Transcriptomic Changes Associated with Loss of Cell Viability Induced by Oxysterol Treatment of a Retinal Photoreceptor-Derived Cell Line: An In Vitro Model of Smith–Lemli–Opitz Syndrome
Abstract
1. Introduction
2. Results
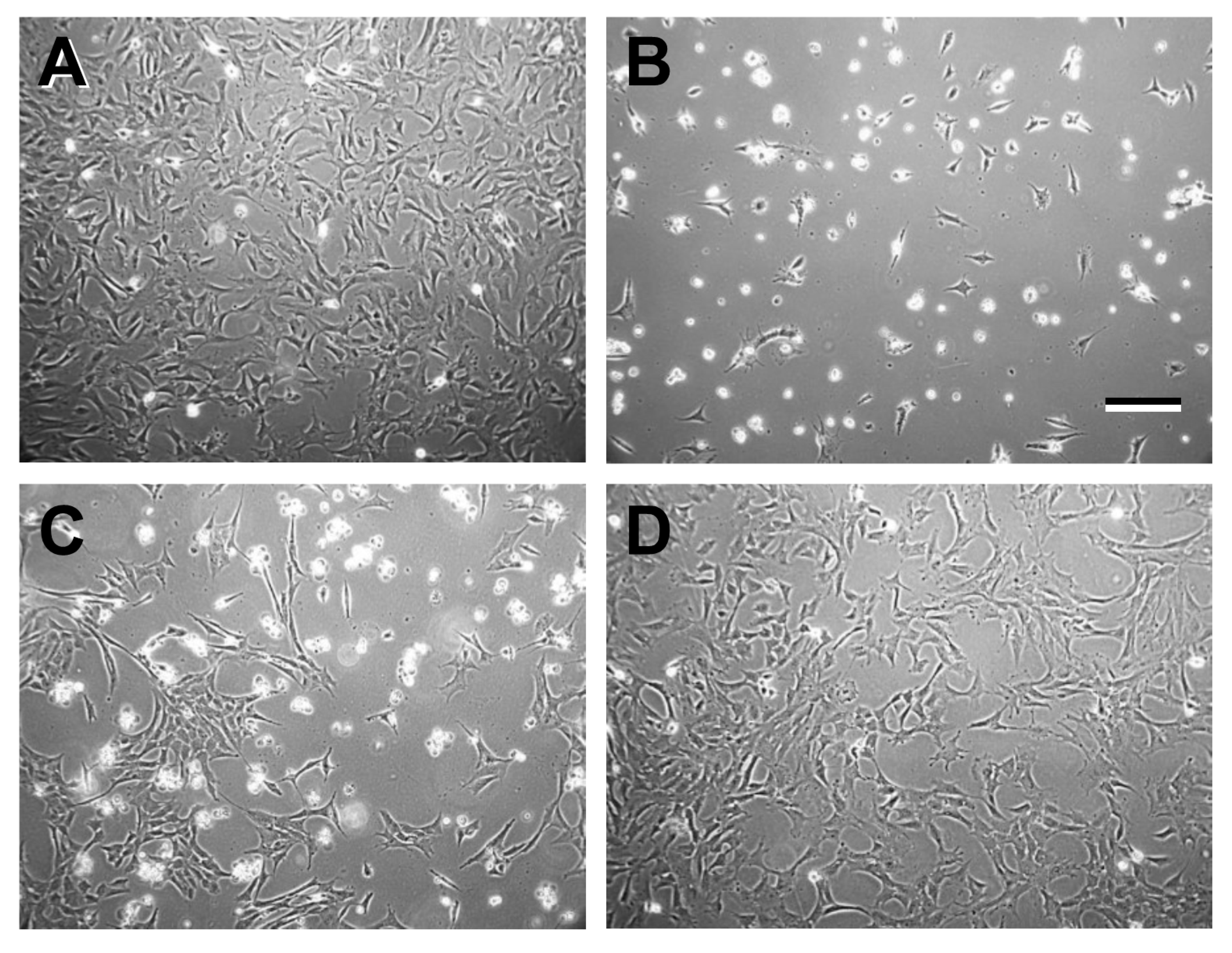
2.1. Validation of 661W Cell Culture Response to Treatments
2.1.1. Isolation of RNA: Rationale
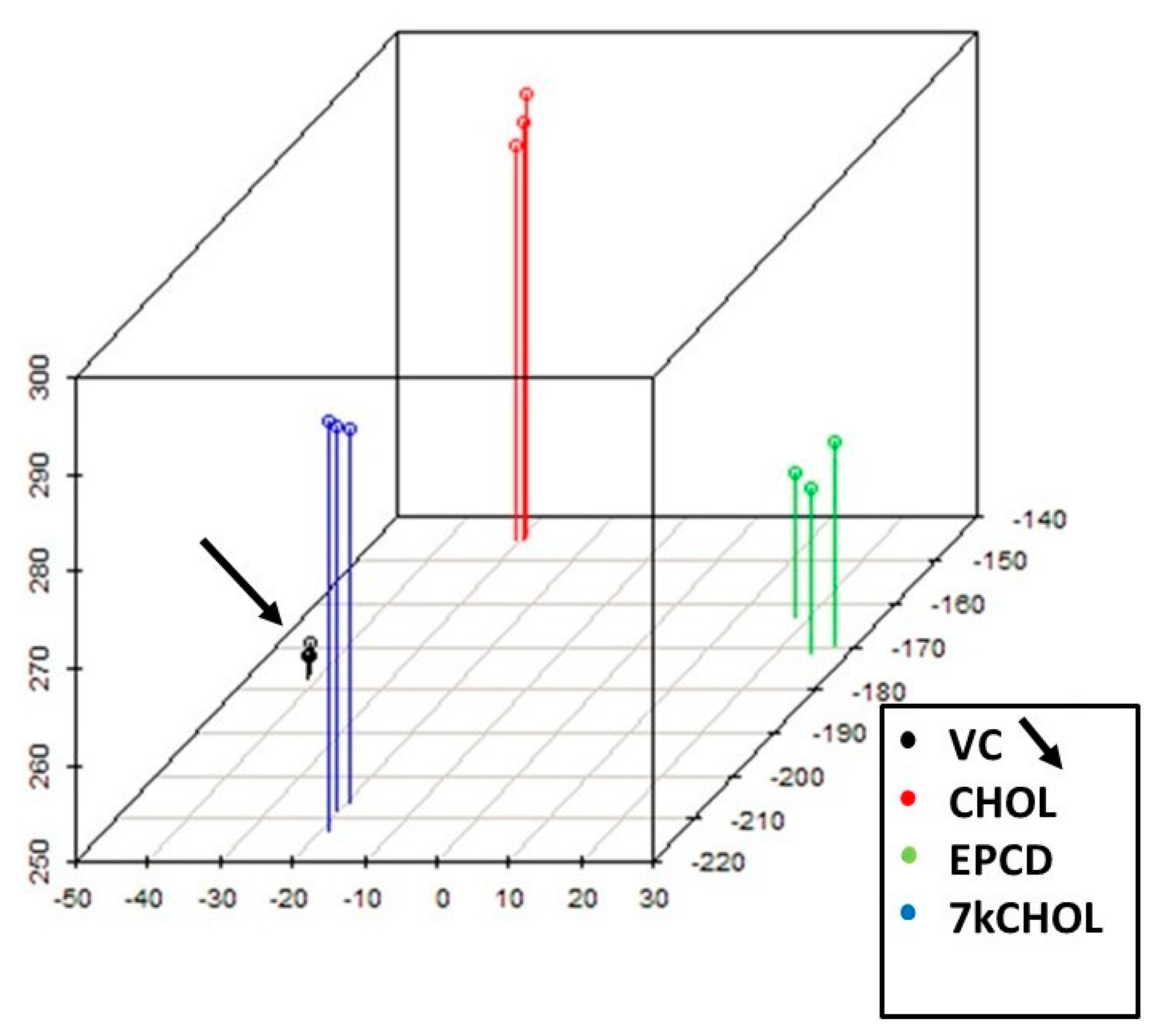
2.1.2. Principal Component Analysis (PCA)
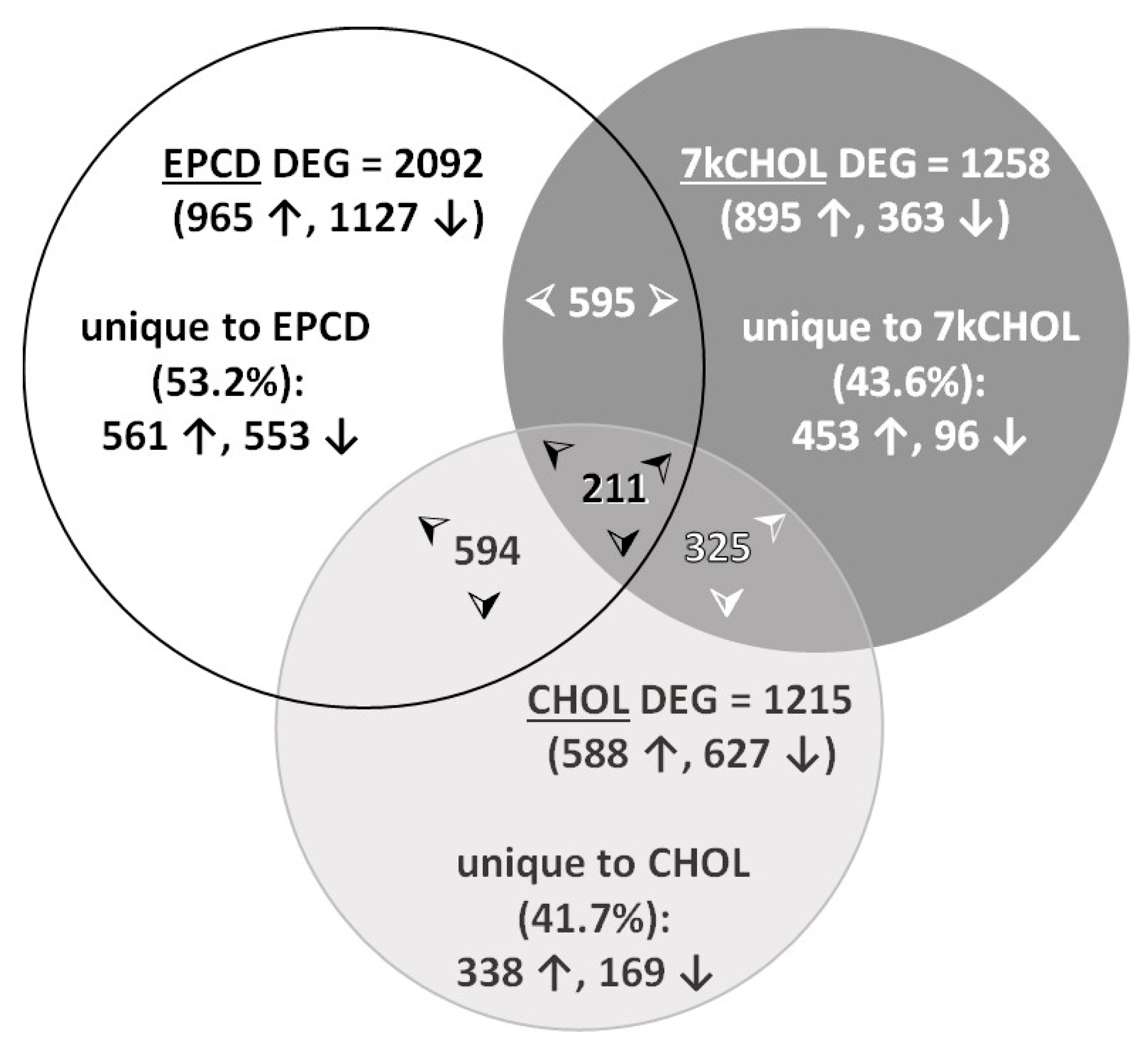
2.1.3. Identification of DEGs for Each Treatment vs. VC
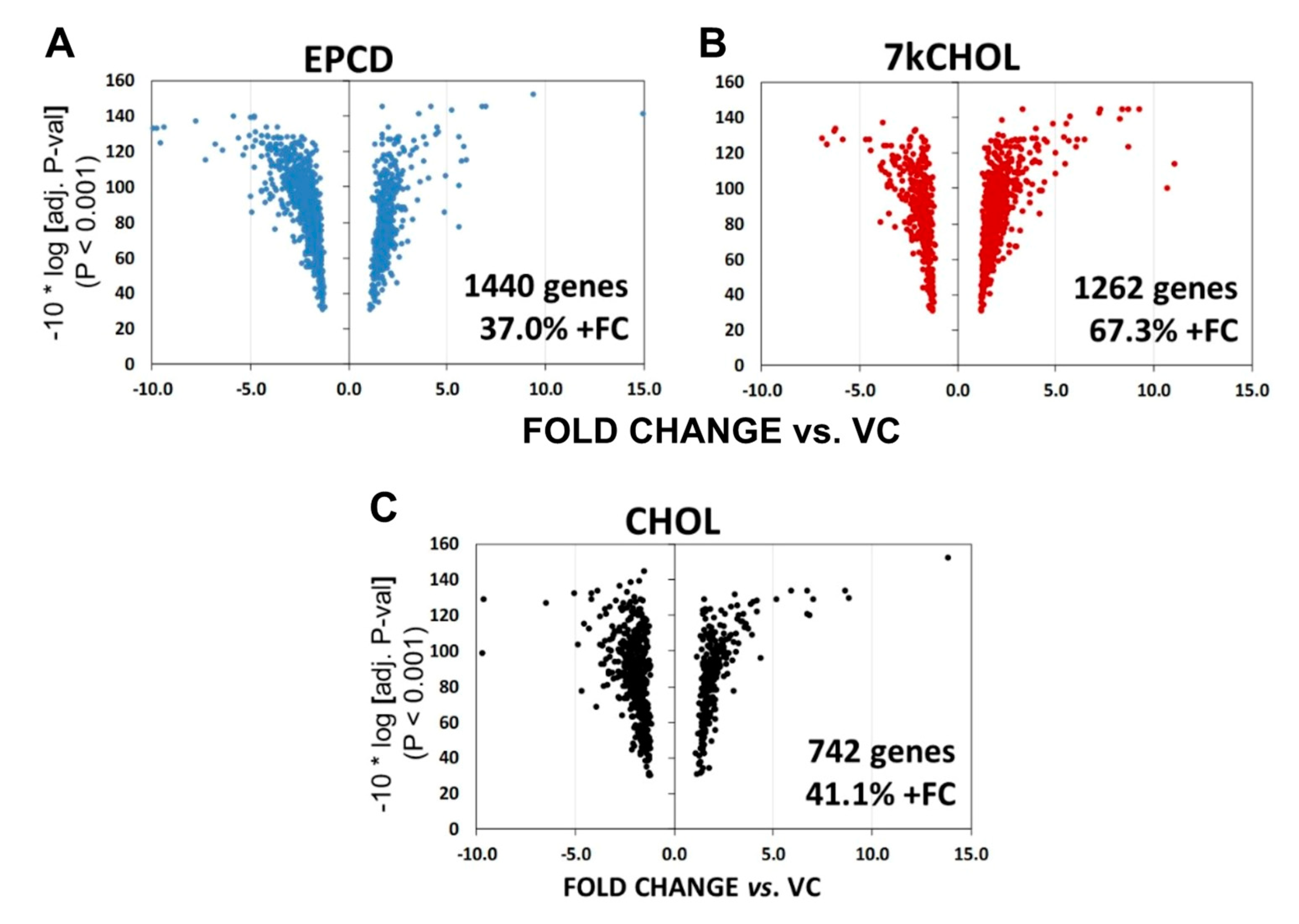
2.1.4. Volcano Plot
2.2. Gene Enrichment Analysis
2.2.1. Rationale
- (1)
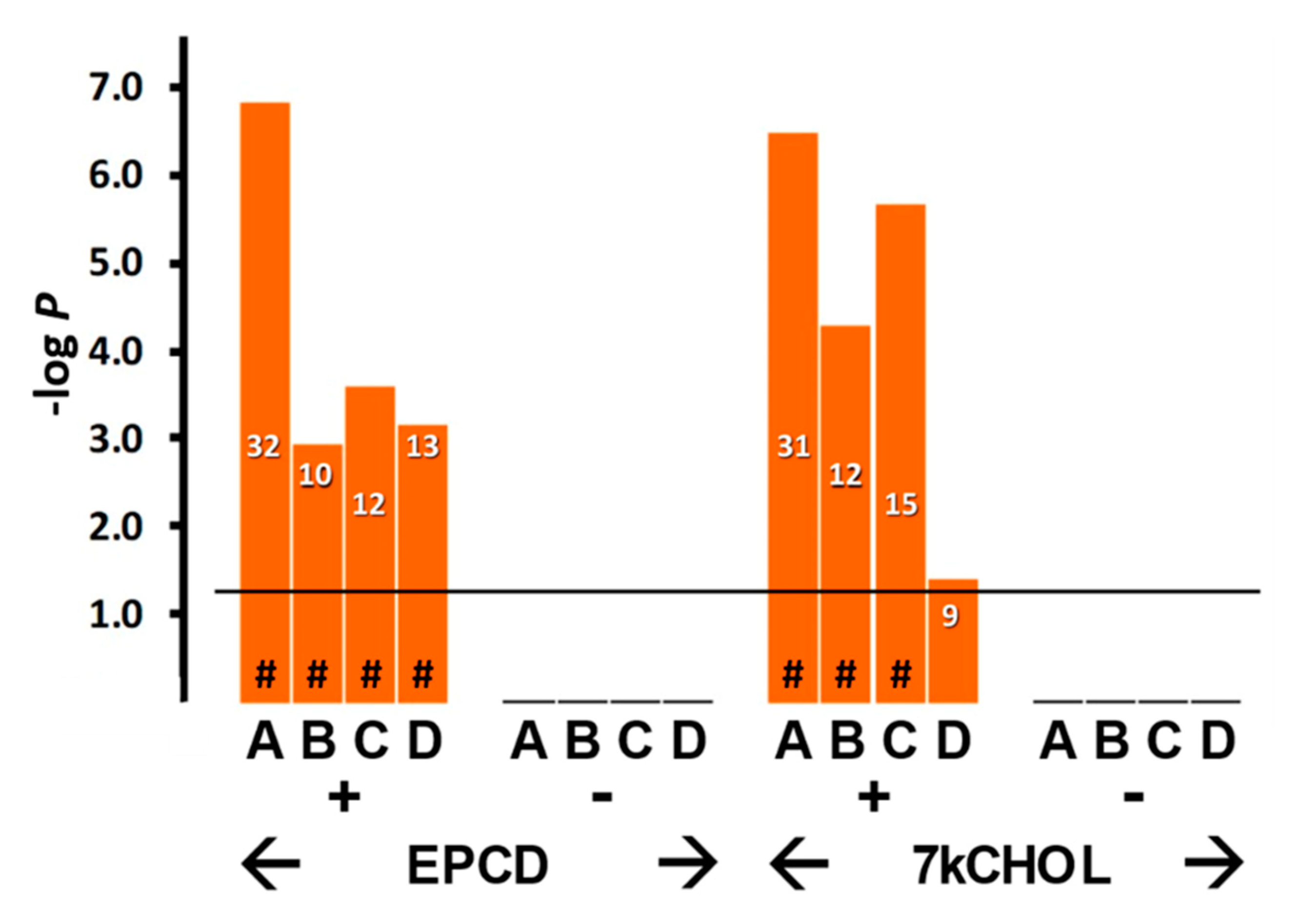
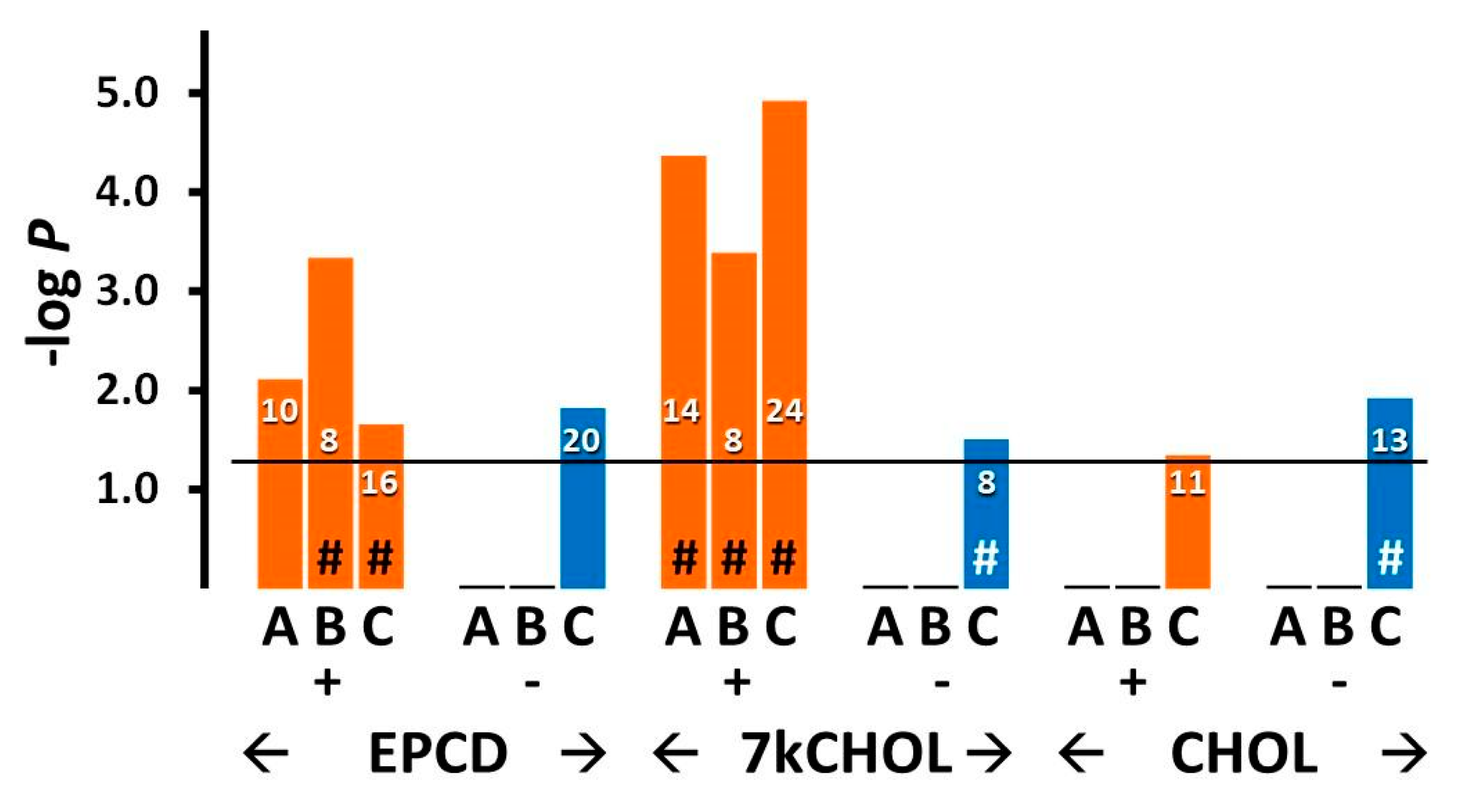
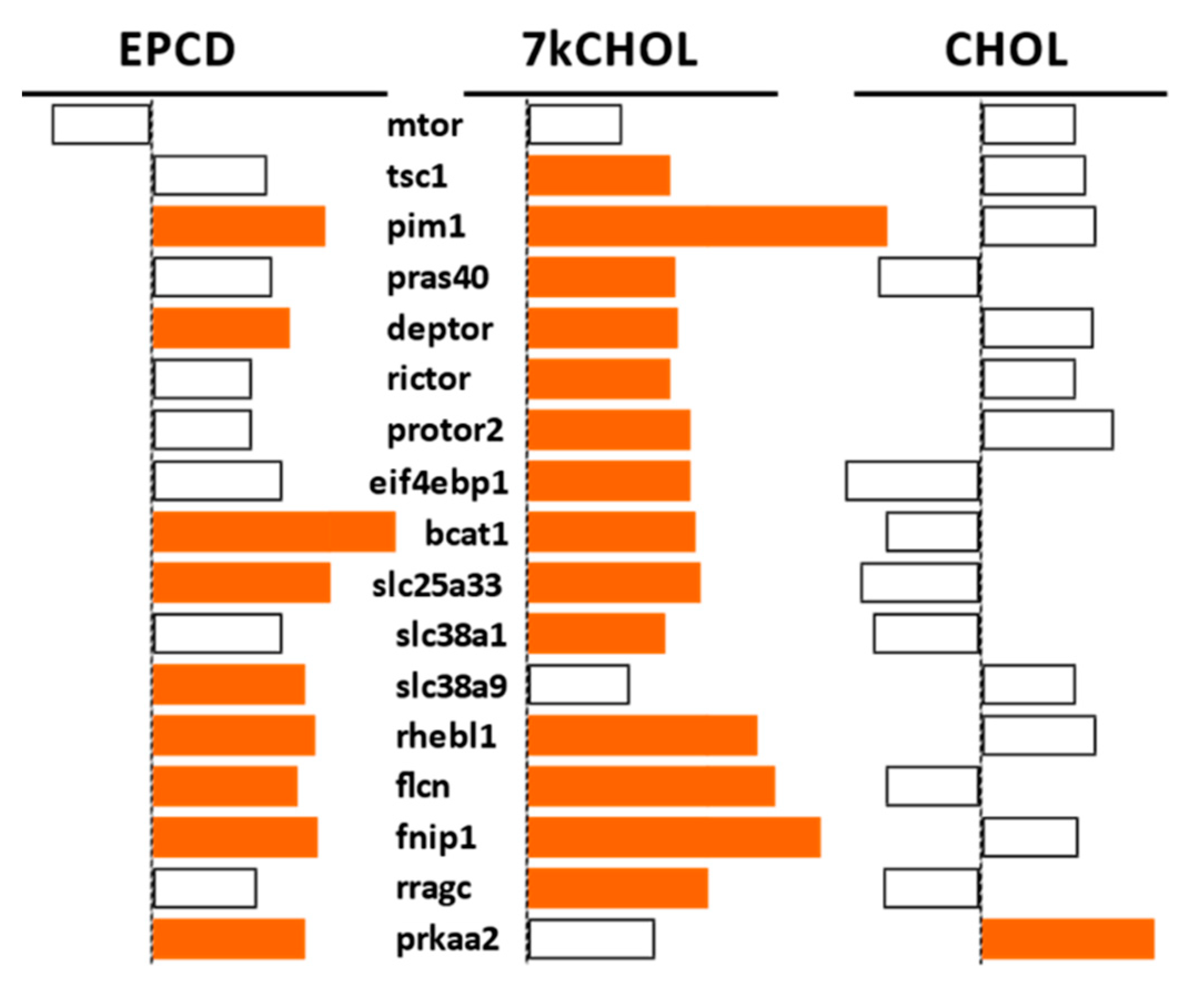
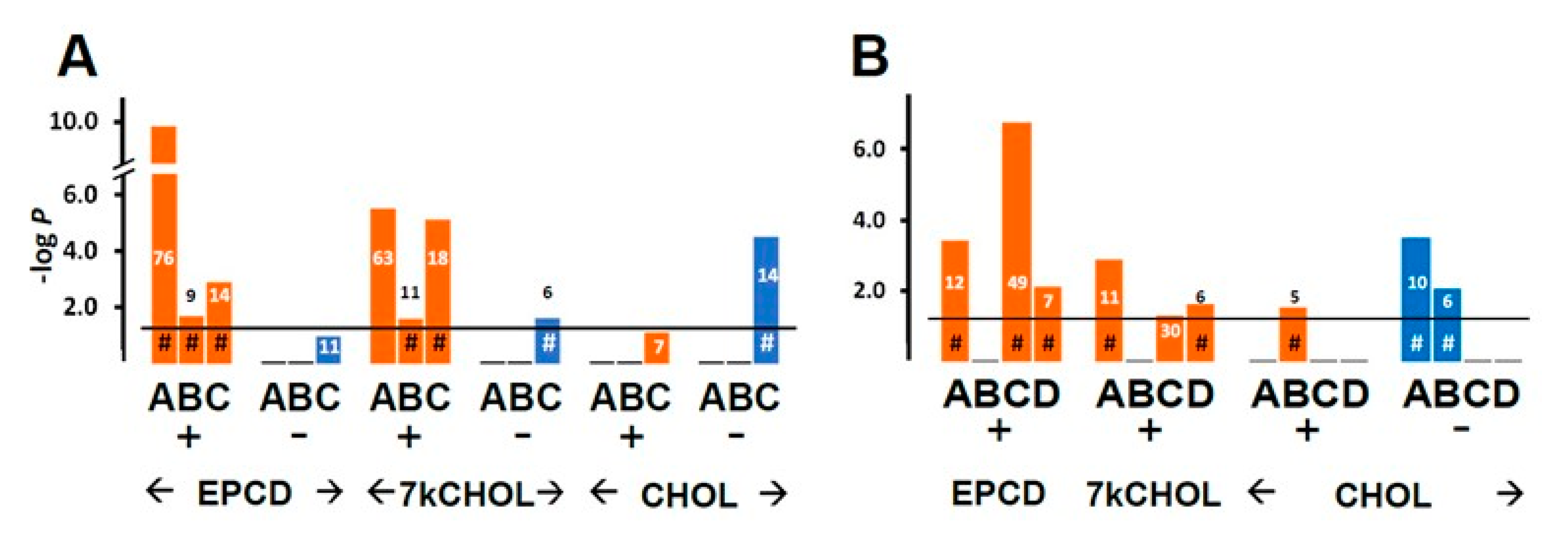
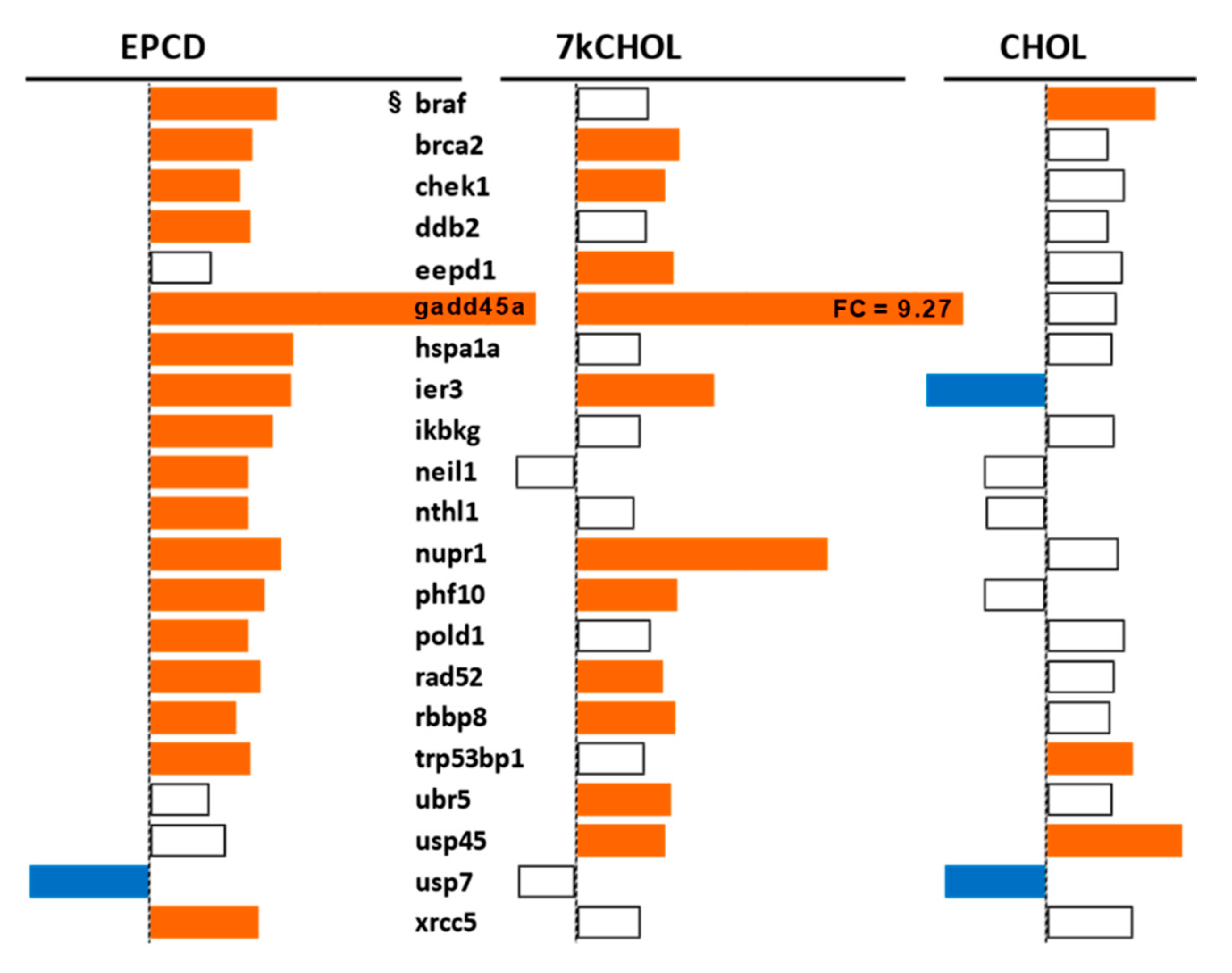
- From the enrichment analysis were derived the vertical bar charts indicating the statistical significance (with the number of DEGs from our array also indicated) for the relevant GO terms. These charts always included separate results for DEGs with both positive and negative FC, as subgroups. In many cases, we queried both positive and negative regulation of appropriate pathways and processes; therefore, with the division of DEGs into positive and negative FC overlaid, the expanded results depicted were more informative and mechanistically detailed, and possibly even predictive.
- (2)
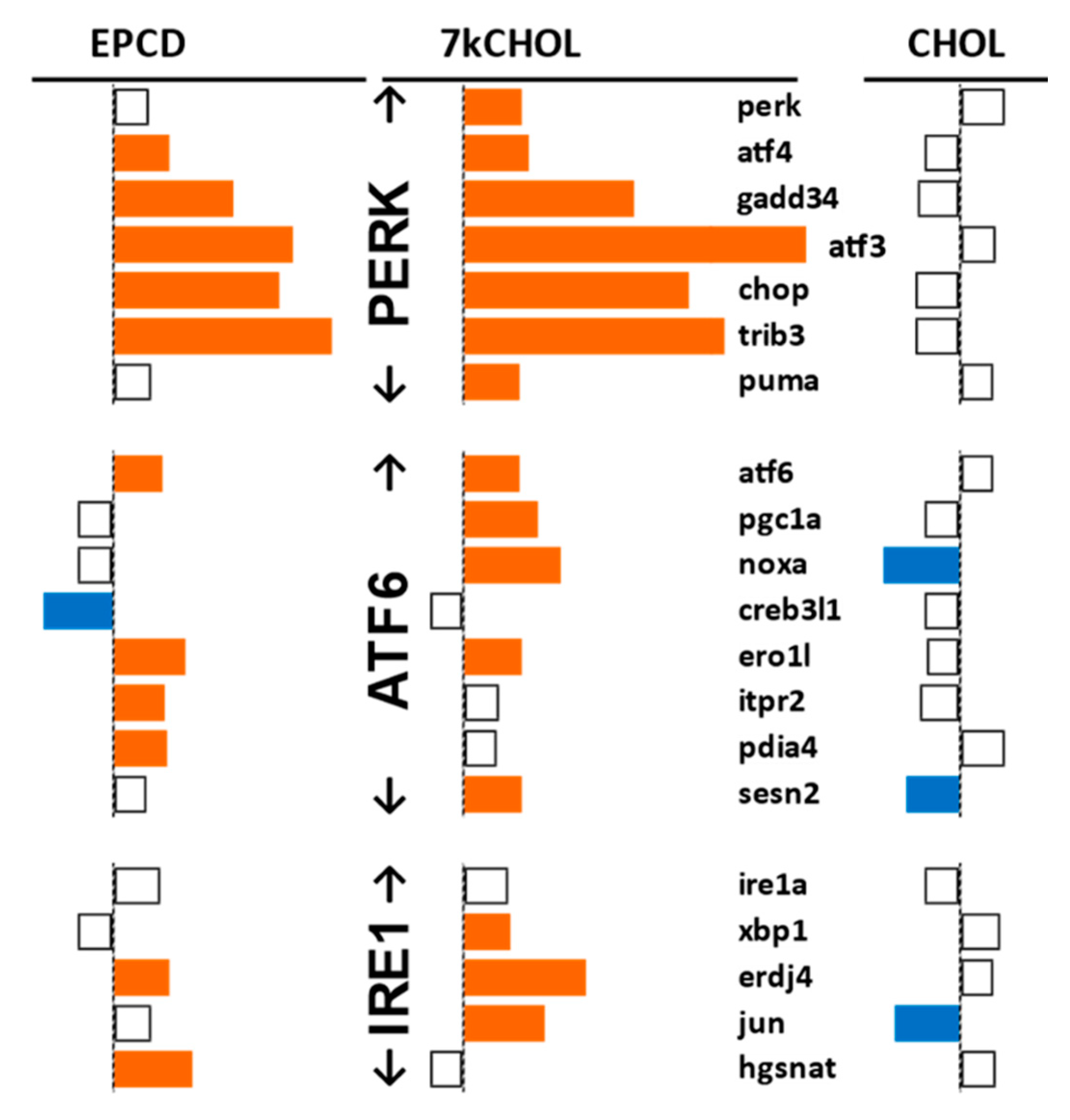
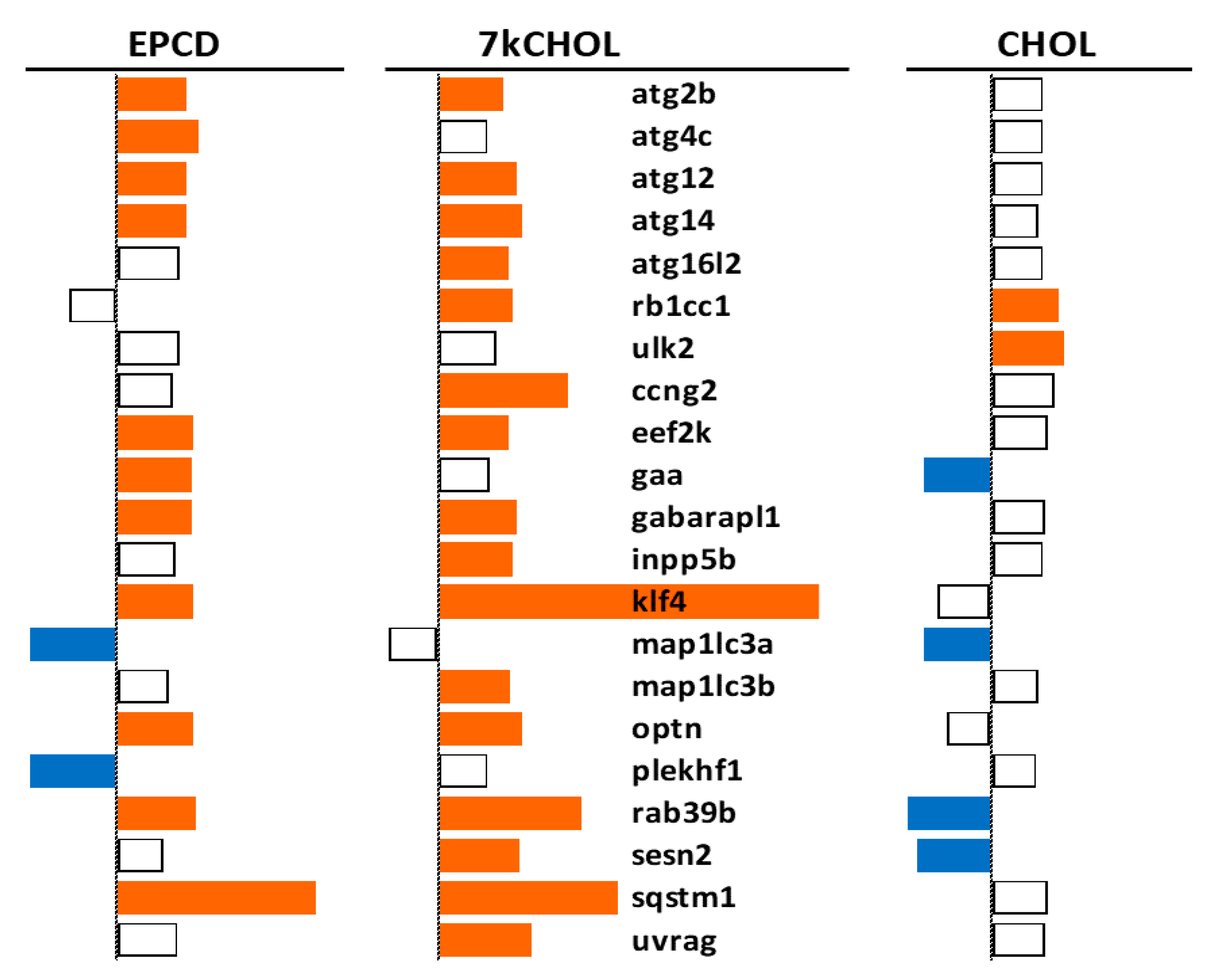
- From our own curation, we created charts with horizontal flags representing the magnitude of expression changes for individual, selected, signature genes for the pathways/processes of interest. Genes with differential expression meeting our FC and AdjP criteria were directionally indicated by orange and blue flags to denote positive or negative FC, respectively. In addition to the ability to assess such results on a gene-by-gene basis, and to distinguish between the often contrasting pattern for the two oxysterols plus CHOL, we found that the general appearance of these charts would provide a qualitative overview of the extent of gene expression changes governing said pathway or process. Such an overall visual result is in keeping with the concept embodied in gene enrichment analysis, namely that the greater the fraction of relevant DEGs within a selected (functional, etc.) gene set is calculated to be (i.e., is overrepresented), compared to the proportion of total DEGs out of all genes in the mouse array, the more likely it is that the process/pathway in question has been affected by the experimental treatment in statistically significant fashion, and the more reliable the conclusion that it may underlie the phenotype or pathophysiology that the treatment is modeling.
2.2.2. Endoplasmic Reticulum (ER) Stress
2.2.3. Oxidative Stress
2.2.4. Autophagy
2.2.5. mTORC Pathways
2.2.6. DNA Damage and Repair
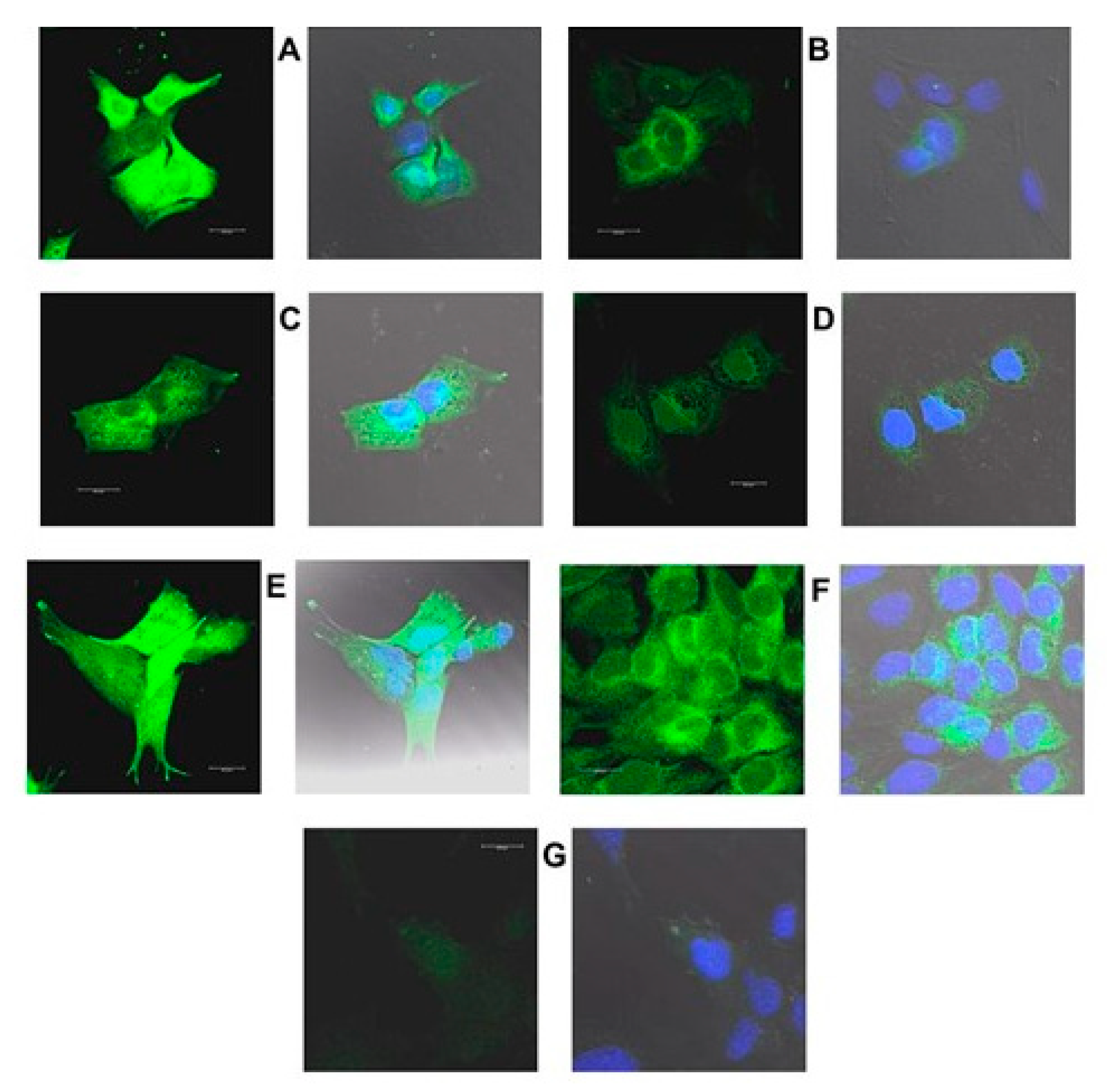
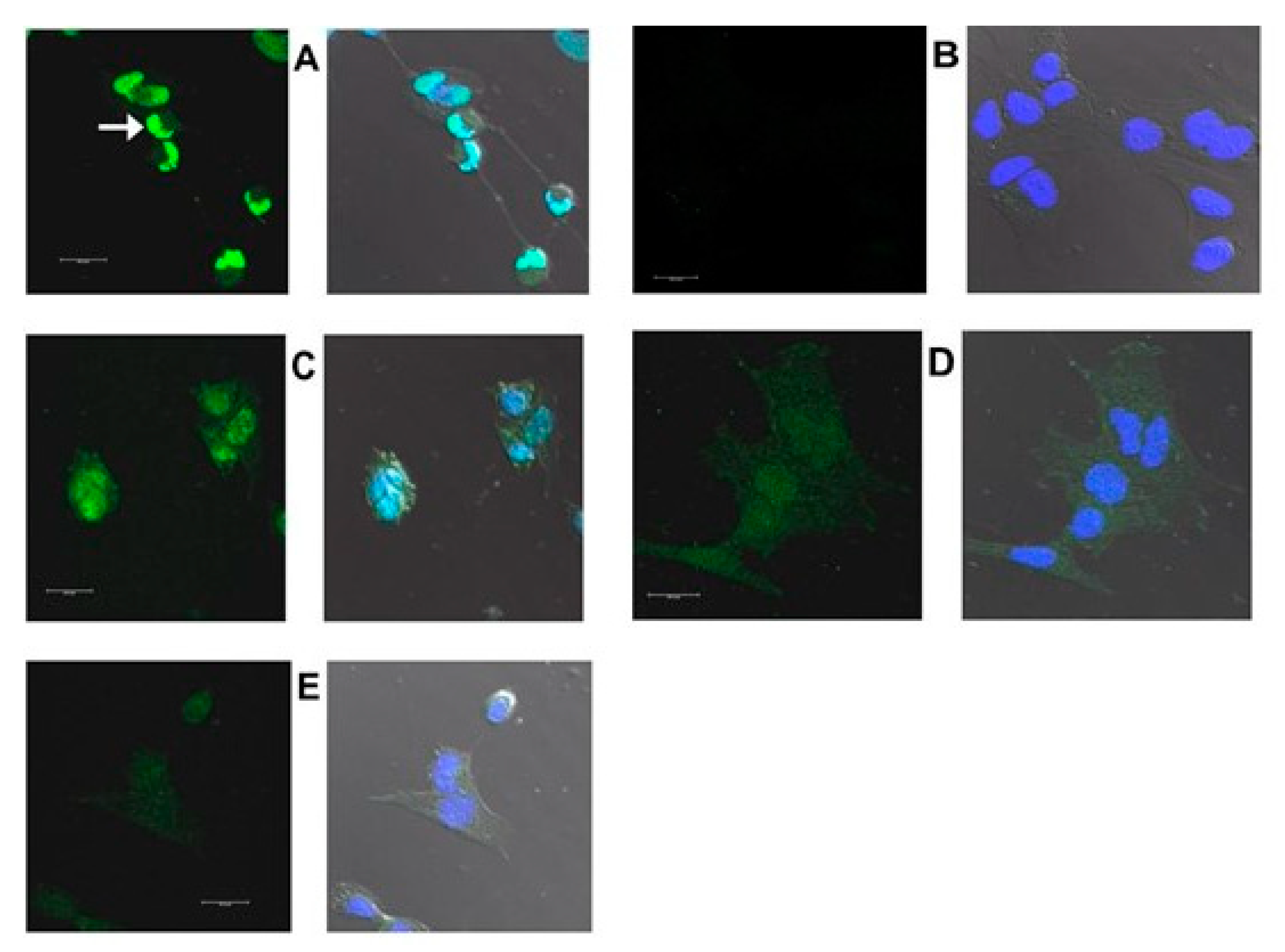
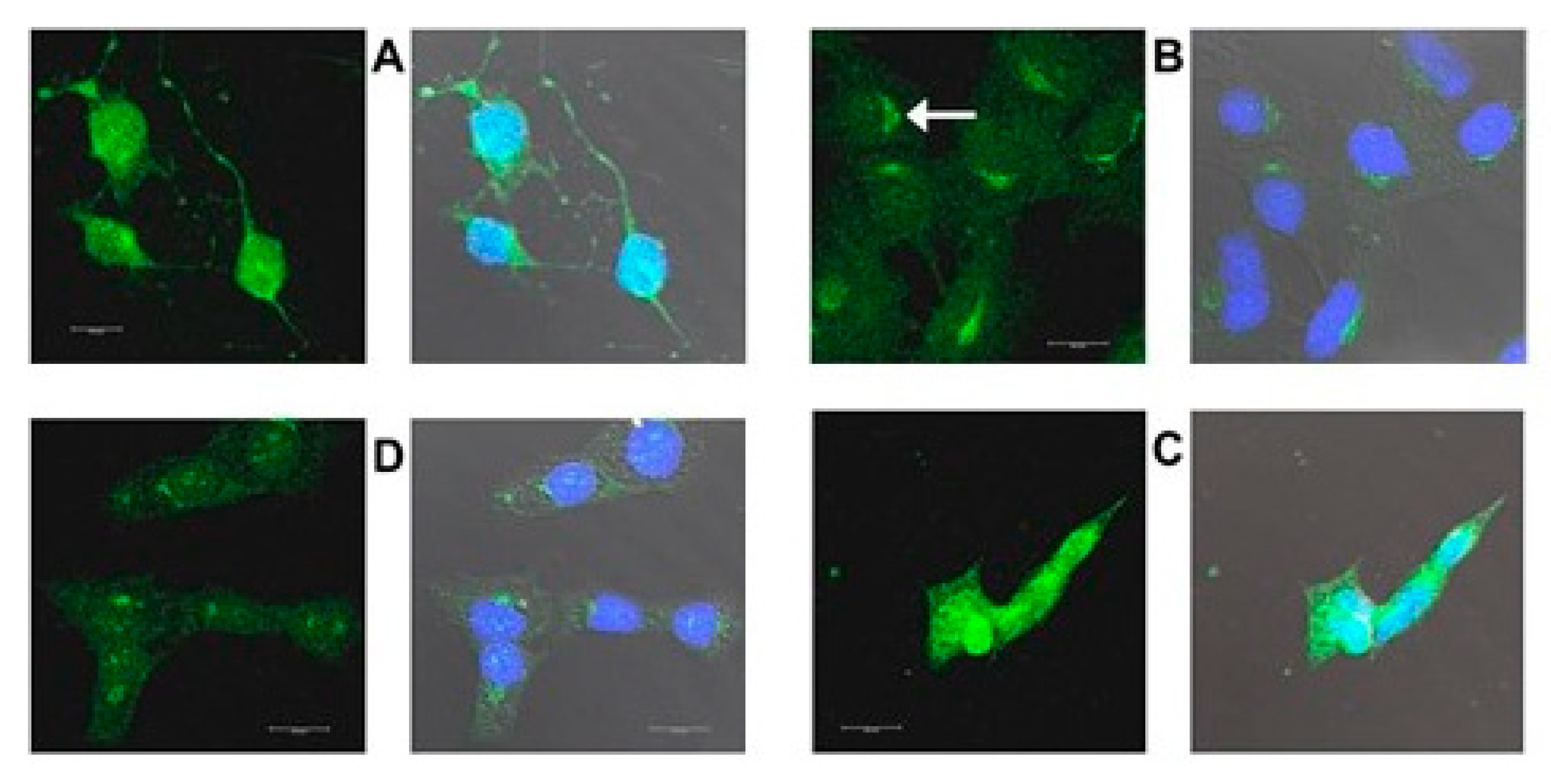
2.3. Increased Expression of Proteins Coded by Selected DEGs Following Oxysterol Treatments
2.3.1. Introduction
2.3.2. HMOX1
2.3.3. CHOP
2.3.4. TRIB3
2.3.5. HERPUD1
3. Discussion
- Cellular responses to oxidative and nitrosative stresses,
- Regulation of cellular homeostasis,
- Apoptotic process,
- Cysteine endopeptidase activity,
- ERAD,
- PERK arm of ER stress,
- Autophagy,
- Mitophagy, and
- Cell cycle regulation.
4. Materials and Methods
4.1. Sterol and Oxysterol Reagents
4.2. Cell Culture Methods
4.3. Experimental Treatments
- (1)
- EPCD, 23 h (final concentration, 6 µM): A 10 mM source stock of EPCD in DMSO was thawed, vortexed, and initially diluted with vortexing, at 1:167, to 60 μM (10× the desired final concentration) in the required volume of incubation medium (in this and all following preparations the incubation medium had been briefly warmed and gassed in the cell culture incubator). The final DMSO concentration in the medium incubated with cells was 0.08% (v/v).
- (2)
- 7kCHOL, 5 h (final concentration, 16 µM): 2 μL (10 μmol) aliquots of 5 mM 7kCHOL in absolute ethanol were dried in amber Eppendorf tubes and stored under Ar at −20 °C until further use. Source stock: To the 7kCHOL was added 1 mL of a 1:9 (v/v) mixture of absolute EtOH and 45% (w/v) hydroxypropyl-β-cyclodextrin (hpβCD; Sigma-Aldrich) in water [246]; this mixture was sealed with Ar, protected from light, and vortexed periodically at room temperature for 1 h, when a clear solution was formed. This 10 mM aqueous stock in complexation with hpβCD was diluted with vortexing, at 1:62.5, to 160 μM (10× the desired final concentration) in incubation medium. The final hpβCD concentration exposed to cells was 720 µg/mL, and the nominal final ethanol concentration was 0.0016%.
- (3)
- CHOL, 23 h (final concentration, 8 µM): A 5 mM stock in DMSO was thawed, vortexed, and diluted with vortexing, at 1:62.5 to 80 μM (10× the desired final concentration) in the required volume of incubation medium. The final DMSO concentration exposed to cells was 0.16%.
- (4)
- hpβCD vehicle control (VC), 24 h: The hpβCD/EtOH-only stock otherwise used to formulate the 7kCHOL treatment ((2), above) was diluted to a 10× stock in incubation medium by 1:62.5 (equivalently to the 7kCHOL stock), for final concentrations with cells of 720 µg/mL hpβCD and 0.0016% EtOH.
4.4. Isolation and Quality Assessment of Total RNA
4.5. Processing of RNA Samples, Reading of Hybridized Arrays, and Processing of Raw Data
4.6. Immunofluorescence Detection of Proteins Corresponding to Selected DEGs
4.6.1. Preparation and Treatments of 661W Cells for Confocal Microscopy
4.6.2. Fixation of Treated 661W Cells
Formaldehyde Fixation
Methacarn Fixation
4.6.3. Immunohistochemistry
4.7. Gene Enrichment and Other Analyses
5. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| 7DHC | 7-dehydrocholesterol |
| 7kCHOL | 7-ketocholesterol |
| AdjP | adjusted p-value |
| ARE | antioxidant response element |
| BCS | bovine calf serum |
| BLRB | bilirubin |
| BLVD | biliverdin |
| BVRD | biliverdin reductase |
| BSA | bovine serum albumin |
| BSS | balanced salt solution |
| CDK | cyclin-dependent kinase |
| CHOL | cholesterol |
| CHOP | C/EBP homologous protein (also known as: Ddit3; Gadd 153) |
| CNS | central nervous system |
| CO | carbon monoxide |
| DAPI | 4′,6-diamido-2-phenylindole |
| DAVID | database for annotation, visualization and integrated discovery |
| DEG | differentially expressed gene |
| DHCR7 | sterol Δ7-reductase |
| DIC | differential interference contrast |
| DMSO | dimethyl sulfoxide |
| EPCD | 5,9-endoperoxycholest-7-ene-3β,6α-diol |
| ER | endoplasmic reticulum |
| ERAD | endoplasmic reticulum-associated protein degradation |
| FC | fold change |
| GO | gene ontology |
| GSH | reduced glutathione |
| Herpud1 | homocysteine-responsive ER protein with ubiquitin-like domain 1 |
| Hmox1 | heme oxygenase-1; HO-1 |
| hpβCD | hydroxypropyl-beta-cyclodextrin |
| PBS | phosphate-buffered saline |
| PCA | principal component analysis |
| RIN | RNA integrity score |
| RPE | retinal pigment epithelium |
| SLOS | Smith–Lemli–Opitz syndrome |
| SV40 | simian virus 40 |
| Trib3 | tribbles homologue 3; Trb3 |
| UPR | unfolded protein response |
| VC | vehicle control |
| WAG | weighted average difference |
References
- Kelley, R.I.; Hennekam, R.C.M. The Smith-Lemli-Opitz syndrome. J. Med. Genet. 2004, 37, 321–325. [Google Scholar] [CrossRef]
- Salen, G.; Shefer, S.; Batta, A.K.; Tint, G.S.; Xu, G.; Honda, A.; Irons, M.; Elias, E.R. Abnormal cholesterol biosynthesis in the Smith-Lemli-Opitz syndrome. J. Lipid. Res. 1996, 37, 1169–1180. [Google Scholar] [CrossRef]
- Smith, D.W.; Lemli, L.; Opitz, J.M. A newly recognized syndrome of multiple congenital anomalies. J. Pediatr. 1964, 64, 210–217. [Google Scholar] [CrossRef]
- Waterham, H.R.; Hennekam, R.C. Mutational spectrum of Smith-Lemli-Opitz syndrome. Am. J. Med. Genet. 2012, 160C, 263–284. [Google Scholar] [CrossRef]
- Witsch-Baumgartner, M.; Löffler, J.; Utermann, G. Mutations in the human DHCR7 gene. Hum. Mutat. 2001, 17, 172–182. [Google Scholar] [CrossRef]
- Fitzky, B.U.; Moebius, F.F.; Asaoka, H.; Waage-Baudet, H.; Xu, L.; Xu, G.; Maeda, N.; Kluckman, K.; Hiller, S.; Yu, H.; et al. 7-Dehydrocholesterol-dependent proteolysis of HMG-CoA reductase suppresses sterol biosynthesis in a mouse model of Smith-Lemli-Optiz/RSH syndrome. J. Clin. Investig. 2001, 108, 905–915. [Google Scholar] [CrossRef]
- Porter, F.D. Smith-Lemli-Opitz syndrome: Pathogenesis, diagnosis and management. Eur. J. Hum. Genet. 2008, 16, 535–541. [Google Scholar] [CrossRef] [PubMed]
- DeBarber, A.E.; Eroglu, Y.; Merkens, L.S.; Pappu, A.S.; Steiner, R.D. Smith-Lemli-Opitz syndrome. Expert Rev. Mol. Med. 2011, 13, e24. [Google Scholar] [CrossRef]
- Wassif, C.A.; Zhu, P.; Kratz, L.I.; Krakowiak, P.A.; Battaile, K.P.; Weight, F.F.; Grinberg, A.; Steiner, R.D.; Nwokoro, N.A.; Kelley, R.I.; et al. Biochemical, phenotypic and neurophysiological characterization of a genetic mouse model of RSH/Smith-Lemli-Opitz syndrome. Hum. Mol. Genet. 2001, 10, 555–564. [Google Scholar] [CrossRef]
- Fliesler, S.J. Retinal degeneration in a rat model of Smith-Lemli-Opitz Syndrome: Thinking beyond cholesterol deficiency. Adv. Exp. Med. Biol. 2010, 664, 481–489. [Google Scholar] [CrossRef]
- Boesze-Battaglia, K.; Damek-Poprawa, M.; Mitchell, D.C.; Greeley, L.; Brush, R.S.; Anderson, R.E.; Richards, M.J.; Fliesler, S.J. Alteration of retinal rod outer segment membrane fluidity in a rat model of Smith-Lemli-Opitz syndrome. J. Lipid Res. 2008, 49, 1488–1499. [Google Scholar] [CrossRef]
- Francis, K.R.; Ton, A.N.; Xin, Y.; O’Halloran, P.E.; Wassif, C.A.; Malik, N.; Williams, I.M.; Cluzeau, C.V.; Trivedi, N.S.; Pavan, W.J.; et al. Modeling Smith-Lemli-Opitz syndrome with induced pluripotent stem cells reveals a causal role for Wnt/β-catenin defects in neuronal cholesterol synthesis phenotypes. Nat. Med. 2016, 22, 388–396. [Google Scholar] [CrossRef]
- Xu, L.; Davis, T.A.; Porter, N.A. Rate constants for peroxidation of polyunsaturated fatty acids and sterols in solution and in liposomes. J. Am. Chem. Soc. 2009, 131, 13037–13044. [Google Scholar] [CrossRef]
- Korade, Z.; Xu, L.; Shelton, R.; Porter, N.A. Biological activities of 7-dehydrocholesterol-derived oxysterols: Implication for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2010, 51, 3259–3269. [Google Scholar] [CrossRef]
- Xu, L.; Mirnics, K.; Bowman, A.B.; Liu, W.; Da, J.; Porter, N.A.; Korade, Z. DHCEO accumulation is a critical mediator of pathophysiology in a Smith-Lemli-Opitz syndrome model. Neurobiol. Dis. 2012, 45, 923–929. [Google Scholar] [CrossRef]
- Fliesler, S.J.; Peachey, N.S.; Richards, M.J.; Nagel, B.A.; Vaughan, D.K. Retinal degeneration in a rodent model of Smith-Lemli-Opitz syndrome: Electrophysiologic, biochemical, and morphologic features. Arch. Ophthalmol. 2004, 122, 1190–1200. [Google Scholar] [CrossRef] [PubMed]
- Ford, D.A.; Monda, J.K.; Brush, R.S.; Anderson, R.E.; Richards, M.J.; Fliesler, S.J. Lipidomic analysis of the retina in a rat model of Smith-Lemli-Opitz syndrome: Alterations in docosahexaenoic acid content of phospholipid molecular species. J. Neurochem. 2008, 105, 1032–1047. [Google Scholar] [CrossRef]
- Goulah, C.; Struys, E.; Jansen, E.; Fliesler, S.J. Metabolomic analysis of a rat model of the Smith-Lemli-Opitz syndrome (SLOS). Investig. Ophthalmol. Vis. Sci. 2013, 54, 710. [Google Scholar]
- Tu, C.; Li, J.; Jiang, X.; Sheflin, L.G.; Pfeffer, B.A.; Behringer, M.; Fliesler, S.J.; Qu, J. Ion-current-based proteomic profiling of the retina in a rat model of Smith-Lemli-Opitz syndrome. Mol. Cell. Proteom. 2013, 12, 3583–3598. [Google Scholar] [CrossRef]
- Xu, L.; Sheflin, L.G.; Porter, N.A.; Fliesler, S.J. 7-dehydrocholesterol-derived oxysterols and retinal degeneration in a rat model of Smith-Lemli-Opitz syndrome. Biochim. Biophys. Acta 2012, 1821, 877–883. [Google Scholar] [CrossRef]
- Pfeffer, B.A.; Xu, L.; Porter, N.A.; Ramachandra Rao, S.; Fliesler, S.J. Differential cytotoxic effects of 7-dehydrocholesterol-derived oxysterols on cultured retina-derived cells: Dependence on sterol structure, cell type, and density. Exp. Eye Res. 2016, 145, 297–316. [Google Scholar] [CrossRef]
- Xu, L.; Korade, Z.; Porter, N.A. Oxysterols from free radical chain oxidation of 7-dehydrocholesterol: Product and mechanistic studies. J. Am. Chem. Soc. 2010, 132, 2222–2232. [Google Scholar] [CrossRef]
- Smyth, G.K. limma: Linear models for microarray data. In Bioinformatics and Computational Biology Solutions Using R and Bioconductor; Gentleman, R., Carey, V.J., Huber, W., Irizarry, R.A., Dudoit, S., Eds.; Statistics for Biology and Health; Springer: New York, NY, USA, 2005. [Google Scholar] [CrossRef]
- Ringnér, M. What is principal component analysis? Nat. Biotechnol. 2008, 26, 303–304. [Google Scholar] [CrossRef]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Statist. Soc. B. 1995, 57, 289–300, MR1325392. [Google Scholar] [CrossRef]
- Kadota, K.; Nakai, Y.; Shimizu, K. Ranking differentially expressed genes from Affymetrix gene expression data: Methods with reproducibility, sensitivity, and specificity. Algorithms Mol. Biol. 2009, 4, 7. [Google Scholar] [CrossRef]
- .Kapphahn, R.J.; Richards, M.J.; Ferrington, D.A.; Fliesler, S.J. Lipid-derived and other oxidative modifications of retinal proteins in a rat model of Smith-Lemli-Opitz syndrome. Exp. Eye Res. 2019, 178, 247–254. [Google Scholar] [CrossRef]
- Berthier, A.; Lemaire-Ewing, S.; Prunet, C.; Montange, T.; Vejux, A.; De Barros, J.P.P.; Monier, S.; Gambert, P.; Lizard, G.; Néel, D. 7-Ketocholesterol-induced apoptosis: Involvement of several pro-apoptotic but also anti-apoptotic calcium-dependent transduction pathways. FEBS J. 2005, 272, 3093–3104. [Google Scholar] [CrossRef]
- Pedruzzi, E.; Guichard, C.; Ollivier, V.; Driss, F.; Fay, M.; Prunet, C.; Marie, J.-C.; Pouzet, C.; Samadi, M.; Elbim, C.; et al. NAD(P)H oxidase Nox-4 mediates 7-ketocholesterol-induced endoplasmic reticulum stress and apoptosis in human aortic smooth muscle cells. Mol. Cell. Biol. 2004, 24, 10703–10717. [Google Scholar] [CrossRef]
- Rodríguez, I.R.; Larrayoz, I.M. Cholesterol oxidation in the retina: Implications of 7KCh formation in chronic inflammation and age-related macular degeneration. J. Lipid Res. 2010, 51, 2847–2862. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Systematic and integrative analysis of large gene lists using DAVID Bioinformatics Resources. Nature Protoc. 2009, 4, 44–57. [Google Scholar] [CrossRef]
- Huang, D.W.; Sherman, B.T.; Lempicki, R.A. Bioinformatics enrichment tools: Paths toward the comprehensive functional analysis of large gene lists. Nucleic Acids Res. 2009, 37, 1–13. [Google Scholar] [CrossRef]
- Urra, H.; Dufey, E.; Lisbona, F.; Rojas-Rivera, D.; Hetz, C. When ER stress reaches a dead end. Biochim. Biophys. Acta 2013, 1833, 3507–3517. [Google Scholar] [CrossRef]
- Zhang, S.X.; Sanders, E.; Fliesler, S.J.; Wang, J.L. Endoplasmic reticulum stress and the unfolded protein responses in retinal degeneration. Exp. Eye Res. 2014, 125, 30–40. [Google Scholar] [CrossRef]
- Chiang, W.-C.; Kroeger, H.; Sakami, S.; Messah, C.; Yasumura, D.; Matthes, M.T.; Coppinger, J.A.; Palczewski, K.; Lavail, M.M.; Lin, J.H. Robust Endoplasmic Reticulum-Associated Degradation of Rhodopsin Precedes Retinal Degeneration. Mol. Neurobiol. 2015, 52, 679–695. [Google Scholar] [CrossRef] [PubMed]
- Arensdorf, A.M.; Diedrichs, D.; Rutkowski, D.T. Regulation of the transcriptome by ER stress: Non-canonical mechanisms and physiological consequences. Front. Genet. 2013, 4, 256. [Google Scholar] [CrossRef] [PubMed]
- Jegal, K.H.; Park, S.M.; Cho, S.S.; Byun, S.H.; Ku, S.K.; Kim, S.C.; Ki, S.H.; Cho, I.J. Activating transcription factor 6-dependent sestrin 2 induction ameliorates ER stress-mediated liver injury. Biochim. Biophys. Acta-Mol. Cell Res. 2017, 1864, 1295–1307. [Google Scholar] [CrossRef]
- Lee, J.H.; Bodmer, R.; Bier, E.; Karin, M. Sestrins at the crossroad between stress and aging. Aging 2010, 2, 369–374. [Google Scholar] [CrossRef]
- Dikic, I. Open questions: Why should we care about ER-phagy and ER remodelling? BMC Biol. 2018, 16, 131. [Google Scholar] [CrossRef] [PubMed]
- Grumati, P.; Dikic, I.; Stolz, A. ER-phagy at a glance. J. Cell Sci. 2018, 131, jcs217364. [Google Scholar] [CrossRef]
- Kristiansen, M.; Menghi, F.; Hughes, R.; Hubank, M.; Ham, J. Global analysis of gene expression in NGF-deprived sympathetic neurons identifies molecular pathways associated with cell death. BMC Genom. 2011, 12, 551. [Google Scholar] [CrossRef]
- Pobre, K.F.R.; Poet, G.J.; Hendershot, L.M. The endoplasmic reticulum (ER) chaperone BiP is a master regulator of ER functions: Getting by with a little help from ERdj friends. J. Biol. Chem. 2019, 294, 2098–2108. [Google Scholar] [CrossRef]
- Doycheva, D.; Kaur, H.; Tang, J.; Zhang, J.H. The characteristics of the ancient cell death suppressor, TMBIM6, and its related signaling pathways after endoplasmic reticulum stress. J. Neurosci. Res. 2020, 98, 77–86. [Google Scholar] [CrossRef]
- McLaughlin, T.; Falkowski, M.; Wang, J.J.; Zhang, S.X. Molecular chaperone ERp29: A potential target for cellular protection in retinal and neurodegenerative diseases. Adv. Exp. Med. Biol. 2018, 1074, 421–427. [Google Scholar] [CrossRef]
- Sok, J.; Wang, X.Z.; Batchvarova, N.; Kuroda, M.; Harding, H.; Ron, D. CHOP-dependent stress-inducible expression of a novel form of carbonic anhydrase VI. Mol. Cell Biol. 1999, 19, 495–504. [Google Scholar] [CrossRef]
- Matthews, T.A.; Abel, A.; Demme, C.; Sherman, T.; Pan, P.W.; Halterman, M.W.; Parkkila, S.; Nehrke, K. Expression of the CHOP-inducible carbonic anhydrase CAVI-b is required for BDNF-mediated protection from hypoxia. Brain Res. 2014, 1543, 28–37. [Google Scholar] [CrossRef][Green Version]
- Smith, S.B.; Wang, J.; Cui, X.; Mysona, B.; Zhao, J.; Bollinger, K.E. Sigma 1 Receptor: A novel therapeutic target in retinal disease. Prog. Retin. Eye Res. 2018, 67, 130–149. [Google Scholar] [CrossRef] [PubMed]
- Alam, S.; Abdullah, C.S.; Aishwarya, R.; Orr, A.W.; Traylor, J.; Miriyala, S.; Panchatcharam, M.; Pattillo, C.B.; Bhuiyan, M.S. Sigmar1 regulates endoplasmic reticulum stress-induced C/EBP-homologous protein expression in cardiomyocytes. Biosci. Rep. 2017, 37, BSR20170898. [Google Scholar] [CrossRef]
- Suzuki, T.; Huang, C.; Fujihira, H. The cytoplasmic peptide:N-glycanase (NGLY1); structure, expression and cellular functions. Gene 2016, 577, 1–7. [Google Scholar] [CrossRef] [PubMed]
- Richards, M.J.; Nagel, B.A.; Fliesler, S.J. Lipid hydroperoxide formation in the retina: Correlation with retinal degeneration and light damage in a rat model of Smith–Lemli–Opitz syndrome. Exp. Eye Res. 2006, 82, 538–541. [Google Scholar] [CrossRef]
- Navas-Madroñal, M.; Rodriguez, C.; Kassan, M.; Fité, J.; Escudero, J.R.; Cañes, L.; Martínez-González, J.; Camacho, M.; Galán, M. Enhanced endoplasmic reticulum and mitochondrial stress in abdominal aortic aneurysm. Clin. Sci. 2019, 133, 1421–1438. [Google Scholar] [CrossRef]
- Nury, T.; Zarrouk, A.; Yammine, A.; Mackrill, J.J.; Vejux, A.; Lizard, G. Oxiapoptophagy: A type of cell death induced by some oxysterols. Br. J. Pharmacol. 2020, 2020, 1–9. [Google Scholar] [CrossRef]
- Bokhari, B.; Sharma, S. Stress Marks on the Genome: Use or Lose? Int. J. Mol. Sci. 2019, 20, 364. [Google Scholar] [CrossRef] [PubMed]
- Cullinan, S.B.; Diehl, J.A. Coordination of ER and oxidative stress signaling: The PERK/Nrf2 signaling pathway. Int. J. Biochem. Cell Biol. 2006, 38, 317–332. [Google Scholar] [CrossRef]
- Flynn, J.M.; Czerwieniec, G.A.; Choi, S.W.; Day, N.U.; Gibson, B.W.; Hubbard, A.; Melov, S. Proteogenomics of synaptosomal mitochondrial oxidative stress. Free Radic. Biol. Med. 2012, 53, 1048–1060. [Google Scholar] [CrossRef] [PubMed]
- Button, R.W.; Luo, S.; Rubinsztein, D.C. Autophagic activity in neuronal cell death. Neurosci. Bull. 2015, 31, 382–394. [Google Scholar] [CrossRef] [PubMed]
- Peker, N.; Gozuacik, D. Autophagy as a cellular stress response mechanism in the nervous system. J. Mol. Biol. 2020, 432, 2560–2588. [Google Scholar] [CrossRef] [PubMed]
- Kroemer, G.; Levine, B. Autophagic cell death: The story of a misnomer. Nat. Rev. Mol. Cell Biol. 2008, 9, 1004–1010. [Google Scholar] [CrossRef] [PubMed]
- Frost, L.S.; Mitchell, C.H.; Boesze-Battaglia, K. Autophagy in the eye: Implications for ocular cell health. Exp. Eye Res. 2014, 124, 56–66. [Google Scholar] [CrossRef] [PubMed]
- Ramachandra Rao, S.; Pfeffer, B.A.; Gómez, N.M.; Skelton, L.A.; Keiko, U.; Sparrow, J.R.; Rowsam, A.M.; Mitchell, C.H.; Fliesler, S.J. Compromised phagosome maturation underlies RPE pathology in cell culture and whole animal models of Smith-Lemli-Opitz Syndrome. Autophagy 2018, 14, 1796–1817. [Google Scholar] [CrossRef] [PubMed]
- Jung, C.H.; Seo, M.; Otto, N.M.; Kim, D.H. ULK1 inhibits the kinase activity of mTORC1 and cell proliferation. Autophagy 2011, 7, 1212–1221. [Google Scholar] [CrossRef]
- Korac, J.; Schaeffer, V.; Kovacevic, I.; Clement, A.M.; Jungblut, B.; Behl, C.; Terzic, J.; Dikic, I. Ubiquitin-independent function of optineurin in autophagic clearance of protein aggregates. J. Cell Sci. 2013, 126, 580–592. [Google Scholar] [CrossRef] [PubMed]
- Pankiv, S.; Clausen, T.H.; Lamark, T.; Brech, A.; Bruun, J.A.; Outzen, H.; Øvervatn, A.; Bjørkøy, G.; Johansen, T. p62/SQSTM1 binds directly to Atg8/LC3 to facilitate degradation of ubiquitinated protein aggregates by autophagy. J. Biol. Chem. 2007, 282, 24131–24145. [Google Scholar] [CrossRef]
- Rogov, V.; Dötsch, V.; Johansen, T.; Kirkin, V. Interactions between autophagy receptors and ubiquitin-like proteins form the molecular basis for selective autophagy. Mol. Cell 2014, 53, 167–1178. [Google Scholar] [CrossRef] [PubMed]
- Wang, C.W.; Klionsky, D.J. The molecular mechanism of autophagy. Mol. Med. 2003, 9, 65–76. [Google Scholar] [CrossRef] [PubMed]
- Le Grand, J.N.; Bon, K.; Fraichard, A.; Zhang, J.; Jouvenot, M.; Risold, P.Y.; Boyer-Guittaut, M.; Delage-Mourroux, R. Specific distribution of the autophagic protein GABARAPL1/GEC1 in the developing and adult mouse brain and identification of neuronal populations expressing GABARAPL1/GEC1. PLoS ONE 2013, 8, e63133. [Google Scholar] [CrossRef]
- Liu, C.; DeRoo, E.P.; Stecyk, C.; Wolsey, M.; Szuchnicki, M.; Hagos, E.G. Impaired autophagy in mouse embryonic fibroblasts null for Krüppel-like Factor 4 promotes DNA damage and increases apoptosis upon serum starvation. Mol. Cancer 2015, 14, 101. [Google Scholar] [CrossRef] [PubMed]
- Liang, C.; Sir, D.; Lee, S.; Ou, J.H.J.; Jung, J.U. Beyond autophagy: The role of UVRAG in membrane trafficking. Autophagy 2008, 4, 817–820. [Google Scholar] [CrossRef][Green Version]
- Egan, D.; Kim, J.; Shaw, R.J.; Guan, K.L. The autophagy initiating kinase ULK1 is regulated via opposing phosphorylation by AMPK and mTOR. Autophagy 2011, 7, 643–644. [Google Scholar] [CrossRef] [PubMed]
- Lee, J.W.; Park, S.; Takahashi, Y.; Wang, H.G. The association of AMPK with ULK1 regulates autophagy. PLoS ONE 2010, 5, e15394. [Google Scholar] [CrossRef]
- Cheng, Y.; Ren, X.; Zhang, Y.; Shan, Y.; Huber-Keener, K.J.; Zhang, L.; Kimball, S.R.; Harvey, H.; Jefferson, L.S.; Yang, J.M. Integrated regulation of autophagy and apoptosis by EEF2K controls cellular fate and modulates the efficacy of curcumin and velcade against tumor cells. Autophagy 2013, 9, 208–219. [Google Scholar] [CrossRef] [PubMed]
- Ambrosio, S.; Saccà, C.D.; Amente, S.; Paladino, S.; Lania, L.; Majello, B. Lysine-specific demethylase LSD1 regulates autophagy in neuroblastoma through SESN2-dependent pathway. Oncogene 2017, 36, 6701–6711. [Google Scholar] [CrossRef] [PubMed]
- Roux, P.R.; Topisirovic, I. Signaling pathways involved in the regulation of mRNA translation. Mol. Cell. Biol. 2018, 38, e00070-18. [Google Scholar] [CrossRef]
- Sakai, Y.; Kassai, H.; Nakayama, H.; Fukaya, M.; Maeda, T.; Nakao, K.; Hashimoto, K.; Sakagami, H.; Kano, M.; Aiba, A. Hyperactivation of mTORC1disrupts cellular homeostasis in cerebellar Purkinje cells. Sci. Rep. 2019, 9, 2799. [Google Scholar] [CrossRef] [PubMed]
- Sarbassov, D.D.; Guertin, D.A.; Ali, S.M.; Sabatini, D.M. Phosphorylation and regulation of Akt/PKB by the rictor-mTOR complex. Science 2005, 307, 1098–1101. [Google Scholar] [CrossRef] [PubMed]
- Menon, S.; Dibble, C.C.; Talbott, G.; Hoxhaj, G.; Valvezan, A.J.; Takahashi, H.; Cantley, L.C.; Manning, B.D. Spatial control of the TSC complex integrates insulin and nutrient regulation of mTORC1 at the lysosome. Cell 2014, 156, 771–785. [Google Scholar] [CrossRef] [PubMed]
- Bar-Peled, L.; Sabatini, D.M. Regulation of mTORC1 by amino acids. Trends Cell Biol. 2014, 24, 400–406. [Google Scholar] [CrossRef]
- Schmidt, L.S.; Linehan, W.M. FLCN: The causative gene for Birt-Hogg-Dubé syndrome. Gene 2018, 640, 28–42. [Google Scholar] [CrossRef] [PubMed]
- Tee, A.R.; Blenis, J.; Proud, C.G. Analysis of mTOR signaling by the small G-proteins, Rheb and RhebL1. FEBS Lett. 2005, 579, 4763–4768. [Google Scholar] [CrossRef] [PubMed]
- Tsun, Z.Y.; Bar-Peled, L.; Chantranupong, L.; Zoncu, R.; Wang, T.; Kim, C.; Spooner, E.; Sabatini, D.M. The Folliculin tumor suppressor is a GAP for RagC/D GTPases that signal amino acid levels to mTORC1. Mol. Cell 2013, 52, 495–505. [Google Scholar] [CrossRef]
- Wan, X.; Harkavy, B.; Shen, N.; Grohar, P.; Helman, L.J. Rapamycin induces feedback activation of Akt signaling through anIGF-1R-dependent mechanism. Oncogene 2007, 26, 1932–1940. [Google Scholar] [CrossRef]
- Yang, H.; Jiang, X.; Buren Li, B.; Yang, H.J.; Miller, M.; Yang, A.; Dhar, A.; Pavletich, N.P. Mechanisms of mTORC1 activation by RHEB and inhibition by PRAS40. Nature 2017, 552, 368–373. [Google Scholar] [CrossRef] [PubMed]
- Zhang, K.S.; Schecker, J.; Krull, A.; Riechert, E.; Jürgensen, L.; Kamuf-Schenk, V.; Burghaus, J.; Kiper, L.; Ho, T.C.; Wöltje, K.; et al. PRAS40 suppresses atherogenesis through inhibition of mTORC1-dependent pro-inflammatory signaling in endothelial cells. Sci. Rep. 2019, 9, 16787. [Google Scholar] [CrossRef] [PubMed]
- Laplante, M.; Sabatini, D.M. mTOR signaling at a glance. J. Cell Sci. 2009, 122, 3589–3594. [Google Scholar] [CrossRef] [PubMed]
- Agarwal, S.; Bell, C.M.; Rothbart, S.B.; Moran, R.G. AMP-activated protein kinase (AMPK) control of mTORC1 is p53- and TSC2-independent in pemetrexed-treated carcinoma cells. J. Biol. Chem. 2015, 290, 27473–27486. [Google Scholar] [CrossRef] [PubMed]
- Carroll, B.; Dunlop, E.A. The lysosome: A crucial hub for AMPK and mTORC1 signalling. Biochem. J. 2017, 474, 1453–1466. [Google Scholar] [CrossRef]
- Floyd, S.; Favre, C.; Lasorsa, F.M.; Leahy, M.; Trigiante, G.; Stroebel, P.; Marx, A.; Loughran, G.; O’Callaghan, K.; Marobbio, C.M.T.; et al. The insulin-like growth factor-I–mTOR signaling pathway induces the mitochondrial pyrimidine nucleotide carrier to promote cell growth. Mol. Biol. Cell 2007, 18, 3545–3555. [Google Scholar] [CrossRef]
- Shafei, M.A.; Matthew Harris, M.; Conway, M.E. Divergent metabolic regulation of autophagy and mTORC1—early events in Alzheimer’s disease? Front. Aging Neurosci. 2017, 9, 173. [Google Scholar] [CrossRef]
- Fitch, M.E.; Nakajima, S.; Yasui, A.; Ford, J.M. In vivo recruitment of XPC to UV-induced cyclobutane pyrimidine dimers by the DDB2 gene product. J. Biol. Chem. 2003, 278, 46906–46910. [Google Scholar] [CrossRef]
- Mortensen, U.H.; Lisby, M.; Rothstein, R. Rad52. Curr. Biol. 2009, 19, R676–R677. [Google Scholar] [CrossRef]
- Wang, H.; Dharmalingam, P.; Vasquez, V.; Mitra, J.; Boldogh, I.; Rao, K.S.; Kent, T.A.; Mitra, S.; Hegde, M.L. Chronic oxidative damage together with genome repair deficiency in the neurons is a double whammy for neurodegeneration: Is damage response signaling a potential therapeutic target? Mech. Ageing Dev. 2017, 161A, 163–176. [Google Scholar] [CrossRef]
- Schwertman, P.; Vermeulen, W.; Marteijn, J.A. UVSSA and USP7, a new couple in transcription-coupled DNA repair. Chromosoma 2013, 122, 275–284. [Google Scholar] [CrossRef] [PubMed]
- Zhu, Q.; Wani, A.A. Nucleotide excision repair: Finely tuned molecular orchestra of early pre-incision events. Photochem. Photobiol. 2017, 93, 166–177. [Google Scholar] [CrossRef] [PubMed]
- Zhan, Q. Gadd45a, a p53- and BRCA1-regulated stress protein, in cellular response to DNA damage. Mutat. Res. 2005, 569, 133–143. [Google Scholar] [CrossRef] [PubMed]
- Pawlikowska, P.; Leray, I.; de Laval, B.; Guihard, S.; Kumar, R.; Rosselli, F.; Porteu, F. ATM-dependent expression of IEX-1 controls nuclear accumulation of Mcl-1 and the DNA damage response. Cell Death Differ. 2010, 17, 1739–1750. [Google Scholar] [CrossRef]
- Lin, Q.; Weis, S.; Yang, G.; Weng, Y.-H.; Helston, R.; Rish, K.; Smith, A.; Bordner, J.; Polte, T.; Gaunitz, F.; et al. Heme oxygenase-1 protein localizes to the nucleus and activates transcription factors important in oxidative stress. J. Biol. Chem. 2007, 282, 20621–20633. [Google Scholar] [CrossRef] [PubMed]
- Xu, L.; Korade, Z.; Rosado Jr, D.A.; Mirnics, K.; Porter, N.A. Metabolism of oxysterols derived from nonenzymatic oxidation of 7-dehydrocholesterol in cells. J. Lipid Res. 2013, 54, 1135–1143. [Google Scholar] [CrossRef] [PubMed]
- Shinkyo, R.; Xu, L.; Tallman, K.A.; Cheng, Q.; Porter, N.A.; Guengerich, F.P. Conversion of 7-dehydrocholesterol to 7-ketocholesterol Is catalyzed by human cytochrome P450 7A1 and occurs by direct oxidation without an epoxide intermediate. J. Biol. Chem. 2011, 286, 33021–33028. [Google Scholar] [CrossRef]
- Xu, L.; Liu, W.; Sheflin, L.G.; Fliesler, S.J.; Porter, N.A. Novel oxysterols observed in tissues and fluids of AY9944-treated rats: A model for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2011, 52, 1810–1820. [Google Scholar] [CrossRef] [PubMed]
- Al-Ubaidi, M.R.; Font, R.L.; Quiambao, A.B.; Keener, M.J.; Liou, G.I.; Overbeek, P.A.; Baehr, W. Bilateral retinal and brain tumors in transgenic mice expressing simian virus 40 large T antigen under control of the human interphotoreceptor retinoid-binding protein promoter. J. Cell Biol. 1992, 119, 1681–1687. [Google Scholar] [CrossRef]
- Wenzel, A.; Grimm, C.; Samardzija, M.; Remé, C.E. Molecular mechanisms of light-induced photoreceptor apoptosis and neuroprotection for retinal degeneration. Prog. Retin. Eye Res. 2005, 24, 275–306. [Google Scholar] [CrossRef]
- Arango-Gonzalez, B.; Trifunović, D.; Sahaboglu, A.; Kranz, K.; Michalakis, S.; Farinelli, P.; Koch, S.; Koch, F.; Cottet, S.; Janssen-Bienhold, U.; et al. Identification of a common non-apoptotic cell death mechanism in hereditary retinal degeneration. PLoS ONE 2014, 9, e112142. [Google Scholar] [CrossRef] [PubMed]
- Simón, M.V.; Prado Spalm, F.H.; Vera, M.S.; Rotstein, N.P. Sphingolipids as emerging mediators in retina degeneration. Front. Cell. Neurosci. 2019, 13, 246. [Google Scholar] [CrossRef]
- Kakavand, K.; Jobling, A.I.; Greferath, U.; Vessey, K.A.; de Iongh, R.U.; Fletcher, E.L. Photoreceptor degeneration in Pro23His transgenic rats (Line 3) involves autophagic and necroptotic mechanisms. Front. Neurosci. 2020, 14, 581579. [Google Scholar] [CrossRef]
- Power, M.J.; Rogerson, L.E.; Schubert, T.; Berens, P.; Euler, T.; Paquet-Durand, F. Systematic spatiotemporal mapping reveals divergent cell death pathways in three mouse models of hereditary retinal degeneration. J. Comp. Neurol. 2020, 528, 1113–1139. [Google Scholar] [CrossRef] [PubMed]
- Dalman, M.R.; Deeter, A.; Nimishakavi, G.; Duan, Z.H. Fold change and p-value cutoffs significantly alter microarray interpretations. BMC Bioinform. 2012, 13, S11. [Google Scholar] [CrossRef]
- Zhao, B.; Erwin, A.; Xue, B. How many differentially expressed genes: A perspective from the comparison of genotypic and phenotypic distances. Genomics 2018, 110, 67–73. [Google Scholar] [CrossRef]
- Goldstein, J.L.; Brown, M.S. Regulation of the mevalonate pathway. Nature 1990, 343, 425–430. [Google Scholar] [CrossRef]
- Edfors, F.; Danielsson, F.; Hallström, B.M.; Käll, L.; Lundberg, E.; Pontén, F.; Forsström, B.; Uhlén, M. Gene-specific correlation of RNA and protein levels in human cells and tissues. Mol. Syst. Biol. 2016, 12, 883. [Google Scholar] [CrossRef] [PubMed]
- Zheng-Bradley, X.; Rung, J.; Parkinson, H.; Brazma, A. Large scale comparison of global gene expression patterns in human and mouse. Genome Biol. 2010, 11, R124. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Beyer, A.; Aebersold, R. On the dependency of cellular protein levels on mRNA abundance. Cell 2016, 165, 535–550. [Google Scholar] [CrossRef]
- Scapagnini, G.; D’Agata, V.; Calabrese, V.; Pascale, A.; Colombrita, C.; Alkon, D.; Cavallaro, S. Gene expression profiles of heme oxygenase isoforms in the rat brain. Brain Res. 2002, 954, 51–59. [Google Scholar] [CrossRef]
- Ewing, J.F.; Maines, M.D. Glutathione depletion induces heme oxygenase-1 (HSP32) mRNA and protein in rat brain. J. Neurochem. 1993, 60, 1512–1519. [Google Scholar] [CrossRef]
- Liu, X.; Peyton, K.J.; Ensenat, D.; Wang, H.; Schafer, A.I.; Alam, J.; Durante, W. Endoplasmic reticulum stress stimulates heme oxygenase-1 gene expression in vascular smooth muscle. J. Biol. Chem. 2005, 280, 872–877. [Google Scholar] [CrossRef]
- Osburn, W.O.; Kensler, T.W. Nrf2 signaling: An adaptive response pathway for protection against environmental toxic insults. Mutat. Res. 2008, 659, 31–39. [Google Scholar] [CrossRef]
- Bryan, H.K.; Olayanju, A.; Goldring, C.E.; Park, B.K. The Nrf2 cell defence pathway: Keap1-dependent and -independent mechanisms of regulation. Biochem. Pharmacol. 2013, 85, 705–717. [Google Scholar] [CrossRef]
- Anwar, A.A.; Li, F.Y.; Leake, D.S.; Ishii, T.; Mann, G.E.; Siow, R.C. Induction of heme oxygenase 1 by moderately oxidized low-density lipoproteins in human vascular smooth muscle cells: Role of mitogen-activated protein kinases and Nrf2. Free Radic. Biol. Med. 2005, 39, 227–236. [Google Scholar] [CrossRef] [PubMed]
- Martinet, W.; De Bie, M.; Schrijvers, D.M.; De Meyer, G.R.Y.; Herman, A.G.; Kockx, M.M. 7-Ketocholesterol induces protein ubiquitination, myelin figure formation, and light chain 3 processing in vascular smooth muscle cells. Arterioscler. Thromb. Vasc. Biol. 2004, 24, 2296–2301. [Google Scholar] [CrossRef] [PubMed]
- Elbirt, K.K.; Bonkovsky, H.L. Heme oxygenase: Recent advances in understanding its regulation and role. Proc. Assoc. Am. Physicians 1999, 111, 438–447. [Google Scholar] [CrossRef]
- Lee, J.M.; Li, J.; Johnson, D.A.; Stein, T.D.; Kraft, A.D.; Calkins, M.J.; Jakel, R.J.; Johnson, J.A. Nrf2, a multi-organ protector? FASEB J. 2005, 19, 1061–1066. [Google Scholar] [CrossRef]
- Tebay, L.E.; Robertson, H.; Durant, S.T.; Vitale, S.R.; Penning, T.M.; Dinkova-Kostova, A.T.; Hayes, J.D. Mechanisms of activation of the transcription factor Nrf2 by redox stressors, nutrient cues, and energy status and the pathways through which it attenuates degenerative disease. Free Radic. Biol. Med. 2015, 88, 108–146. [Google Scholar] [CrossRef] [PubMed]
- Kourula, S.; Ang, J.; Zhao, H.; Kalish, F.; Vandenabeele, P.; Sylvester, K.G.; Wong, R.J.; Stevenson, D.K. Heme oxygenase activity and heme binding in a neonatal mouse model. Neonatology 2017, 112, 376–383. [Google Scholar] [CrossRef]
- Ryter, S.W.; Alam, J.; Choi, A.M.K. Heme oxygenase-1/carbon monoxide: From basic science to therapeutic applications. Physiol. Rev. 2006, 86, 583–650. [Google Scholar] [CrossRef]
- Chen, Z.; Wang, R.; Wu, J.; Xia, F.; Sun, Q.; Xu, J.; Liu, L. Low-dose carbon monoxide inhalation protects neuronal cells from apoptosis after optic nerve crush. Biochem. Biophys. Res. Commun. 2016, 469, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Sedlak, T.W.; Snyder, S.H. Bilirubin benefits: Cellular protection by a biliverdin reductase antioxidant cycle. Pediatrics 2004, 113, 1776–1782. [Google Scholar] [CrossRef]
- Miao, W.; Hu, L.; Scrivens, P.J.; Batist, G. Transcriptional regulation of NF-E2 p45-related factor (NRF2) expression by the aryl hydrocarbon receptor-xenobiotic response element signaling pathway: Direct cross-talk between phase I and II drug-metabolizing enzymes. J. Biol. Chem. 2005, 280, 20340–20348. [Google Scholar] [CrossRef] [PubMed]
- Savouret, J.F.; Antenos, M.; Quesne, M.; Xu, J.; Milgrom, E.; Casper, R.F. 7-Ketocholesterol is an endogenous modulator for the arylhydrocarbon receptor. J. Biol. Chem. 2001, 276, 3054–3059. [Google Scholar] [CrossRef] [PubMed]
- Schipper, H.M.; Song, W.; Tavitian, A.; Cressatti, M. The sinister face of heme oxygenase-1 in brain aging and disease. Prog. Neurobiol. 2019, 172, 40–70. [Google Scholar] [CrossRef]
- Zukor, H.; Song, W.; Liberman, A.; Mui, J.; Vali, H.; Fillebeen, C.; Pantopoulos, K.; Wu, T.-D.; Guerquin-Kern, J.-L.; Schipper, H.M. HO-1-mediated macroautophagy: A mechanism for unregulated iron deposition in aging and degenerating neural tissues. J. Neurochem. 2009, 109, 776–791. [Google Scholar] [CrossRef] [PubMed]
- Lymboussaki, A.; Pignatti, E.; Montosi, G.; Garuti, C.; Haile, D.J.; Pietrangelo, A. The role of the iron responsive element in the control of ferroportin1/IREG1/MTP1 gene expression. J. Hepatol. 2003, 39, 710–715. [Google Scholar] [CrossRef]
- Kajarabille, N.; Latunde-Dada, G.O. Programmed cell-death by ferroptosis: Antioxidants as mitigators. Int. J. Mol. Sci. 2019, 20, 4968. [Google Scholar] [CrossRef] [PubMed]
- Fabiani, C.; Zulueta, A.; Bonezzi, F.; Casas, J.; Ghidoni, R.; Signorelli, P.; Caretti, A. 2-Acetyl-5-tetrahydroxybutyl imidazole (THI) protects 661W cells against oxidative stress. Naunyn Schmiedebergs Arch. Pharmacol. 2017, 390, 741–751. [Google Scholar] [CrossRef] [PubMed]
- Kutty, G.; Hayden, B.; Osawa, Y.; Wiggert, B.; Chader, G.J.; Kutty, R.K. Heme oxygenase: Expression in human retina and modulation by stress agents in a human retinoblastoma cell model system. Curr. Eye Res. 1992, 11, 153–160. [Google Scholar] [CrossRef]
- Sun, M.H.; Pang, J.H.S.; Chen, S.L.; Kuo, P.C.; Chen, K.J.; Kao, L.Y.; Wu, J.Y.; Lin, K.K.; Tsao, Y.P. Photoreceptor protection against light damage by AAV-mediated overexpression of heme oxygenase-1. Investig. Ophthalmol. Vis. Sci. 2007, 48, 5699–5707. [Google Scholar] [CrossRef] [PubMed]
- Orozco-Ibarra, M.; Estrada-Sánchez, A.M.; Massieu, L.; Pedraza-Chaverrí, J. Heme oxygenase-1 induction prevents neuronal damage triggered during mitochondrial inhibition: Role of CO and bilirubin. Int. J. Biochem. Cell Biol. 2009, 41, 1304–1314. [Google Scholar] [CrossRef]
- Wang, X.Z.; Lawson, B.; Brewer, J.W.; Zinszner, H.; Sanjay, A.; Mi, L.J.; Boorstein, R.; Kreibich, G.; Hendershot, L.M.; Ron, D. Signals from the stressed endoplasmic reticulum induce C/EBP-homologous protein (CHOP/GADD153). Mol. Cell. Biol. 1996, 16, 4273–4280. [Google Scholar] [CrossRef] [PubMed]
- Yang, Y.; Liu, L.; Naik, I.; Braunstein, Z.; Zhong, J.; Ren, B. Transcription factor C/EBP homologous protein in health and diseases. Front. Immunol. 2017, 8, 1612. [Google Scholar] [CrossRef]
- Jauhiainen, A.; Thomsen, C.; Strömbom, L.; Grundevik, P.; Andersson, C.; Danielsson, A.; Andersson, M.K.; Nerman, O.; Rörkvist, L.; Ståhlberg, A.; et al. Distinct cytoplasmic and nuclear functions of the stress induced protein DDIT3/CHOP/GADD153. PLoS ONE 2012, 7, e33208. [Google Scholar] [CrossRef]
- Miyazaki-Anzai, S.; Masuda, M.; Demos-Davies, K.M.; Keenan, A.L.; Saunders, S.J.; Masuda, R.; Jablonski, K.; Cavasin, M.A.; Kendrick, J.; Chonchol, M.; et al. Endoplasmic reticulum stress effector CCAAT/enhancer-binding protein homologous protein (CHOP) regulates chronic kidney disease-induced vascular calcification. J. Am. Heart Assoc. 2014, 3, e000949. [Google Scholar] [CrossRef] [PubMed]
- Ohoka, N.; Yoshii, S.; Hattori, T.; Onozaki, K.; Hayashi, H. TRB3, a novel ER stress-inducible gene, is induced via ATF4–CHOP pathway and is involved in cell death. EMBO J. 2005, 24, 1243–1255. [Google Scholar] [CrossRef]
- Li, G.; Mongillo, M.; Chin, K.T.; Harding, H.; Ron, D.; Marks, A.R.; Tabas, I. Role of ERO1-α–mediated stimulation of inositol 1,4,5-triphosphate receptor activity in endoplasmic reticulum stress–induced apoptosis. J. Cell Biol. 2009, 186, 783–792. [Google Scholar] [CrossRef]
- Marciniak, S.J.; Yun, C.Y.; Oyadomari, S.; Novoa, I.; Zhang, Y.; Jungreis, R.; Nagata, K.; Harding, H.P.; Ron, D. CHOP induces death by promoting protein synthesis and oxidation in the stressed endoplasmic reticulum. Genes Devel. 2004, 18, 3066–3077. [Google Scholar] [CrossRef] [PubMed]
- B’Chir, W.; Maurin, A.-C.; Carraro, V.; Averous, J.; Jousse, C.; Muranishi, Y.; Parry, L.; Stepien, G.; Fafournoux, P.; Bruhat, A. The eIF2α/ATF4 pathway is essential for stress-induced autophagy gene expression. Nucleic Acids Res. 2013, 41, 7683–7699. [Google Scholar] [CrossRef]
- Han, J.; Back, S.H.; Hur, J.; Lin, Y.-H.; Gildersleeve, R.; Shan, J.; Yuan, C.L.; Krokowski, D.; Wang, S.; Hatzoglou, M.; et al. ER-stress-induced transcriptional regulation increases protein synthesis leading to cell death. Nat. Cell Biol. 2013, 15, 481–490. [Google Scholar] [CrossRef]
- Do, J.H. Neurotoxin-induced pathway perturbation in human neuroblastoma SH-EP cells. Mol. Cells 2014, 37, 672–684. [Google Scholar] [CrossRef]
- Wan, X.-S.; Lu, X.-H.; Xiao, Y.-C.; Lin, Y.; Zhu, H.; Ding, T.; Yang, Y.; Huang, Y.; Zhang, Y.; Liu, Y.-L.; et al. ATF4- and CHOP-dependent induction of FGF21 through endoplasmic reticulum stress. BioMed Res. Intl. 2014, 2014, 807874. [Google Scholar] [CrossRef] [PubMed]
- Gómez-Sámano, M.Á.; Grajales-Gómez, M.; Zuarth-Vázquez, J.M.; Navarro-Flores, M.F.; Martínez-Saavedra, M.; Juárez-León, Ó.A.; Morales-García, M.G.; Enríquez-Estrada, V.M.; Gómez-Pérez, F.J.; Cuevas-Ramos, D. Fibroblast growth factor 21 and its novel association with oxidative stress. Redox Biol. 2017, 11, 335–341. [Google Scholar] [CrossRef] [PubMed]
- Lee, M.Y.; Kim, H.J.; Kim, M.A.; Jee, H.J.; Kim, A.J.; Bae, Y.S.; Park, J.I.; Chung, J.H.; Yun, J. Nek6 is involved in G2/M phase cell cycle arrest through DNA damage-induced phosphorylation. Cell Cycle 2008, 7, 2705–2709. [Google Scholar] [CrossRef]
- Li, J.; Lee, B.; Lee, A.S. Endoplasmic reticulum stress-induced apoptosis: Multiple pathways and activation of p53-up-regulated modulator of apoptosis (PUMA) and NOXA by p53. J. Biol. Chem. 2006, 281, 7260–7270. [Google Scholar] [CrossRef]
- Galehdar, Z.; Swan, P.; Fuerth, B.; Callaghan, S.M.; Park, D.S.; Cregan, S.P. Neuronal apoptosis induced by endoplasmic reticulum stress is regulated by ATF4–CHOP-mediated induction of the Bcl-2 homology 3-only member PUMA. J. Neurosci. 2010, 30, 16938–16948. [Google Scholar] [CrossRef] [PubMed]
- Xia, P.; Liu, Y.; Chen, J.; Cheng, Z. Cell cycle proteins as key regulators of postmitotic cell death. Yale J. Biol. Med. 2019, 92, 641–650. [Google Scholar]
- Niida, H.; Nakanishi, M. DNA damage checkpoints in mammals. Mutagenesis 2006, 21, 3–9. [Google Scholar] [CrossRef] [PubMed]
- Lee, D.; Hokinson, D.; Park, S.; Elvira, R.; Kusuma, F.; Lee, J.M.; Yun, M.; Lee, S.G.; Han, J. ER stress induces cell cycle arrest at the G2/M phase through eIF2α phosphorylation and GADD45α. Int. J. Mol. Sci. 2019, 20, 6309. [Google Scholar] [CrossRef]
- Barone, M.V.; Crozat, A.; Tabaee, A.; Philipson, L.; Ron, D. CHOP (GADD153) and its oncogenic variant, TLS-CHOP, have opposing effects on the induction of Gj/S arrest. Genes Dev. 1994, 8, 453–464. [Google Scholar] [CrossRef] [PubMed]
- Igase, M.; Okura, T.; Nakamura, M.; Takata, Y.; Kitami, Y.; Hiwada, K. Role of GADD153 (growth arrest- and DNA damage-inducible gene 153) in vascular smooth muscle cell apoptosis. Clin. Sci. 2001, 100, 275–281. [Google Scholar] [CrossRef]
- Wang, Y.; Kuramitsu, Y.; Baron, B.; Kitagawa, T.; Tokuda, K.; Akada, J.; Nakamura, K. CGK733-induced LC3 II formation is positively associated with the expression of cyclin-dependent kinase inhibitor p21Waf1/Cip1 through modulation of the AMPK and PERK/CHOP signaling pathways. Oncotarget 2015, 6, 39692–39701. [Google Scholar] [CrossRef]
- Zhu, J.; Chen, M.; Chen, N.; Ma, A.; Zhu, C.; Zhao, R.; Jiang, M.; Zhou, J.; Ye, L.; Fu, H.; et al. Glycyrrhetinic acid induces G1-phase cell cycle arrest in human non-small cell lung cancer cells through endoplasmic reticulum stress pathway. Int. J. Oncol. 2015, 46, 981–988. [Google Scholar] [CrossRef]
- Kreis, N.N.; Louwen, F.; Yuan, J. The multifaceted p21 (Cip1/Waf1/CDKN1A) in cell differentiation, migration and cancer therapy. Cancers 2019, 11, 1220. [Google Scholar] [CrossRef]
- Sanli, T.; Steinberg, G.R.; Singh, G.; Tsakiridis, T. AMP-activated protein kinase (AMPK) beyond metabolism: A novel genomic stress sensor participating in the DNA damage response pathway. Cancer Biol. Ther. 2014, 15, 156–169. [Google Scholar] [CrossRef] [PubMed]
- Tokarz, P.; Kaarniranta, K.; Blasiak, J. Role of the cell cycle re-initiation in DNA damage response of post-mitotic cells and its implication in the pathogenesis of neurodegenerative diseases. Rejuv. Res. 2016, 19, 131–139. [Google Scholar] [CrossRef]
- Herrup, K.; Neve, R.; Ackerman, S.L.; Copani, A. Divide and die: Cell cycle events as triggers of nerve cell death. J. Neurosci. 2004, 24, 9232–9239. [Google Scholar] [CrossRef] [PubMed]
- Parker, S.B.; Eicele, G.; Zhang, P.; Rawls, A.; Sands, A.T.; Bradley, A. p53-independent expression of p21Cip1 in muscle and other terminally differentiating cells. Science 1995, 267, 1024–1027. [Google Scholar] [CrossRef]
- De Luca, P.; Moiola, C.P.; Zalazar, F.; Gardner, K.; Vazquez, E.S.; De Siervi, A. BRCA1 and p53 regulate critical prostate cancer pathways. Prostate Cancer Prostatic Dis. 2013, 16, 233–238. [Google Scholar] [CrossRef]
- Wang, X.Z.; Ron, D. Stress-induced phosphorylation and activation of the transcription factor CHOP (GADD153) by p38 MAP Kinase. Science 1996, 272, 1347–1349. [Google Scholar] [CrossRef] [PubMed]
- Cherasse, Y.; Maurin, A.-C.; Chaveroux, C.; Jousse, C.; Carraro, V.; Parry, L.; Deval, C.; Chambon, C.; Fafournoux, P.; Bruhat, A. The p300/CBP-associated factor (PCAF) is a cofactor of ATF4 for amino acid-regulated transcription of CHOP. Nucleic Acids Res. 2007, 35, 5954–5965. [Google Scholar] [CrossRef] [PubMed]
- Çelik, H.; Bulut, G.; Han, J.; Graham, G.T.; Minas, T.Z.; Conn, E.J.; Hong, S.-H.; Pauly, G.T.; Hayran, M.; Li, X.; et al. Ezrin inhibition up-regulates stress response gene expression. J. Biol. Chem. 2016, 291, 13257–13270. [Google Scholar] [CrossRef] [PubMed]
- Rashid, H.O.; Yadav, R.K.; Kim, H.R.; Chae, H.J. ER stress: Autophagy induction, inhibition and selection. Autophagy 2015, 11, 1956–1977. [Google Scholar] [CrossRef] [PubMed]
- Nakanishi, T.; Shimazawa, M.; Sugitani, S.; Kudo, T.; Imai, S.; Inokuchi, Y.; Tsuruma, K.; Hara, H. Role of endoplasmic reticulum stress in light-induced photoreceptor degeneration in mice. J. Neurochem. 2013, 125, 111–124. [Google Scholar] [CrossRef] [PubMed]
- Zhu, H.; Qian, J.; Wang, W.; Yan, Q.; Xu, Y.; Jiang, Y.; Zhang, L.; Lu, F.; Hu, W.; Zhang, X.; et al. RNA interference of GADD153 protects photoreceptors from endoplasmic reticulum stress-mediated apoptosis after retinal detachment. PLoS ONE 2013, 8, e59339. [Google Scholar] [CrossRef]
- Rozpędek-Kamińska, W.; Siwecka, N.; Wawrzynkiewicz, A.; Wojtczak, R.; Pytel, D.; Diehl, J.A.; Majsterek, I. The PERK-dependent molecular mechanisms as a novel therapeutic target for neurodegenerative diseases. Int. J. Mol. Sci. 2020, 21, 2108. [Google Scholar] [CrossRef]
- Ma, Y.; Brewer, J.W.; Diehl, J.A.; Hendershot, L.M. Two distinct stress signaling pathways converge upon the CHOP promoter during the mammalian unfolded protein response. J. Mol. Biol. 2002, 318, 1351–1365. [Google Scholar] [CrossRef]
- Enyedi, B.; Várnai, P.; Geiszt, M. Redox state of the endoplasmic reticulum is controlled by Ero1L-alpha and intraluminal calcium. Antioxid. Redox Signal. 2010, 13, 721–729. [Google Scholar] [CrossRef] [PubMed]
- Croteau, D.L.; Bohr, V.A. Repair of oxidative damage to nuclear and mitochondrial DNA in mammalian cells. J. Biol. Chem. 1997, 272, 25409–25412. [Google Scholar] [CrossRef] [PubMed]
- Cooke, M.S.; Evans, M.D.; Dizdaroglu, M.; Lunec, J. Oxidative DNA damage: Mechanisms, mutation, and disease. FASEB J. 2003, 17, 1195–1214. [Google Scholar] [CrossRef]
- Huggins, C.J.; Mayekar, M.K.; Martin, N.; Saylor, K.L.; Gonit, M.; Jailwala, P.; Kasoji, M.; Haines, D.C.; Quinones, O.A.; Johnson, P.F. C/EBPγ is a critical regulator of cellular stress response networks through heterodimerization with ATF4. Mol. Cell. Biol. 2016, 36, 693–713. [Google Scholar] [CrossRef]
- Chen, B.P.C.; Wolfgang, C.D.; Hai, T. Analysis of ATF3, a transcription factor induced by physiological stresses and modulated by gadd153/Chop10. Mol. Cell. Biol. 1996, 16, 1157–1168. [Google Scholar] [CrossRef] [PubMed]
- Pereira, R.C.; Delany, A.M.; Canalis, E. CCAAT/enhancer binding protein homologous protein (DDIT3) induces osteoblastic cell differentiation. Endocrinology 2004, 145, 1952–1960. [Google Scholar] [CrossRef] [PubMed]
- Fawcett, T.W.; Martindale, J.L.; Guyton, K.Z.; Hai, T.; Holbrook, N.J. Complexes containing activating transcription factor (ATF)/cAMP-responsive element-binding protein (CREB) interact with the CCAAT/enhancer-binding protein (C/EBP)–ATF composite site to regulate Gadd153 expression during the stress response. Biochem. J. 1999, 339, 135–141. [Google Scholar] [PubMed]
- Peña-Altamira, E.; Polazzi, E.; Moretto, E.; Lauriola, M.; Monti, B. The transcription factor CCAAT enhancer-binding protein β protects rat cerebellar granule neurons from apoptosis through its transcription-activating isoforms. Eur. J. Neurosci. 2014, 39, 176–185. [Google Scholar] [CrossRef]
- Halterman, M.W.; De Jesus, C.; Rempe, D.A.; Schor, N.F.; Federoff, H.J. Loss of c/EBP-β activity promotes the adaptive to apoptotic switch in hypoxic cortical neurons. Mol. Cell. Neurosci. 2008, 38, 125–137. [Google Scholar] [CrossRef][Green Version]
- Reisman, D.; Takahashi, P.; Polson, A.; Boggs, K. Transcriptional regulation of the p53 tumor suppressor gene in S-phase of the cell-cycle and the cellular response to DNA damage. Biochem. Res. Int. 2012, 2012, 808934. [Google Scholar] [CrossRef]
- Vuong, L.; Conley, S.M.; Al-Ubaidi, M.R. Expression and role of p53 in the retina. Investig. Ophthalmol. Vis. Sci. 2012, 53, 1362–1371. [Google Scholar] [CrossRef] [PubMed]
- Cregan, S.P.; Arbour, N.A.; MacLaurin, J.G.; Callaghan, S.M.; Fortin, A.; Cheung, E.C.C.; Guberman, D.S.; Park, D.S.; Slack, R.S. p53 activation domain 1 is essential for PUMA upregulation and p53-mediated neuronal cell death. J. Neurosci. 2004, 24, 10003–10012. [Google Scholar] [CrossRef]
- Engel, T.; Sanz-Rodgriguez, A.; Jimenez-Mateos, E.M.; Concannon, C.G.; Jimenez-Pacheco, A.; Moran, C.; Mesuret, G.; Petit, E.; Delanty, N.; Farrell, M.A.; et al. CHOP regulates the p53–MDM2 axis and is required for neuronal survival after seizures. Brain 2013, 136, 577–592. [Google Scholar] [CrossRef]
- Cunard, R. Mammalian tribbles homologs at the crossroads of endoplasmic reticulum stress and mammalian target of rapamycin pathways. Scientifica 2013, 2013, 750871. [Google Scholar] [CrossRef] [PubMed]
- Mondal, D.; Mathur, A.; Chandra, P.K. Tripping on TRIB3 at the junction of health, metabolic dysfunction and cancer. Biochimie 2016, 124, 34–52. [Google Scholar] [CrossRef]
- Corcoran, C.A.; Luo, X.; He, Q.; Jiang, C.; Huang, Y.; Sheikh, M.S. Genotoxic and endoplasmic reticulum stresses differentially regulate TRB3 expression. Cancer Biol. 2005, 4, 1063–1067. [Google Scholar] [CrossRef] [PubMed][Green Version]
- Cunha, D.A.; Igoillo-Esteve, M.; Gurzov, E.N.; Germano, C.M.; Naamane, N.; Marhfour, I.; Fukaya, M.; Vanderwinden, J.-M.; Gysemans, C.; Mathieu, C.; et al. Death protein 5 and p53-upregulated modulator of apoptosis mediate the endoplasmic reticulum stress-mitochondrial dialog triggering lipotoxic rodent and human β-cell apoptosis. Diabetes 2012, 61, 2763–2775. [Google Scholar] [CrossRef] [PubMed]
- Aynaud, M.M.; Suspène, R.; Vidalain, P.O.; Mussil, B.; Guétard, D.; Tangy, F.; Wain-Hobson, S.; Vartanian, J.P. Human tribbles 3 protects nuclear DNA from cytidine deamination by APOBEC3A. J. Biol. Chem. 2012, 287, 39182–39192. [Google Scholar] [CrossRef] [PubMed]
- Sakai, S.; Ohoka, N.; Onozaki, K.; Kitagawa, M.; Nakanishi, M.; Hayashi, H. Dual mode of regulation of cell division cycle 25 A protein by TRB3. Biol. Pharm. Bull. 2010, 33, 1112–1116. [Google Scholar] [CrossRef]
- Shimizu, K.; Takahama, S.; Endo, Y.; Sawasaki, T. Stress-inducible caspase substrate TRB3 promotes nuclear translocation of procaspase-3. PLoS ONE 2012, 7, e42721. [Google Scholar] [CrossRef]
- Shang, Y.Y.; Wang, Z.H.; Zhang, L.P.; Zhong, M.; Zhang, Y.; Deng, J.T.; Zhang, W. TRB3, upregulated by ox-LDL, mediates human monocyte-derived macrophage apoptosis. FEBS J. 2009, 276, 2752–2761. [Google Scholar] [CrossRef]
- Van der Krieken, S.E.; Popeijus, H.E.; Mensink, R.P.; Plat, J. CCAAT/enhancer binding protein β in relation to ER stress, inflammation, and metabolic disturbances. BioMed. Res. Int. 2015, 2015, 324845. [Google Scholar] [CrossRef] [PubMed]
- Selim, E.; Frkanec, J.T.; Cunard, R. Fibrates upregulate TRB3 in lymphocytes independent of PPAR alpha by augmenting CCAAT/enhancer-binding protein beta (C/EBP beta) expression. Mol. Immunol. 2007, 44, 1218–1229. [Google Scholar] [CrossRef]
- Hua, F.; Ki, K.; Yu, J.J.; Hu, Z.W. The TRIB3-SQSTM1 interaction mediates metabolic stress-promoted tumorigenesis and progression via suppressing autophagic and proteasomal degradation. Autophagy 2015, 11, 1929–1931. [Google Scholar] [CrossRef]
- Zhang, X.-W.; Zhou, J.-C.; Peng, D.; Hua, F.; Li, K.; Yu, J.-J.; Lv, X.-X.; Cui, B.; Liu, S.-S.; Yu, J.-M.; et al. Disrupting the TRIB3-SQSTM1 interaction reduces liver fibrosis by restoring autophagy and suppressing exosome-mediated HSC activation. Autophagy 2020, 16, 782–796. [Google Scholar] [CrossRef]
- Saleem, S.; Biswas, S.C. Tribbles pseudokinase 3 induces both apoptosis and autophagy in amyloid-β-induced neuronal death. J. Biol. Chem. 2017, 292, 2571–2585. [Google Scholar] [CrossRef] [PubMed]
- Chan, S.L.; Fu, W.; Zhang, P.; Cheng, A.; Lee, J.; Kokame, K.; Mattson, M.P. Herp stabilizes neuronal Ca2+ homeostasis and mitochondrial function during endoplasmic reticulum stress. J. Biol. Chem. 2004, 279, 28733–28743. [Google Scholar] [CrossRef]
- Kokame, K.; Agarwala, K.L.; Kato, H.; Miyata, T. Herp, a new ubiquitin-like membrane protein induced by endoplasmic reticulum stress. J. Biol. Chem. 2000, 275, 32846–32853. [Google Scholar] [CrossRef]
- Bergmann, T.J.; Fregno, I.; Fumagalli, F.; Rinaldi, A.; Bertoni, F.; Boersema, P.J.; Picotti, P.; Molinari, M. Chemical stresses fail to mimic the unfolded protein response resulting from luminal load with unfolded polypeptides. J. Biol. Chem. 2018, 293, 5600–5612. [Google Scholar] [CrossRef] [PubMed]
- Leitman, J.; Shenkman, M.; Gofman, Y.; Shtern, N.O.; Ben-Tal, N.; Hendershot, L.M.; Lederkremer, G.Z. Herp coordinates compartmentalization and recruitment of HRD1 and misfolded proteins for ERAD. Mol. Biol. Cell 2018, 25, 1050–1060. [Google Scholar] [CrossRef]
- Belal, C.; Ameli, N.J.; El Kommos, A.; Bezalel, S.; Al’Khafaji, A.M.; Mughal, M.R.; Mattson, M.P.; Kyriazis, G.A.; Tyrberg, B.; Chan, S.L. The homocysteine-inducible endoplasmic reticulum (ER) stress protein Herp counteracts mutant α-synuclein-induced ER stress via the homeostatic regulation of ER-resident calcium release channel proteins. Hum. Mol. Genet. 2012, 21, 963–977. [Google Scholar] [CrossRef]
- Chigurupati, S.; Wei, Z.; Belal, C.; Vandermey, M.; Kyriazis, G.A.; Arumugam, T.V.; Chan, S.L. The homocysteine-inducible endoplasmic reticulum stress protein counteracts calcium store depletion and induction of CCAAT enhancer-binding protein homologous protein in a neurotoxin model of Parkinson disease. J. Biol. Chem. 2009, 284, 18323–18333. [Google Scholar] [CrossRef]
- Liang, G.; Li, Q.; Tang, Y.; Kokame, K.; Kikuchi, T.; Wu, G.; Chen, X.Z. Polycystin-2 is regulated by endoplasmic reticulum-associated degradation. Hum. Mol. Genet. 2008, 17, 1109–1119. [Google Scholar] [CrossRef] [PubMed]
- Tulenko, T.N.; Boeze-Battaglia, K.; Mason, R.P.; Tint, G.S.; Steiner, R.D.; Connor, W.E.; Labelle, E.F. A membrane defect in the pathogenesis of the Smith-Lemli-Opitz syndrome. J. Lipid Res. 2006, 47, 134–143. [Google Scholar] [CrossRef]
- Shim, S.M.; Choi, H.R.; Joonsung, H.; Lee, Y.J.; Kim, S.T.; Kim, D.; Mun, S.R.; Hwang, J.; Cha-Molstad, H.; Ciechanover, A.; et al. The endoplasmic reticulum–residing chaperone BiP is short-lived and metabolized through N-terminal arginylation. Sci. Signal. 2018, 11, eaan0630. [Google Scholar] [CrossRef]
- Okuda-Shimizu, Y.; Hendershot, L.M. Characterization of an ERAD pathway for non-glycosylated BiP substrates which requires Herp. Mol. Cell 2007, 28, 544–554. [Google Scholar] [CrossRef] [PubMed]
- Schäuble, N.; Lang, S.; Jung, M.; Cappel, S.; Schorr, S.; Ulucan, Ö.; Linxweiler, J.; Dudek, J.; Blum, R.; Helms, V.; et al. BiP-mediated closing of the Sec61 channel limits Ca2+ leakage from the ER. EMBO J. 2012, 31, 3282–3296. [Google Scholar] [CrossRef]
- Bengoechea-Alonso, M.T.; Ericsson, J. SREBP in signal transduction: Cholesterol metabolism and beyond. Curr. Opin. Cell Biol. 2007, 19, 215–222. [Google Scholar] [CrossRef] [PubMed]
- Liu, Y.; Li, P.; Fan, L.; Wu, M. The nuclear transportation routes of membrane-bound transcription factors. Cell Comm. Signal. 2018, 16, 12. [Google Scholar] [CrossRef]
- Song, L.; Ma, L.; Cong, F.; Shen, X.; Jing, P.; Ying, X.; Zhou, H.; Jiang, J.; Fu, Y.; Yan, H. Radioprotective effects of genistein on HL-7702 cells via the inhibition of apoptosis and DNA damage. Cancer Lett. 2015, 366, 100–111. [Google Scholar] [CrossRef] [PubMed]
- Upadhya, S.C.; Hegde, A.N. A potential proteasome-interacting motif within the ubiquitin-like domain of parkin and other proteins. Trends Biochem. Sci. 2003, 28, 280–283. [Google Scholar] [CrossRef]
- Kaur, M.; Pop, M.; Shi, D.; Brignone, C.; Grossman, S.R. hHR23B is required for genotoxic-specific activation of p53 and apoptosis. Oncogene 2007, 26, 1231–1237. [Google Scholar] [CrossRef] [PubMed]
- Kao, S.Y. DNA damage induces nuclear translocation of parkin. J. Biomed. Sci. 2009, 16, 67. [Google Scholar] [CrossRef]
- Jones, R.D.; Gardner, R.G. Protein quality control in the nucleus. Curr. Opin. Cell Biol. 2016, 40, 81–89. [Google Scholar] [CrossRef]
- Korade, Ž.; Kenworthy, A.K.; Mirnics, K. Molecular consequences of altered neuronal cholesterol biosynthesis. J. Neurosci. Res. 2009, 87, 866–875. [Google Scholar] [CrossRef] [PubMed]
- Brechalov, A.V.; Georgieva, S.G.; Soshnikova, N.V. Mammalian cells contain two functionally distinct PBAF complexes incorporating different isoforms of PHF10 signature subunit. Cell Cycle 2014, 13, 1970–1979. [Google Scholar] [CrossRef] [PubMed]
- Peng, S.; Sen, B.; Mazumdar, T.; Byers, L.A.; Diao, L.; Wang, J.; Tong, P.; Giri, U.; Heymach, J.V.; Kadara, H.N.; et al. Dasatinib induces DNA damage and activates DNA repair pathways leading to senescence in non-small cell lung cancer cell lines with kinase-inactivating BRAF mutations. Oncotarget 2015, 7, 565–579. [Google Scholar] [CrossRef]
- Waage-Baudet, H.; Dunty, W.C., Jr.; Dehart, D.B.; Hiller, S.; Sulik, K.K. Immunohistochemical and microarray analyses of a mouse model for the Smith-Lemli-Opitz syndrome. Dev. Neurosci. 2005, 27, 378–396. [Google Scholar] [CrossRef]
- Biasi, F.; Chiarpotto, E.; Sottero, B.; Maina, M.; Mascia, C.; Guina, T.; Gamba, P.F.; Gargiulo, S.; Testa, G.; Leonarduzzi, G.M.; et al. Evidence of cell damage induced by major components of a diet-compatible mixture of oxysterols in human colon cancer CaCo-2 cell line. Biochimie 2013, 95, 632–640. [Google Scholar] [CrossRef]
- Chang, S.; Ren, G.; Steiner, R.D.; Merkens, L.; Roullet, J.B.; Korade, Z.; DiMuzio, P.J.; Tulenko, T.N. Elevated autophagy and mitochondrial dysfunction in the Smith-Lemli-Opitz syndrome. Mol. Genet. Metab. Rep. 2014, 1, 431–442. [Google Scholar] [CrossRef]
- Svoboda, M.D.; Christie, J.M.; Eroglu, Y.; Freeman, K.A.; Steiner, R.D. Treatment of Smith-Lemli-Opitz syndrome and other sterol disorders. Am. J. Med. Genet. C Semin. Med. Genet. 2012, 160, 285–294. [Google Scholar] [CrossRef]
- Fliesler, S.J.; Peachey, N.S.; Herron, J.; Hines, K.M.; Weinstock, N.I.; Ramachandra Rao, S.; Xu, L. Prevention of retinal degeneration in a rat model of Smith-Lemli-Opitz Syndrome. Sci. Rep. 2018, 8, 1286. [Google Scholar] [CrossRef]
- Chen, Y.; Brandizzi, F. IRE1: ER stress sensor and cell fate executor. Trends Cell Biol. 2013, 23, 547–555. [Google Scholar] [CrossRef] [PubMed]
- Wheway, G.; Nazlamova, L.; Turner, D.; Cross, S. 661W photoreceptor cell line as a cell model for studying retinal ciliopathies. Front. Genet. 2019, 10, 308. [Google Scholar] [CrossRef] [PubMed]
- Tan, E.; Ding, X.Q.; Saadi, A.; Agarwal, N.; Naash, M.I.; Al-Ubaidi, M.R. Expression of cone-photoreceptor-specific antigens in a cell line derived from retinal tumors in transgenic mice. Investig. Ophthalmol. Vis. Sci. 2004, 45, 764–768. [Google Scholar] [CrossRef] [PubMed]
- Kanan, Y.; Gordon, W.C.; Mukherjee, P.K.; Bazan, N.G.; Al-Ubaidi, M.R. Neuroprotectin D1 is synthesized in the cone photoreceptor cell line 661W and elicits protection against light-induced stress. Cell. Mol. Neurobiol. 2015, 35, 197–204. [Google Scholar] [CrossRef][Green Version]
- Krishnamoorthy, R.R.; Crawford, M.J.; Chaturvedi, M.M.; Jain, S.K.; Aggarwal, B.B.; Al-Ubaidi, M.R.; Agarwal, N. Photo-oxidative stress down-modulates the activity of nuclear factor-κB via involvement of caspase-1, leading to apoptosis of photoreceptor cells. J. Biol. Chem. 1999, 274, 3734–3743. [Google Scholar] [CrossRef] [PubMed]
- Lin, C.W.; Yang, C.M.; Yang, C.H. Effects of the emitted light spectrum of liquid crystal displays on light-induced retinal photoreceptor cell damage. Int. J. Mol. Sci. 2019, 20, 2318. [Google Scholar] [CrossRef] [PubMed]
- Tao, J.X.; Zhou, W.C.; Zhu, X.G. Mitochondria as potential targets and initiators of the blue light hazard to the retina. Oxid. Med. Cell. Longev. 2019, 2019, 6435364. [Google Scholar] [CrossRef]
- Kayatz, P.; Heimann, K.; Schraermeyer, U. Ultrastructural localization of light-induced lipid peroxides in the rat retina. Investig. Ophthalmol. Vis. Sci. 1999, 40, 2314–2321. [Google Scholar]
- Winkler, B.S. An hypothesis to account for the renewal of outer segments in rod and cone photoreceptor cells: Renewal as a surrogate antioxidant. Investig. Ophthalmol. Vis. Sci. 2008, 49, 3259–3261. [Google Scholar] [CrossRef]
- Organisciak, D.T.; Vaughan, D.K. Retinal light damage: Mechanisms and protection. Prog. Retin. Eye Res. 2010, 29, 113–134. [Google Scholar] [CrossRef] [PubMed]
- Campochiaro, P.A.; Mir, T.A. The mechanism of cone cell death in retinitis pigmentosa. Prog. Retin. Eye Res. 2018, 62, 24–37. [Google Scholar] [CrossRef] [PubMed]
- Yang, J.H.; Basinger, S.F.; Gross, R.L.; Wu, S.M. Blue light–induced generation of reactive oxygen species in photoreceptor ellipsoids requires mitochondrial electron transport. Investig. Ophthalmol. Vis. Sci. 2003, 44, 1312–1319. [Google Scholar] [CrossRef] [PubMed]
- Lefevere, E.; Toft-Kehler, A.K.; Vohra, R.; Kolko, M.; Moons, L.; Van Hove, I. Mitochondrial dysfunction underlying outer retinal diseases. Mitochondrion 2017, 36, 66–76. [Google Scholar] [CrossRef]
- Yang, L.P.; Wu, L.M.; Guo, X.J.; Li, Y.; Tso, M.O.M. Endoplasmic reticulum stress is activated in light-induced retinal degeneration. J. Neurosci. Res. 2008, 86, 910–919. [Google Scholar] [CrossRef]
- Cai, X.; Chen, L.; McGinnis, J.F. Correlation of ER stress and retinal degeneration in tubby mice. Exp. Eye Res. 2015, 140, 130–138. [Google Scholar] [CrossRef] [PubMed]
- Bhatia, V.; Valdés-Sánchez, L.; Rodriguez-Martinez, D.; Bhattacharya, S.S. Formation of 53BP1 foci and ATM activation under oxidative stress is facilitated by RNA: DNA hybrids and loss of ATM-53BP1 expression promotes photoreceptor cell survival in mice. F1000Research 2018, 7, 1233. [Google Scholar] [CrossRef]
- Pan, Y.R.; Song, J.Y.; Fan, B.; Wang, Y.; Che, L.; Zhang, S.M.; Chang, Y.X.; He, C.; Li, G.Y. mTOR may interact with PARP-1 to regulate visible light-induced parthanatos in photoreceptors. Cell Comm. Signal. 2020, 18, 27. [Google Scholar] [CrossRef]
- Xu, L.; Korade, Z.; Rosado, D.A., Jr.; Liu, W.; Lamberson, C.R.; Porter, N.A. An oxysterol biomarker for 7-dehydrocholesterol oxidation in cell/mouse models for Smith-Lemli-Opitz syndrome. J. Lipid Res. 2011, 52, 1222–1233. [Google Scholar] [CrossRef] [PubMed]
- Pfeffer, B.A.; Fliesler, S.J. Streamlined duplex live-dead microplate assay for cultured cells. Exp. Eye Res. 2017, 161, 17–29. [Google Scholar] [CrossRef] [PubMed]
- Brewer, G.J.; Torricelli, J.R.; Evege, E.K.; Price, P.J. Optimized survival of hippocampal neurons in B27-supplemented Neurobasal, a new serum-free medium combination. J. Neurosci. Res. 1993, 35, 567–576. [Google Scholar] [CrossRef]
- Chen, Y.; Stevens, B.; Chang, J.; Milbrandt, J.; Barres, B.A.; Hell, J.W. NS21: Redefined and modified supplement B27 for neuronal cultures. J. Neurosci. Methods 2008, 171, 239–247. [Google Scholar] [CrossRef] [PubMed]
- Bajpai, R.; Lesperance, J.; Kim, M.; Terskikh, A.V. Efficient propagation of single cells Accutase-dissociated human embryonic stem cells. Mol. Reprod. Dev. 2008, 75, 818–827. [Google Scholar] [CrossRef]
- Larrayoz, I.M.; Huang, J.D.; Lee, J.W.; Pascual, I.; Rodriguez, I.R. 7-ketocholesterol-induced inflammation: Involvement of multiple kinase signaling pathways via NFκB but independently of reactive oxygen species formation. Investig. Ophthalmol. Vis. Sci. 2010, 51, 4942–4955. [Google Scholar] [CrossRef]
- Mueller, O.; Lightfoot, S.; Schroeder, A. RNA Integrity number (RIN)—Standardization of RNA quality control. In Agilent Application Note; Publication No. 5989-1165EN; Agilent: Santa Clara, CA, USA, 2004. [Google Scholar]
- Kauffmann, A.; Gentleman, R.; Huber, W. arrayQualityMetrics—A bioconductor package for quality assessment of microarray data. Bioinformatics 2009, 25, 415–416. [Google Scholar] [CrossRef]
- Heber, S.; Sick, B. Quality Assessment of Affymetrix GeneChip Data. OMICS 2006, 10, 358–368. [Google Scholar] [CrossRef] [PubMed]
- Gentleman, R.C.; Carey, V.J.; Bates, D.M.; Bolstad, B.; Dettling, M.; Dudoit, S.; Ellis, B.; Gautier, L.; Ge, Y.; Gentry, J.; et al. Bioconductor: Open software development for computational biology and bioinformatics. Genome Biol. 2004, 5, R80. [Google Scholar] [CrossRef]
- Gautier, L.; Cope, L.; Bolstad, B.M.; Irizarry, R.A. affy—Analysis of Affymetrix GeneChip data at the probe level. Bioinformatics 2004, 20, 307–315. [Google Scholar] [CrossRef] [PubMed]
- Kleinfeld, D.; Kahler, K.H.; Hockberger, P.E. Controlled outgrowth of dissociated neurons on patterned substrates. J. Neurosci. 1988, 8, 4098–4120. [Google Scholar] [CrossRef]
- Ishihara, N.; Jofuku, A.; Eura, Y.; Mihara, K. Regulation of mitochondrial morphology by membrane potential, and DRP1-dependent division and FZO1-dependent fusion reaction in mammalian cells. Biochem. Biophys. Res. Comm. 2003, 301, 891–898. [Google Scholar] [CrossRef]
- Lane, C.F. Sodium cyanoborohydride—A highly selective reducing agent for organic functional groups. Synthesis 1975, 1975, 135–146. [Google Scholar] [CrossRef]
- Puchtler, H.; Waldrop, F.S.; Meloan, S.N.; Terry, M.S.; Conner, H.M. Methacarn (methanol-Carnoy) fixation. Practical and theoretical considerations. Histochemie 1970, 21, 97–116. [Google Scholar] [CrossRef]
- Hale, I.L.; Matsumoto, B. Resolution of subcellular detail in thick tissue sections: Immunohistochemical preparation and fluorescence confocal microscopy. Methods Cell Biol. 2002, 70, 301–335. [Google Scholar] [CrossRef] [PubMed]





| EPCD | 7kCHOL | CHOL | Number of Genes |
|---|---|---|---|
| ↑ | ↑ | ↑ | 49 |
| ↑ | ↑ | ↓ | 15 |
| ↓ | ↑ | ↑ | 7 |
| ↓ | ↑ | ↓ | 15 |
| ↓ | ↓ | ↑ | 5 |
| ↓ | ↓ | ↓ | 120 |
| Dilution | Cat. No. | Source | Host Species 1 | FC, Array vs. VC | Subcellular Localization | Pathways | Symbol | Protein | |
|---|---|---|---|---|---|---|---|---|---|
| 7kCHOL | EPCD | ||||||||
| 2 μg/mL | 13243 | Abcam | R PC | 5.646 | 10.743 | ER, caveolae, nucleus | Oxidative stress | HMOX1 | Heme oxygenase-1 |
| 1:1000 | 2895 (L63F7) | Cell Signaling | M MC | 5.296 | 7.261 | Nucleus | ER stress; apoptosis | CHOP | DNA damage-inducible transcript 3 |
| 2 μg/mL | R12-2387 | Assay Biotech | R PC | 7.056 | 8.424 | Nucleus, cytoplasm | ER stress | TRIB3 | Tribbles homolg-3 |
| 2 μg/mL | PA5-29469 | Thermo Scientific | R PC | 2.561 | 4.061 | ER, nucleus | ER stress | HERPUD1 | Homocysteine ER stress-inducible |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Pfeffer, B.A.; Xu, L.; Fliesler, S.J. Transcriptomic Changes Associated with Loss of Cell Viability Induced by Oxysterol Treatment of a Retinal Photoreceptor-Derived Cell Line: An In Vitro Model of Smith–Lemli–Opitz Syndrome. Int. J. Mol. Sci. 2021, 22, 2339. https://doi.org/10.3390/ijms22052339
Pfeffer BA, Xu L, Fliesler SJ. Transcriptomic Changes Associated with Loss of Cell Viability Induced by Oxysterol Treatment of a Retinal Photoreceptor-Derived Cell Line: An In Vitro Model of Smith–Lemli–Opitz Syndrome. International Journal of Molecular Sciences. 2021; 22(5):2339. https://doi.org/10.3390/ijms22052339
Chicago/Turabian StylePfeffer, Bruce A., Libin Xu, and Steven J. Fliesler. 2021. "Transcriptomic Changes Associated with Loss of Cell Viability Induced by Oxysterol Treatment of a Retinal Photoreceptor-Derived Cell Line: An In Vitro Model of Smith–Lemli–Opitz Syndrome" International Journal of Molecular Sciences 22, no. 5: 2339. https://doi.org/10.3390/ijms22052339
APA StylePfeffer, B. A., Xu, L., & Fliesler, S. J. (2021). Transcriptomic Changes Associated with Loss of Cell Viability Induced by Oxysterol Treatment of a Retinal Photoreceptor-Derived Cell Line: An In Vitro Model of Smith–Lemli–Opitz Syndrome. International Journal of Molecular Sciences, 22(5), 2339. https://doi.org/10.3390/ijms22052339







