Glucocorticoid Receptor Antagonist Mifepristone Does Not Alter Innate Anxiety-Like Behavior in Genetically-Selected Marchigian Sardinian (msP) Rats
Abstract
1. Introduction
2. Results
2.1. Effects of Mifepristone on Anxiety-Like Behavior
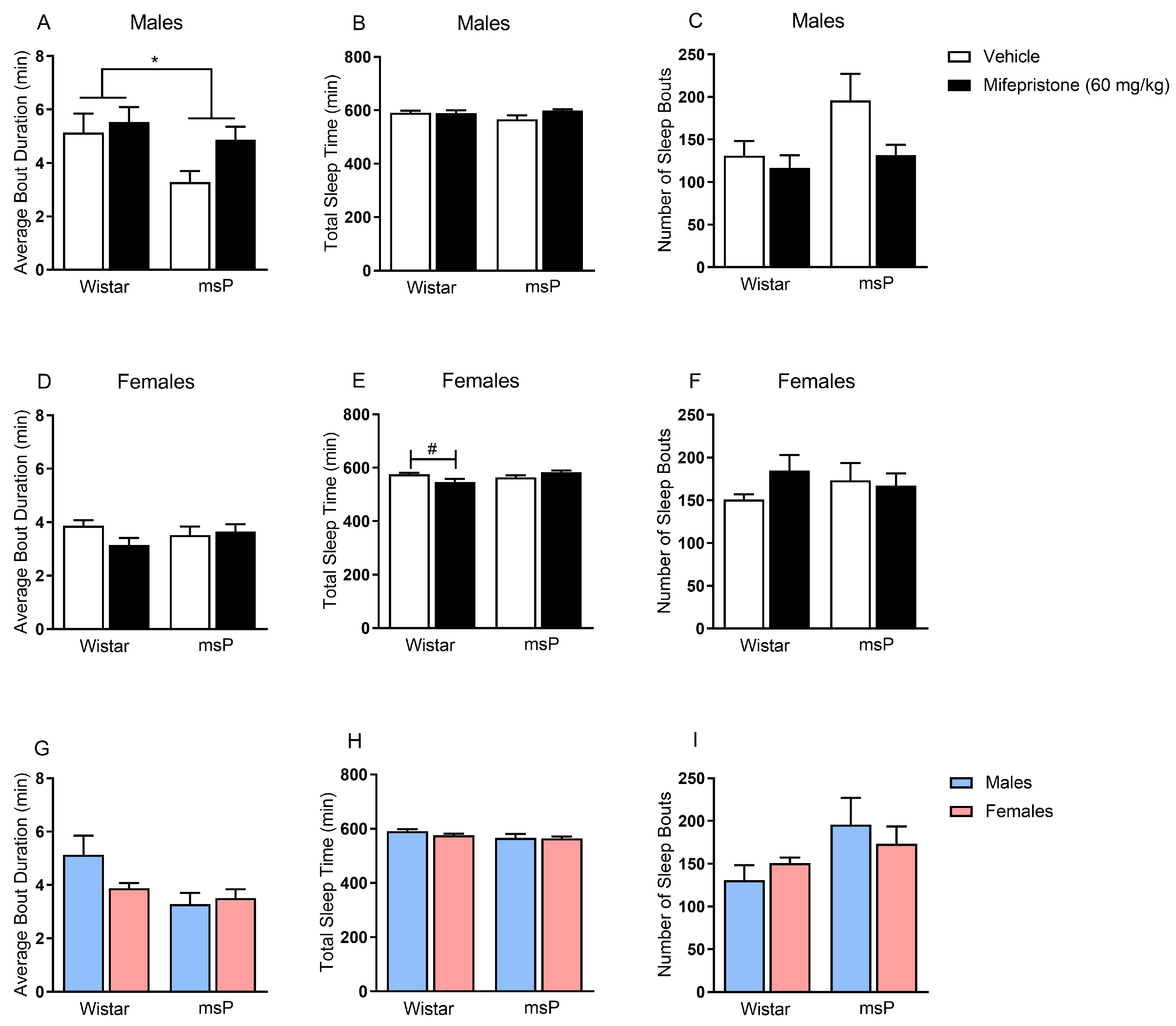
2.2. Effects of Mifepristone on Sleep Disturbances
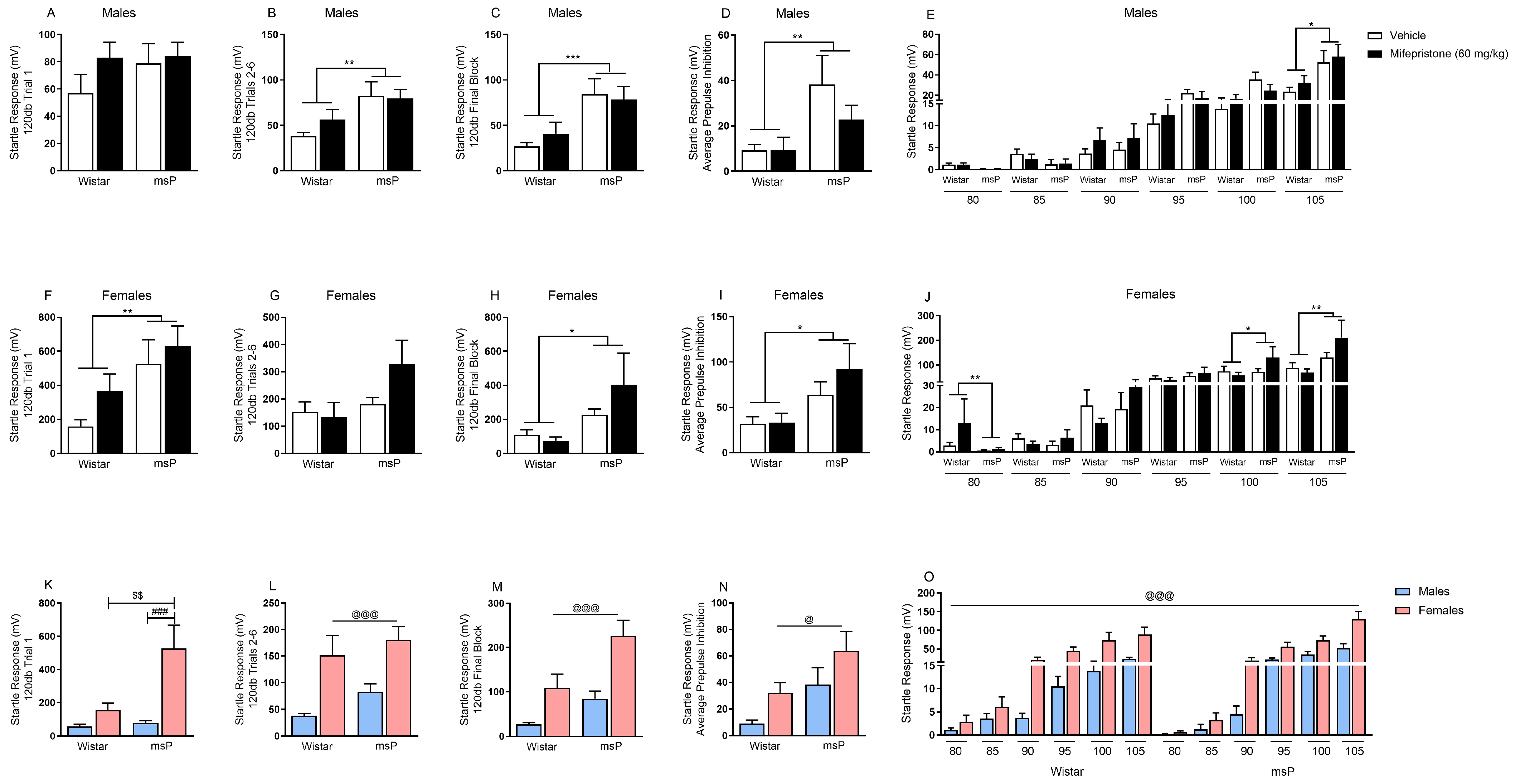
2.3. Effects of Mifepristone on Hyperarousal States
3. Discussion
4. Materials and Methods
4.1. Animals
4.2. Drug Preparation and Treatment
4.3. Novelty-Induced Hypophagia (NIH)
4.4. Comprehensive Lab Animal Monitoring System (CLAMS)
4.5. Acoustic Startle
4.6. Statistical Analyses
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Stephens, M.A.; Wand, G. Stress and the HPA axis: Role of glucocorticoids in alcohol dependence. Alcohol Res. Curr. Rev. 2012, 34, 468–483. [Google Scholar]
- Vale, W.; Spiess, J.; Rivier, C.; Rivier, J. Characterization of a 41-residue ovine hypothalamic peptide that stimulates secretion of corticotropin and beta-endorphin. Science 1981, 213, 1394–1397. [Google Scholar] [CrossRef]
- Herman, J.P.; McKlveen, J.M.; Ghosal, S.; Kopp, B.; Wulsin, A.; Makinson, R.; Scheimann, J.; Myers, B. Regulation of the Hypothalamic-Pituitary-Adrenocortical Stress Response. Compr. Physiol. 2016, 6, 603–621. [Google Scholar] [CrossRef]
- Reul, J.M.; de Kloet, E.R. Anatomical resolution of two types of corticosterone receptor sites in rat brain with in vitro autoradiography and computerized image analysis. J. Steroid Biochem. 1986, 24, 269–272. [Google Scholar] [CrossRef]
- Edwards, S.; Little, H.J.; Richardson, H.N.; Vendruscolo, L.F. Divergent regulation of distinct glucocorticoid systems in alcohol dependence. Alcohol 2015, 49, 811–816. [Google Scholar] [CrossRef]
- Makino, S.; Gold, P.W.; Schulkin, J. Effects of corticosterone on CRH mRNA and content in the bed nucleus of the stria terminalis; comparison with the effects in the central nucleus of the amygdala and the paraventricular nucleus of the hypothalamus. Brain Res. 1994, 657, 141–149. [Google Scholar] [CrossRef]
- Zorrilla, E.P.; Koob, G.F. The therapeutic potential of CRF1 antagonists for anxiety. Expert Opin. Investig. Drugs 2004, 13, 799–828. [Google Scholar] [CrossRef] [PubMed]
- Smith, S.M.; Vale, W.W. The role of the hypothalamic-pituitary-adrenal axis in neuroendocrine responses to stress. Dialogues Clin. Neurosci. 2006, 8, 383–395. [Google Scholar]
- Roberto, M.; Cruz, M.T.; Gilpin, N.W.; Sabino, V.; Schweitzer, P.; Bajo, M.; Cottone, P.; Madamba, S.G.; Stouffer, D.G.; Zorrilla, E.P.; et al. Corticotropin releasing factor-induced amygdala gamma-aminobutyric Acid release plays a key role in alcohol dependence. Biol. Psychiatry 2010, 67, 831–839. [Google Scholar] [CrossRef] [PubMed]
- Schulkin, J.; Gold, P.W.; McEwen, B.S. Induction of corticotropin-releasing hormone gene expression by glucocorticoids: Implication for understanding the states of fear and anxiety and allostatic load. Psychoneuroendocrinology 1998, 23, 219–243. [Google Scholar] [CrossRef]
- Ciccocioppo, R.; Economidou, D.; Cippitelli, A.; Cucculelli, M.; Ubaldi, M.; Soverchia, L.; Lourdusamy, A.; Massi, M. Genetically selected Marchigian Sardinian alcohol-preferring (msP) rats: An animal model to study the neurobiology of alcoholism. Addict. Biol. 2006, 11, 339–355. [Google Scholar] [CrossRef]
- Borruto, A.M.; Stopponi, S.; Li, H.; Weiss, F.; Roberto, M.; Ciccocioppo, R. Genetically selected alcohol-preferring msP rats to study alcohol use disorder: Anything lost in translation? Neuropharmacology 2021, 186, 108446. [Google Scholar] [CrossRef] [PubMed]
- Ayanwuyi, L.O.; Carvajal, F.; Lerma-Cabrera, J.M.; Domi, E.; Bjork, K.; Ubaldi, M.; Heilig, M.; Roberto, M.; Ciccocioppo, R.; Cippitelli, A. Role of a genetic polymorphism in the corticotropin-releasing factor receptor 1 gene in alcohol drinking and seeking behaviors of marchigian sardinian alcohol-preferring rats. Front. Psychiatry 2013, 4, 23. [Google Scholar] [CrossRef]
- Cippitelli, A.; Ayanwuyi, L.O.; Barbier, E.; Domi, E.; Lerma-Cabrera, J.M.; Carvajal, F.; Scuppa, G.; Li, H.; Ubaldi, M.; Heilig, M.; et al. Polymorphism in the corticotropin-releasing factor receptor 1 (CRF1-R) gene plays a role in shaping the high anxious phenotype of Marchigian Sardinian alcohol-preferring (msP) rats. Psychopharmacology 2015, 232, 1083–1093. [Google Scholar] [CrossRef] [PubMed]
- Hansson, A.C.; Cippitelli, A.; Sommer, W.H.; Fedeli, A.; Bjork, K.; Soverchia, L.; Terasmaa, A.; Massi, M.; Heilig, M.; Ciccocioppo, R. Variation at the rat Crhr1 locus and sensitivity to relapse into alcohol seeking induced by environmental stress. Proc. Natl. Acad. Sci. USA 2006, 103, 15236–15241. [Google Scholar] [CrossRef]
- Logrip, M.L.; Walker, J.R.; Ayanwuyi, L.O.; Sabino, V.; Ciccocioppo, R.; Koob, G.F.; Zorrilla, E.P. Evaluation of Alcohol Preference and Drinking in msP Rats Bearing a Crhr1 Promoter Polymorphism. Front. Psychiatry 2018, 9, 28. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.; Kallupi, M.; Luu, G.; Oleata, C.S.; Heilig, M.; Koob, G.F.; Ciccocioppo, R.; Roberto, M. Enhanced GABAergic transmission in the central nucleus of the amygdala of genetically selected Marchigian Sardinian rats: Alcohol and CRF effects. Neuropharmacology 2013, 67, 337–348. [Google Scholar] [CrossRef] [PubMed]
- Herman, M.A.; Varodayan, F.P.; Oleata, C.S.; Luu, G.; Kirson, D.; Heilig, M.; Ciccocioppo, R.; Roberto, M. Glutamatergic transmission in the central nucleus of the amygdala is selectively altered in Marchigian Sardinian alcohol-preferring rats: Alcohol and CRF effects. Neuropharmacology 2016, 102, 21–31. [Google Scholar] [CrossRef]
- Cannella, N.; Ubaldi, M.; Masi, A.; Bramucci, M.; Roberto, M.; Bifone, A.; Ciccocioppo, R. Building better strategies to develop new medications in Alcohol Use Disorder: Learning from past success and failure to shape a brighter future. Neurosci. Biobehav. Rev. 2019, 103, 384–398. [Google Scholar] [CrossRef]
- Natividad, L.A.; Steinman, M.Q.; McGinn, M.A.; Sureshchandra, S.; Kerr, T.M.; Ciccocioppo, R.; Messaoudi, I.; Edwards, S.; Roberto, M. Impaired hypothalamic feedback dysregulates brain glucocorticoid signaling in genetically-selected Marchigian Sardinian alcohol-preferring rats. Addict. Biol. 2020, e12978. [Google Scholar] [CrossRef]
- Vendruscolo, L.F.; Estey, D.; Goodell, V.; Macshane, L.G.; Logrip, M.L.; Schlosburg, J.E.; McGinn, M.A.; Zamora-Martinez, E.R.; Belanoff, J.K.; Hunt, H.J.; et al. Glucocorticoid receptor antagonism decreases alcohol seeking in alcohol-dependent individuals. J. Clin. Investig. 2015, 125, 3193–3197. [Google Scholar] [CrossRef]
- Sitruk-Ware, R.; Spitz, I.M. Pharmacological properties of mifepristone: Toxicology and safety in animal and human studies. Contraception 2003, 68, 409–420. [Google Scholar] [CrossRef]
- Vendruscolo, L.F.; Barbier, E.; Schlosburg, J.E.; Misra, K.K.; Whitfield, T.W., Jr.; Logrip, M.L.; Rivier, C.; Repunte-Canonigo, V.; Zorrilla, E.P.; Sanna, P.P.; et al. Corticosteroid-dependent plasticity mediates compulsive alcohol drinking in rats. J. Neurosci. 2012, 32, 7563–7571. [Google Scholar] [CrossRef] [PubMed]
- Somkuwar, S.S.; Vendruscolo, L.F.; Fannon, M.J.; Schmeichel, B.E.; Nguyen, T.B.; Guevara, J.; Sidhu, H.; Contet, C.; Zorrilla, E.P.; Mandyam, C.D. Abstinence from prolonged ethanol exposure affects plasma corticosterone, glucocorticoid receptor signaling and stress-related behaviors. Psychoneuroendocrinology 2017, 84, 17–31. [Google Scholar] [CrossRef] [PubMed]
- Simms, J.A.; Haass-Koffler, C.L.; Bito-Onon, J.; Li, R.; Bartlett, S.E. Mifepristone in the central nucleus of the amygdala reduces yohimbine stress-induced reinstatement of ethanol-seeking. Neuropsychopharmacology 2012, 37, 906–918. [Google Scholar] [CrossRef]
- Calvo, N.; Volosin, M. Glucocorticoid and mineralocorticoid receptors are involved in the facilitation of anxiety-like response induced by restraint. Neuroendocrinology 2001, 73, 261–271. [Google Scholar] [CrossRef]
- Jakovcevski, M.; Schachner, M.; Morellini, F. Susceptibility to the long-term anxiogenic effects of an acute stressor is mediated by the activation of the glucocorticoid receptors. Neuropharmacology 2011, 61, 1297–1305. [Google Scholar] [CrossRef] [PubMed]
- Kim, K.S.; Han, P.L. Mice lacking adenylyl cyclase-5 cope badly with repeated restraint stress. J. Neurosci. Res. 2009, 87, 2983–2993. [Google Scholar] [CrossRef]
- Suh, J.; Ressler, K.J. Common Biological Mechanisms of Alcohol Use Disorder and Post-Traumatic Stress Disorder. Alcohol Res. 2018, 39, 131–145. [Google Scholar]
- Natividad, L.A.; Buczynski, M.W.; Herman, M.A.; Kirson, D.; Oleata, C.S.; Irimia, C.; Polis, I.; Ciccocioppo, R.; Roberto, M.; Parsons, L.H. Constitutive Increases in Amygdalar Corticotropin-Releasing Factor and Fatty Acid Amide Hydrolase Drive an Anxious Phenotype. Biol. Psychiatry 2017, 82, 500–510. [Google Scholar] [CrossRef]
- Stopponi, S.; Fotio, Y.; Domi, A.; Borruto, A.M.; Natividad, L.; Roberto, M.; Ciccocioppo, R.; Cannella, N. Inhibition of fatty acid amide hydrolase in the central amygdala alleviates co-morbid expression of innate anxiety and excessive alcohol intake. Addict. Biol. 2018, 23, 1223–1232. [Google Scholar] [CrossRef]
- Zeng, T.; Mott, C.; Mollicone, D.; Sanford, L.D. Automated determination of wakefulness and sleep in rats based on non-invasively acquired measures of movement and respiratory activity. J. Neurosci. Methods 2012, 204, 276–287. [Google Scholar] [CrossRef]
- Golub, Y.; Mauch, C.P.; Dahlhoff, M.; Wotjak, C.T. Consequences of extinction training on associative and non-associative fear in a mouse model of Posttraumatic Stress Disorder (PTSD). Behav. Brain Res. 2009, 205, 544–549. [Google Scholar] [CrossRef]
- Koenig, H.N.; Olive, M.F. The glucocorticoid receptor antagonist mifepristone reduces ethanol intake in rats under limited access conditions. Psychoneuroendocrinology 2004, 29, 999–1003. [Google Scholar] [CrossRef]
- Boero, G.; Pisu, M.G.; Biggio, F.; Muredda, L.; Carta, G.; Banni, S.; Paci, E.; Follesa, P.; Concas, A.; Porcu, P.; et al. Impaired glucocorticoid-mediated HPA axis negative feedback induced by juvenile social isolation in male rats. Neuropharmacology 2018, 133, 242–253. [Google Scholar] [CrossRef] [PubMed]
- Fox, L.C.; Davies, D.R.; Scholl, J.L.; Watt, M.J.; Forster, G.L. Differential effects of glucocorticoid and mineralocorticoid antagonism on anxiety behavior in mild traumatic brain injury. Behav. Brain Res. 2016, 312, 362–365. [Google Scholar] [CrossRef] [PubMed]
- Wang, W.; Liu, L.; Yang, X.; Gao, H.; Tang, Q.K.; Yin, L.Y.; Yin, X.Y.; Hao, J.R.; Geng, D.Q.; Gao, C. Ketamine improved depressive-like behaviors via hippocampal glucocorticoid receptor in chronic stress induced- susceptible mice. Behav. Brain Res. 2019, 364, 75–84. [Google Scholar] [CrossRef] [PubMed]
- Groeneweg, F.L.; Karst, H.; de Kloet, E.R.; Joels, M. Rapid non-genomic effects of corticosteroids and their role in the central stress response. J. Endocrinol. 2011, 209, 153–167. [Google Scholar] [CrossRef]
- Dwyer, D.B.; Kalman, J.L.; Budde, M.; Kambeitz, J.; Ruef, A.; Antonucci, L.A.; Kambeitz-Ilankovic, L.; Hasan, A.; Kondofersky, I.; Anderson-Schmidt, H.; et al. An Investigation of Psychosis Subgroups With Prognostic Validation and Exploration of Genetic Underpinnings: The PsyCourse Study. JAMA Psychiatry 2020, 77, 523–533. [Google Scholar] [CrossRef] [PubMed]
- Kirson, D.; Oleata, C.S.; Parsons, L.H.; Ciccocioppo, R.; Roberto, M. CB1 and ethanol effects on glutamatergic transmission in the central amygdala of male and female msP and Wistar rats. Addict. Biol. 2018, 23, 676–688. [Google Scholar] [CrossRef]
- Silva, A.F.; Sousa, D.S.; Medeiros, A.M.; Macedo, P.T.; Leao, A.H.; Ribeiro, A.M.; Izidio, G.S.; Silva, R.H. Sex and estrous cycle influence diazepam effects on anxiety and memory: Possible role of progesterone. Prog. Neuropsychopharmacol. Biol. Psychiatry 2016, 70, 68–76. [Google Scholar] [CrossRef] [PubMed]
- Galeeva, A.Y.; Pivina, S.G.; Tuohimaa, P.; Ordyan, N.E. Involvement of nuclear progesterone receptors in the formation of anxiety in female mice. Neurosci. Behav. Physiol. 2007, 37, 843–848. [Google Scholar] [CrossRef] [PubMed]
- Flores, R.J.; Cruz, B.; Uribe, K.P.; Correa, V.L.; Arreguin, M.C.; Carcoba, L.M.; Mendez, I.A.; O’Dell, L.E. Estradiol promotes and progesterone reduces anxiety-like behavior produced by nicotine withdrawal in female rats. Psychoneuroendocrinology 2020, 119, 104694. [Google Scholar] [CrossRef]
- Steinman, M.Q.; Kirson, D.; Wolfe, S.A.; Khom, S.; D’Ambrosio, S.R.; Spierling Bagsic, S.R.; Bajo, M.; Vlkolinsky, R.; Hoang, N.K.; Singhal, A.; et al. Importance of sex and trauma context on circulating cytokines and amygdalar GABAergic signaling in a comorbid model of posttraumatic stress and alcohol use disorders. Mol. Psychiatry 2020. [Google Scholar] [CrossRef]
- Dong, L.; Wang, S.; Li, Y.; Zhao, Z.; Shen, Y.; Liu, L.; Xu, G.; Ma, C.; Li, S.; Zhang, X.; et al. RU486 Reverses Emotional Disorders by Influencing Astrocytes and Endoplasmic Reticulum Stress in Chronic Restraint Stress Challenged Rats. Cell Physiol. Biochem. 2017, 42, 1098–1108. [Google Scholar] [CrossRef] [PubMed]
- Bechtholt, A.J.; Hill, T.E.; Lucki, I. Anxiolytic effect of serotonin depletion in the novelty-induced hypophagia test. Psychopharmacology 2007, 190, 531–540. [Google Scholar] [CrossRef]
- Bluett, R.J.; Gamble-George, J.C.; Hermanson, D.J.; Hartley, N.D.; Marnett, L.J.; Patel, S. Central anandamide deficiency predicts stress-induced anxiety: Behavioral reversal through endocannabinoid augmentation. Transl. Psychiatry 2014, 4, e408. [Google Scholar] [CrossRef]
- Dulawa, S.C.; Hen, R. Recent advances in animal models of chronic antidepressant effects: The novelty-induced hypophagia test. Neurosci. Biobehav. Rev. 2005, 29, 771–783. [Google Scholar] [CrossRef]
- Ross, R.J.; Ball, W.A.; Sullivan, K.A.; Caroff, S.N. Sleep disturbance as the hallmark of posttraumatic stress disorder. Am. J. Psychiatry 1989, 146, 697–707. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (http://creativecommons.org/licenses/by/4.0/).
Share and Cite
Vozella, V.; Cruz, B.; Natividad, L.A.; Benvenuti, F.; Cannella, N.; Edwards, S.; Zorrilla, E.P.; Ciccocioppo, R.; Roberto, M. Glucocorticoid Receptor Antagonist Mifepristone Does Not Alter Innate Anxiety-Like Behavior in Genetically-Selected Marchigian Sardinian (msP) Rats. Int. J. Mol. Sci. 2021, 22, 3095. https://doi.org/10.3390/ijms22063095
Vozella V, Cruz B, Natividad LA, Benvenuti F, Cannella N, Edwards S, Zorrilla EP, Ciccocioppo R, Roberto M. Glucocorticoid Receptor Antagonist Mifepristone Does Not Alter Innate Anxiety-Like Behavior in Genetically-Selected Marchigian Sardinian (msP) Rats. International Journal of Molecular Sciences. 2021; 22(6):3095. https://doi.org/10.3390/ijms22063095
Chicago/Turabian StyleVozella, Valentina, Bryan Cruz, Luis A. Natividad, Federica Benvenuti, Nazzareno Cannella, Scott Edwards, Eric P. Zorrilla, Roberto Ciccocioppo, and Marisa Roberto. 2021. "Glucocorticoid Receptor Antagonist Mifepristone Does Not Alter Innate Anxiety-Like Behavior in Genetically-Selected Marchigian Sardinian (msP) Rats" International Journal of Molecular Sciences 22, no. 6: 3095. https://doi.org/10.3390/ijms22063095
APA StyleVozella, V., Cruz, B., Natividad, L. A., Benvenuti, F., Cannella, N., Edwards, S., Zorrilla, E. P., Ciccocioppo, R., & Roberto, M. (2021). Glucocorticoid Receptor Antagonist Mifepristone Does Not Alter Innate Anxiety-Like Behavior in Genetically-Selected Marchigian Sardinian (msP) Rats. International Journal of Molecular Sciences, 22(6), 3095. https://doi.org/10.3390/ijms22063095





