Expression of Flavodiiron Proteins Flv2-Flv4 in Chloroplasts of Arabidopsis and Tobacco Plants Provides Multiple Stress Tolerance
Abstract
:1. Introduction
2. Results
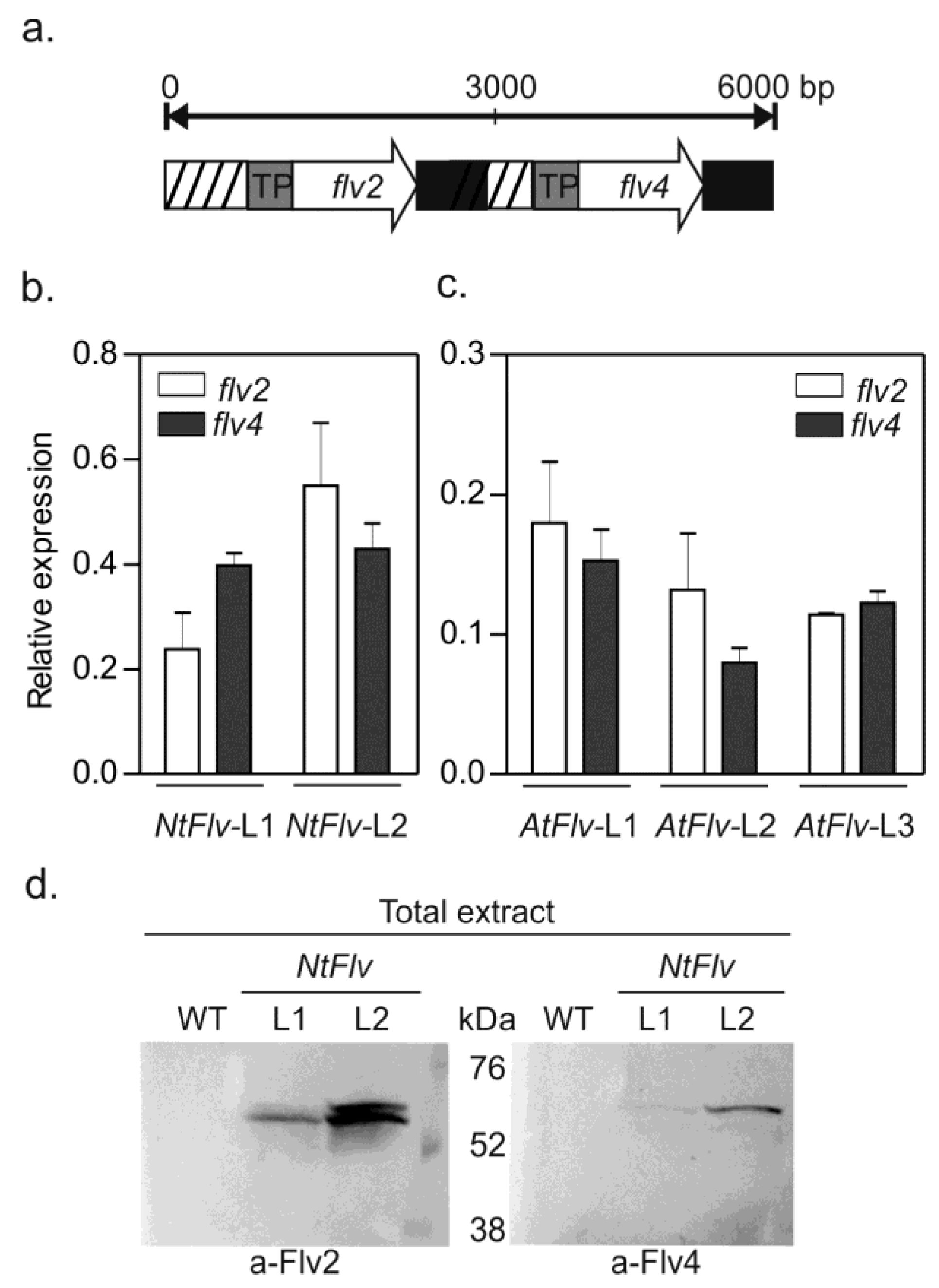
2.1. Preparation of Transgenic Plants Expressing Plastid-Targeted Synechocystis Flv2-Flv4
2.2. Flv2-Flv4 Protected Photosystem II from High Light Intensity in Tobacco Leaves
2.3. Flv2-Flv4 Provided Increased Tolerance to Salt and Oxidative Stress in Tobacco and Arabidopsis
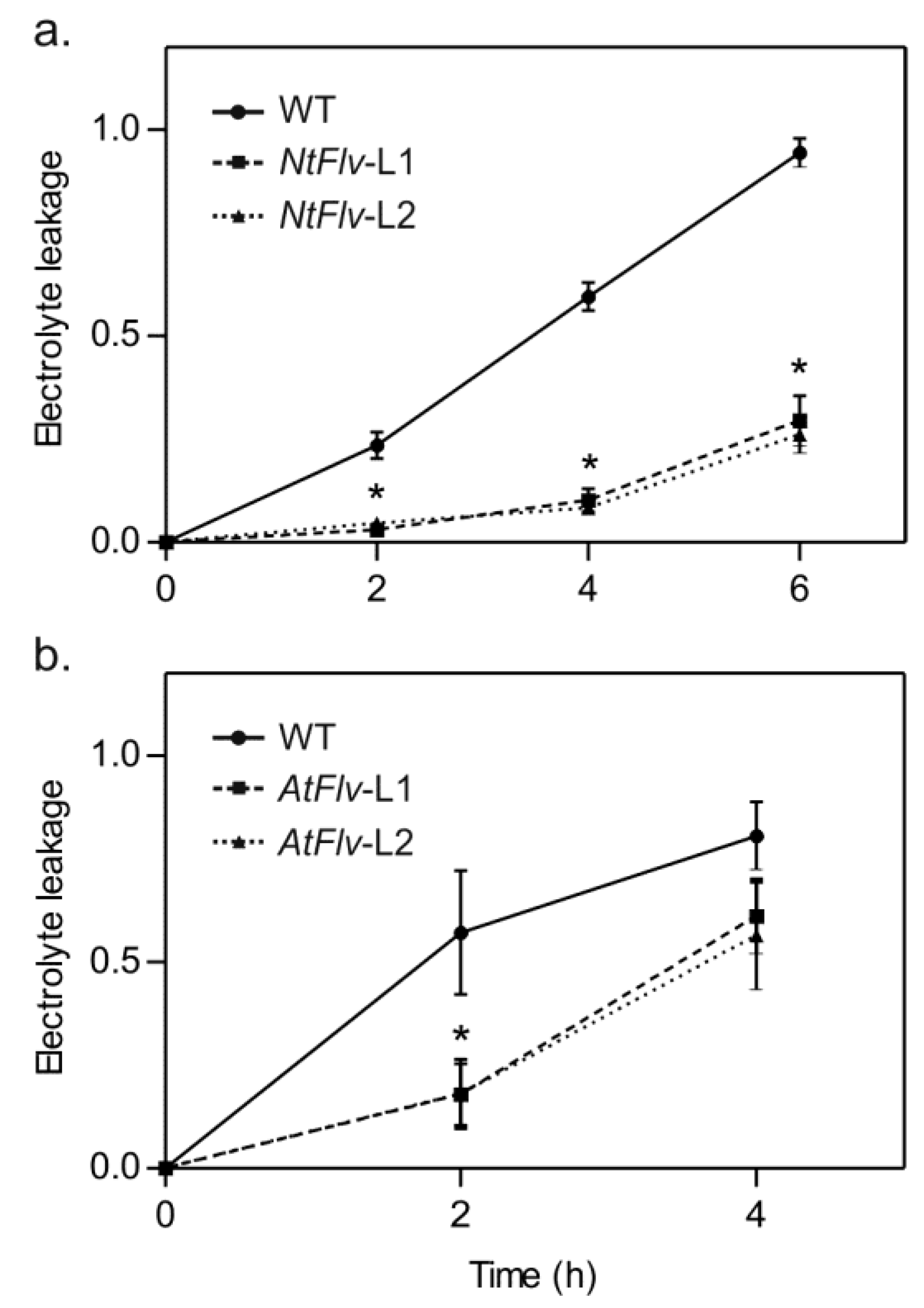
2.4. Chloroplast Flv2-Flv4 Enhanced Drought Tolerance in Tobacco and Arabidopsis Plants
2.5. Metabolic Profiling in Leaves of Drought-Stressed Arabidopsis Plants Expressing Flv2-Flv4
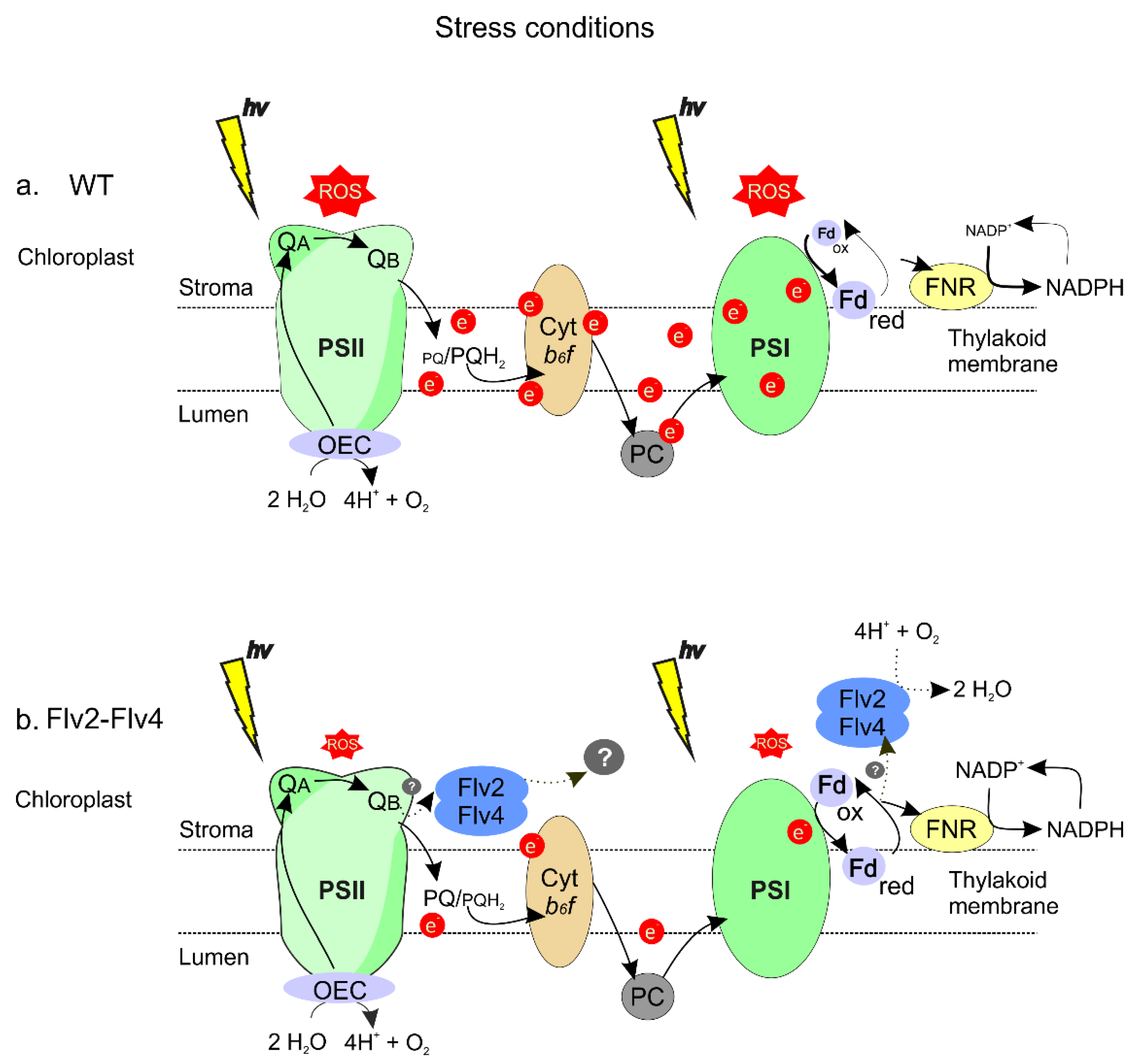
3. Discussion
4. Materials and Methods
4.1. Construction of Binary Vectors
4.2. Plant Transformation and Growth Conditions
4.3. RNA Extraction, cDNA Synthesis, and qRT-PCR Reactions
4.4. Western Blot Analysis of Flv-Expressing Tobacco Lines
4.5. Photoinhibitory Treatment
4.6. Thylakoid Isolation and D1 Degradation Assay
4.7. In Situ Detection of Reactive Oxygen Species
4.8. Salt Stress Treatment
4.9. Oxidative Stress Treatment
4.10. Drought Stress
4.11. Photosynthetic Measurements
4.12. Carbohydrate and Amino Acid Profiling
4.13. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Acknowledgments
Conflicts of Interest
Abbreviations
| PETC | Photosynthetic electron transport chain |
| ROS | Reactive oxygen species |
| AET | Alternative electron transport |
| PS | Photosystem |
| CET | Cyclic electron transport |
| NPQ | Non-photochemical quenching |
| FDPs | Flavodiiron proteins |
| Fd | Ferredoxin |
| QB | Quinone-B |
| WT | Wild type |
| TP | Transit peptide |
| FNR | Ferredoxin-NADP+ reductase |
| CaMV-35S | Cauliflower mosaic virus 35S |
| SDS-PAGE | Sodium dodecyl sulfate polyacrylamide gel electrophoresis |
| GFP | Green fluorescent protein |
| Chl | Chlorophyll |
| CLSM | Confocal laser scanning microscopy |
| Fv/Fm | Maximum efficiency of PSII |
| DCFDA | 2′,7′-dichlorofluorescein diacetate |
| AU | Arbitrary units |
| 0.5xMS-agar | Half-strength Murashige and Skoog medium |
| MV | Methyl viologen |
| FC | Field capacity |
| GABA | γ-amino butyric acid |
| EEE | Excess excitation energy |
| OEC | Oxygen evolving complex |
| PC | Plastocyanin |
| Cyt b6f | Cytochrome b6/f |
| DTT | Dithiothreitol |
| PMSF | Phenylmethylsulfonyl fluoride |
References
- Noctor, G.; Reichheld, J.-P.; Foyer, C.H. ROS-Related Redox Regulation and Signaling in Plants. Semin. Cell Dev. Biol. 2018, 80, 3–12. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Czarnocka, W.; Karpiński, S. Friend or Foe? Reactive Oxygen Species Production, Scavenging and Signaling in Plant Response to Environmental Stresses. Free Radic. Biol. Med. 2018, 122, 4–20. [Google Scholar] [CrossRef] [PubMed]
- Gollan, P.J.; Lima-Melo, Y.; Tiwari, A.; Tikkanen, M.; Aro, E.-M. Interaction between Photosynthetic Electron Transport and Chloroplast Sinks Triggers Protection and Signalling Important for Plant Productivity. Philos. Trans. R. Soc. B Biol. Sci. 2017, 372, 20160390. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gómez, R.; Vicino, P.; Carrillo, N.; Lodeyro, A.F. Manipulation of Oxidative Stress Responses as a Strategy to Generate Stress-Tolerant Crops. From Damage to Signaling to Tolerance. Crit. Rev. Biotechnol. 2019, 39. [Google Scholar] [CrossRef] [PubMed]
- Rantala, S.; Lempiäinen, T.; Gerotto, C.; Tiwari, A.; Aro, E.-M.; Tikkanen, M. PGR5 and NDH-1 Systems Do Not Function as Protective Electron Acceptors but Mitigate the Consequences of PSI Inhibition. Biochim. Biophys. Acta Bioenerg. 2020, 1861, 148154. [Google Scholar] [CrossRef]
- Vicente, J.B.; Justino, M.C.; Gonçalves, V.L.; Saraiva, L.M.; Teixeira, M. Chapter 2—Biochemical, Spectroscopic, and Thermodynamic Properties of Flavodiiron Proteins. In Globins and Other Nitric Oxide-Reactive Proteins, Part, B; Poole, R., Ed.; Academic Press: Cambridge, MA, USA, 2008; Volume 437, pp. 21–45. [Google Scholar]
- Allahverdiyeva, Y.; Isojärvi, J.; Zhang, P.; Aro, E.-M. Cyanobacterial Oxygenic Photosynthesis Is Protected by Flavodiiron Proteins. Life 2015, 5. [Google Scholar] [CrossRef]
- Ilík, P.; Pavlovič, A.; Kouřil, R.; Alboresi, A.; Morosinotto, T.; Allahverdiyeva, Y.; Aro, E.-M.; Yamamoto, H.; Shikanai, T. Alternative Electron Transport Mediated by Flavodiiron Proteins Is Operational in Organisms from Cyanobacteria up to Gymnosperms. New Phytol. 2017, 214, 967–972. [Google Scholar] [CrossRef] [Green Version]
- Folgosa, F.; Martins, M.C.; Teixeira, M. Diversity and Complexity of Flavodiiron NO/O2 Reductases. FEMS Microbiol. Lett. 2017, 365. [Google Scholar] [CrossRef] [Green Version]
- Bersanini, L.; Battchikova, N.; Jokel, M.; Rehman, A.; Vass, I.; Allahverdiyeva, Y.; Aro, E.-M. Flavodiiron Protein Flv2/Flv4-Related Photoprotective Mechanism Dissipates Excitation Pressure of PSII in Cooperation with Phycobilisomes in Cyanobacteria. Plant Physiol. 2014, 164, 805–818. [Google Scholar] [CrossRef] [Green Version]
- Santana-Sánchez, A.; Solymosi, D.; Mustila, H.; Bersanini, L.; Aro, E.-M.; Allahverdiyeva, Y. Flavodiiron Proteins 1-to-4 Function in Versatile Combinations in O2 Photoreduction in Cyanobacteria. eLife 2019, 8, e45766. [Google Scholar] [CrossRef]
- Alboresi, A.; Storti, M.; Morosinotto, T. Balancing Protection and Efficiency in the Regulation of Photosynthetic Electron Transport across Plant Evolution. New Phytol. 2019, 221, 105–109. [Google Scholar] [CrossRef] [Green Version]
- Allahverdiyeva, Y.; Mustila, H.; Ermakova, M.; Bersanini, L.; Richaud, P.; Ajlani, G.; Battchikova, N.; Cournac, L.; Aro, E.-M. Flavodiiron Proteins Flv1 and Flv3 Enable Cyanobacterial Growth and Photosynthesis under Fluctuating Light. Proc. Natl. Acad. Sci. USA 2013, 110, 4111–4116. [Google Scholar] [CrossRef] [Green Version]
- Chaux, F.; Burlacot, A.; Mekhalfi, M.; Auroy, P.; Blangy, S.; Richaud, P.; Peltier, G. Flavodiiron Proteins Promote Fast and Transient O2 Photoreduction in Chlamydomonas. Plant Physiol. 2017, 174, 1825–1836. [Google Scholar] [CrossRef] [Green Version]
- Shimakawa, G.; Ishizaki, K.; Tsukamoto, S.; Tanaka, M.; Sejima, T.; Miyake, C. The Liverwort, Marchantia, Drives Alternative Electron Flow Using a Flavodiiron Protein to Protect PSI. Plant Physiol. 2017, 173, 1636–1647. [Google Scholar] [CrossRef] [Green Version]
- Jokel, M.; Johnson, X.; Peltier, G.; Aro, E.-M.; Allahverdiyeva, Y. Hunting the Main Player Enabling Chlamydomonas reinhardtii Growth under Fluctuating Light. Plant J. 2018, 94, 822–835. [Google Scholar] [CrossRef] [Green Version]
- Storti, M.; Alboresi, A.; Gerotto, C.; Aro, E.-M.; Finazzi, G.; Morosinotto, T. Role of Cyclic and Pseudo-Cyclic Electron Transport in Response to Dynamic Light Changes in Physcomitrella patens. Plant Cell Environ. 2019, 42, 1590–1602. [Google Scholar] [CrossRef]
- Storti, M.; Segalla, A.; Mellon, M.; Alboresi, A.; Morosinotto, T. Regulation of Electron Transport Is Essential for Photosystem I Stability and Plant Growth. New Phytol. 2020, 228, 1316–1326. [Google Scholar] [CrossRef]
- Yamamoto, H.; Takahashi, S.; Badger, M.R.; Shikanai, T. Artificial Remodelling of Alternative Electron Flow by Flavodiiron Proteins in Arabidopsis. Nat. Plants 2016, 2, 16012. [Google Scholar] [CrossRef]
- Gómez, R.; Carrillo, N.; Morelli, M.P.; Tula, S.; Shahinnia, F.; Hajirezaei, M.-R.; Lodeyro, A.F. Faster Photosynthetic Induction in Tobacco by Expressing Cyanobacterial Flavodiiron Proteins in Chloroplasts. Photosynth. Res. 2018, 136, 129–138. [Google Scholar] [CrossRef]
- Wada, S.; Yamamoto, H.; Suzuki, Y.; Yamori, W.; Shikanai, T.; Makino, A. Flavodiiron Protein Substitutes for Cyclic Electron Flow without Competing CO2 Assimilation in Rice. Plant Physiol. 2018, 176, 1509–1518. [Google Scholar] [CrossRef] [Green Version]
- Tula, S.; Shahinnia, F.; Melzer, M.; Rutten, T.; Gómez, R.; Lodeyro, A.F.; von Wirén, N.; Carrillo, N.; Hajirezaei, M.-R. Providing an Additional Electron Sink by the Introduction of Cyanobacterial Flavodiirons Enhances the Growth of Arabidopsis thaliana in Varying Light. Front. Plant Sci. 2020, 11, 902. [Google Scholar] [CrossRef] [PubMed]
- Zhang, P.; Eisenhut, M.; Brandt, A.-M.; Carmel, D.; Silén, H.M.; Vass, I.; Allahverdiyeva, Y.; Salminen, T.A.; Aro, E.-M. Operon flv4-flv2 Provides Cyanobacterial Photosystem II with Flexibility of Electron Transfer. Plant Cell 2012, 24, 1952–1971. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, P.; Allahverdiyeva, Y.; Eisenhut, M.; Aro, E.-M. Flavodiiron Proteins in Oxygenic Photosynthetic Organisms: Photoprotection of Photosystem II by Flv2 and Flv4 in Synechocystis sp. PCC 6803. PLoS ONE 2009, 4, e5331. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chukhutsina, V.; Bersanini, L.; Aro, E.-M.; van Amerongen, H. Cyanobacterial flv4-2 Operon-Encoded Proteins Optimize Light Harvesting and Charge Separation in Photosystem II. Mol. Plant 2015, 8, 747–761. [Google Scholar] [CrossRef] [Green Version]
- Bersanini, L.; Allahverdiyeva, Y.; Battchikova, N.; Heinz, S.; Lespinasse, M.; Ruohisto, E.; Mustila, H.; Nickelsen, J.; Vass, I.; Aro, E.-M. Dissecting the Photoprotective Mechanism Encoded by the flv4-2 Operon: A Distinct Contribution of Sll0218 in Photosystem II Stabilization. Plant. Cell Environ. 2017, 40, 378–389. [Google Scholar] [CrossRef] [Green Version]
- Pätsikkä, E.; Aro, E.-M.; Tyystjärvi, E. Increase in the Quantum Yield of Photoinhibition Contributes to Copper Toxicity In Vivo. Plant Physiol. 1998, 117, 619–627. [Google Scholar] [CrossRef] [Green Version]
- Kato, Y.; Sun, X.; Zhang, L.; Sakamoto, W. Cooperative D1 Degradation in the Photosystem II Repair Mediated by Chloroplastic Proteases in Arabidopsis. Plant Physiol. 2012, 159, 1428–1439. [Google Scholar] [CrossRef] [Green Version]
- Baker, N.R. Chlorophyll Fluorescence: A Probe of Photosynthesis In Vivo. Annu. Rev. Plant Biol. 2008, 59, 89–113. [Google Scholar] [CrossRef] [Green Version]
- Khorobrykh, S.; Havurinne, V.; Mattila, H.; Tyystjärvi, E. Oxygen and ROS in Photosynthesis. Plants 2020, 9. [Google Scholar] [CrossRef] [Green Version]
- Daghastanli, N.A.; Itri, R.; Baptista, M.S. Singlet Oxygen Reacts with 2′,7′-dichlorodihydrofluorescein and Contributes to the Formation of 2′,7′-dichlorofluorescein. Photochem. Photobiol. 2008, 84, 1238–1243. [Google Scholar] [CrossRef]
- Reiniers, M.J.; van Golen, R.F.; Bonnet, S.; Broekgaarden, M.; van Gulik, T.M.; Egmond, M.R.; Heger, M. Preparation and Practical Applications of 2′,7′-dichlorodihydrofluorescein in Redox Assays. Anal. Chem. 2017, 89, 3853–3857. [Google Scholar] [CrossRef]
- Murashige, T.; Skoog, F. A Revised Medium for Rapid Growth and Bioassays with Tobacco Tissue Culture. Physiol. Plant 1962, 15, 473–497. [Google Scholar] [CrossRef]
- Nelson, N.; Ben-Shem, A. The Complex Architecture of Oxygenic Photosynthesis. Nat. Rev. Mol. Cell Biol. 2004, 5, 971–982. [Google Scholar] [CrossRef]
- Tietz, S.; Hall, C.C.; Cruz, J.A.; Kramer, D.M. NPQ(T): A Chlorophyll Fluorescence Parameter for Rapid Estimation and Imaging of Non-Photochemical Quenching of Excitons in Photosystem-II-Associated Antenna Complexes. Plant Cell Environ. 2017, 40, 1243–1255. [Google Scholar] [CrossRef]
- Lodeyro, A.F.; Carrillo, N. Salt Stress in Higher Plants: Mechanisms of Toxicity and Defensive Responses. In Stress Responses in Plants; Tripathi, B.N., Müller, M., Eds.; Springer: Berlin, Germany, 2015; pp. 1–33. [Google Scholar]
- Loutfy, N.; El-Tayeb, M.A.; Hassanen, A.M.; Moustafa, M.F.M.; Sakuma, Y.; Inouhe, M. Changes in the Water Status and Osmotic Solute Contents in Response to Drought and Salicylic Acid Treatments in Four Different Cultivars of Wheat (Triticum aestivum). J. Plant Res. 2012, 125, 173–184. [Google Scholar] [CrossRef]
- Guo, R.; Shi, L.; Jiao, Y.; Li, M.; Zhong, X.; Gu, F.; Liu, Q.; Xia, X.; Li, H. Metabolic Responses to Drought Stress in the Tissues of Drought-Tolerant and Drought-Sensitive Wheat Genotype Seedlings. AoB PLANTS 2018, 10. [Google Scholar] [CrossRef] [Green Version]
- Fàbregas, N.; Fernie, A.R. The Metabolic Response to Drought. J. Exp. Bot. 2019, 70, 1077–1085. [Google Scholar] [CrossRef] [Green Version]
- Batista-Silva, W.; Heinemann, B.; Rugen, N.; Nunes-Nesi, A.; Araújo, W.L.; Braun, H.-P.; Hildebrandt, T.M. The Role of Amino Acid Metabolism during Abiotic Stress Release. Plant Cell Environ. 2019, 42, 1630–1644. [Google Scholar] [CrossRef] [Green Version]
- Gibson, D.G.; Young, L.; Chuang, R.-Y.; Venter, J.C.; Hutchison, C.A.; Smith, H.O. Enzymatic Assembly of DNA Molecules up to Several Hundred Kilobases. Nat. Methods 2009, 6, 343–345. [Google Scholar] [CrossRef]
- Clough, S.J.; Bent, A.F. Floral Dip: A Simplified method for Agrobacterium-Mediated Transformation of Arabidopsis thaliana. Plant J. 1998, 16, 735–743. [Google Scholar] [CrossRef] [Green Version]
- Gallois, P.; Marinho, P. Leaf Disk Transformation Using Agrobacterium tumefaciens—Expression of Heterologous Genes in Tobacco. In Plant Gene Transfer and Expression Protocols; Jones, H., Ed.; Springer: Berlin, Germany, 1995; pp. 39–48. [Google Scholar]
- Logemann, J.; Shell, J.; Willmitzer, L. Improved Method for the Isolation of RNA from Plant Tissues. Anal. Biochem. 1987, 20, 16–20. [Google Scholar] [CrossRef]
- Schmittgen, T.D.; Livak, K.J. Analyzing Real-Time PCR Data by the Comparative C T Method. Nat. Protoc. 2008, 3, 1101. [Google Scholar] [CrossRef] [PubMed]
- Camm, E.L.; Green, B.R. Fractionation of Thylakoid Membranes with the Nonionic Detergent Octyl-β-D-Glucopyranoside. Plant Physiol. 1980, 66, 428–432. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Porra, R.J.; Thompson, W.A.; Kriedemann, P.E. Determination of Accurate Extinction Coefficients and Simultaneous Equations for Assaying Chlorophylls a and b Extracted with Four Different Solvents: Verification of the Concentration of Chlorophyll Standards by Atomic Absorption Spectroscopy. Biochim. Biophys. Acta Bioenerg. 1989, 975, 384–394. [Google Scholar] [CrossRef]
- Schindelin, J.; Arganda-Carreras, I.; Frise, E.; Kaynig, V.; Longair, M.; Pietzsch, T.; Preibisch, S.; Rueden, C.; Saalfeld, S.; Schmid, B.; et al. Fiji: An Open-Source Platform for Biological-Image Analysis. Nat. Methods 2012, 9, 676–682. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mayta, M.L.; Lodeyro, A.F.; Guiamet, J.J.; Tognetti, V.B.; Melzer, M.; Hajirezaei, M.R.; Carrillo, N. Expression of a Plastid-Targeted Flavodoxin Decreases Chloroplast Reactive Oxygen Species Accumulation and Delays Senescence in Aging Tobacco Leaves. Front. Plant Sci. 2018, 9, 1039. [Google Scholar] [CrossRef] [PubMed]
- Lichtenthaler, H.K.B.T.-M. Chlorophylls and Carotenoids: Pigments of Photosynthetic Biomembranes. In Methods in Enzymology; Academic Press: Orlando, FL, USA, 1987; Volume 148, pp. 350–382. [Google Scholar]
- Tognetti, V.B.; Palatnik, J.F.; Fillat, M.F.; Melzer, M.; Hajirezaei, M.-R.; Valle, E.M.; Carrillo, N. Functional Replacement of Ferredoxin by a Cyanobacterial Flavodoxin in Tobacco Confers Broad-Range Stress Tolerance. Plant Cell 2006, 18, 2035–2050. [Google Scholar] [CrossRef] [Green Version]
- Kuhlgert, S.; Austic, G.; Zegarac, R.; Osei-Bonsu, I.; Hoh, D.; Chilvers, M.I.; Roth, M.G.; Bi, K.; TerAvest, D.; Weebadde, P.; et al. MultispeQ Beta: A Tool for Large-Scale Plant Phenotyping Connected to the Open photosynQ Network. R. Soc. Open Sci. 2016, 3, 160592. [Google Scholar] [CrossRef] [Green Version]
- Ahkami, A.H.; Melzer, M.; Ghaffari, M.R.; Pollmann, S.; Ghorbani Javid, M.; Shahinnia, F.; Hajirezaei, M.R.; Druege, U. Distribution of Indole-3-acetic Acid in Petunia hybrida Shoot Tip Cuttings and Relationship between Auxin Transport, Carbohydrate Metabolism and Adventitious Root Formation. Planta 2013, 238, 499–517. [Google Scholar] [CrossRef] [Green Version]






Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vicino, P.; Carrillo, J.; Gómez, R.; Shahinnia, F.; Tula, S.; Melzer, M.; Rutten, T.; Carrillo, N.; Hajirezaei, M.-R.; Lodeyro, A.F. Expression of Flavodiiron Proteins Flv2-Flv4 in Chloroplasts of Arabidopsis and Tobacco Plants Provides Multiple Stress Tolerance. Int. J. Mol. Sci. 2021, 22, 1178. https://doi.org/10.3390/ijms22031178
Vicino P, Carrillo J, Gómez R, Shahinnia F, Tula S, Melzer M, Rutten T, Carrillo N, Hajirezaei M-R, Lodeyro AF. Expression of Flavodiiron Proteins Flv2-Flv4 in Chloroplasts of Arabidopsis and Tobacco Plants Provides Multiple Stress Tolerance. International Journal of Molecular Sciences. 2021; 22(3):1178. https://doi.org/10.3390/ijms22031178
Chicago/Turabian StyleVicino, Paula, Julieta Carrillo, Rodrigo Gómez, Fahimeh Shahinnia, Suresh Tula, Michael Melzer, Twan Rutten, Néstor Carrillo, Mohammad-Reza Hajirezaei, and Anabella F. Lodeyro. 2021. "Expression of Flavodiiron Proteins Flv2-Flv4 in Chloroplasts of Arabidopsis and Tobacco Plants Provides Multiple Stress Tolerance" International Journal of Molecular Sciences 22, no. 3: 1178. https://doi.org/10.3390/ijms22031178
APA StyleVicino, P., Carrillo, J., Gómez, R., Shahinnia, F., Tula, S., Melzer, M., Rutten, T., Carrillo, N., Hajirezaei, M.-R., & Lodeyro, A. F. (2021). Expression of Flavodiiron Proteins Flv2-Flv4 in Chloroplasts of Arabidopsis and Tobacco Plants Provides Multiple Stress Tolerance. International Journal of Molecular Sciences, 22(3), 1178. https://doi.org/10.3390/ijms22031178





