Label-Free Multiphoton Microscopy: Much More Than Fancy Images
Abstract
:1. Introduction
2. Multiphoton Technique
3. Label-Free Multiphoton Microscopy in Biomedical Research
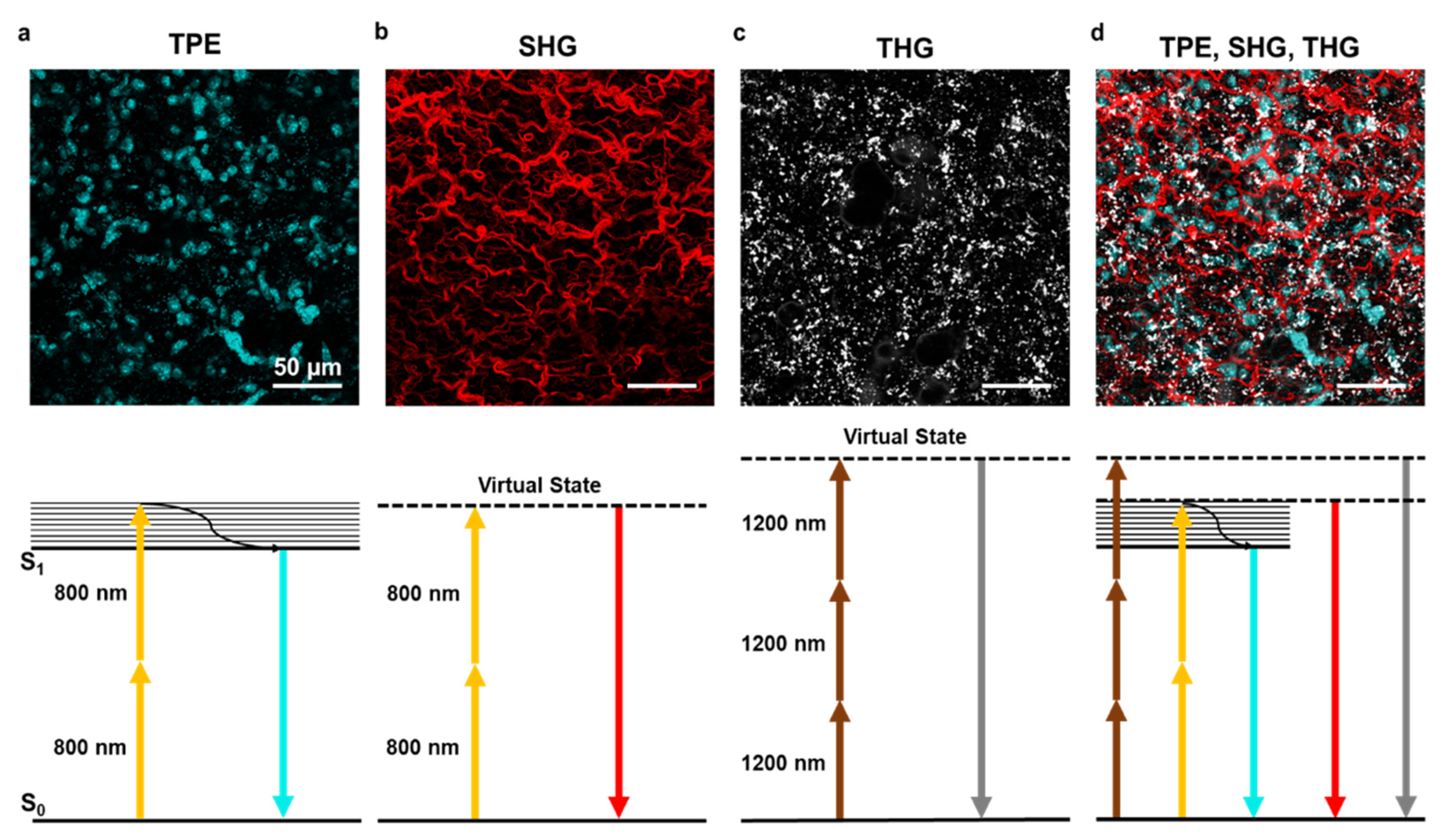
3.1. Autofluorescence
3.2. Second Harmonic Generation
3.3. Third Harmonic Generation
3.4. Research and Clinical Applications
4. Sample Preparation
4.1. Fixation
4.2. Sectioning
4.3. Mounting
5. Quantitative SHG Image Methods
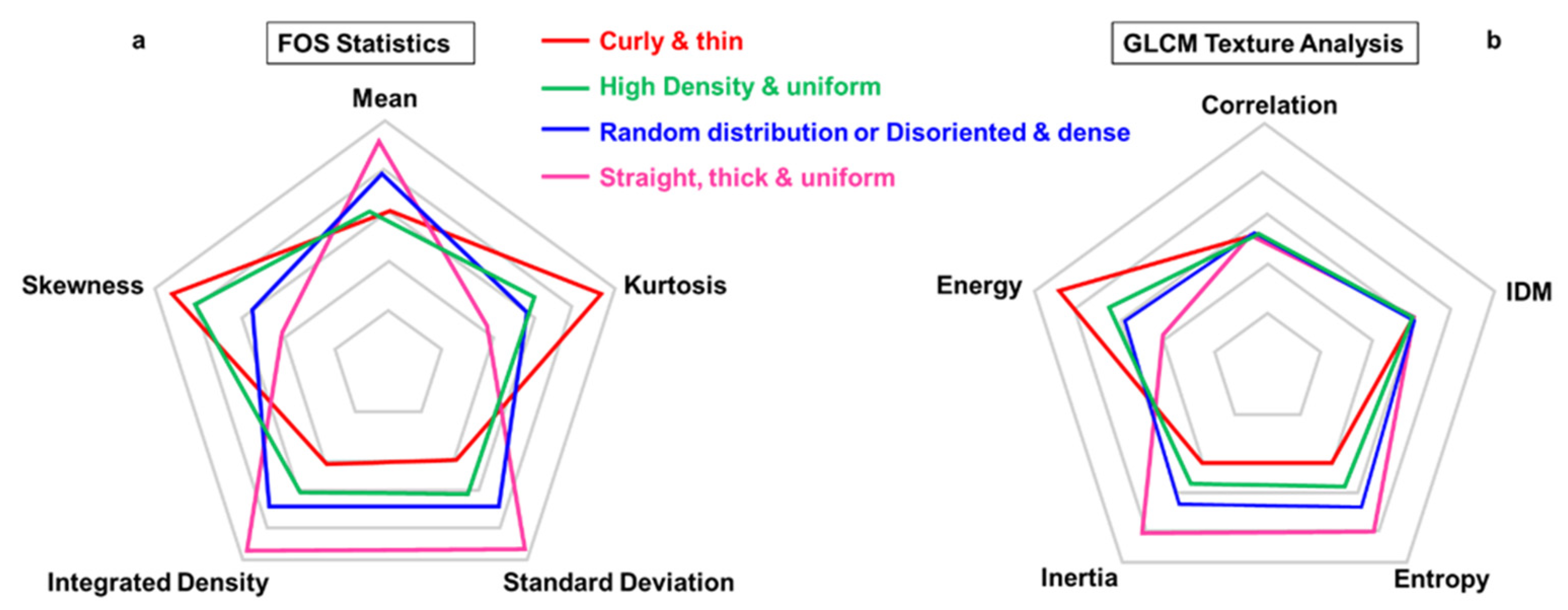
5.1. Amount and Texture Description
5.1.1. Intensity-Based Analysis
- →
- First-Order Statistics (FOS)
- →
- Second-Order Statistics (Gray Level Co-Occurrence Matrix, GLCM)
5.1.2. Transform-Based Methods
- →
- 2D Fast Fourier Transformation (2D-FFT)
- →
- Wavelet Transformation
5.2. Fibers Orientation
- →
- Forward–Backward SHG-Signal (F-SHG/B-SHG)
- →
- Polarization
- →
- Coherency (C)
5.3. Fibers Waviness
5.4. Fiber Thickness and Distance
6. Limitations and New Perspectives
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| STED | Stimulated Emission Depletion |
| STORM | Stochastic Optical Reconstruction Microscopy |
| PALM | Photo Activated Localization Microscopy |
| NADH | 1,4-DiHydroNicotinamide Adenine Dinucleotide |
| SHG | Second Harmonic Generation |
| THG | Third Harmonic Generation |
| CARS | Coherent Anti-Stokes Raman Spectroscopy |
| SRS | Stimulated Raman Spectroscopy |
| MPM | Multiphoton Microscopy |
| TPE | Two-Photon Excitation |
| OPO | Optical Parametric Oscillator |
| MPF | Multiphoton Fluorescence |
| LFM | Label-Free Multiphoton |
| YFP | Yellow Fluorescent Protein |
| FAD | Flavin Adenine Dinucleotide |
| FA | Formaldehyde |
| PFA | Paraformaldehyde |
| FOS | First-Order Statistics |
| GLCM | Gray Level Co-Occurrence Matrix |
| FFT | Fast Fourier Transformation |
| IDM | Inverse Difference Moment |
| iSCAT | Interferometric Scattering |
| PSHG | Polarization-Sensitive Second Harmonic Generation |
References
- Zipfel, W.R.; Williams, R.M.; Webb, W.W. Nonlinear magic: Multiphoton microscopy in the biosciences. Nat. Biotechnol. 2003, 21, 1369–1377. [Google Scholar] [CrossRef]
- Lefort, C. A review of biomedical multiphoton microscopy and its laser sources. J. Phys. D Appl. Phys. 2017, 50. [Google Scholar] [CrossRef] [Green Version]
- Huff, J. The Airyscan detector from ZEISS: Confocal imaging with improved signal-to-noise ratio and super-resolution. Nat. Methods 2015. [Google Scholar] [CrossRef]
- Carriles, R.; Sheetz, K.E.; Hoover, E.E.; Squier, J.A.; Barzda, V. Simultaneous multifocal, multiphoton, photon counting microscopy. Opt. Express 2008, 16, 10364. [Google Scholar] [CrossRef] [PubMed]
- Moneron, G.; Hell, S.W. Two-photon excitation STED microscopy. Opt. Express 2009. [Google Scholar] [CrossRef] [Green Version]
- Borile, G.; De Mauro, C.; Urbani, A.; Alfieri, D.; Pavone, F.S.; Mongillo, M. Multispot multiphoton Ca2+ imaging in acute myocardial slices. J. Biomed. Opt. 2015, 20. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Filippi, A.; Dal Sasso, E.; Iop, L.; Armani, A.; Gintoli, M.; Sandri, M.; Gerosa, G.; Romanato, F.; Borile, G. Multimodal label-free ex vivo imaging using a dual-wavelength microscope with axial chromatic aberration compensation. J. Biomed. Opt. 2018, 23. [Google Scholar] [CrossRef]
- Silvestri, L.; Costantini, I.; Sacconi, L.; Pavone, F.S. Clearing of fixed tissue: A review from a microscopist’s perspective. J. Biomed. Opt. 2016. [Google Scholar] [CrossRef] [Green Version]
- Chen, F.; Tillberg, P.W.; Boyden, E.S. Expansion microscopy. Science 2015, 80. [Google Scholar] [CrossRef] [Green Version]
- Jonkman, J.; Brown, C.M.; Wright, G.D.; Anderson, K.I.; North, A.J. Tutorial: Guidance for quantitative confocal microscopy. Nat. Protoc. 2020, 15, 1585–1611. [Google Scholar] [CrossRef] [PubMed]
- Huang, S.; Heikal, A.A.; Webb, W.W. Two-photon fluorescence spectroscopy and microscopy of NAD(P)H and flavoprotein. Biophys. J. 2002. [Google Scholar] [CrossRef] [Green Version]
- Masters, B.R.; So, P.T.C.; Gratton, E. Multiphoton excitation fluorescence microscopy and spectroscopy of in vivo human skin. Biophys. J. 1997, 72, 2405–2412. [Google Scholar] [CrossRef] [Green Version]
- So, P.T.C.; Dong, C.Y.; Masters, B.R. Two-photon excitation fluorescence microscopy. Biomed. Photonics Handb. 2003, 2667–2671. [Google Scholar] [CrossRef]
- Lakowicz, J.R.; Szmacinski, H.; Nowaczyk, K.; Johnson, M.L. Fluorescence lifetime imaging of free and protein-bound NADH. Proc. Natl. Acad. Sci. USA 1992, 89, 1271–1275. [Google Scholar] [CrossRef] [Green Version]
- Blacker, T.S.; Mann, Z.F.; Gale, J.E.; Ziegler, M.; Bain, A.J.; Szabadkai, G.; Duchen, M.R. Separating NADH and NADPH fluorescence in live cells and tissues using FLIM. Nat. Commun. 2014, 5. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, Y.; Periasamy, A. Characterization of two-photon excitation fluorescence lifetime imaging microscopy for protein localization. Microsc. Res. Tech. 2004, 63, 72–80. [Google Scholar] [CrossRef]
- Cao, R.; Wallrabe, H.K.; Periasamy, A. Multiphoton FLIM imaging of NAD(P)H and FAD with one excitation wavelength. J. Biomed. Opt. 2020, 25, 1. [Google Scholar] [CrossRef]
- Mostaço-Guidolin, L.; Rosin, N.L.; Hackett, T.L. Imaging collagen in scar tissue: Developments in second harmonic generation microscopy for biomedical applications. Int. J. Mol. Sci. 2017, 18, 1772. [Google Scholar] [CrossRef] [PubMed]
- Psilodimitrakopoulos, S.; Santos, S.I.C.O.; Amat-Roldan, I.; Thayil, A.K.N.; Artigas, D.; Loza-Alvarez, P. In vivo, pixel-resolution mapping of thick filaments’ orientation in nonfibrilar muscle using polarization-sensitive second harmonic generation microscopy. J. Biomed. Opt. 2009, 14, 014001. [Google Scholar] [CrossRef] [Green Version]
- Psilodimitrakopoulos, S.; Artigas, D.; Soria, G.; Amat-Roldan, I.; Planas, A.M.; Loza-Alvarez, P. Quantitative discrimination between endogenous SHG sources in mammalian tissue, based on their polarization response. Opt. Express 2009, 17, 10168. [Google Scholar] [CrossRef] [Green Version]
- Weigelin, B.; Bakker, G.J.; Friedl, P. Third harmonic generation microscopy of cells and tissue organization. J. Cell Sci. 2016. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hall, G.; Tilbury, K.B.; Campbell, K.R.; Eliceiri, K.W.; Campagnola, P.J. Experimental and simulation study of the wavelength dependent second harmonic generation of collagen in scattering tissues. Opt. Lett. 2014, 39, 1897. [Google Scholar] [CrossRef]
- Sandoz, P.A.; Tremblay, C.; Gisou van der Goot, F.; Frechin, M. Image-based analysis of living mammalian cells using label-free 3D refractive index maps reveals new organelle dynamics and dry mass flux. PLoS Biol. 2019. [Google Scholar] [CrossRef] [Green Version]
- Fürhapter, S.; Jesacher, A.; Bernet, S.; Ritsch-Marte, M. Spiral phase contrast imaging in microscopy. Opt. Express 2005, 13, 689. [Google Scholar] [CrossRef] [Green Version]
- Beard, P. Biomedical photoacoustic imaging. Interface Focus 2011, 1, 602–631. [Google Scholar] [CrossRef] [PubMed]
- Kim, T.-K.; Lee, B.-W.; Fujii, F.; Kim, J.K.; Pack, C.-G. Physicochemical properties of nucleoli in live cells analyzed by label-free optical diffraction tomography. Cells 2019, 8, 699. [Google Scholar] [CrossRef] [Green Version]
- Young, G.; Kukura, P. Interferometric scattering microscopy. Annu. Rev. Phys. Chem. 2019, 70, 301–322. [Google Scholar] [CrossRef]
- Gemeinhardt, A.; McDonald, M.P.; König, K.; Aigner, M.; Mackensen, A.; Sandoghdar, V. Label-free imaging of single proteins secreted from living cells via iscat microscopy. J. Vis. Exp. 2018, 2018, 1–10. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sheppard, C.J.R. Multiphoton microscopy: A personal historical review, with some future predictions. J. Biomed. Opt. 2020, 25, 1. [Google Scholar] [CrossRef] [PubMed]
- Denk, W.; Strickler, J.H.; Webb, W.W. Two-photon laser scanning fluorescence microscopy. Science 1990, 80. [Google Scholar] [CrossRef] [Green Version]
- Patterson, G.H.; Piston, D.W. Photobleaching in two-photon excitation microscopy. Biophys. J. 2000, 78, 2159–2162. [Google Scholar] [CrossRef] [Green Version]
- Duemani Reddy, G.; Kelleher, K.; Fink, R.; Saggau, P. Three-dimensional random access multiphoton microscopy for functional imaging of neuronal activity. Nat. Neurosci. 2008. [Google Scholar] [CrossRef]
- Marigo, I.; Trovato, R.; HOFER, F.; Ingangi, V.; De Sanctis, F.; Ugel, S.; Cane, S.; Simonelli, A.; Lamolinara, A.; Iezzi, M.; et al. The Disabled homolog 2 controls pro-metastatic activity of tumor-associated macrophages. Cancer Discov. 2020. [Google Scholar] [CrossRef] [PubMed]
- Franken, P.A.; Hill, A.E.; Peters, C.W.; Weinreich, G. Generation of optical harmonics. Phys. Rev. Lett. 1961, 7, 118–119. [Google Scholar] [CrossRef] [Green Version]
- Mahou, P.; Zimmerley, M.; Loulier, K.; Matho, K.S.; Labroille, G.; Morin, X.; Supatto, W.; Livet, J.; Débarre, D.; Beaurepaire, E. Multicolor two-photon tissue imaging by wavelength mixing. Nat. Methods 2012. [Google Scholar] [CrossRef] [PubMed]
- Helmchen, F.; Denk, W. Deep tissue two-photon microscopy. Nat. Methods 2005, 2, 932–940. [Google Scholar] [CrossRef]
- Chu, S.W. Quantitative analysis of backward generation and backscattering for epi-collected second harmonic generation in biological tissues. J. Med. Biol. Eng. 2007, 27, 177–182. [Google Scholar]
- Centonze, V.E.; White, J.G. Multiphoton excitation provides optical sections from deeper within scattering specimens than confocal imaging. Biophys. J. 1998. [Google Scholar] [CrossRef] [Green Version]
- Zouhair, S.; Sasso, E.D.; Tuladhar, S.R.; Fidalgo, C.; Vedovelli, L.; Filippi, A.; Borile, G.; Bagno, A.; Marchesan, M.; De Rossi, G.; et al. A comprehensive comparison of bovine and porcine decellularized pericardia: New insights for surgical applications. Biomolecules 2020, 10, 371. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- D’Angelo, E.; Natarajan, D.; Sensi, F.; Ajayi, O.; Fassan, M.; Mammano, E.; Pilati, P.; Pavan, P.; Bresolin, S.; Preziosi, M.; et al. Patient-derived scaffolds of colorectal cancer metastases as an organotypic 3D model of the liver metastatic microenvironment. Cancers 2020, 12, 364. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Nadiarynkh, O.; Plotnikov, S.; Campagnola, P.J. Second harmonic generation microscopy for quantitative analysis of collagen fibrillar structure. Nat. Protoc. 2012, 7, 654–669. [Google Scholar] [CrossRef]
- Débarre, D.; Supatto, W.; Pena, A.M.; Fabre, A.; Tordjmann, T.; Combettes, L.; Schanne-Klein, M.C.; Beaurepaire, E. Imaging lipid bodies in cells and tissues using third-harmonic generation microscopy. Nat. Methods 2006. [Google Scholar] [CrossRef] [PubMed]
- Dimitrow, E.; Riemann, I.; Ehlers, A.; Koehler, M.J.; Norgauer, J.; Elsner, P.; König, K.; Kaatz, M. Spectral fluorescence lifetime detection and selective melanin imaging by multiphoton laser tomography for melanoma diagnosis. Exp. Dermatol. 2009, 18, 509–515. [Google Scholar] [CrossRef] [PubMed]
- Koenig, K.; Riemann, I. High-resolution multiphoton tomography of human skin with subcellular spatial resolution and picosecond time resolution. J. Biomed. Opt. 2003, 8, 432. [Google Scholar] [CrossRef] [Green Version]
- Pouli, D.; Genega, E.M.; Sullivan, T.B.; Rieger-Christ, K.M.; Wright, V.; Georgakoudi, I.; Schnelldorfer, T. Two-photon images reveal unique texture features for label-free identification of ovarian cancer peritoneal metastases. Biomed. Opt. Express 2019. [Google Scholar] [CrossRef]
- Alexander, S.; Weigelin, B.; Winkler, F.; Friedl, P. Preclinical intravital microscopy of the tumour-stroma interface: Invasion, metastasis, and therapy response. Curr. Opin. Cell Biol. 2013, 25, 659–671. [Google Scholar] [CrossRef] [PubMed]
- Wu, P.C.; Hsieh, T.Y.; Tsai, Z.U.; Liu, T.M. In vivo quantification of the structural changes of collagens in a melanoma microenvironment with second and third harmonic generation microscopy. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Passaro, D.; Abarrategi, A.; Foster, K.; Ariza-McNaughton, L.; Bonnet, D. Bioengineering of humanized bone marrow microenvironments in mouse and their visualization by live imaging. J. Vis. Exp. 2017. [Google Scholar] [CrossRef] [Green Version]
- Abarrategi, A.; Foster, K.; Hamilton, A.; Mian, S.A.; Passaro, D.; Gribben, J.; Mufti, G.; Bonnet, D. Versatile humanized niche model enables study of normal and malignant human hematopoiesis. J. Clin. Invest. 2017. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Small, D.M.; Jones, J.S.; Tendler, I.I.; Miller, P.E.; Ghetti, A.; Nishimura, N. Label-free imaging of atherosclerotic plaques using third-harmonic generation microscopy. Biomed. Opt. Express 2018. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Baria, E.; Nesi, G.; Santi, R.; Maio, V.; Massi, D.; Pratesi, C.; Cicchi, R.; Pavone, F.S. Improved label-free diagnostics and pathological assessment of atherosclerotic plaques through nonlinear microscopy. J. Biophoton. 2018. [Google Scholar] [CrossRef] [Green Version]
- Mostaço-Guidolin, L.B.; Ko, A.C.T.; Popescu, D.P.; Smith, M.S.D.; Kohlenberg, E.K.; Shiomi, M.; Major, A.; Sowa, M.G. Evaluation of texture parameters for the quantitative description of multimodal nonlinear optical images from atherosclerotic rabbit arteries. Phys. Med. Biol. 2011, 56, 5319–5334. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Caorsi, V.; Toepfer, C.; Sikkel, M.B.; Lyon, A.R.; MacLeod, K.; Ferenczi, M.A. Non-linear optical microscopy sheds light on cardiovascular disease. PLoS ONE 2013. [Google Scholar] [CrossRef] [Green Version]
- Lim, H.; Sharoukhov, D.; Kassim, I.; Zhang, Y.; Salzer, J.L.; Melendez-Vasquez, C.V. Label-free imaging of Schwann cell myelination by third harmonic generation microscopy. Proc. Natl. Acad. Sci. USA 2014. [Google Scholar] [CrossRef] [Green Version]
- Farrar, M.J.; Wise, F.W.; Fetcho, J.R.; Schaffer, C.B. In vivo imaging of myelin in the vertebrate central nervous system using third harmonic generation microscopy. Biophys. J. 2011. [Google Scholar] [CrossRef] [Green Version]
- Kwan, A.C.; Duff, K.; Gouras, G.K.; Webb, W.W. Optical visualization of Alzheimer’s pathology via multiphoton-excited intrinsic fluorescence and second harmonic generation. Opt. Express 2009. [Google Scholar] [CrossRef] [PubMed]
- Lopez, A.L.; Larina, I.V. Second harmonic generation microscopy of early embryonic mouse hearts. Biomed. Opt. Express 2019. [Google Scholar] [CrossRef]
- Aviles-Espinosa, R. Third-harmonic generation for the study of Caenorhabditis elegans embryogenesis. J. Biomed. Opt. 2010. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- You, S.; Tu, H.; Chaney, E.J.; Sun, Y.; Zhao, Y.; Bower, A.J.; Liu, Y.Z.; Marjanovic, M.; Sinha, S.; Pu, Y.; et al. Intravital imaging by simultaneous label-free autofluorescence-multiharmonic microscopy. Nat. Commun. 2018. [Google Scholar] [CrossRef] [Green Version]
- Gibson, E.A.; Masihzadeh, O.; Lei, T.C.; Ammar, D.A.; Kahook, M.Y. Multiphoton microscopy for ophthalmic imaging. J. Ophthalmol. 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kottmann, R.M.; Sharp, J.; Owens, K.; Salzman, P.; Xiao, G.Q.; Phipps, R.P.; Sime, P.J.; Brown, E.B.; Perry, S.W. Second harmonic generation microscopy reveals altered collagen microstructure in usual interstitial pneumonia versus healthy lung. Respir. Res. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ochoa, L.F.; Kholodnykh, A.; Villarreal, P.; Tian, B.; Pal, R.; Freiberg, A.N.; Brasier, A.R.; Motamedi, M.; Vargas, G. Imaging of murine whole lung fibrosis by large scale 3D microscopy aided by tissue optical clearing. Sci. Rep. 2018. [Google Scholar] [CrossRef] [Green Version]
- Rothstein, E.C.; Carroll, S.; Combs, C.A.; Jobsis, P.D.; Balaban, R.S. Skeletal muscle NAD(P)H two-photon fluorescence microscopy in vivo: Topology and optical inner filters. Biophys. J. 2005. [Google Scholar] [CrossRef] [Green Version]
- Rehberg, M.; Krombach, F.; Pohl, U.; Dietzel, S. Label-free 3D visualization of cellular and tissue structures in intact muscle with second and third harmonic generation microscopy. PLoS ONE 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ranjit, S.; Dobrinskikh, E.; Montford, J.; Dvornikov, A.; Lehman, A.; Orlicky, D.J.; Nemenoff, R.; Gratton, E.; Levi, M.; Furgeson, S. Label-free fluorescence lifetime and second harmonic generation imaging microscopy improves quantification of experimental renal fibrosis. Kidney Int. 2016. [Google Scholar] [CrossRef] [Green Version]
- Dilipkumar, A.; Al-Shemmary, A.; Kreiß, L.; Cvecek, K.; Carlé, B.; Knieling, F.; Gonzales Menezes, J.; Thoma, O.M.; Schmidt, M.; Neurath, M.F.; et al. Label-free multiphoton endomicroscopy for minimally invasive in vivo imaging. Adv. Sci. 2019. [Google Scholar] [CrossRef] [Green Version]
- Gailhouste, L.; Le Grand, Y.; Odin, C.; Guyader, D.; Turlin, B.; Ezan, F.; Désille, Y.; Guilbert, T.; Bessard, A.; Frémin, C.; et al. Fibrillar collagen scoring by second harmonic microscopy: A new tool in the assessment of liver fibrosis. J. Hepatol. 2010. [Google Scholar] [CrossRef] [PubMed]
- Spitzer, N.; Sammons, G.S.; Price, E.M. Autofluorescent cells in rat brain can be convincing impostors in green fluorescent reporter studies. J. Neurosci. Methods 2011. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Van Steenbergen, V.; Boesmans, W.; Li, Z.; de Coene, Y.; Vints, K.; Baatsen, P.; Dewachter, I.; Ameloot, M.; Clays, K.; Vanden Berghe, P. Molecular understanding of label-free second harmonic imaging of microtubules. Nat. Commun. 2019. [Google Scholar] [CrossRef] [Green Version]
- Monaghan, M.G.; Kroll, S.; Brucker, S.Y.; Schenke-Layland, K. Enabling multiphoton and second harmonic generation imaging in paraffin-embedded and histologically stained sections. Tissue Eng. Part. C Methods 2016. [Google Scholar] [CrossRef] [Green Version]
- Mostaço-Guidolin, L.B.; Ko, A.C.T.; Wang, F.; Xiang, B.; Hewko, M.; Tian, G.; Major, A.; Shiomi, M.; Sowa, M.G. Collagen morphology and texture analysis: From statistics to classification. Sci. Rep. 2013, 3. [Google Scholar] [CrossRef] [PubMed]
- Schindelin, J.; Arganda-Carrera, I.; Frise, E.; Verena, K.; Mark, L.; Tobias, P.; Stephan, P.; Curtis, R.; Stephan, S.; Benjamin, S.; et al. Fiji—An open platform for biological image analysis. Nat. Methods 2009, 9. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Haralick, R.M.; Shanmugam, K.; Dinstein, I. Textural features for image classification. IEEE Trans. Syst. Man. Cybern. 1973, 3, 610–621. [Google Scholar] [CrossRef] [Green Version]
- Indahl, U.G.; Naes, T. Evaluation of alternative spectral feature extraction methods of textural images for multivariate modelling. J. Chemom. 1998, 12, 261–278. [Google Scholar] [CrossRef]
- Aït-Belkacem, D. Microscopic structural study of collagen aging in isolated fibrils using polarized second harmonic generation. J. Biomed. Opt. 2012, 17, 080506. [Google Scholar] [CrossRef]
- Sivaguru, M.; Durgam, S.; Ambekar, R.; Luedtke, D.; Fried, G.; Stewart, A.; Toussaint, K.C. Quantitative analysis of collagen fiber organization in injured tendons using Fourier transform-second harmonic generation imaging. Opt. Express 2010, 18, 24983. [Google Scholar] [CrossRef]
- Rao, R.A.R.; Mehta, M.R.; Leithem, S.; Toussaint, K.C., Jr. Quantitative analysis of forward and backward second-harmonic images of collagen fibers using Fourier transform second-harmonic-generation microscopy. Opt. Lett. 2009, 34, 3779. [Google Scholar] [CrossRef] [PubMed]
- Unser, M. Texture Classification and segmentation using wavelet frames. IEEE Trans. Image Process. 1995. [Google Scholar] [CrossRef] [Green Version]
- Arivazhagan, S.; Ganesan, L. Texture segmentation using wavelet transform. Pattern Recognit. Lett. 2003, 24, 3197–3203. [Google Scholar] [CrossRef]
- Laine, A.; Huda, W.; Steinbach, B.G.; Honeyman, J.C. Mammographic image processing using wavelet processing techniques. Eur. Radiol. 1995, 5, 518–523. [Google Scholar] [CrossRef]
- Chang, T.; Kuo, C.-J. Texture analysis and classification with tree-structured wavelet transform. IEEE Trans. Image Process. 1993, 2, 429–441. [Google Scholar] [CrossRef] [Green Version]
- Liu, Y.; Zhu, X.; Huang, Z.; Cai, J.; Chen, R.; Xiong, S.; Chen, G.; Zeng, H. Texture analysis of collagen second-harmonic generation images based on local difference local binary pattern and wavelets differentiates human skin abnormal scars from normal scars. J. Biomed. Opt. 2015, 20, 016021. [Google Scholar] [CrossRef] [PubMed]
- Légaré, F.; Pfeffer, C.; Olsen, B.R. The role of backscattering in SHG tissue imaging. Biophys. J. 2007, 93, 1312–1320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, R.M.; Zipfel, W.R.; Webb, W.W. Interpreting second-harmonic generation images of collagen I fibrils. Biophys. J. 2005, 88, 1377–1386. [Google Scholar] [CrossRef] [Green Version]
- Stoller, P.; Kim, B.-M.; Rubenchik, A.M.; Reiser, K.M.; Da Silva, L.B. Polarization-dependent optical second-harmonic imaging of a rat-tail tendon. J. Biomed. Opt. 2002, 7, 205. [Google Scholar] [CrossRef]
- Turcotte, R.; Mattson, J.M.; Wu, J.W.; Zhang, Y.; Lin, C.P. Molecular order of arterial collagen using circular polarization second-harmonic generation imaging. Biophys. J. 2016, 110, 530–533. [Google Scholar] [CrossRef] [Green Version]
- Plotnikov, S.V.; Millard, A.C.; Campagnola, P.J.; Mohler, W.A. Characterization of the myosin-based source for second-harmonic generation from muscle sarcomeres. Biophys. J. 2006, 90, 693–703. [Google Scholar] [CrossRef] [Green Version]
- Campagnola, P.J.; Millard, A.C.; Terasaki, M.; Hoppe, P.E.; Malone, C.J.; Mohler, W.A. Three-dimensional high-resolution second-harmonic generation imaging of endogenous structural proteins in biological tissues. Biophys. J. 2002, 82, 493–508. [Google Scholar] [CrossRef] [Green Version]
- Psilodimitrakopoulos, S.; Amat-Roldan, I.; Loza-Alvarez, P.; Artigas, D. Estimating the helical pitch angle of amylopectin in Starch using polarization second harmonic generation microscopy. J. Opt. 2010, 12. [Google Scholar] [CrossRef]
- Rezakhaniha, R.; Agianniotis, A.; Schrauwen, J.T.C.; Griffa, A.; Sage, D.; Bouten, C.V.C.; Van De Vosse, F.N.; Unser, M.; Stergiopulos, N. Experimental investigation of collagen waviness and orientation in the arterial adventitia using confocal laser scanning microscopy. Biomech. Model. Mechanobiol. 2012, 11, 461–473. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ariel, P. A beginner’s guide to tissue clearing. Int. J. Biochem. Cell Biol. 2017, 84, 35–39. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Williams, M.P.I.; Rigon, M.; Straka, T.; Hörner, S.J.; Thiel, M.; Gretz, N.; Hafner, M.; Reischl, M.; Rudolf, R. A novel optical tissue clearing protocol for mouse skeletal muscle to visualize endplates in their tissue context. Front. Cell. Neurosci. 2019, 13, 49. [Google Scholar] [CrossRef] [Green Version]
- Costantini, I.; Ghobril, J.-P.; Di Giovanna, A.P.; Allegra Mascaro, A.L.; Silvestri, L.; Müllenbroich, M.C.; Onofri, L.; Conti, V.; Vanzi, F.; Sacconi, L.; et al. A versatile clearing agent for multi-modal brain imaging. Sci. Rep. 2015, 5, 9808. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Costantini, I.; Cicchi, R.; Silvestri, L.; Vanzi, F.; Pavone, F.S. In-vivo and ex-vivo optical clearing methods for biological tissues: Review. Biomed. Opt. Express 2019, 10, 5251–5267. [Google Scholar] [CrossRef]
- Tian, N.; Fu, L.; Gu, M. Resolution and contrast enhancement of subtractive second harmonic generation microscopy with a circularly polarized vortex beam. Sci. Rep. 2015. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Winter, P.W.; York, A.G.; Nogare, D.D.; Ingaramo, M.; Christensen, R.; Chitnis, A.; Patterson, G.H.; Shroff, H. Two-photon instant structured illumination microscopy improves the depth penetration of super-resolution imaging in thick scattering samples. Optica 2014, 1, 181. [Google Scholar] [CrossRef] [Green Version]
- Gregor, I.; Spiecker, M.; Petrovsky, R.; Großhans, J.; Ros, R.; Enderlein, J. Rapid nonlinear image scanning microscopy. Nat. Methods 2017, 14, 1087–1089. [Google Scholar] [CrossRef] [PubMed]


| Biomedical Field | Representative Results |
|---|---|
| Regenerative medicine and tissue engineering |
|
| |
| Cancer |
|
| |
| |
| |
| Cardiovascular |
|
| |
| Neuroscience |
|
| |
| In vitro 3D models |
|
| |
| Development and embryogenesis |
|
| |
| Immunology |
|
| |
| Ophthalmology |
|
| Respiratory disease |
|
| |
| Muscle physiology and pathology |
|
| |
| Kidney, Colon, and Liver |
|
| |
|
| Features | Methods | Set-Up Sophistication | Analysis of Complexity |
|---|---|---|---|
| Amount and texture description | First-order statistics (FOS) | + | + |
| Second-order statistics (gray level co-occurrence matrix, GLCM) | + | ++ | |
| 2D-Fast Fourier Transformation (2D-FFT) | + | ++ | |
| Wavelet transformation | + | +++ | |
| Fibers orientation | Forward–backward SHG signal (F-SHG/B-SHG) | ++ | ++ |
| Polarization | +++ | ++ | |
| Coherency (C) | + | +++ | |
| Fibers waviness | Straightness parameter (Ps) | + | ++ |
| Fibers thickness and distance | Plot profile | + | + |
| Parameters | Meaning | Interpretation in the Image |
|---|---|---|
| Mean | Average value | The average value of gray tones |
| Standard Deviation | The standard deviation of the gray values used to generate the mean gray value | Contrast |
| Integrated Density | Product of the image’s area and mean gray value | Lightness/darkness |
| Skewness | It quantifies how symmetrical the distribution is relative to the mean value | The imbalance between the extent of areas (or number of pixels) that are darker or brighter than the mean |
| Kurtosis | It quantifies whether the shape of the data distribution matches the Gaussian distribution | The spread of gray tones around the mean value |
| Parameters | Meaning | Interpretation in the Image |
|---|---|---|
| Inverse difference moment (IDM) | Quantifies the local similarities present in the image | Homogeneity |
| Energy | Probabilities of different gray levels in the image | Uniformity |
| Inertia | The similarity in gray levels between neighboring pixels | Contrast |
| Entropy | Measure the lack of spatial organization inside the image | Randomness |
| Correlation | Dependence of gray levels between two pixels separated by a certain distance | Regularity in repetition patterns |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Borile, G.; Sandrin, D.; Filippi, A.; Anderson, K.I.; Romanato, F. Label-Free Multiphoton Microscopy: Much More Than Fancy Images. Int. J. Mol. Sci. 2021, 22, 2657. https://doi.org/10.3390/ijms22052657
Borile G, Sandrin D, Filippi A, Anderson KI, Romanato F. Label-Free Multiphoton Microscopy: Much More Than Fancy Images. International Journal of Molecular Sciences. 2021; 22(5):2657. https://doi.org/10.3390/ijms22052657
Chicago/Turabian StyleBorile, Giulia, Deborah Sandrin, Andrea Filippi, Kurt I. Anderson, and Filippo Romanato. 2021. "Label-Free Multiphoton Microscopy: Much More Than Fancy Images" International Journal of Molecular Sciences 22, no. 5: 2657. https://doi.org/10.3390/ijms22052657
APA StyleBorile, G., Sandrin, D., Filippi, A., Anderson, K. I., & Romanato, F. (2021). Label-Free Multiphoton Microscopy: Much More Than Fancy Images. International Journal of Molecular Sciences, 22(5), 2657. https://doi.org/10.3390/ijms22052657







