Design of RGDS Peptide-Immobilized Self-Assembling β-Strand Peptide from Barnacle Protein
Abstract
1. Introduction
2. Results and Discussion
2.1. Peptide Synthesis
2.2. Secondary Structure Analysis by Infrared Absorption (IR) Spectrum
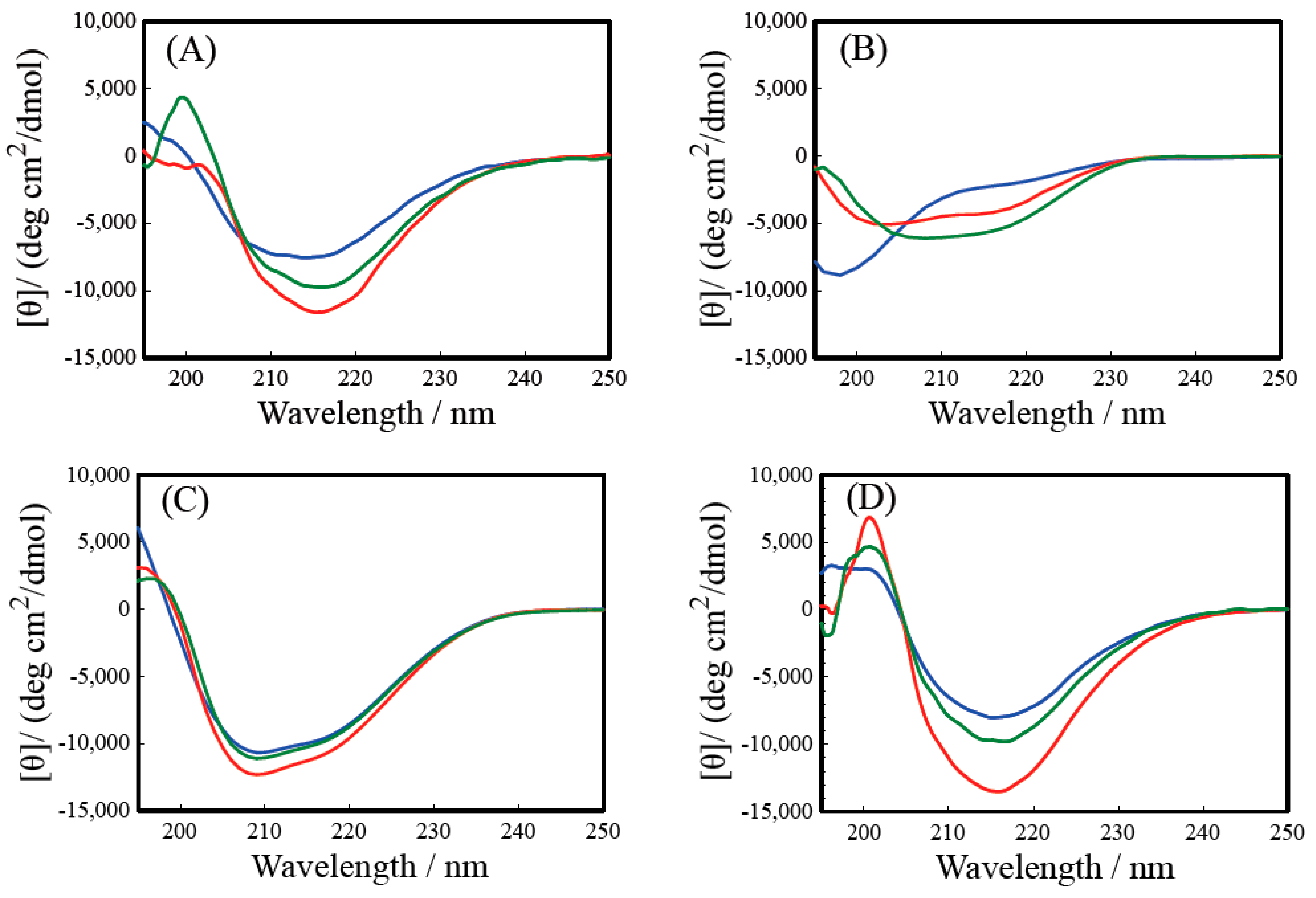
2.3. Secondary Structure Analysis by Circular Dichroism (CD) Spectrum
2.4. Preparation of Peptide Hydrogel and Evaluation of Physical Properties
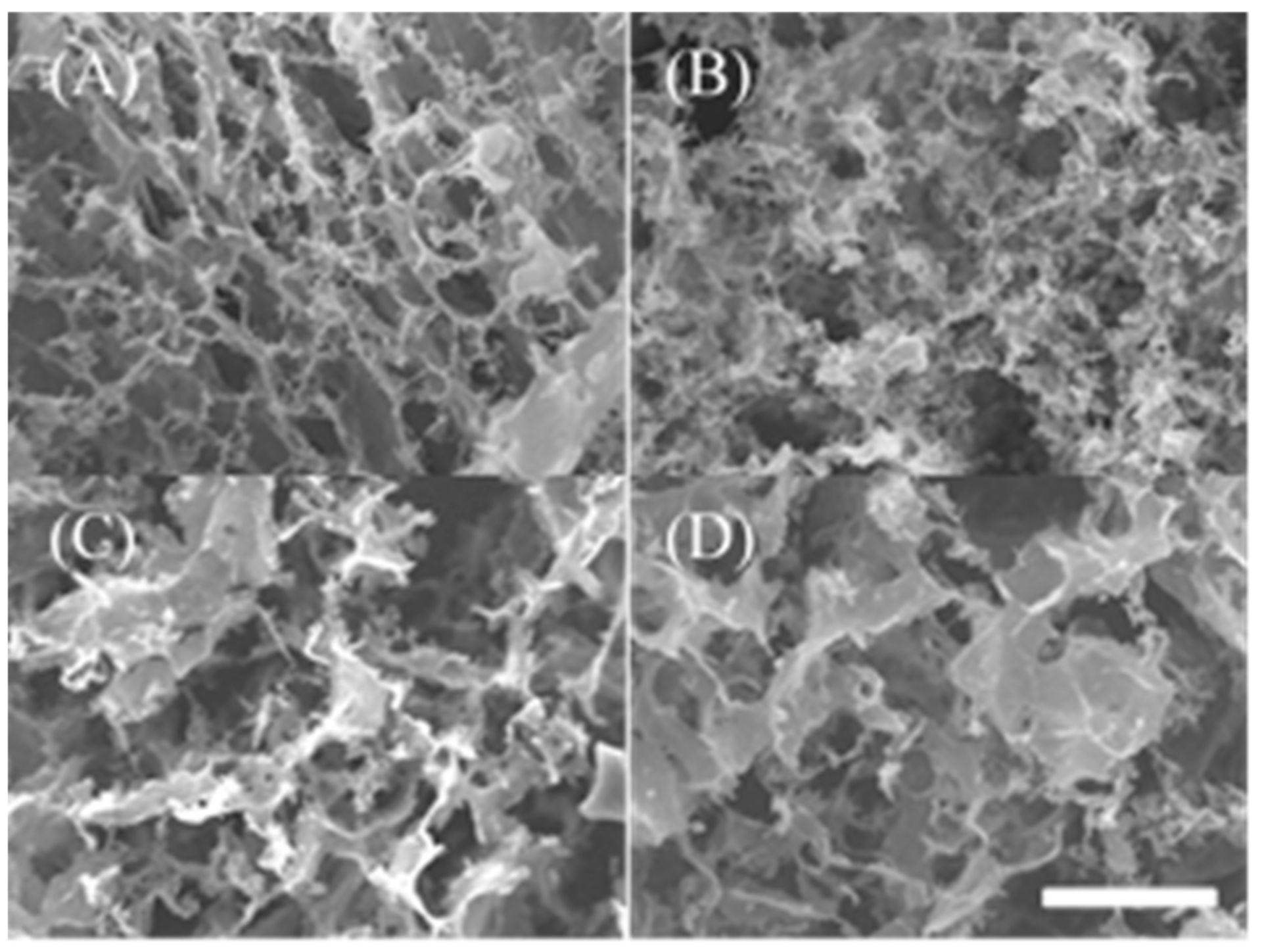
2.5. Observation of Microstructure of Peptide Hydrogel with Scanning Electron Microscope (SEM)
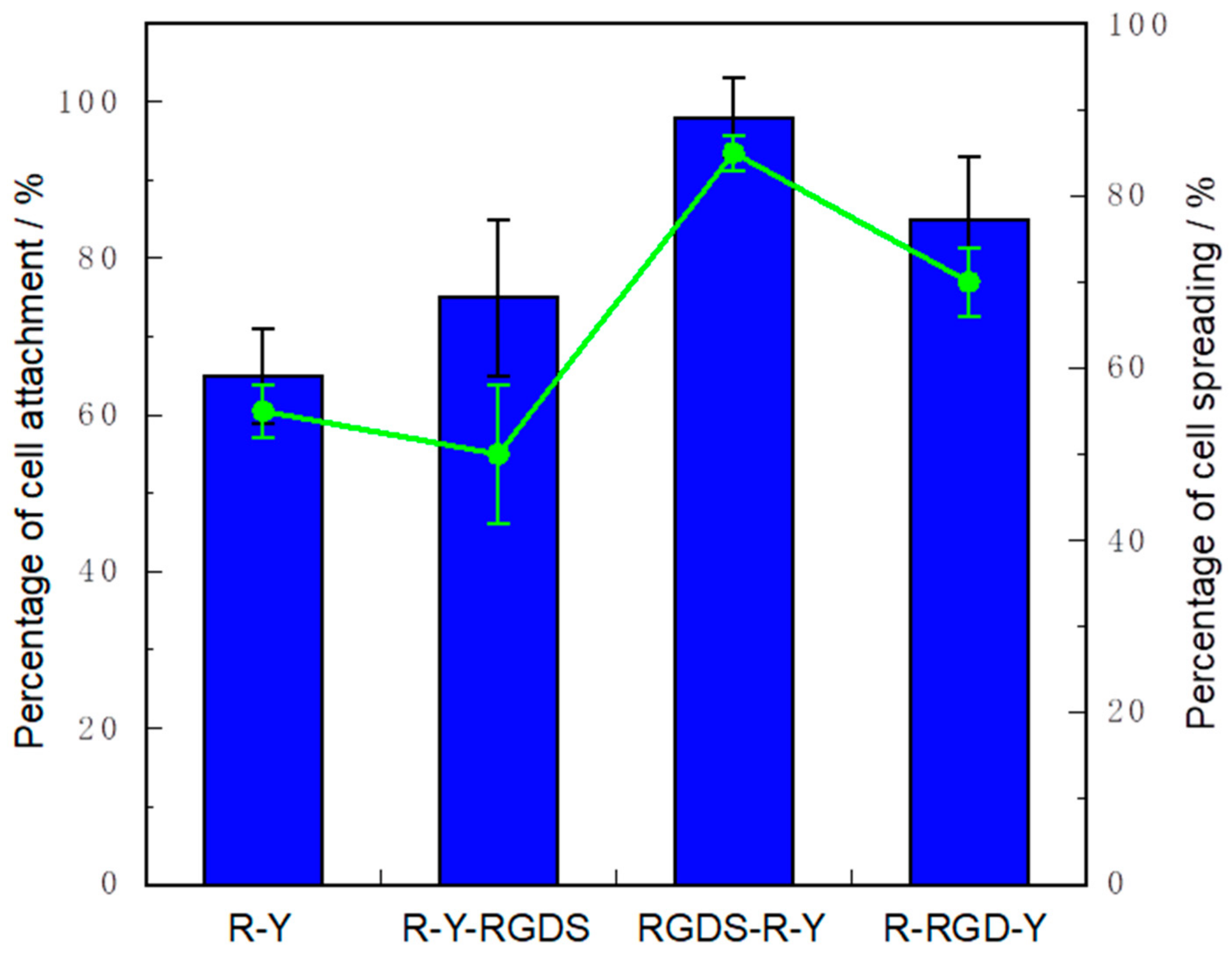
2.6. Cell Culture on a Peptide-Immobilized Substrate
3. Materials and Method
3.1. Materials
3.2. Peptide Synthesis
3.3. Secondary Structure Analysis by Infrared Absorption (IR) Spectrum
3.4. Secondary Structure Analysis by Circular Dichroism (CD) Spectrum
3.5. Preparation of Peptide Hydrogel and Evaluation of Physical Properties
3.6. Observation of Microstructure of Peptide Hydrogel with Scanning Electron Microscope (SEM)
3.6.1. Cells and Culture
3.6.2. Preparation of Peptide-Immobilized Cell Culture Plate
3.6.3. Cell Culture on a Peptide-Immobilized Substrate
4. Summary
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Chen, J.; Zou, X. Self-assemble peptide biomaterials and their biomedical applications. Bioact. Mater. 2019, 4, 120–131. [Google Scholar] [CrossRef]
- Liu, J.; Song, H.; Zhang, L.; Xu, H.; Zhao, X. Self-Assembly-Peptide Hydrogels as Tissue-Engineering Scaffolds for Three-Dimensional Culture of Chondrocytes in vitro. Macromol. Biosci. 2010, 10, 1164–1170. [Google Scholar] [CrossRef] [PubMed]
- Hendricks, M.P.; Sato, K.; Palmer, L.C.; Stupp, S.I. Supramolecular Assembly of Peptide Amphiphiles. Acc. Chem. Res. 2017, 50, 2440–2448. [Google Scholar] [CrossRef] [PubMed]
- Lombardo, D.; Calandra, P.; Pasqua, L.; Magazù, S. Self-Assembly of Organic Nanomaterials and Biomaterials: The Bottom-Up Approach for Functional Nanostructures Formation and Advanced Applications. Materials 2020, 13, 1048. [Google Scholar] [CrossRef] [PubMed]
- Tang, J.D.; Mura, C.; Lampe, K.J. Stimuli-Responsive, Pentapeptide, Nanofiber Hydrogel for Tissue Engineering. J. Am. Chem. Soc. 2019, 141, 4886–4899. [Google Scholar] [CrossRef] [PubMed]
- Sheffield, C.; Meyers, K.; Johnson, E.; Rajachar, R.M. Application of Composite Hydrogels to Control Physical Properties in Tissue Engineering and Regenerative Medicine. Gels 2018, 4, 51. [Google Scholar] [CrossRef]
- Pugliese, R.; Gelain, F. Peptidic Biomaterials: From Self-Assembling to Regenerative Medicine. Trends Biotechnol. 2017, 35, 145–158. [Google Scholar] [CrossRef]
- Matsuura, K. Synthetic approaches to construct viral capsid-like spherical nanomaterials. Chem. Commun. 2018, 54, 8944–8959. [Google Scholar] [CrossRef]
- Inaba, H.; Matsuura, K. Peptide Nanomaterials Designed from Natural Supramolecular Systems. Chem. Rec. 2019, 19, 843–858. [Google Scholar] [CrossRef]
- Yokoi, H.; Kinoshita, T.; Zhang, S. Dynamic reassembly of peptide RADA16 nanofiber scaffold. Proc. Natl. Acad. Sci. USA 2005, 102, 8414–8419. [Google Scholar] [CrossRef]
- Zhang, S.; Holmes, T.C.; DiPersio, C.; Hynes, R.O.; Su, X.; Rich, A. Self-complementary oligopeptide matrices support mammalian cell attachment. Biomaterials 1995, 16, 1385–1393. [Google Scholar] [CrossRef]
- Luo, Z.; Zhao, X.; Zhang, S. Structural Dynamic of a Self-Assembling Peptide d-EAK16 Made of Only D-Amino Acids. PLoS ONE 2008, 3, e2364. [Google Scholar] [CrossRef]
- Schneider, J.P.; Pochan, D.J.; Ozbas, B.; Rajagopal, K.; Pakstis, L.; Kretsinger, J. Responsive hydrogels from the intramolecu-lar folding and self-assembly of a designed peptide. J. Am. Chem. Soc. 2002, 124, 15030–15037. [Google Scholar] [CrossRef]
- Pochan, D.J.; Schneider, J.P.; Kretsinger, J.; Ozbas, B.; Rajagopal, K.; Haines, L. Thermally reversible hydrogels via intramo-lecular folding and consequent self-assembly of a de novo designed peptide. J. Am. Chem. Soc. 2003, 125, 11802–11803. [Google Scholar] [CrossRef] [PubMed]
- Ozbas, B.; Rajagopal, K.; Schneider, J.P.; Pochan, D.J. Semiflexible chain networks formed via self-assembly of beta-hairpin molecules. Phys. Rev. Lett. 2004, 93, 268106. [Google Scholar] [CrossRef]
- Kretsinger, J.K.; Haines, L.A.; Ozbas, B.; Pochan, D.J.; Schneider, J.P. Cytocompatibility of self-assembled beta-hairpin pep-tide hydrogel surfaces. Biomaterials 2005, 26, 5177–5186. [Google Scholar] [CrossRef] [PubMed]
- Haines-Butterick, L.; Rajagopal, K.; Branco, M.; Salick, D.; Rughani, R.; Pilarz, M.; Lamm, M.S.; Pochan, D.J.; Schneider, J.P. Controlling hydrogelation kinetics by peptide design for three-dimensional encapsulation and injectable delivery of cells. Proc. Natl. Acad. Sci. USA 2007, 104, 7791–7796. [Google Scholar] [CrossRef] [PubMed]
- Haines-Butterick, L.A.; Salick, D.A.; Pochan, D.J.; Schneider, J.P. In vitro assessment of the pro-inflammatory potential of beta-hairpin peptide hydrogels. Biomaterials 2008, 9, 4164–4169. [Google Scholar] [CrossRef] [PubMed]
- Rajagopal, L.A.; Lamm, M.S.; Haines-Butterick, L.A.; Pochan, D.J.L.A.; Schneider, J.P.L.A. Tuning the pH responsiveness of beta-hairpin peptide folding, self-assembly, and hydrogel material formation. Biomacromolecules 2009, 10, 2619–2625. [Google Scholar] [CrossRef]
- Yan, C.; Altunbas, A.; Yucel, T.; Nagarkar, R.P.; Schneider, J.P.; Pochan, D.J. Injectable solid hydrogel: Mechanism of shear-thinning and immediate recovery of injectable β-hairpin peptide hydrogels. Soft Matter 2010, 6, 5143–5156. [Google Scholar] [CrossRef]
- Yan, C.; Mackay, M.E.; Czymmek, K.; Nagarkar, R.P.; Schneider, J.P.; Pochan, D.J. Injectable Solid Peptide Hydrogel as a Cell Carrier: Effects of Shear Flow on Hydrogels and Cell Payload. Langmuir 2012, 28, 6076–6087. [Google Scholar] [CrossRef] [PubMed]
- Smith, D.J.; Brat, G.A.; Medina, S.H.; Tong, D.; Huang, Y.; Grahammer, J.; Furtmüller, G.J.; Oh, B.C.; Nagy-Smith, K.J.; Walczak, P.; et al. A multiphase transitioning peptide hydrogel for suturing ultrasmall vessels. Nat. Nanotechnol. 2016, 11, 95–102. [Google Scholar] [CrossRef]
- Yamada, Y.; Patel, N.L.; Kalen, J.D.; Schneider, J.P. Design of a Peptide-Based Electronegative Hydrogel for the Direct Encapsulation, 3D Culturing, in Vivo Syringe-Based Delivery, and Long-Term Tissue Engraftment of Cells. ACS Appl. Mater. Interfaces 2019, 11, 34688–34697. [Google Scholar] [CrossRef] [PubMed]
- Jayawarna, V.; Ali, M.; Jowitt, T.A.; Miller, A.F.; Saiani, A.; Gough, J.E.; Ulijn, R.V. Nanostructured Hydrogels for Three-Dimensional Cell Culture Through Self-Assembly of Fluorenylmethoxycarbonyl–Dipeptides. Adv. Mater. 2006, 18, 611–614. [Google Scholar] [CrossRef]
- Mahler, A.; Reches, M.; Rechter, M.; Cohen, S.; Gazit, E. Rigid, Self-Assembled Hydrogel Composed of a Modified Aromatic Dipeptide. Adv. Mater. 2006, 18, 1365–1370. [Google Scholar] [CrossRef]
- Zhou, M.; Smith, A.M.; Das, A.K.; Hodson, N.W.; Collins, R.F.; Ulijn, R.V.; Gough, J.E. Self-assembled peptide-based hydrogels as scaffolds for anchorage-dependent cells. Biomaterials 2009, 30, 2523–2530. [Google Scholar] [CrossRef] [PubMed]
- Ischakov, R.; Adler-Abramovich, L.; Buzhansky, L.; Shekhter, T.; Gazit, E. Peptide-based hydrogel nanoparticles as effective drug delivery agents. Bioorg. Med. Chem. 2013, 21, 3517–3522. [Google Scholar] [CrossRef]
- Diaferia, C.; Morelli, G.; Accardo, A. Fmoc-diphenylalanine as a suitable building block for the preparation of hybrid materials and their potential applications. J. Mater. Chem. B 2019, 7, 5142–5155. [Google Scholar] [CrossRef]
- Oyen, E.; Martin, C.; Caveliers, V.; Madder, A.; Van Mele, B.; Hoogenboom, R.; Hernot, S.; Ballet, S. In Vivo Imaging of the Stability and Sustained Cargo Release of an Injectable Amphipathic Peptide-Based Hydrogel. Biomacromolecules 2017, 18, 994–1001. [Google Scholar] [CrossRef]
- Francis, N.L.; Zhao, N.; Calvelli, H.R.; Saini, A.; Gifford, J.J.; Wagner, G.C.; Cohen, R.I.; Pang, Z.P.; Moghe, P.V. Peptide-Based Scaffolds for the Culture and Transplantation of Human Dopaminergic Neurons. Tissue Eng. Part A 2019. [Google Scholar] [CrossRef]
- Davies, R.P.W.; Liu, B.; Maude, S.; Carrick, L.M.; Nyrkova, I.; McLeish, T.C.; Harris, S.A. Peptide strand length controls the energetics of self-assembly and morphology of β-sheet fibrils. Pept. Sci. 2018, 110, e23073. [Google Scholar] [CrossRef] [PubMed]
- Lee, N.R.; Bowerman, C.J.; Nilsson, B.L. Sequence length determinants for self-assembly of amphipathic beta-sheet peptides. Biopolymers 2013, 100, 738–850. [Google Scholar] [CrossRef] [PubMed]
- Janek, K.; Behlke, J.; Zipper, J.; Fabian, H.; Georgalis, Y.; Beyermann, M.; Bienert, M.; Krause, E. Water-soluble beta-sheet models which self-assemble into fibrillar structures. Biochemistry 1999, 38, 8246–8252. [Google Scholar] [CrossRef] [PubMed]
- Zhang, Y.; Cui, P.; Wang, Y.; Zhang, S. Identification and bioactivity analysis of a newly identified defensin from the oyster Magallana gigas. Dev. Comp. Immunol. 2018, 85, 177–187. [Google Scholar] [CrossRef] [PubMed]
- Zhang, S. Discovery of the first self-assembling peptide, study of peptide dynamic behaviors, and G protein-coupled receptors using an Aviv circular dichroism spectropolarimeter. Biopolymers 2018, 109, e23235. [Google Scholar] [CrossRef] [PubMed]
- King, P.J.; Giovanna, L.M.; Booth, A.; Collins, R.F.; Gough, J.E.; Miller, A.F.; Webb, S.J. A modular self-assembly approach to functionalised beta-sheet peptide hydrogel biomaterials. Soft Matter 2016, 12, 1915–1923. [Google Scholar] [CrossRef]
- Gasiorowski, J.Z.; Collier, J.H. Directed Intermixing in Multicomponent Self-Assembling Biomaterials. Biomacromolecules 2011, 12, 3549–3558. [Google Scholar] [CrossRef]
- Gambaretto, R.; Tonin, L.; Di Bello, C.; Dettin, M. Self-assembling peptides: Sequence, secondary structure in solution and film formation. Biopolymers 2008, 89, 906–915. [Google Scholar] [CrossRef]
- Hirano, Y.; Kando, Y.; Hayashi, T.; Goto, K.; Nakajima, A. Synthesis and cell attachment activity of bioactive oligopeptides: RGD, RGDS, RGDV, and RGDT. J. Biomed. Mater. Res. 1991, 25, 1523–1534. [Google Scholar] [CrossRef]
- Castelletto, V.; Gouveia, R.M.; Connon, C.J.; Hamley, I.W.; Seitsonen, J.; Nykänen, A.; Ruokolainen, J. Alanine-rich am-phiphilic peptide containing the RGD cell adhesion motif: A coating material for human fibroblast attachment and culture. Biomater. Sci. 2014, 2, 362–369. [Google Scholar] [CrossRef]
- Jung, J.P.; Jones, J.L.; Cronier, S.A.; Collier, J.H. Modulating the mechanical properties of self-assembled peptide hydrogels via native chemical ligation. Biomaterials 2008, 29, 2143–2151. [Google Scholar] [CrossRef] [PubMed]
- Cheng, B.; Yan, Y.; Qi, J.; Deng, L.; Shao, Z.-W.; Zhang, K.-Q.; Li, B.; Sun, Z.; Li, X. Cooperative Assembly of a Peptide Gelator and Silk Fibroin Afford an Injectable Hydrogel for Tissue Engineering. ACS Appl. Mater. Interfaces 2018, 10, 12474–12484. [Google Scholar] [CrossRef] [PubMed]
- Kamino, K. Underwater Adhesive of Marine Organisms as the Vital Link between Biological Science and Material Science. Mar. Biotechnol. 2008, 10, 111–121. [Google Scholar] [CrossRef] [PubMed]
- Kamino, K. Molecular Design of Barnacle Cement in Comparison with Those of Mussel and Tubeworm. J. Adhes. 2010, 86, 96–110. [Google Scholar] [CrossRef]
- Kamino, K.; Inoue, K.; Maruyama, T.; Takamatsu, N.; Harayama, S.; Shizuri, Y. Barnacle cement proteins: Importance of di-sulfide bonds in their insolubility. J. Biol. Chem. 2000, 275, 27360–27365. [Google Scholar] [CrossRef]
- Kamino, K. Mini-review: Barnacle adhesives and adhesion. Biofouling 2013, 29, 735–749. [Google Scholar] [CrossRef]
- Kamino, K.; Nakano, M.; Kanai, S. Significance of the conformation of building blocks in curing of barnacle underwater ad-hesive. FEBS J. 2012, 279, 1750–1760. [Google Scholar] [CrossRef]
- Nakano, M.; Kamino, K. Amyloid-like Conformation and Interaction for the Self-Assembly in Barnacle Underwater Cement. Biochemistry 2015, 54, 826–835. [Google Scholar] [CrossRef]
- Benoiton, N.L. Chemistry of Peptide Synthesis; CRC Press: Boca Raton, FL, USA, 2016. [Google Scholar]
- Chan, W.C.; White, P.D. Fmoc Solid Peptide Synthesis: A Practical Approach; Oxford University Press: New York, NY, USA, 2004. [Google Scholar]
- Kunishima, M.; Kawachi, C.; Monta, J.; Terao, K.; Iwasaki, F.; Tani, S. 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methyl-morpholinium chloride: An efficient condensing agent leading to the for-mation of amides and esters. Tetrahedron 1999, 55, 13159–13170. [Google Scholar] [CrossRef]
- Kunishima, M.; Kitao, A.; Kawachi, C.; Watanabe, Y.; Iguchi, S.; Hioki, K.; Tani, S. A racemization test in peptide synthesis using 4-(4,6-dimethoxy-1,3,5-triazin-2-yl)-4-methylmorpholinium chloride (DMT-MM). Chem. Pharm. Bull. 2002, 50, 549–550. [Google Scholar] [CrossRef]
- Ridgley, D.M.; Claunch, E.C.; Barone, J.R. Characterization of large amyloid fibers and tapes with Fourier transform infra-red (FT-IR) and Raman spectroscopy. Appl. Spectrosc. 2013, 67, 1417–1426. [Google Scholar] [CrossRef] [PubMed]
- Melck: Cell Culture. Available online: https://www.sigmaaldrich.com/life-science/cell-culture/cell-culture-products.html (accessed on 22 January 2021).

| Code | Amino Acid Sequences (One Letter Amino Acid Code) |
|---|---|
| R-Y (control) | RRKYSGILGDLIQVAVIRYY |
| R-Y-RGDS | RRKYSGILGDLIQVAVIRYYGRGDS |
| RGDS-R- | RGDSGRRKYSGILGDLIQVAVIRYY |
| R-RGD-Y | RRKYSGIRGDLIQVAVIRYY |
| Code | Date of MALDI-TOF-MS | % of Secondary Structure | |||||
|---|---|---|---|---|---|---|---|
| Fw (Cal.) | [M]+ or [M+H]+ | [M+Na]+ | [M+K]+ | β-Sheet | β-Turn | Other | |
| R-Y (control) | 2383.79 | 2383.9 | - | - | 67 | 16 | 17 |
| R-Y-RGDS | 2856.24 | 2853.2 | - | - | 50 | 25 | 25 |
| RGDS-R-Y | 2856.24 | 2858.7 | 2881.26 | - | 68 | 16 | 16 |
| R-RGD-Y | 2426.82 | 2424.7 | - | - | 64 | 17 | 19 |
| R-Y (Pa) | R-Y-RGDS (Pa) | RGDS-R-Y (Pa) | R-RGD-Y (Pa) | |
|---|---|---|---|---|
| 1 wt% | 290.9 (±18.4) | *1 | 179.5 (±12.2) | 219.1 (±12.4) |
| 2 wt% | 912.2 (±49.9) | 8.5 (±6.0) | 990.2 (±60.1) | 924.5 (±46.8) |
| 3 wt% | 3125.1 (±152.2) | 3.8 (±2.7) | 2444.2 (±15.8) | 3872.1 (±21.4) |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Fujii, D.; Takase, K.; Takagi, A.; Kamino, K.; Hirano, Y. Design of RGDS Peptide-Immobilized Self-Assembling β-Strand Peptide from Barnacle Protein. Int. J. Mol. Sci. 2021, 22, 1240. https://doi.org/10.3390/ijms22031240
Fujii D, Takase K, Takagi A, Kamino K, Hirano Y. Design of RGDS Peptide-Immobilized Self-Assembling β-Strand Peptide from Barnacle Protein. International Journal of Molecular Sciences. 2021; 22(3):1240. https://doi.org/10.3390/ijms22031240
Chicago/Turabian StyleFujii, Daisuke, Kento Takase, Ami Takagi, Kei Kamino, and Yoshiaki Hirano. 2021. "Design of RGDS Peptide-Immobilized Self-Assembling β-Strand Peptide from Barnacle Protein" International Journal of Molecular Sciences 22, no. 3: 1240. https://doi.org/10.3390/ijms22031240
APA StyleFujii, D., Takase, K., Takagi, A., Kamino, K., & Hirano, Y. (2021). Design of RGDS Peptide-Immobilized Self-Assembling β-Strand Peptide from Barnacle Protein. International Journal of Molecular Sciences, 22(3), 1240. https://doi.org/10.3390/ijms22031240






