Protective Effect of Quercetin on Sodium Iodate-Induced Retinal Apoptosis through the Reactive Oxygen Species-Mediated Mitochondrion-Dependent Pathway
Abstract
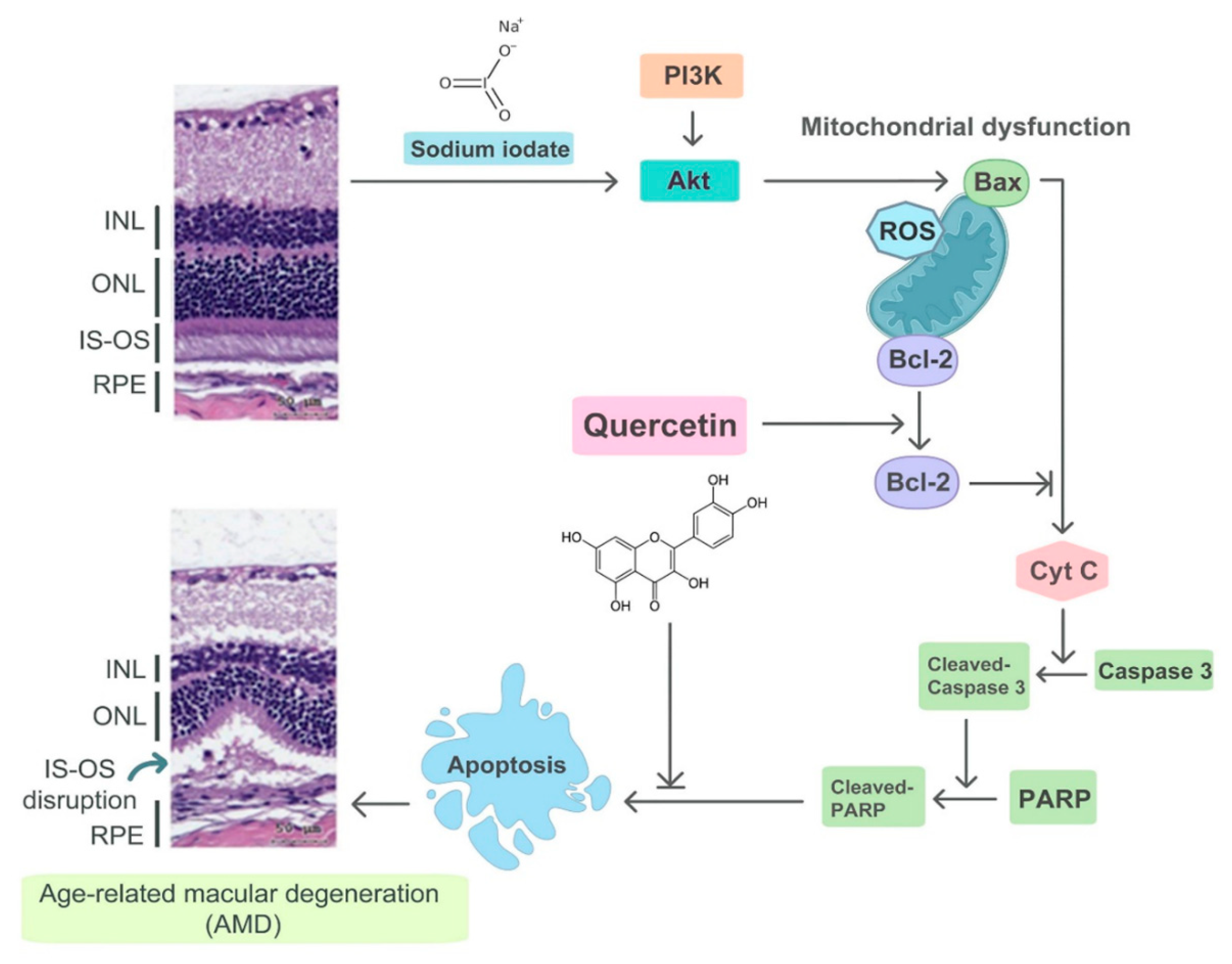
:1. Introduction
2. Materials and Methods
2.1. Cell Culture
2.2. Cell Viability Assay
2.3. Measurement of Intracellular ROS and H2O2 Production
2.4. Measurement of Mitochondrial Damage
2.5. Measurements of Antioxidative Capacities and H2O2 Production
2.6. Western Blot Analysis
2.7. Animal Model
- Mock group: Animals pretreated with an intraperitoneal (IP) injection of PBS and then a single intravenous (IV) injection of PBS.
- Vehicle-treated group: Animals pretreated with an IP injection of PBS and then a single IV injection of 40 mg/kg NaIO3 [31].
- Experimental group: Animals pretreated with an IP injection of 100 mg/kg quercetin and then a single IV injection of 40 mg/kg NaIO3.
2.8. Histology and Immunohistochemistry
2.9. Retinal Imaging
2.10. Statistical Analysis
3. Results
3.1. Effect of Quercetin Pretreatment on the Viability and NaIO3-Induced Apoptosis of ARPE-19 Cells
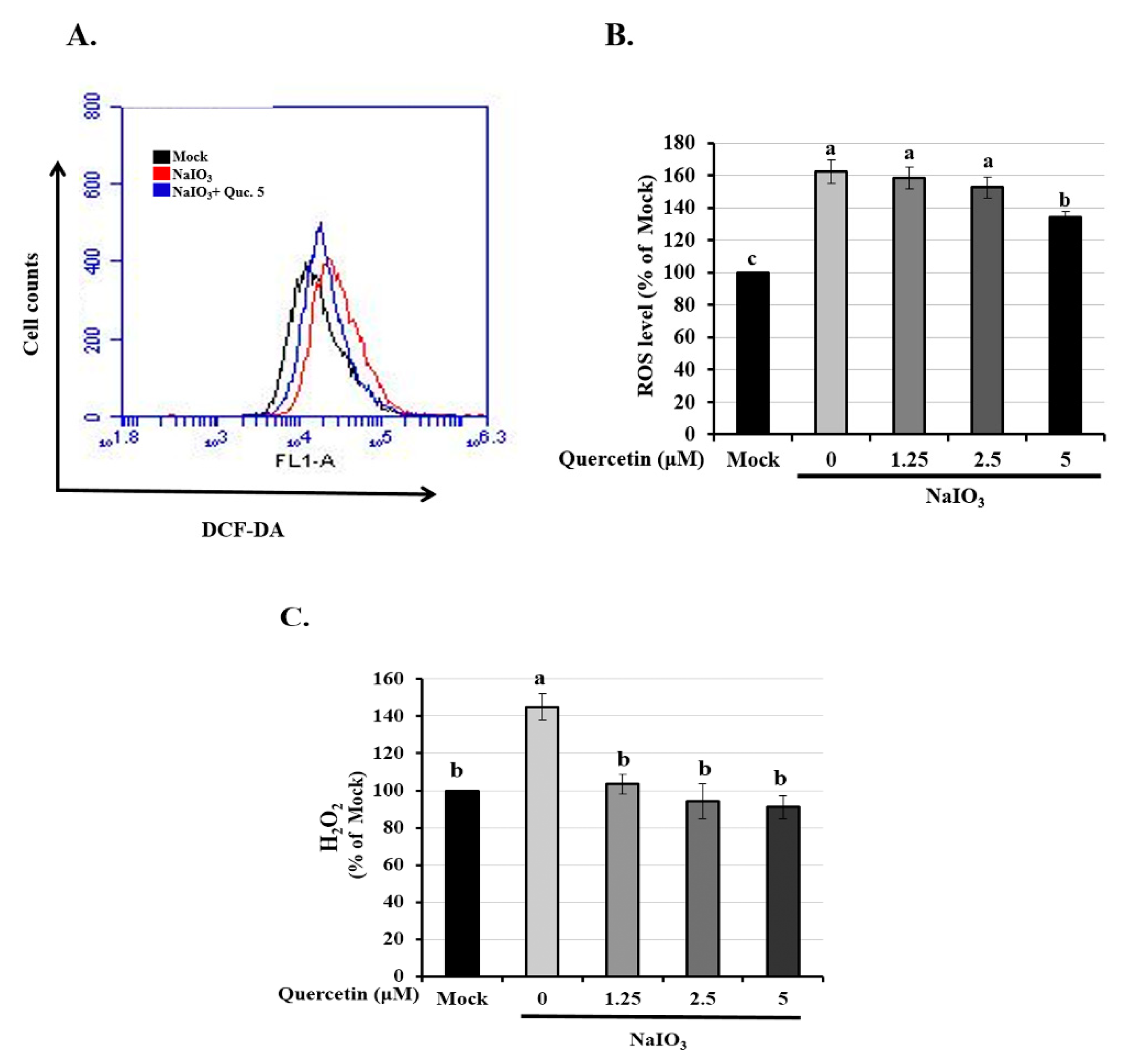
3.2. Quercetin Reduced the Expression of Intracellular ROS and H2O2 Production
3.3. Quercetin Inhibited the Reduction of Mitochondrial Membrane Potential (∆Ψm) via Modulated the Activity of Anti-Oxidants
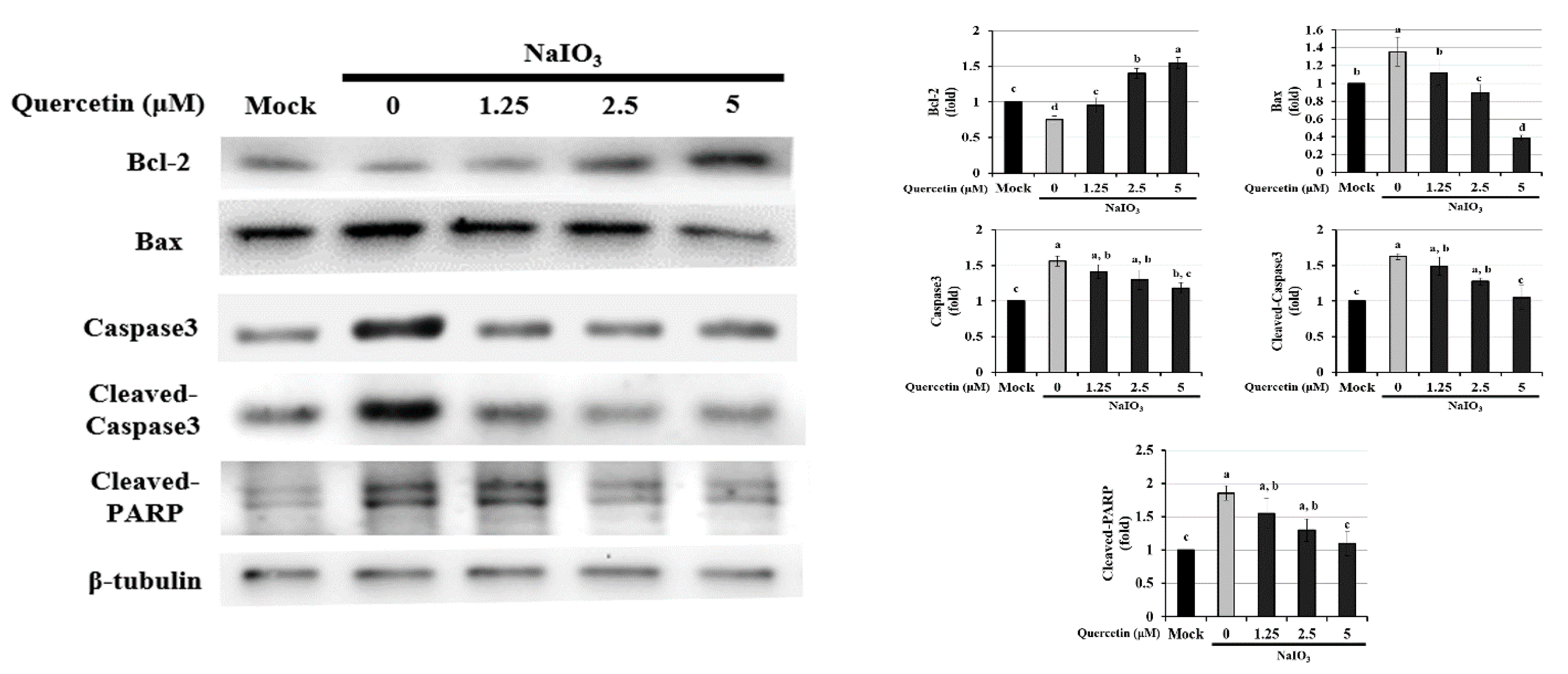
3.4. Effect of Quercetin on the Endogenous NaIO3-Induced Apoptotic Pathway
3.5. Quercetin Suppressed Apoptosis via the PI3K/AKT Signaling Pathway
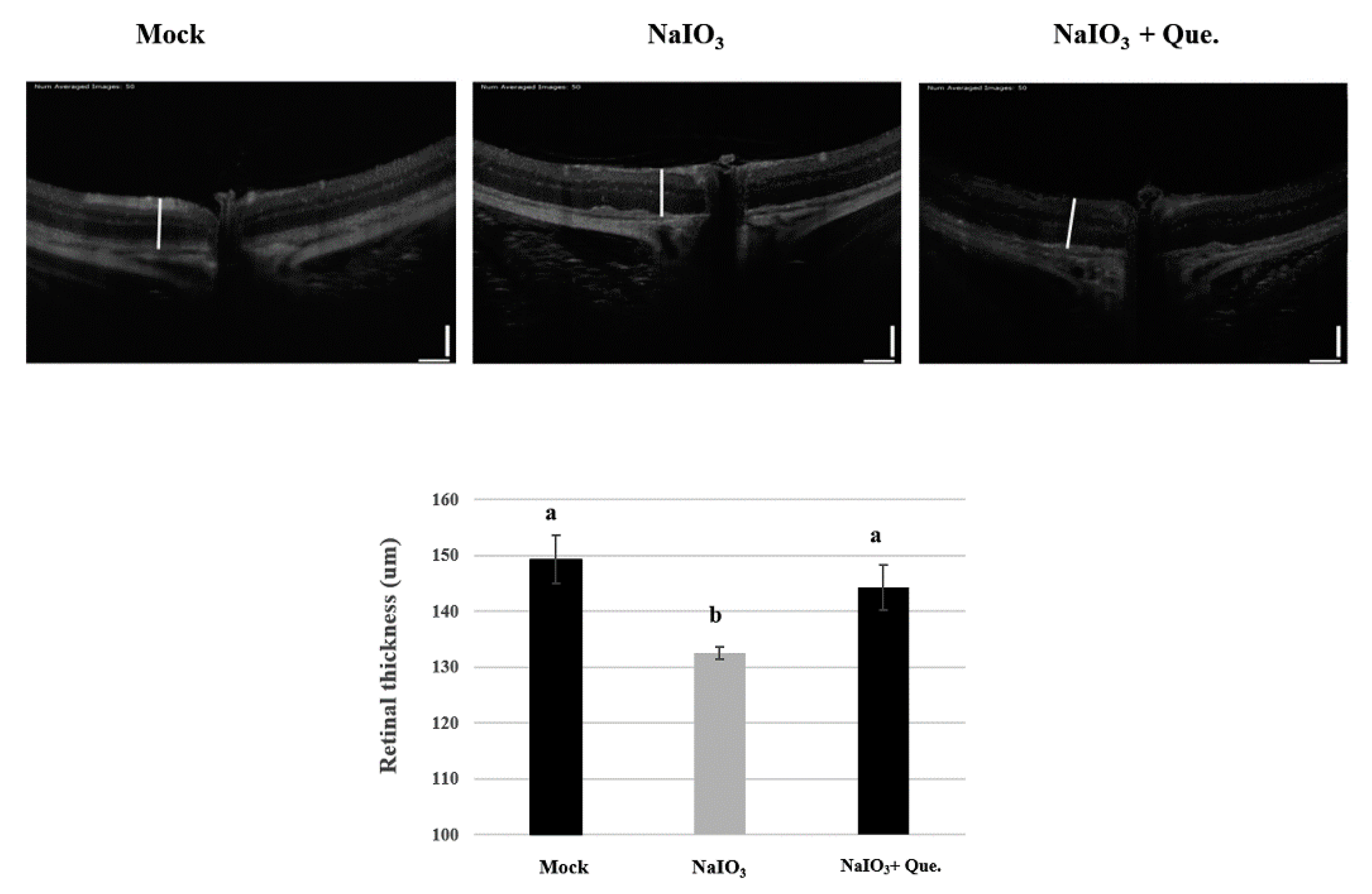
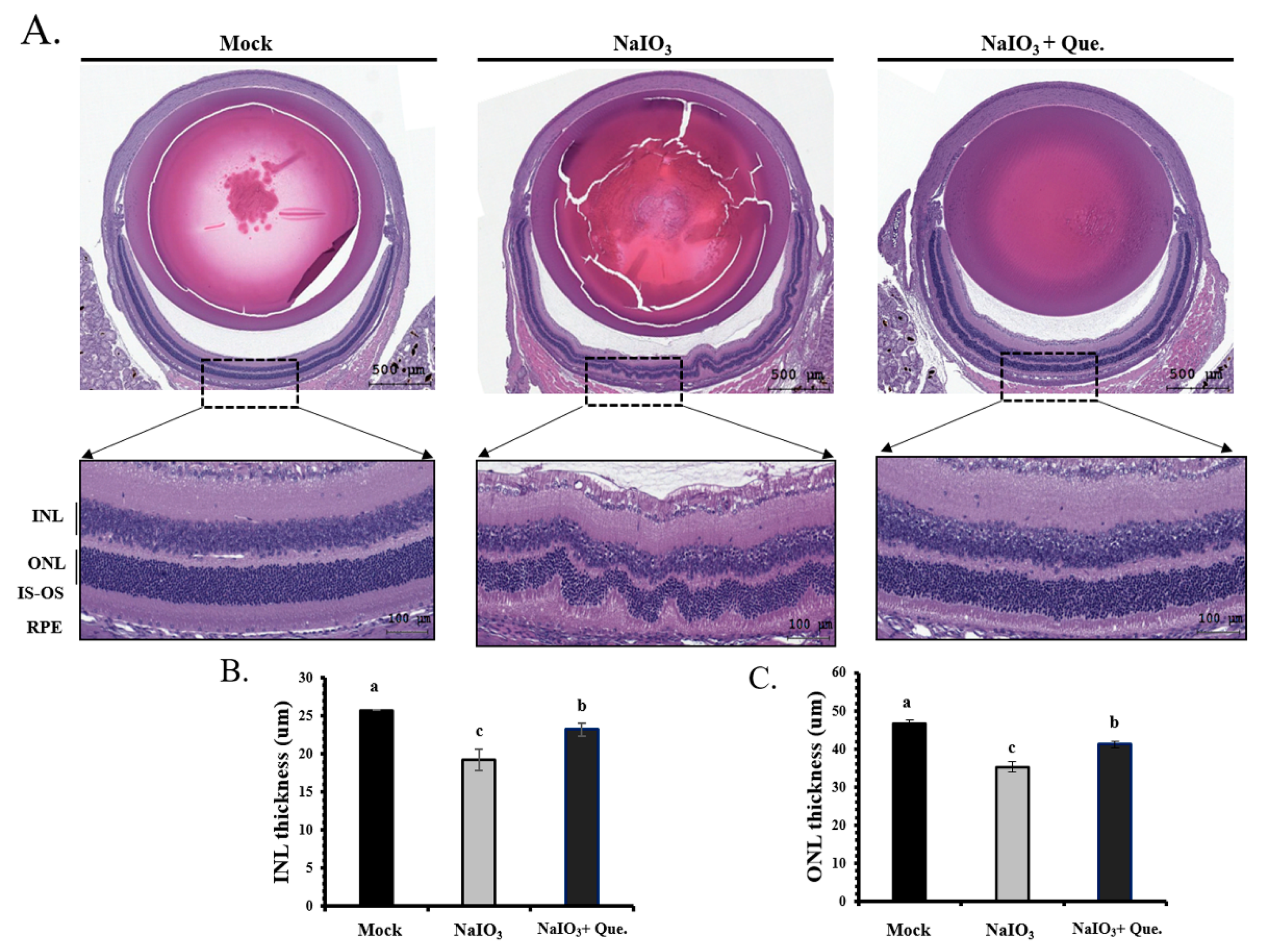
3.6. Protective Effects of Quercetin on Retinal Degeneration
3.7. Quercetin Reduced NaIO3-Induced Retinal Apoptosis in Mice
4. Discussion
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Gibbons, A.; Ali, T.K.; Waren, D.P.; Donaldson, K.E. Causes and correction of dissatisfaction after implantation of presbyopia-correcting intraocular lenses. Clin. Ophthalmol. 2016, 10, 1965–1970. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ambati, J.; Atkinson, J.P.; Gelfand, B.D. Immunology of age-related macular degeneration. Nat. Rev. Immunol. 2013, 13, 438–451. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Klein, R.; Myers, C.E.; Buitendijk, G.H.; Rochtchina, E.; Gao, X.; de Jong, P.T.; Sivakumaran, T.A.; Burlutsky, G.; McKean-Cowdin, R.; Hofman, A.; et al. Lipids, lipid genes, and incident age-related macular degeneration: The three continent age-related macular degeneration consortium. Am. J. Ophthalmol. 2014, 158, 513–524.e3. [Google Scholar] [CrossRef] [Green Version]
- Mahr, M.A.; Hodge, D.O.; Erie, J.C. Racial Differences in Age-Related Macular Degeneration and Associated Anti-Vascular Endothelial Growth Factor Intravitreal Injections among Medicare Beneficiaries. Ophthalmol. Retin. 2018, 2, 1188–1195. [Google Scholar] [CrossRef] [PubMed]
- Rozing, M.P.; Durhuus, J.A.; Krogh Nielsen, M.; Subhi, Y.; Kirkwood, T.B.; Westendorp, R.G.; Sorensen, T.L. Age-related macular degeneration: A two-level model hypothesis. Prog. Retin. Eye Res. 2020, 76, 100825. [Google Scholar] [CrossRef]
- Acar, I.E.; Lores-Motta, L.; Colijn, J.M.; Meester-Smoor, M.A.; Verzijden, T.; Cougnard-Gregoire, A.; Ajana, S.; Merle, B.M.J.; de Breuk, A.; Heesterbeek, T.J.; et al. Integrating Metabolomics, Genomics, and Disease Pathways in Age-Related Macular Degeneration: The EYE-RISK Consortium. Ophthalmology 2020, 127, 1693–1709. [Google Scholar] [CrossRef]
- Feng, C.; Krogh Nielsen, M.; Sorensen, T.L.; Subhi, Y. Systemic levels of C-reactive protein in patients with age-related macular degeneration: A systematic review with meta-analyses. Mech. Ageing Dev. 2020, 191, 111353. [Google Scholar] [CrossRef]
- Shin, H.T.; Yoon, B.W.; Seo, J.H. Comparison of risk allele frequencies of single nucleotide polymorphisms associated with age-related macular degeneration in different ethnic groups. BMC Ophthalmol. 2021, 21, 97. [Google Scholar] [CrossRef] [PubMed]
- Donato, L.; D’Angelo, R.; Alibrandi, S.; Rinaldi, C.; Sidoti, A.; Scimone, C. Effects of A2E-Induced Oxidative Stress on Retinal Epithelial Cells: New Insights on Differential Gene Response and Retinal Dystrophies. Antioxidants 2020, 9, 307. [Google Scholar] [CrossRef] [Green Version]
- Donato, L.; Scimone, C.; Alibrandi, S.; Pitruzzella, A.; Scalia, F.; D’Angelo, R.; Sidoti, A. Possible A2E Mutagenic Effects on RPE Mitochondrial DNA from Innovative RNA-Seq Bioinformatics Pipeline. Antioxidants 2020, 9, 1158. [Google Scholar] [CrossRef] [PubMed]
- Scimone, C.; Alibrandi, S.; Scalinci, S.Z.; Trovato Battagliola, E.; D’Angelo, R.; Sidoti, A.; Donato, L. Expression of Pro-Angiogenic Markers Is Enhanced by Blue Light in Human RPE Cells. Antioxidants 2020, 9, 1154. [Google Scholar] [CrossRef]
- Donato, L.; Scimone, C.; Alibrandi, S.; Abdalla, E.M.; Nabil, K.M.; D’Angelo, R.; Sidoti, A. New Omics-Derived Perspectives on Retinal Dystrophies: Could Ion Channels-Encoding or Related Genes Act as Modifier of Pathological Phenotype? Int. J. Mol. Sci. 2020, 22, 70. [Google Scholar] [CrossRef] [PubMed]
- Beatty, S.; Koh, H.; Phil, M.; Henson, D.; Boulton, M. The Role of Oxidative Stress in the Pathogenesis of Age-Related Macular Degeneration. Surv. Ophthalmol. 2000, 45, 115–134. [Google Scholar] [CrossRef] [Green Version]
- Chew, E.Y.; Clemons, T.E.; SanGiovanni, J.P.; Danis, R.; Ferris, F.L., 3rd; Elman, M.; Antoszyk, A.; Ruby, A.; Orth, D.; Bressler, S.; et al. Lutein + zeaxanthin and omega-3 fatty acids for age-related macular degeneration: The Age-Related Eye Disease Study 2 (AREDS2) randomized clinical trial. JAMA 2013, 309, 2005–2015. [Google Scholar]
- Balmer, J.; Zulliger, R.; Roberti, S.; Enzmann, V. Retinal cell death caused by sodium iodate involves multiple caspase-dependent and caspase-independent cell-death pathways. Int. J. Mol. Sci. 2015, 16, 15086–15103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Kiuchi, K.; Yoshizawa, K.; Shikata, N.; Moriguchi, K.; Tsubura, A. Morphologic characteristics of retinal degeneration induced by sodium iodate in mice. Curr Eye Res. 2002, 25, 373–379. [Google Scholar] [CrossRef] [PubMed]
- Nadal-Nicolas, F.M.; Becerra, S.P. Pigment epithelium-derived factor protects retinal pigment epithelial cells against cytotoxicity “in vitro”. Adv. Exp. Med. Biol. 2018, 1074, 457–464. [Google Scholar] [PubMed]
- Wang, J.; Iacovelli, J.; Spencer, C.; Saint-Geniez, M. Direct effect of sodium iodate on neurosensory retina. Investig. Ophthalmol. Vis. Sci. 2014, 55, 1941–1953. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hanus, J.; Anderson, C.; Sarraf, D.; Ma, J.; Wang, S. Retinal pigment epithelial cell necroptosis in response to sodium iodate. Cell Death Discov. 2016, 2, 16054. [Google Scholar] [CrossRef] [PubMed]
- Hanus, J.; Anderson, C.; Wang, S. RPE necroptosis in response to oxidative stress and in AMD. Ageing Res. Rev. 2016, 24, 286–298. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Juel, H.B.; Faber, C.; Svendsen, S.G.; Vallejo, A.N.; Nissen, M.H. Inflammatory cytokines protect retinal pigment epithelial cells from oxidative stress-induced death. PLoS ONE 2013, 8, e64619. [Google Scholar] [CrossRef]
- Mao, X.; Pan, T.; Shen, H.; Xi, H.; Yuan, S.; Liu, Q. The rescue effect of mesenchymal stem cell on sodium iodate-induced retinal pigment epithelial cell death through deactivation of NF-kappaB-mediated NLRP3 inflammasome. Biomed. Pharmacother. 2018, 103, 517–523. [Google Scholar] [CrossRef]
- Hwang, N.; Kwon, M.Y.; Woo, J.M.; Chung, S.W. Oxidative Stress-Induced Pentraxin 3 Expression Human Retinal Pigment Epithelial Cells Is Involved in the Pathogenesis of Age-Related Macular Degeneration. Int. J. Mol. Sci. 2019, 20, 6028. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tressera-Rimbau, A.; Arranz, S.; Eder, M.; Vallverdú-Queralt, A. Dietary Polyphenols in the Prevention of Stroke. Oxid. Med. Cell Longev. 2017, 7467962. [Google Scholar] [CrossRef] [Green Version]
- Zhao, Y.; Chen, B.; Shen, J.; Wan, L.; Zhu, Y.; Yi, T.; Xiao, Z. The Beneficial Effects of Quercetin, Curcumin, and Resveratrol in Obesity. Oxid. Med. Cell Longev. 2017, 2017, 1459497. [Google Scholar] [CrossRef]
- Ferlemi, A.-V.; Makri, O.E.; Mermigki, P.G.; Lamari, F.N.; Georgakopoulos, C.D. Quercetin glycosides and chlorogenic acid in highbush blueberry leaf decoction prevent cataractogenesis in vivo and in vitro: Investigation of the effect on calpains, antioxidant and metal chelating properties. Exp. Eye Res. 2016, 145, 258–268. [Google Scholar] [CrossRef] [PubMed]
- Wang, Y.; Zhao, L.; Wang, C.T.; Hu, J.M.; Guo, X.X.; Zhang, D.; Wu, W.; Zhou, F.; Ji, B. Protective effect of quercetin and chlorogenic acid, two polyphenols widely present in edible plant varieties, on visible light-induced retinal degeneration in vivo. J. Funct. Foods 2017, 33, 103–111. [Google Scholar] [CrossRef]
- Sharmila, G.; Bhat, F.A.; Arunkumar, R.; Elumalai, P.; Raja Singh, P.; Senthilkumar, K.; Arunakaran, J. Chemopreventive effect of quercetin, a natural dietary flavonoid on prostate cancer in in vivo model. Clin. Nutr. 2014, 33, 718–726. [Google Scholar] [CrossRef]
- Ossola, B.; Kääriäinen, T.M.; Männistö, P.T. The Multiple Faces of Quercetin in Neuroprotection. Expert Opin. Drug Saf. 2009, 8, 397–409. [Google Scholar] [CrossRef]
- Weng, S.; Mao, L.; Gong, Y.; Sun, T.; Gu, Q. Role of quercetin in protecting ARPE 19 cells against H2O2 induced injury via nuclear factor erythroid 2 like 2 pathway activation and endoplasmic reticulum stress inhibition. Mol. Med. Rep. 2017, 16, 3461–3468. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moriguchi, M.; Nakamura, S.; Inoue, Y.; Nishinaka, A.; Nakamura, M.; Shimazawa, M.; Hara, H. Irreversible Photoreceptors and RPE Cells Damage by Intravenous Sodium Iodate in Mice Is Related to Macrophage Accumulation. Invest Ophthalmol. Vis. Sci. 2018, 59, 3476–3487. [Google Scholar] [CrossRef] [Green Version]
- Latendresse, J.R.; Warbrittion, A.R.; Jonassen, H.; Creasy, D.M. Fixation of testes and eyes using a modified Davidson’s fluid: Comparison with Bouin’s fluid and conventional Davidson’s fluid. Toxicol. Pathol. 2002, 30, 524–533. [Google Scholar] [CrossRef] [PubMed]
- Jia, Y.; Bailey, S.T.; Wilson, D.J.; Tan, O.; Klein, M.L.; Flaxel, C.J.; Potsaid, B.; Liu, J.J.; Lu, C.D.; Kraus, M.F.; et al. Quantitative optical coherence tomography angiography of choroidal neovascularization in age-related macular degeneration. Ophthalmology 2014, 121, 1435–1444. [Google Scholar] [CrossRef] [Green Version]
- Ishibazawa, A.; Nagaoka, T.; Takahashi, A.; Omae, T.; Tani, T.; Sogawa, K.; Yokota, H.; Yoshida, A. Optical Coherence Tomography Angiography in Diabetic Retinopathy: A Prospective Pilot Study. Am. J. Ophthalmol. 2015, 160, 35–44. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Diwanji, N.; Bergmann, A. An unexpected friend-ROS in apoptosis-induced compensatory proliferation: Implications for regeneration and cancer. Semin. Cell Dev. Biol. 2018, 80, 74–82. [Google Scholar] [CrossRef] [PubMed]
- Chan, C.M.; Huang, D.Y.; Sekar, P.; Hsu, S.H.; Lin, W.W. Reactive oxygen species-dependent mitochondrial dynamics and autophagy confer protective effects in retinal pigment epithelial cells against sodium iodate-induced cell death. J. Biomed. Sci. 2019, 26, 40. [Google Scholar] [CrossRef]
- Wnek, E.; Andrzejewska, J.; Kobos, K.; Taran, P. Przewratil. Molecular and immunohistochemical expression of apoptoticproteins Bax, Bcl-2 and Caspase 3 in infantile hemangioma tissues asan effect of propranolol treatment. Immunol. Lett. 2017, 185, 27–31. [Google Scholar] [CrossRef] [PubMed]
- Zhang, X.; Gu, W.; Ma, Z.; Liu, Y.; Ru, H.; Zhou, J.; Zhang, Y.; Xu, Z.; Qian, G. Short-term exposure to ZnO/MCB persistent free radical particles causes mouse lung lesions via inflammatory reactions and apoptosis pathways. Environ. Pollut. 2020, 261, 114039. [Google Scholar] [CrossRef]
- Chamcheu, J.C.; Roy, T.; Uddin, M.B.; Banang-Mbeumi, S.; Chamcheu, R.N.; Walker, A.L.; Liu, Y.Y.; Huang, S. Role and Therapeutic Targeting of the PI3K/Akt/mTOR Signaling Pathway in Skin Cancer: A Review of Current Status and Future Trends on Natural and Synthetic Agents Therapy. Cells 2019, 8, 803. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Yeung, L.; Lima, V.C.; Garcia, P.; Landa, G.; Rosen, R.B. Correlation between spectral domain optical coherence tomography findings and fluorescein angiography patterns in diabetic macular edema. Ophthalmology 2009, 116, 1158–1167. [Google Scholar] [CrossRef]
- Gehrs, K.M.; Anderson, D.H.; Johnson, L.V.; Hageman, G.S. Age-related macular degeneration–emerging pathogenetic and therapeutic concepts. Ann. Med. 2006, 38, 450–471. [Google Scholar] [CrossRef]
- Girmens, J.F.; Sahel, J.A.; Marazova, K. Dry age-related macular degeneration: A currently unmet clinical need. Intractable Rare Dis. Res. 2012, 1, 103–114. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, Y.C.; Horng, L.Y.; Sung, H.C.; Wu, R.T. Sodium iodate disrupted the mitochondrial-lysosomal axis in cultured retinal pigment epithelial cells. J. Ocul. Pharmacol. Ther. 2018, 34, 500–511. [Google Scholar] [CrossRef]
- Zhou, P.; Kannan, R.; Spee, C.; Sreekumar, P.G.; Dou, G.; Hinton, D.R. Protection of retina by alphaB crystallin in sodium iodate induced retinal degeneration. PLoS ONE 2014, 9, e98275. [Google Scholar]
- Jin, H.L.; Choung, S.-Y.; Jeong, K.W. Protective mechanisms of polyphenol-enriched fraction of Vaccinium uliginosum L. Against blue light-induced cell death of human retinal pigmented epithelial cells. J. Funct. Foods 2017, 39, 28–36. [Google Scholar] [CrossRef]
- Yoon, S.M.; Lee, B.L.; Guo, Y.R.; Choung, S.Y. Preventive effect of Vaccinium uliginosum L. extract and its fractions on age-related macular degeneration and its action mechanisms. Arch. Pharmacal. Res. 2016, 39, 21–32. [Google Scholar] [CrossRef]
- Sorsby, A. Experimental pigmentary degeneration of the retina by sodium iodate. Br. J. Ophthalmol. 1941, 25, 58–62. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Machalińska, A.; Lubiński, W.; Kłos, P.; Kawa, M.; Baumert, B.; Krzysztof Penkala Grzegrzółka, R.; Karczewicz, D.; Wiszniewska, B.; Machaliński, B. Sodium iodate selectively injuries the posterior pole of the retina in a dosedependent manner: Morphological and electrophysiological study. Neurochem. Res. 2010, 35, 1819–1827. [Google Scholar] [CrossRef] [Green Version]
- Hariri, S.; Tam, M.C.; Lee, D.; Hileeto, D.; Moayed, A.A.; Bizheva, K. Noninvasive Imaging of the Early Effect of Sodium Iodate Toxicity in a Rat Model of Outer Retina Degeneration With Spectral Domain Optical Coherence Tomography. J. Biomed. Opt. 2013, 18, 26017. [Google Scholar] [CrossRef]
- Jian, Q.; Tao, Z.; Li, Y.; Yin, Z.Q. Acute retinal injury and the relationship between nerve growth factor, Notch1 transcription and short-lived dedifferentiation transient changes of mammalian Müller cells. Vision Res. 2015, 110, 107–117. [Google Scholar] [CrossRef] [Green Version]
- Mones, J.; Leiva, M.; Pena, T.; Martínez, G.; Biarnés, M.; Garcia, M.; Serrano, A.; Fernandez, E. A swine model of selective geographic atrophy of outer retinal layers mimicking atrophic AMD: A phase I escalating dose of subretinal sodium iodate. Investig. Ophthalmol. Vis. Sci. 2016, 57, 3974–3983. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wen, J.J.; Garg, N.J. Manganese superoxide dismutase deficiency exacerbates the mitochondrial ROS production and oxidative damage in Chagas disease. PLoS Negl. Trop. Dis. 2018, 12, e0006687. [Google Scholar] [CrossRef] [PubMed]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Chang, Y.-Y.; Lee, Y.-J.; Hsu, M.-Y.; Wang, M.; Tsou, S.-C.; Chen, C.-C.; Lin, J.-A.; Hsiao, Y.-P.; Lin, H.-W. Protective Effect of Quercetin on Sodium Iodate-Induced Retinal Apoptosis through the Reactive Oxygen Species-Mediated Mitochondrion-Dependent Pathway. Int. J. Mol. Sci. 2021, 22, 4056. https://doi.org/10.3390/ijms22084056
Chang Y-Y, Lee Y-J, Hsu M-Y, Wang M, Tsou S-C, Chen C-C, Lin J-A, Hsiao Y-P, Lin H-W. Protective Effect of Quercetin on Sodium Iodate-Induced Retinal Apoptosis through the Reactive Oxygen Species-Mediated Mitochondrion-Dependent Pathway. International Journal of Molecular Sciences. 2021; 22(8):4056. https://doi.org/10.3390/ijms22084056
Chicago/Turabian StyleChang, Yuan-Yen, Yi-Ju Lee, Min-Yen Hsu, Meilin Wang, Shang-Chun Tsou, Ching-Chung Chen, Jer-An Lin, Yai-Ping Hsiao, and Hui-Wen Lin. 2021. "Protective Effect of Quercetin on Sodium Iodate-Induced Retinal Apoptosis through the Reactive Oxygen Species-Mediated Mitochondrion-Dependent Pathway" International Journal of Molecular Sciences 22, no. 8: 4056. https://doi.org/10.3390/ijms22084056
APA StyleChang, Y.-Y., Lee, Y.-J., Hsu, M.-Y., Wang, M., Tsou, S.-C., Chen, C.-C., Lin, J.-A., Hsiao, Y.-P., & Lin, H.-W. (2021). Protective Effect of Quercetin on Sodium Iodate-Induced Retinal Apoptosis through the Reactive Oxygen Species-Mediated Mitochondrion-Dependent Pathway. International Journal of Molecular Sciences, 22(8), 4056. https://doi.org/10.3390/ijms22084056








