The T Cell Repertoires from Nickel Sensitized Joint Implant Failure Patients
Abstract
:1. Introduction
2. Results
2.1. Characterization of Subject Clinical Features
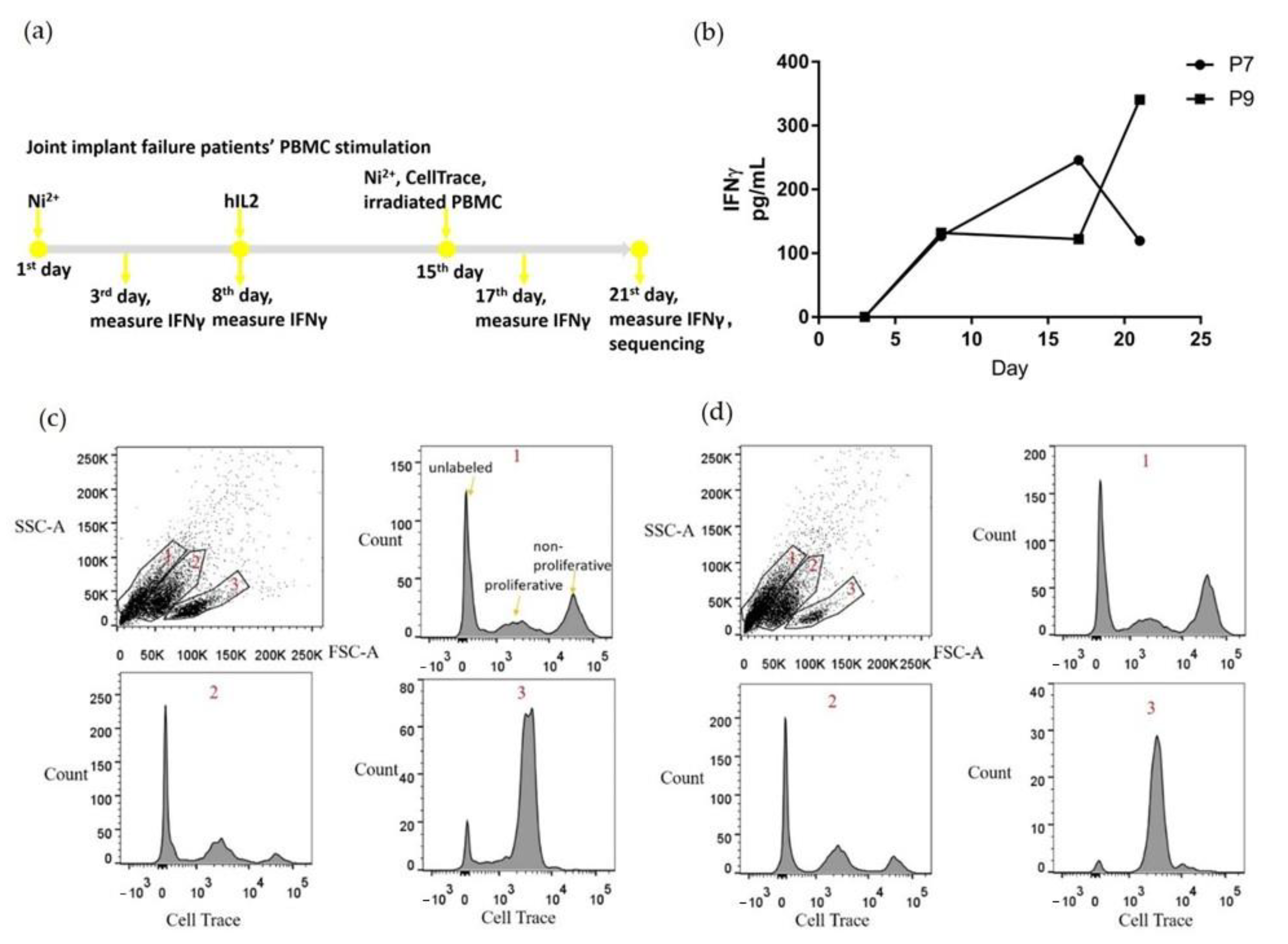
2.2. PBMCs Proliferated and Secreted IFNγ with Ni2+ Stimulation In Vitro
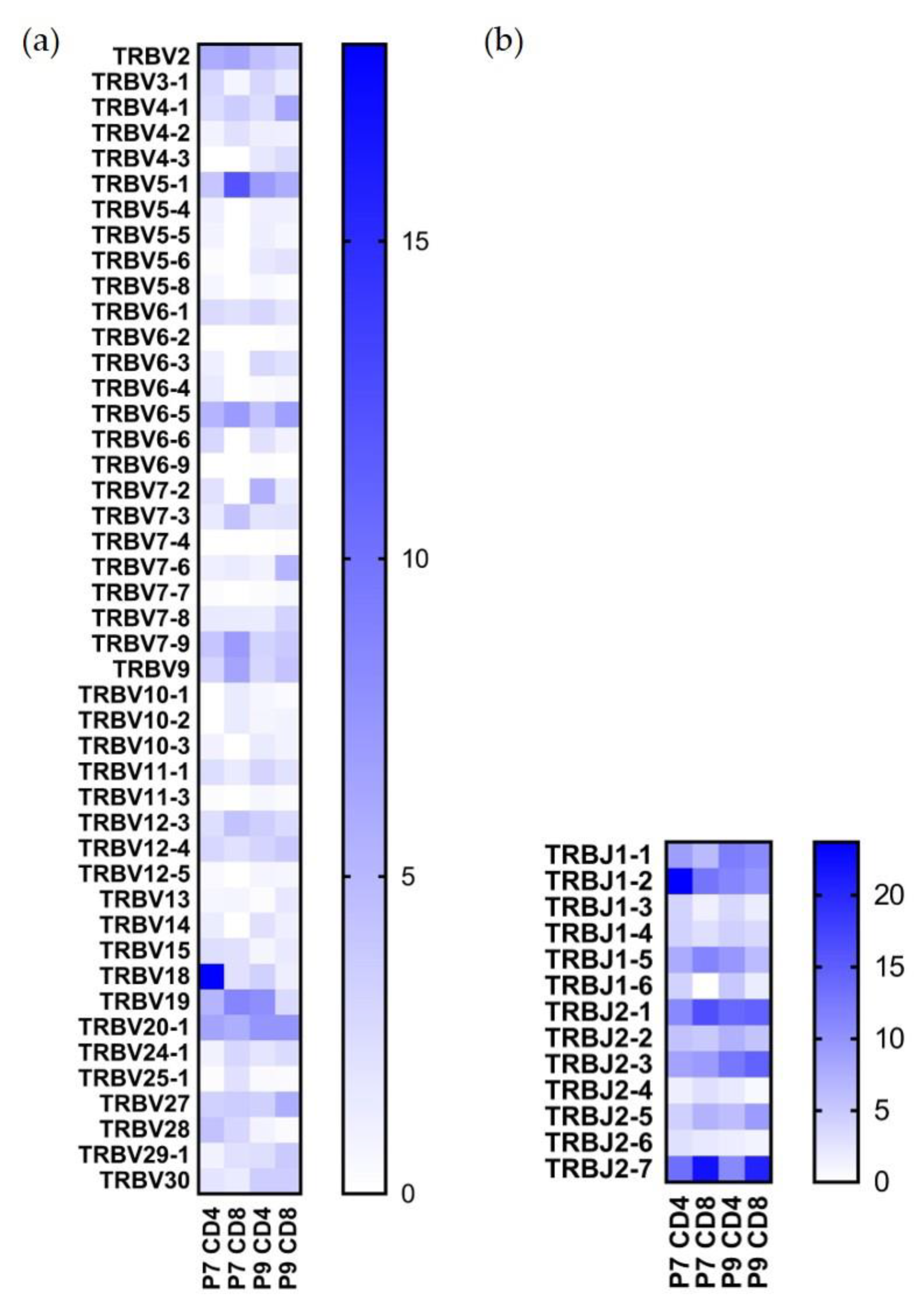
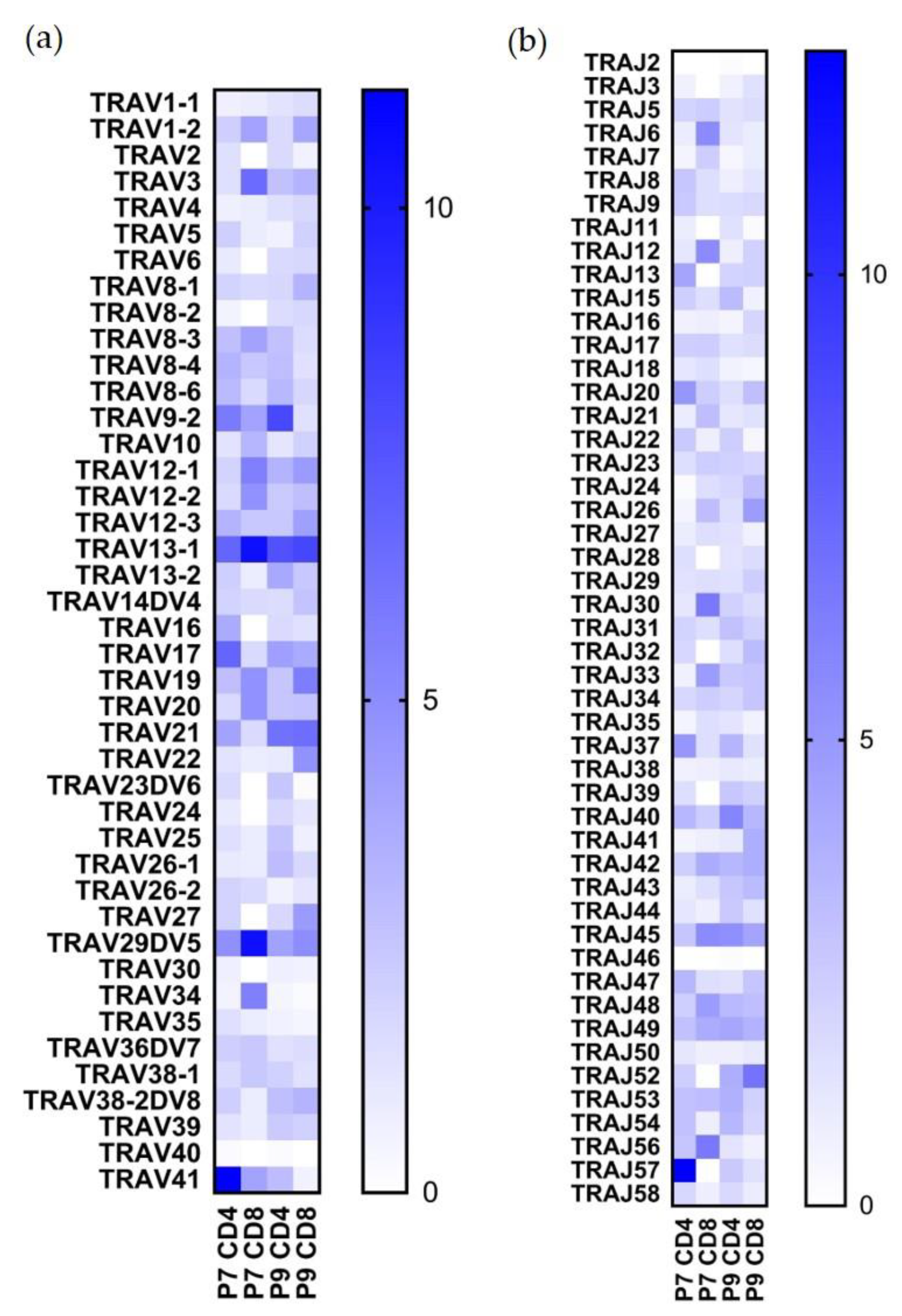
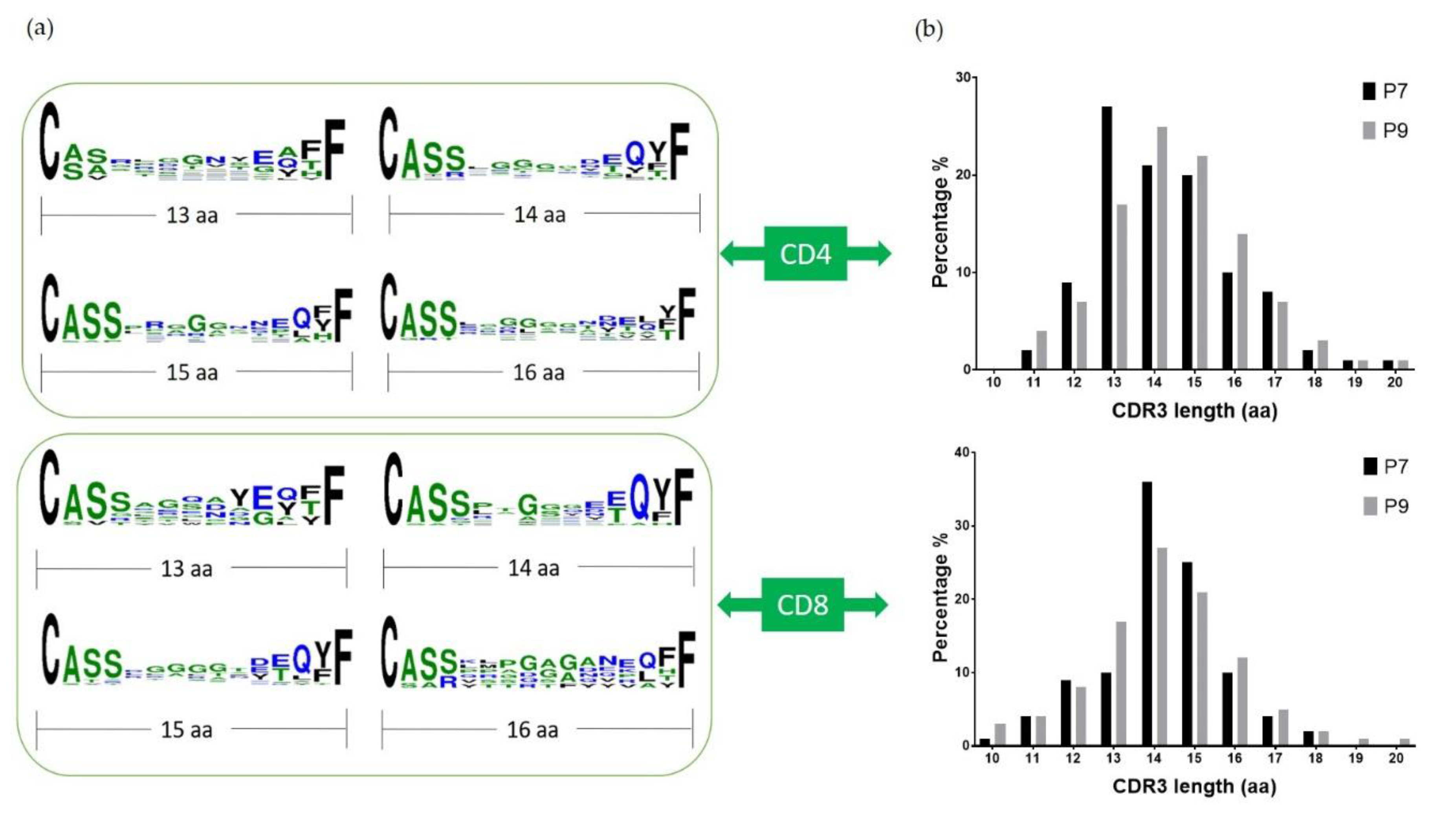
2.3. Preferential TCR Usage in the Repertoire of Ni2+-Recognizing CD4+ and CD8+ T Cells
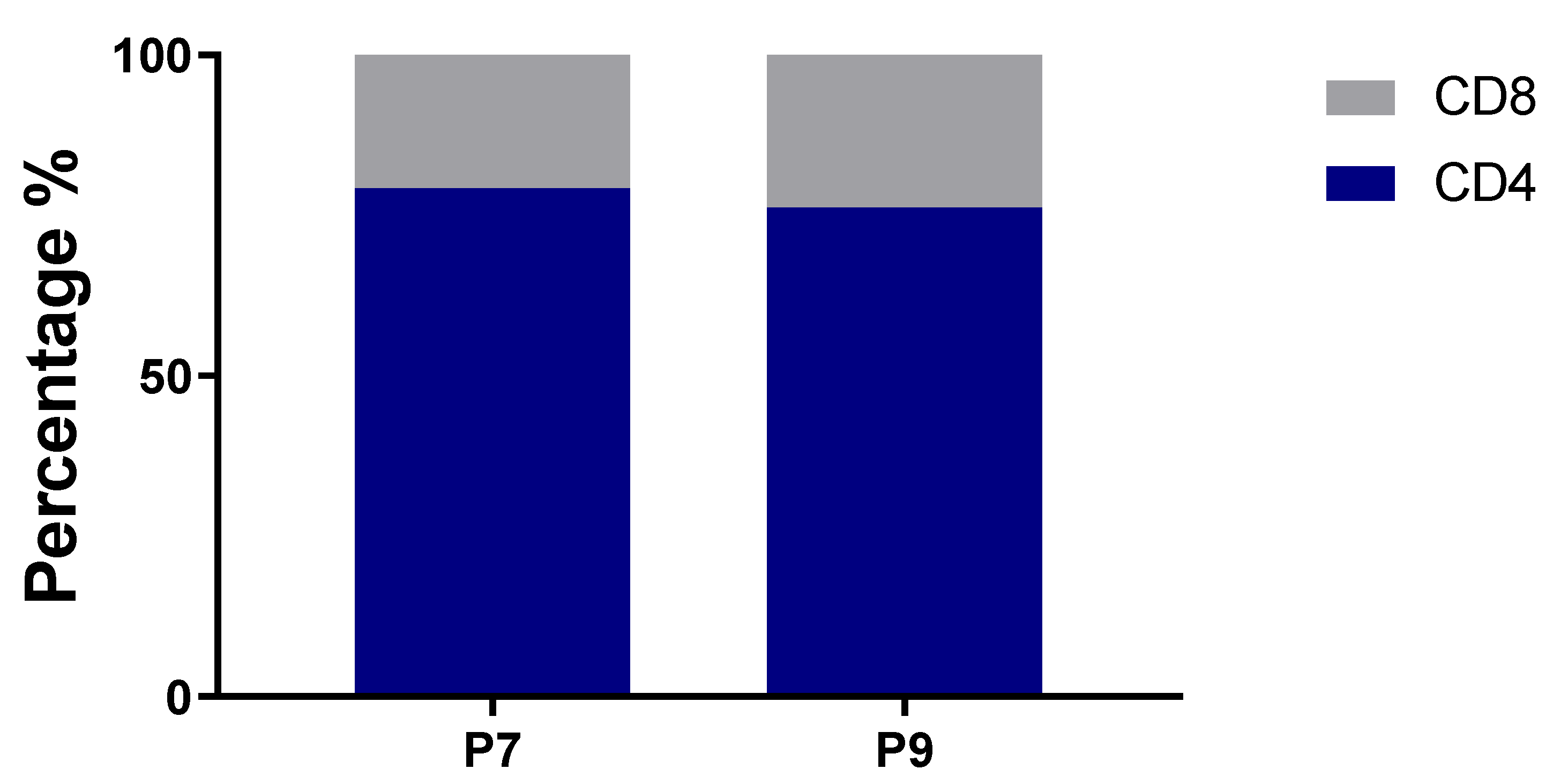
2.4. Highly Expanded TCR Clones Are Detected in Both CD4+ and CD8+ T Cells
2.5. Conserved Glu Is Found in the CDR3 Sequences
3. Discussion
4. Materials and Methods
4.1. Subject Selection
4.2. PBMC Culture and Stimulation
4.3. HLA Genotyping
4.4. IFNγ Measurement
4.5. Cell Staining and Single-Cell Sequencing
4.6. Data Processing
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| PBMC | peripheral blood mononuclear cell |
| P7 | patient #7 |
| P9 | patient #9 |
| APC MHC TCR VDJ CDR LPT Postop PSI THA TKA TRAV TRAJ TRBV TRBJ Th1 Tc1 | antigen-presenting cell major histocompatibility complex T cell receptor variable (V), diversity (D) and joining (J) gene segments Complementarity-determining region lymphocyte proliferation test Post-operation peak stimulation index Total hip arthroplasty Total knee arthroplasty T-cell receptor alpha variable T-cell receptor alpha J segment T-cell receptor beta variable T-cell receptor beta J segment Th1 helper cell Type 1 CD8+ T cell |
References
- Williams, S.N.; Wolford, M.L.; Bercovitz, A. Hospitalization for Total Knee Replacement among Inpatients Aged 45 and Over: United States, 2000–2010. NCHS Data Brief 2015, 186, 1–8. [Google Scholar]
- Kurtz, S.; Ong, K.; Lau, E.; Mowat, F.; Halpern, M. Projections of primary and revision hip and knee arthroplasty in the United States from 2005 to 2030. J. Bone Jt. Surg. Am. 2007, 89, 780–785. [Google Scholar] [CrossRef]
- Labek, G.; Janda, W.; Agreiter, M.; Schuh, R.; Bohler, N. Organisation, data evaluation, interpretation and effect of arthroplasty register data on the outcome in terms of revision rate in total hip arthroplasty. Int. Orthop. 2011, 35, 157–163. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ackerman, I.N.; Bohensky, M.A.; Zomer, E.; Tacey, M.; Gorelik, A.; Brand, C.A.; De Steiger, R. The projected burden of primary total knee and hip replacement for osteoarthritis in Australia to the year 2030. BMC Musculoskelet. Disord. 2019, 20, 90. [Google Scholar] [CrossRef] [Green Version]
- Pacheco, K.A. Allergy to Surgical Implants. Clin. Rev. Allergy Immunol. 2019, 56, 72–85. [Google Scholar] [CrossRef] [PubMed]
- Jakobsen, S.S.; Liden, C.; Soballe, K.; Johansen, J.D.; Menne, T.; Lundgren, L.; Bregnbak, D.; Moller, P.; Jellesen, M.S.; Thyssen, J.P. Failure of total hip implants: Metals and metal release in 52 cases. Contact Dermat. 2014, 71, 319–325. [Google Scholar] [CrossRef]
- Schmidt, M.; Raghavan, B.; Muller, V.; Vogl, T.; Fejer, G.; Tchaptchet, S.; Keck, S.; Kalis, C.; Nielsen, P.J.; Galanos, C.; et al. Crucial role for human Toll-like receptor 4 in the development of contact allergy to nickel. Nat. Immunol. 2010, 11, 814–819. [Google Scholar] [CrossRef]
- Grabbe, S.; Schwarz, T. Immunoregulatory mechanisms involved in elicitation of allergic contact hypersensitivity. Immunol. Today 1998, 19, 37–44. [Google Scholar] [CrossRef]
- Gittler, K.J.; Krueger, J.G.; Guttman-Yassky, E. Atopic dermatitis results in intrinsic barrier and immune abnormalities: Implications for contact dermatitis. J. Allergy Clin. Immunol. 2013, 131, 300–313. [Google Scholar] [CrossRef] [Green Version]
- Thomas, P.; Braathen, L.R.; Dorig, M.; Aubock, J.; Nestle, F.; Werfel, T.; Willert, H.G. Increased metal allergy in patients with failed metal-on-metal hip arthroplasty and peri-implant T-lymphocytic inflammation. Allergy 2009, 64, 1157–1165. [Google Scholar] [CrossRef]
- Vollmer, J.; Fritz, M.; Dormoy, A.; Weltzien, H.U.; Moulon, C. Dominance of the BV17 element in nickel-specific human T cell receptors relates to severity of contact sensitivity. Eur. J. Immunol. 1997, 27, 1865–1874. [Google Scholar] [CrossRef]
- Vollmer, J.; Weltzien, H.U.; Gamerdinger, K.; Lang, S.; Choleva, Y.; Moulon, C. Antigen contacts by Ni-reactive TCR: Typical alphass chain cooperation versus alpha chain-dominated specificity. Int. Immunol. 2000, 12, 1723–1731. [Google Scholar] [CrossRef] [Green Version]
- Gamerdinger, K.; Moulon, C.; Karp, D.R.; Van Bergen, J.; Koning, F.; Wild, D.; Pflugfelder, U.; Weltzien, H.U. A new type of metal recognition by human T cells: Contact residues for peptide-independent bridging of T cell receptor and major histocompatibility complex by nickel. J. Exp. Med. 2003, 197, 1345–1353. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lu, L.; Vollmer, J.; Moulon, C.; Weltzien, H.U.; Marrack, P.; Kappler, J. Components of the ligand for a Ni++ reactive human T cell clone. J. Exp. Med. 2003, 197, 567–574. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yin, L.; Crawford, F.; Marrack, P.; Kappler, J.W.; Dai, S. T-cell receptor (TCR) interaction with peptides that mimic nickel offers insight into nickel contact allergy. Proc. Natl. Acad. Sci. USA 2012, 109, 18517–18522. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bechara, R.; Pollastro, S.; Azoury, M.E.; Szely, N.; Maillere, B.; De Vries, N.; Pallardy, M. Identification and Characterization of Circulating Naive CD4+ and CD8+ T Cells Recognizing Nickel. Front. Immunol. 2019, 10, 1331. [Google Scholar] [CrossRef]
- Aparicio-Soto, M.; Riedel, F.; Leddermann, M.; Bacher, P.; Scheffold, A.; Kuhl, H.; Timmermann, B.; Chudakov, D.M.; Molin, S.; Worm, M.; et al. TCRs with segment TRAV9-2 or a CDR3 histidine are overrepresented among nickel-specific CD4+ T cells. Allergy 2020, 75, 2574–2586. [Google Scholar] [CrossRef]
- Zhang, Y.; Wang, Y.; Anderson, K.; Novikov, A.; Liu, Z.; Pacheco, K.; Dai, S. Using DR52c/Ni(2+) mimotope tetramers to detect Ni(2+) reactive CD4(+) T cells in patients with joint replacement failure. Toxicol. Appl. Pharmacol. 2017, 331, 69–75. [Google Scholar] [CrossRef] [PubMed]
- Silvennoinen-Kassinen, S.; Karvonen, J.; Ikaheimo, I. Restricted and individual usage of T-cell receptor beta-gene variables in nickel-induced CD4+ and CD8+ cells. Scand. J. Immunol. 1998, 48, 99–102. [Google Scholar] [CrossRef]
- Budinger, L.; Neuser, N.; Totzke, U.; Merk, H.F.; Hertl, M. Preferential usage of TCR-Vbeta17 by peripheral and cutaneous T cells in nickel-induced contact dermatitis. J. Immunol. 2001, 167, 6038–6044. [Google Scholar] [CrossRef] [Green Version]
- Werfel, T.; Hentschel, M.; Kapp, A.; Renz, H. Dichotomy of blood- and skin-derived IL-4-producing allergen-specific T cells and restricted V beta repertoire in nickel-mediated contact dermatitis. J. Immunol. 1997, 158, 2500–2505. [Google Scholar]
- Kou, C.Z.; Puhr, J.S.; Rojas, M.; McCormack, W.T.; Goodenow, M.M.; Sleasman, J.W. T-Cell receptor Vbeta repertoire CDR3 length diversity differs within CD45RA and CD45RO T-cell subsets in healthy and human immunodeficiency virus-infected children. Clin. Diagn. Lab. Immunol. 2000, 7, 953–959. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Atanaskova Mesinkovska, N.; Tellez, A.; Molina, L.; Honari, G.; Sood, A.; Barsoum, W.; Taylor, J.S. The effect of patch testing on surgical practices and outcomes in orthopedic patients with metal implants. Arch. Dermatol. 2012, 148, 687–693. [Google Scholar] [CrossRef] [Green Version]
- Foussereau, J.; Laugier, P. Allergic eczemas from metallic foreign bodies. Trans. St. John’s Hosp. Dermatol. Soc. 1966, 52, 220–225. [Google Scholar]
- Hallab, N.; Merritt, K.; Jacobs, J.J. Metal sensitivity in patients with orthopaedic implants. J. Bone Jt Surg. Am. 2001, 83, 428–436. [Google Scholar] [CrossRef] [PubMed]
- Symeonides, P.P.; Paschaloglou, C.; Papageorgiou, S. An allergic reaction after internal fixation of a fracture using a vitallium plate. J. Allergy Clin. Immunol. 1973, 51, 251–252. [Google Scholar] [CrossRef]
- Adala, R.; Chakravarthy, M.; Srinivas, V.; Pai, S. Orthopaedic surgery in a patient with metal sensitivity. J. Cutan. Aesthet. Surg. 2011, 4, 67–68. [Google Scholar] [CrossRef]
- Ostendorf, L.; Burns, M.; Durek, P.; Heinz, G.A.; Heinrich, F.; Garantziotis, P.; Enghard, P.; Richter, U.; Biesen, R.; Schneider, U.; et al. Targeting CD38 with Daratumumab in Refractory Systemic Lupus Erythematosus. N. Engl. J. Med. 2020, 383, 1149–1155. [Google Scholar] [CrossRef] [PubMed]
- Ramesh, A.; Schubert, R.D.; Greenfield, A.L.; Dandekar, R.; Loudermilk, R.; Sabatino, J.J., Jr.; Koelzer, M.T.; Tran, E.B.; Koshal, K.; Kim, K.; et al. A pathogenic and clonally expanded B cell transcriptome in active multiple sclerosis. Proc. Natl. Acad. Sci. USA 2020, 117, 22932–22943. [Google Scholar] [CrossRef]
- Traidl, C.; Sebastiani, S.; Albanesi, C.; Merk, H.F.; Puddu, P.; Girolomoni, G.; Cavani, A. Disparate cytotoxic activity of nickel-specific CD8+ and CD4+ T cell subsets against keratinocytes. J. Immunol. 2000, 165, 3058–3064. [Google Scholar] [CrossRef] [Green Version]
- Sebastiani, S.; Albanesi, C.; Nasorri, F.; Girolomoni, G.; Cavani, A. Nickel-specific CD4(+) and CD8(+) T cells display distinct migratory responses to chemokines produced during allergic contact dermatitis. J. Invest. Dermatol. 2002, 118, 1052–1058. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- White, J.; Kappler, J.; Marrack, P. Production and characterization of T cell hybridomas. Methods Mol. Biol. 2000, 134, 185–193. [Google Scholar] [PubMed]
- Moulon, C.; Vollmer, J.; Weltzien, H.U. Characterization of processing requirements and metal cross-reactivities in T cell clones from patients with allergic contact dermatitis to nickel. Eur. J. Immunol. 1995, 25, 3308–3315. [Google Scholar] [CrossRef] [PubMed]
| Patient | HLA Typing | Joint Replaced | Size of Nickel Patch Test (Range is 1+ to 3+ ) | Nickel LPT PSI | ||
|---|---|---|---|---|---|---|
| 7 | DRB1*04:01:01, DRB1*07:01P | DRB4*01:BRF | Postop L THA failure | 1+ | 12.2 | |
| 9 | DRB1*07:01P, DRB1*11:01:02 | DRB3*02:ANCDJ | DRB4*01:BRF | Postop R TKA failure | 3+ | 51.2 |
| Number of Clones | Percentage | TRAV | TRAJ | TRBV | TRBJ |
|---|---|---|---|---|---|
| P7 CD4+ | |||||
| 51 | 13.49% | TRAV41 | TRAJ57 | TRBV18 | TRBJ1-2 |
| 8 | 2.12% | TRAV16 | TRAJ13 | TRBV15 | TRBJ2-7 |
| 4 | 1.06% | TRAV13-1 | TRAJ45 | TRBV12-3 | TRBJ1-1 |
| 3 | 0.79% | TRAV5 | TRAJ22 | TRBV5-1 | TRBJ2-1 |
| 3 | 0.79% | TRAV17 | TRAJ47 | TRBV2;TRBV6-4 | TRBJ2-6;TRBJ1-1 |
| 3 | 0.79% | TRAV13-1 | TRAJ9 | TRBV28 | TRBJ1-2 |
| 2 | 0.53% | TRAV1-2 | TRAJ18 | TRBV9 | TRBJ2-1 |
| 2 | 0.53% | TRAV19 | TRAJ17 | TRBV7-3 | TRBJ2-2 |
| 2 | 0.53% | TRAV29DV5 | TRAJ48 | TRBV7-9 | TRBJ1-2 |
| 2 | 0.53% | TRAV8-1 | TRAJ37 | TRBV6-5 | TRBJ2-7 |
| P7 CD8+ | |||||
| 9 | 7.96% | TRAV29DV5 | TRAJ56 | TRBV6-5 | TRBJ1-5 |
| 5 | 4.42% | TRAV34 | TRAJ26 | TRBV5-1 | TRBJ2-7 |
| 4 | 3.54% | TRAV3 | TRAJ30 | TRBV2 | TRBJ2-3 |
| 4 | 3.54% | TRAV41 | TRAJ49 | TRBV9 | TRBJ2-7 |
| 3 | 2.65% | TRAV10 | TRAJ12 | TRBV19 | TRBJ2-1 |
| 3 | 2.65% | TRAV26-2 | TRAJ48 | TRBV20-1 | TRBJ2-7 |
| 3 | 2.65% | TRAV12-2 | TRAJ8 | TRBV24-1 | TRBJ2-5 |
| 2 | 1.77% | TRAV13-1 | TRAJ21 | TRBV7-3 | TRBJ2-5 |
| 2 | 1.77% | TRAV8-4 | TRAJ49 | TRBV7-9 | TRBJ1-1 |
| 2 | 1.77% | TRAV13-1 | TRAJ6 | TRBV10-1 | TRBJ2-7 |
| 2 | 1.77% | TRAV12-3 | TRAJ18 | TRBV7-9 | TRBJ2-4 |
| P9 CD4+ | |||||
| 24 | 1.47% | TRAV13-1;TRAV23DV6 | TRAJ40;TRAJ15 | TRBV19 | TRBJ1-5 |
| 18 | 1.10% | TRAV23DV6 | TRAJ15 | TRBV19 | TRBJ1-5 |
| 12 | 0.73% | TRAV13-2 | TRAJ45 | TRBV6-3 | TRBJ1-1 |
| 11 | 0.67% | TRAV13-2;TRAV25 | TRAJ45;TRAJ37 | TRBV6-3 | TRBJ1-1 |
| 9 | 0.55% | TRAV39 | TRAJ44 | TRBV7-2 | TRBJ2-3 |
| 7 | 0.43% | TRAV13-1 | TRAJ40 | TRBV19 | TRBJ1-5 |
| 6 | 0.37% | TRAV8-2 | TRAJ40 | TRBV27 | TRBJ2-5 |
| 6 | 0.37% | TRAV9-2 | TRAJ31 | TRBV27 | TRBJ2-7 |
| 5 | 0.31% | TRAV3 | TRAJ23 | TRBV19 | TRBJ1-2 |
| 5 | 0.31% | TRAV9-2 | TRAJ57 | TRBV14 | TRBJ1-1 |
| P9 CD8+ | |||||
| 19 | 3.97% | TRAV22 | TRAJ52 | TRBV7-6 | TRBJ2-7 |
| 9 | 1.88% | TRAV8-1 | TRAJ5 | TRBV5-1 | TRBJ2-3 |
| 8 | 1.67% | TRAV12-3 | TRAJ26 | TRBV7-3 | TRBJ2-3 |
| 6 | 1.26% | TRAV4 | TRAJ20 | TRBV24-1 | TRBJ2-5 |
| 5 | 1.05% | TRAV27 | TRAJ29 | TRBV20-1 | TRBJ2-1 |
| 5 | 1.05% | TRAV36DV7 | TRAJ24 | TRBV20-1 | TRBJ1-2 |
| 5 | 1.05% | TRAV29DV5 | TRAJ33 | TRBV7-8 | TRBJ1-1 |
| 5 | 1.05% | TRAV13-1 | TRAJ32 | TRBV12-3 | TRBJ2-5 |
| 5 | 1.05% | TRAV21 | TRAJ45 | TRBV6-5 | TRBJ1-5 |
| 4 | 0.84% | TRAV12-1;TRAV20 | TRAJ41;TRAJ49 | TRBV6-5 | TRBJ2-7 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chen, L.; Zhang, Y.; Pacheco, K.; Dai, S. The T Cell Repertoires from Nickel Sensitized Joint Implant Failure Patients. Int. J. Mol. Sci. 2021, 22, 2428. https://doi.org/10.3390/ijms22052428
Chen L, Zhang Y, Pacheco K, Dai S. The T Cell Repertoires from Nickel Sensitized Joint Implant Failure Patients. International Journal of Molecular Sciences. 2021; 22(5):2428. https://doi.org/10.3390/ijms22052428
Chicago/Turabian StyleChen, Lan, Yan Zhang, Karin Pacheco, and Shaodong Dai. 2021. "The T Cell Repertoires from Nickel Sensitized Joint Implant Failure Patients" International Journal of Molecular Sciences 22, no. 5: 2428. https://doi.org/10.3390/ijms22052428
APA StyleChen, L., Zhang, Y., Pacheco, K., & Dai, S. (2021). The T Cell Repertoires from Nickel Sensitized Joint Implant Failure Patients. International Journal of Molecular Sciences, 22(5), 2428. https://doi.org/10.3390/ijms22052428





