hartigh, A.B.; Sun, Y.H.; Sondervan, D.; Heuvelmans, N.; Reinders, M.O.; Ficht, T.A.; Tsolis, R.M. Differential requirements for VirB1 and VirB2 during Brucella abortus infection. Infect. Immun. 2004, 72, 5143–5149. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Y.H.; Rolan, H.G.; den Hartigh, A.B.; Sondervan, D.; Tsolis, R.M. Brucella abortus virB12 is expressed during infection but is not an essential component of the type IV secretion system. Infect. Immun. 2005, 73, 6048–6054. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Den Hartigh, A.B.; Rolan, H.G.; de Jong, M.F.; Tsolis, R.M. VirB3 to VirB6 and VirB8 to VirB11, but not VirB7, are essential for mediating persistence of Brucella in the reticuloendothelial system. J. Bacteriol. 2008, 190, 4427–4436. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ward, D.V.; Draper, O.; Zupan, J.R.; Zambryski, P.C. Peptide linkage mapping of the Agrobacterium tumefaciens vir-encoded type IV secretion system reveals protein subassemblies. Proc. Natl. Acad. Sci. USA 2002, 99, 11493–11500. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Berge, C.; Waksman, G.; Terradot, L. Structural and Molecular Biology of Type IV Secretion Systems. Curr. Top. Microbiol. Immunol. 2017, 413, 31–60. [Google Scholar]
- Krause, S.; Pansegrau, W.; Lurz, R.; de la Cruz, F.; Lanka, E. Enzymology of type IV macromolecule secretion systems: The conjugative transfer regions of plasmids RP4 and R388 and the cag pathogenicity island of Helicobacter pylori encode structurally and functionally related nucleoside triphosphate hydrolases. J. Bacteriol. 2000, 182, 2761–2770. [Google Scholar] [CrossRef] [Green Version]
- Hare, S.; Bayliss, R.; Baron, C.; Waksman, G. A large domain swap in the VirB11 ATPase of Brucella suis leaves the hexameric assembly intact. J. Mol. Biol. 2006, 360, 56–66. [Google Scholar] [CrossRef]
- Fronzes, R.; Christie, P.J.; Waksman, G. The structural biology of type IV secretion systems. Nat. Rev. Microbiol. 2009, 7, 703–714. [Google Scholar] [CrossRef] [Green Version]
- Villamil Giraldo, A.M.; Sivanesan, D.; Carle, A.; Paschos, A.; Smith, M.A.; Plesa, M.; Coulton, J.; Baron, C. Type IV secretion system core component VirB8 from Brucella binds to the globular domain of VirB5 and to a periplasmic domain of VirB6. Biochemistry 2012, 51, 3881–3890. [Google Scholar] [CrossRef]
- Villamil Giraldo, A.M.; Mary, C.; Sivanesan, D.; Baron, C. VirB6 and VirB10 from the Brucella type IV secretion system interact via the N-terminal periplasmic domain of VirB6. FEBS Lett. 2015, 589, 1883–1889. [Google Scholar] [CrossRef] [Green Version]
- Terradot, L.; Bayliss, R.; Oomen, C.; Leonard, G.A.; Baron, C.; Waksman, G. Structures of two core subunits of the bacterial type IV secretion system, VirB8 from Brucella suis and ComB10 from Helicobacter pylori. Proc. Natl. Acad. Sci. USA 2005, 102, 4596–4601. [Google Scholar] [CrossRef] [Green Version]
- Sharifahmadian, M.; Arya, T.; Bessette, B.; Lecoq, L.; Ruediger, E.; Omichinski, J.G.; Baron, C. Monomer-to-dimer transition of Brucella suis type IV secretion system component VirB8 induces conformational changes. FEBS J. 2017, 284, 1218–1232. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chandran Darbari, V.; Waksman, G. Structural Biology of Bacterial Type IV Secretion Systems. Annu. Rev. Biochem. 2015, 84, 603–629. [Google Scholar] [CrossRef]
- Fronzes, R.; Schafer, E.; Wang, L.; Saibil, H.R.; Orlova, E.V.; Waksman, G. Structure of a type IV secretion system core complex. Science 2009, 323, 266–268. [Google Scholar] [CrossRef]
- Baron, C.; Thorstenson, Y.R.; Zambryski, P.C. The lipoprotein VirB7 interacts with VirB9 in the membranes of Agrobacterium tumefaciens. J. Bacteriol. 1997, 179, 1211–1218. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keriel, A.; Botella, E.; Estrach, S.; Bragagnolo, G.; Vergunst, A.C.; Feral, C.C.; O’Callaghan, D. Brucella Intracellular Life Relies on the Transmembrane Protein CD98 Heavy Chain. J. Infect. Dis. 2015, 211, 1769–1778. [Google Scholar] [CrossRef]
- Aly, K.A.; Baron, C. The VirB5 protein localizes to the T-pilus tips in Agrobacterium tumefaciens. Microbiology 2007, 153, 3766–3775. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Deng, H.; Zhou, J.; Gong, B.; Xiao, M.; Zhang, M.; Pang, Q.; Zhang, X.; Zhao, B.; Zhou, X. Screening and identification of a human domain antibody against Brucella abortus VirB5. Acta Trop. 2019, 197, 105026. [Google Scholar] [CrossRef]
- Zupan, J.; Hackworth, C.A.; Aguilar, J.; Ward, D.; Zambryski, P. VirB1* promotes T-pilus formation in the vir-Type IV secretion system of Agrobacterium tumefaciens. J. Bacteriol. 2007, 189, 6551–6563. [Google Scholar] [CrossRef] [Green Version]
- Mirkalantari, S.; Zarnani, A.H.; Nazari, M.; Irajian, G.R.; Amirmozafari, N. Brucella melitensis VirB12 recombinant protein is a potential marker for serodiagnosis of human brucellosis. Ann. Clin. Microbiol. Antimicrob. 2017, 16, 8. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Carle, A.; Hoppner, C.; Ahmed Aly, K.; Yuan, Q.; den Dulk-Ras, A.; Vergunst, A.; O’Callaghan, D.; Baron, C. The Brucella suis type IV secretion system assembles in the cell envelope of the heterologous host Agrobacterium tumefaciens and increases IncQ plasmid pLS1 recipient competence. Infect. Immun. 2006, 74, 108–117. [Google Scholar] [CrossRef] [Green Version]
- Hospenthal, M.K.; Costa, T.R.D.; Waksman, G. A comprehensive guide to pilus biogenesis in Gram-negative bacteria. Nat. Rev. Microbiol. 2017, 15, 365–379. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sivanesan, D.; Hancock, M.A.; Villamil Giraldo, A.M.; Baron, C. Quantitative analysis of VirB8-VirB9-VirB10 interactions provides a dynamic model of type IV secretion system core complex assembly. Biochemistry 2010, 49, 4483–4493. [Google Scholar] [CrossRef] [PubMed]
- Cascales, E.; Christie, P.J. Agrobacterium VirB10, an ATP energy sensor required for type IV secretion. Proc. Natl. Acad. Sci. USA 2004, 101, 17228–17233. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yuan, Q.; Carle, A.; Gao, C.; Sivanesan, D.; Aly, K.A.; Hoppner, C.; Krall, L.; Domke, N.; Baron, C. Identification of the VirB4-VirB8-VirB5-VirB2 pilus assembly sequence of type IV secretion systems. J. Biol. Chem. 2005, 280, 26349–26359. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hoppner, C.; Carle, A.; Sivanesan, D.; Hoeppner, S.; Baron, C. The putative lytic transglycosylase VirB1 from Brucella suis interacts with the type IV secretion system core components VirB8, VirB9 and VirB11. Microbiology 2005, 151, 3469–3482. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- De Barsy, M.; Jamet, A.; Filopon, D.; Nicolas, C.; Laloux, G.; Rual, J.F.; Muller, A.; Twizere, J.C.; Nkengfac, B.; Vandenhaute, J.; et al. Identification of a Brucella spp. secreted effector specifically interacting with human small GTPase Rab2. Cell. Microbiol. 2011, 13, 1044–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nkengfac, B.; Pouyez, J.; Bauwens, E.; Vandenhaute, J.; Letesson, J.J.; Wouters, J.; De Bolle, X. Structural analysis of Brucella abortus RicA substitutions that do not impair interaction with human Rab2 GTPase. BMC Biochem. 2012, 13, 16. [Google Scholar] [CrossRef] [Green Version]
- Herrou, J.; Crosson, S. Molecular Structure of the Brucella abortus Metalloprotein RicA, a Rab2-Binding Virulence Effector. Biochemistry 2013, 52, 9020–9028. [Google Scholar] [CrossRef] [Green Version]
- Taguchi, Y.; Imaoka, K.; Kataoka, M.; Uda, A.; Nakatsu, D.; Horii-Okazaki, S.; Kunishige, R.; Kano, F.; Murata, M. Yip1A, a novel host factor for the activation of the IRE1 pathway of the unfolded protein response during Brucella infection. PLoS Pathog. 2015, 11, e1004747. [Google Scholar] [CrossRef]
- Zhi, F.J.; Zhou, D.; Bai, F.R.; Li, J.M.; Xiang, C.X.; Zhang, G.D.; Jin, Y.P.; Wang, A.H. VceC Mediated IRE1 Pathway and Inhibited CHOP-induced Apoptosis to Support Brucella Replication in Goat Trophoblast Cells. Int. J. Mol. Sci. 2019, 20, 4104. [Google Scholar] [CrossRef] [Green Version]
- Zhang, J.; Li, M.; Li, Z.Q.; Shi, J.X.; Zhang, Y.; Deng, X.M.; Liu, L.B.; Wang, Z.; Qi, Y.Y.; Zhang, H. Deletion of the Type IV Secretion System Effector VceA Promotes Autophagy and Inhibits Apoptosis in Brucella-Infected Human Trophoblast Cells. Curr. Microbiol. 2019, 76, 510–519. [Google Scholar] [CrossRef] [PubMed]
- de Jong, M.F.; Sun, Y.H.; den Hartigh, A.B.; van Dijl, J.M.; Tsolis, R.M. Identification of VceA and VceC, two members of the VjbR regulon that are translocated into macrophages by the Brucella type IV secretion system. Mol. Microbiol. 2008, 70, 1378–1396. [Google Scholar] [CrossRef] [Green Version]
- Salcedo, S.P.; Marchesini, M.I.; Lelouard, H.; Fugier, E.; Jolly, G.; Balor, S.; Muller, A.; Lapaque, N.; Demaria, O.; Alexopoulou, L.; et al. Brucella control of dendritic cell maturation is dependent on the TIR-Containing protein btp1. PLoS Path. 2008, 4, e42. [Google Scholar] [CrossRef] [Green Version]
- Sengupta, D.; Koblansky, A.; Gaines, J.; Brown, T.; West, A.P.; Zhang, D.K.; Nishikawa, T.; Park, S.G.; Roop, R.M.; Ghosh, S. Subversion of Innate Immune Responses by Brucella through the Targeted Degradation of the TLR Signaling Adapter, MAL. J. Immunol. 2010, 184, 956–964. [Google Scholar] [CrossRef] [Green Version]
- Alaidarous, M.; Ve, T.; Casey, L.W.; Valkov, E.; Ericsson, D.J.; Ullah, M.O.; Schembri, M.A.; Mansell, A.; Sweet, M.J.; Kobe, B. Mechanism of Bacterial Interference with TLR4 Signaling by Brucella Toll/Interleukin-1 Receptor Domain-containing Protein TcpB. J. Biol. Chem. 2014, 289, 654–668. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Salcedo, S.P.; Marchesini, M.I.; Degos, C.; Terwagne, M.; Von Bargen, K.; Lepidi, H.; Herrmann, C.K.; Lacerda, T.L.S.; Imbert, P.R.C.; Pierre, P.; et al. BtpB, a novel Brucella TIR-containing effector protein with immune modulatory functions. Front. Cell. Infect. Microbiol. 2013, 3, 28. [Google Scholar] [CrossRef] [Green Version]
- Chaudhary, A.; Ganguly, K.; Cabantous, S.; Waldo, G.S.; Micheva-Viteva, S.N.; Nag, K.; Hlavacek, W.S.; Tung, C.S. The Brucella TIR-like protein TcpB interacts with the death domain of MyD88. Biochem. Biophys. Res. Commun. 2012, 417, 299–304. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Coronas-Serna, J.M.; Louche, A.; Rodriguez-Escudero, M.; Roussin, M.; Imbert, P.R.C.; Rodriguez-Escudero, I.; Terradot, L.; Molina, M.; Gorvel, J.P.; Cid, V.J.; et al. The TIR-domain containing effectors BtpA and BtpB from Brucella abortus impact NAD metabolism. PLoS Path. 2020, 16, e1007979. [Google Scholar] [CrossRef] [Green Version]
- Myeni, S.; Child, R.; Ng, T.W.; Kupko, J.J.; Wehrly, T.D.; Porcella, S.F.; Knodler, L.A.; Celli, J. Brucella Modulates Secretory Trafficking via Multiple Type IV Secretion Effector Proteins. PLoS Path. 2013, 9, e1003556. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Marchesini, M.I.; Seijo, S.M.M.; Guaimas, F.F.; Comerci, D.J. A T4SS Effector Targets Host Cell Alpha-Enolase Contributing to Brucella abortus Intracellular Lifestyle. Front. Cell. Infect. Microbiol. 2016, 6, 153. [Google Scholar] [CrossRef]
- Dohmer, P.H.; Valguarnera, E.; Czibener, C.; Ugalde, J.E. Identification of a type IV secretion substrate of Brucella abortus that participates in the early stages of intracellular survival. Cell. Microbiol. 2014, 16, 396–410. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celli, J. The Intracellular Life Cycle of Brucella spp. Microbiol. Spectr. 2019, 7. [Google Scholar] [CrossRef]
- Starr, T.; Ng, T.W.; Wehrly, T.D.; Knodler, L.A.; Celli, J. Brucella intracellular replication requires trafficking through the late endosomal/lysosomal compartment. Traffic 2008, 9, 678–694. [Google Scholar] [CrossRef]
- Von Bargen, K.; Gorvel, J.P.; Salcedo, S.P. Internal affairs: Investigating the Brucella intracellular lifestyle. FEMS Microbiol. Rev. 2012, 36, 533–562. [Google Scholar] [CrossRef] [Green Version]
- Celli, J.; Salcedo, S.P.; Gorvel, J.P. Brucella coopts the small GTPase Sar1 for intracellular replication. Proc. Natl. Acad. Sci. USA 2005, 102, 1673–1678. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Starr, T.; Child, R.; Wehrly, T.D.; Hansen, B.; Hwang, S.; Lopez-Otin, C.; Virgin, H.W.; Celli, J. Selective subversion of autophagy complexes facilitates completion of the Brucella intracellular cycle. Cell Host Microbe 2012, 11, 33–45. [Google Scholar] [CrossRef] [Green Version]
- Hong, P.C.; Tsolis, R.M.; Ficht, T.A. Identification of genes required for chronic persistence of Brucella abortus in mice. Infect. Immun. 2000, 68, 4102–4107. [Google Scholar] [CrossRef] [Green Version]
- Pizarro-Cerda, J.; Meresse, S.; Parton, R.G.; van der Goot, G.; Sola-Landa, A.; Lopez-Goni, I.; Moreno, E.; Gorvel, J.P. Brucella abortus transits through the autophagic pathway and replicates in the endoplasmic reticulum of nonprofessional phagocytes. Infect. Immun. 1998, 66, 5711–5724. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Comerci, D.J.; Martinez-Lorenzo, M.J.; Sieira, R.; Gorvel, J.P.; Ugalde, R.A. Essential role of the VirB machinery in the maturation of the Brucella abortus-containing vacuole. Cell. Microbiol. 2001, 3, 159–168. [Google Scholar] [CrossRef] [PubMed]
- Celli, J.; de Chastellier, C.; Franchini, D.M.; Pizarro-Cerda, J.; Moreno, E.; Gorvel, J.P. Brucella evades macrophage killing via VirB-dependent sustained interactions with the endoplasmic reticulum. J. Exp. Med. 2003, 198, 545–556. [Google Scholar] [CrossRef]
- Scales, S.J.; Pepperkok, R.; Kreis, T.E. Visualization of ER-to-Golgi transport in living cells reveals a sequential mode of action for COPII and COPI. Cell 1997, 90, 1137–1148. [Google Scholar] [CrossRef] [Green Version]
- Barlowe, C. COPII-dependent transport from the endoplasmic reticulum. Curr. Opin. Cell. Biol. 2002, 14, 417–422. [Google Scholar] [CrossRef]
- Sieira, R.; Arocena, G.M.; Bukata, L.; Comerci, D.J.; Ugalde, R.A. Metabolic Control of Virulence Genes in Brucella abortus: HutC Coordinates virB Expression and the Histidine Utilization Pathway by Direct Binding to Both Promoters. J. Bacteriol. 2010, 192, 217–224. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fugier, E.; Salcedo, S.P.; de Chastellier, C.; Pophillat, M.; Muller, A.; Arce-Gorvel, V.; Fourquet, P.; Gorvel, J.P. The Glyceraldehyde-3-Phosphate Dehydrogenase and the Small GTPase Rab 2 Are Crucial for Brucella Replication. PLoS Path. 2009, 5, e1000487. [Google Scholar] [CrossRef] [Green Version]
- De Jong, M.F.; Starr, T.; Winter, M.G.; den Hartigh, A.B.; Child, R.; Knodler, L.A.; van Dijl, J.M.; Celli, J.; Tsolis, R.M. Sensing of bacterial type IV secretion via the unfolded protein response. mBio 2013, 4, e00418-12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keestra-Gounder, A.M.; Byndloss, M.X.; Seyffert, N.; Young, B.M.; Chavez-Arroyo, A.; Tsai, A.Y.; Cevallos, S.A.; Winter, M.G.; Pham, O.H.; Tiffany, C.R.; et al. NOD1/NOD2 signaling links ER stress with inflammation. Nature 2016, 532, 394–397. [Google Scholar] [CrossRef] [Green Version]
- Benitez, P.C.A.; Serantes, D.R.; Herrmann, C.K.; Viglietti, A.I.P.; Vanzulli, S.; Giambartolomei, G.H.; Comerci, D.J.; Delpino, M.V. The Effector Protein BPE005 from Brucella abortus Induces Collagen Deposition and Matrix Metalloproteinase 9 Downmodulation via Transforming Growth Factor beta 1 in Hepatic Stellate Cells. Infect. Immun. 2016, 84, 598–606. [Google Scholar] [CrossRef] [Green Version]
- Zambon, A.C.; Zhang, L.Z.; Minovitsky, S.; Kanter, J.R.; Prabhakar, S.; Salomonis, N.; Vranizan, K.; Dubchak, I.; Conklin, B.R.; Insel, P.A. Gene expression patterns define key transcriptional events in cell-cycle regulation by cAMP and protein kinase A. Proc. Natl. Acad. Sci. USA 2005, 102, 8561–8566. [Google Scholar] [CrossRef] [Green Version]
- Smith, E.P.; Cotto-Rosario, A.; Borghesan, E.; Held, K.; Miller, C.N.; Celli, J. Epistatic Interplay between Type IV Secretion Effectors Engages the Small GTPase Rab2 in the Brucella Intracellular Cycle. mBio 2020, 11, e03350-19. [Google Scholar] [CrossRef] [Green Version]
- Miller, C.N.; Smith, E.P.; Cundiff, J.A.; Knodler, L.A.; Blackburn, J.B.; Lupashin, V.; Celli, J. A Brucella Type IV Effector Targets the COG Tethering Complex to Remodel Host Secretory Traffic and Promote Intracellular Replication. Cell Host Microbe 2017, 22, 317–329. [Google Scholar] [CrossRef] [Green Version]
- Wang, X.G.; Lin, P.F.; Li, Y.; Xiang, C.X.; Yin, Y.L.; Chen, Z.; Du, Y.; Zhou, D.; Jin, Y.P.; Wang, A.H. Brucella suis Vaccine Strain 2 Induces Endoplasmic Reticulum Stress that Affects Intracellular Replication in Goat Trophoblast Cells In vitro. Front. Cell. Infect. Microbiol. 2016, 6, 19. [Google Scholar] [CrossRef] [PubMed]
- Pandey, A.; Lin, F.; Cabello, A.L.; da Costa, L.F.; Feng, X.; Feng, H.Q.; Zhang, M.Z.; Iwawaki, T.; Rice-Ficht, A.; Ficht, T.A.; et al. Activation of Host IRE1alpha-Dependent Signaling Axis Contributes the Intracellular Parasitism of Brucella melitensis. Front. Cell. Infect. Microbiol. 2018, 8, 103. [Google Scholar] [CrossRef]
- Tisdale, E.J.; Kelly, C.; Artalejo, C.R. Glyceraldehyde-3-phosphate dehydrogenase interacts with Rab2 and plays an essential role in endoplasmic reticulum to Golgi transport exclusive of its glycolytic activity. J. Biol. Chem. 2004, 279, 54046–54052. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Smith, E.P.; Miller, C.N.; Child, R.; Cundiff, J.A.; Celli, J. Postreplication Roles of the Brucella VirB Type IV Secretion System Uncovered via Conditional Expression of the VirB11 ATPase. mBio 2016, 7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sieira, R.; Comerci, D.J.; Pietrasanta, L.I.; Ugalde, R.A. Integration host factor is involved in transcriptional regulation of the Brucella abortus virB operon. Mol. Microbiol. 2004, 54, 808–822. [Google Scholar] [CrossRef] [PubMed]
- Rambow-Larsen, A.A.; Petersen, E.M.; Gourley, C.R.; Splitter, G.A. Brucella regulators: Self-control in a hostile environment. Trends Microbiol. 2009, 17, 371–377. [Google Scholar] [CrossRef] [Green Version]
- Kleinman, C.L.; Sycz, G.; Bonomi, H.R.; Rodriguez, R.M.; Zorreguieta, A.; Sieira, R. ChIP-seq analysis of the LuxR-type regulator VjbR reveals novel insights into the Brucella virulence gene expression network. Nucleic Acids Res. 2017, 45, 5757–5769. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Li, P.; Tian, M.; Bao, Y.; Hu, H.; Liu, J.; Yin, Y.; Ding, C.; Wang, S.; Yu, S. Brucella Rough Mutant Induce Macrophage Death via Activating IRE1alpha Pathway of Endoplasmic Reticulum Stress by Enhanced T4SS Secretion. Front. Cell. Infect. Microbiol. 2017, 7, 422. [Google Scholar] [CrossRef] [Green Version]
- Brambila-Tapia, A.J.; Perez-Rueda, E. A functional and phylogenetic comparison of quorum sensing related genes in Brucella melitensis 16M. J. Microbiol. 2014, 52, 709–715. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Y.; Bu, Z.; Qian, J.; Chen, Y.; Qiao, L.; Yang, S.; Chen, S.; Wang, X.; Ren, L.; Yang, Y. The UTP-glucose-1-phosphate uridylyltransferase of Brucella melitensis inhibits the activation of NF-kappaB via regulating the bacterial type IV secretion system. Int. J. Biol. Macromol. 2020, 164, 3098–3104. [Google Scholar] [CrossRef]
- Caswell, C.C.; Gaines, J.M.; Roop, R.M. The RNA Chaperone Hfq Independently Coordinates Expression of the VirB Type IV Secretion System and the LuxR-Type Regulator BabR in Brucella abortus 2308. J. Bacteriol. 2012, 194, 3–14. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lei, S.; Zhong, Z.; Ke, Y.; Yang, M.; Xu, X.; Ren, H.; An, C.; Yuan, J.; Yu, J.; Xu, J.; et al. Deletion of the Small RNA Chaperone Protein Hfq down Regulates Genes Related to Virulence and Confers Protection against Wild-Type Brucella Challenge in Mice. Front. Microbiol. 2015, 6, 1570. [Google Scholar] [CrossRef] [PubMed]

| VirB Protein | Species | Survival in Macrophages | Survival in the Mouse Model |
|---|---|---|---|
| VirB1 | Brucella abortus | Necessary [12] | Dispensable [12] |
| VirB2 | Brucella abortus | Necessary [12] | Necessary [12] |
| VirB3–6 | Brucella abortus | Necessary [14] | Necessary [14] |
| VirB7 | Brucella abortus | Necessary [14] | Dispensable [14] |
| VirB8–11 | Brucella abortus | Necessary [14] | Necessary [14] |
| VirB12 | B. melitensis, B. abortus and B. suis | Dispensable [13] | Dispensable [13] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Xiong, X.; Li, B.; Zhou, Z.; Gu, G.; Li, M.; Liu, J.; Jiao, H. The VirB System Plays a Crucial Role in Brucella Intracellular Infection. Int. J. Mol. Sci. 2021, 22, 13637. https://doi.org/10.3390/ijms222413637
Xiong X, Li B, Zhou Z, Gu G, Li M, Liu J, Jiao H. The VirB System Plays a Crucial Role in Brucella Intracellular Infection. International Journal of Molecular Sciences. 2021; 22(24):13637. https://doi.org/10.3390/ijms222413637
Chicago/Turabian StyleXiong, Xue, Bowen Li, Zhixiong Zhou, Guojing Gu, Mengjuan Li, Jun Liu, and Hanwei Jiao. 2021. "The VirB System Plays a Crucial Role in Brucella Intracellular Infection" International Journal of Molecular Sciences 22, no. 24: 13637. https://doi.org/10.3390/ijms222413637
APA StyleXiong, X., Li, B., Zhou, Z., Gu, G., Li, M., Liu, J., & Jiao, H. (2021). The VirB System Plays a Crucial Role in Brucella Intracellular Infection. International Journal of Molecular Sciences, 22(24), 13637. https://doi.org/10.3390/ijms222413637






