Comparative Transcriptome Analysis Identified Candidate Genes for Late Leaf Spot Resistance and Cause of Defoliation in Groundnut
Abstract
:1. Introduction
2. Results
2.1. RNA Sequencing and Development of Transcriptome Assembly
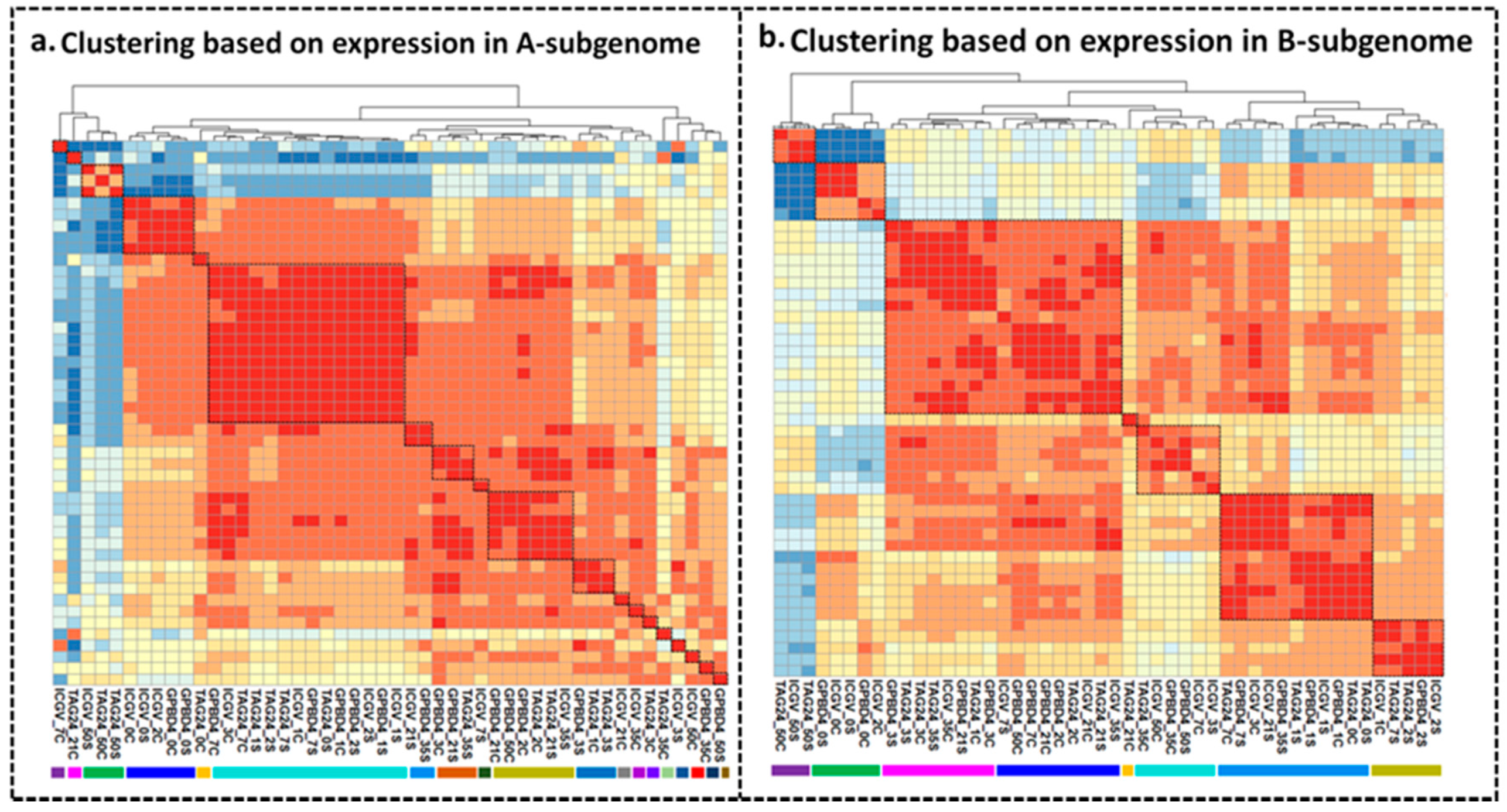
2.2. Genome-Wide Differential Gene Expression Patterns in Resistant and Susceptible Genotypes
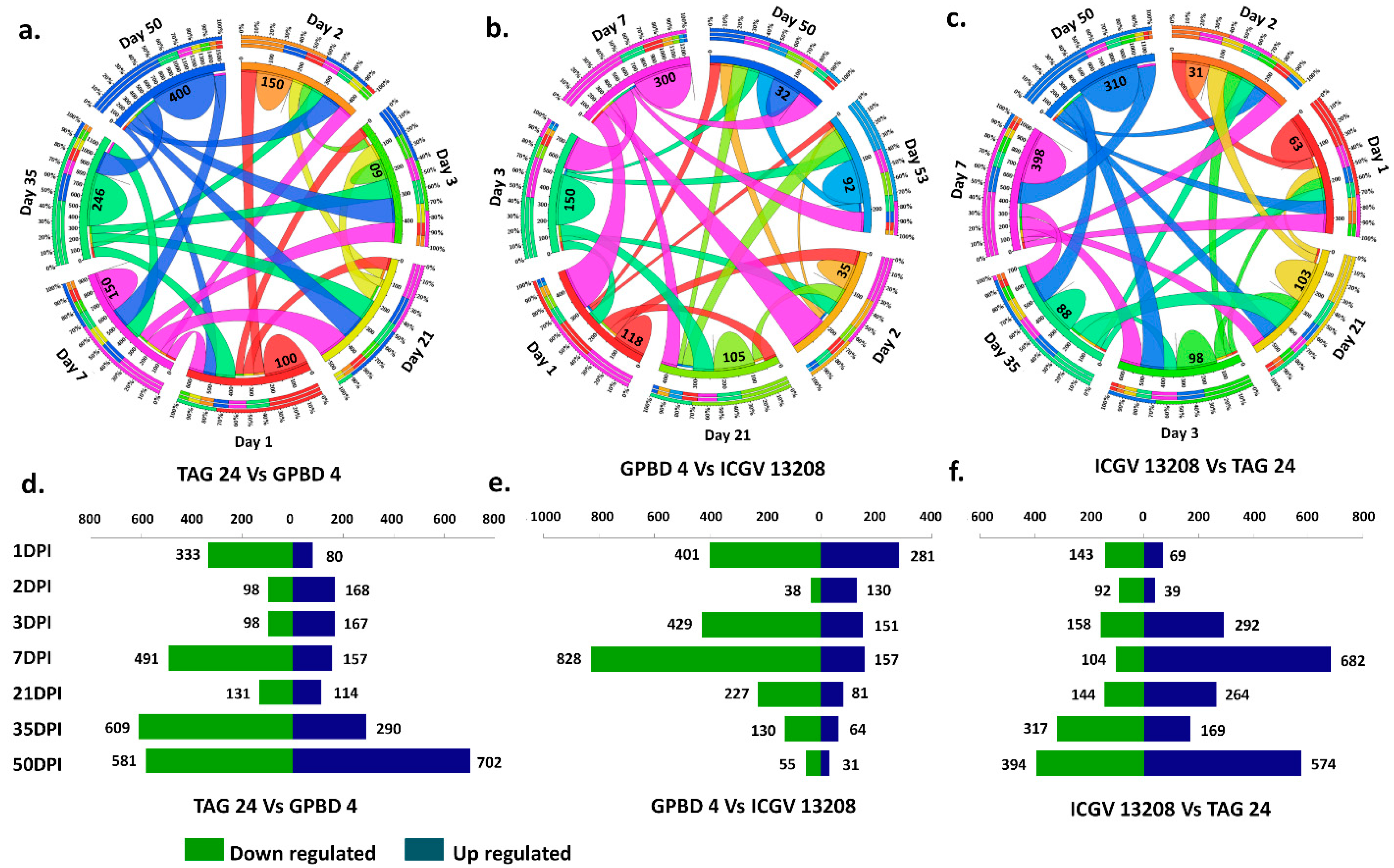
2.3. DEGs Expressed in Each Genotype under Control vs. Stressed
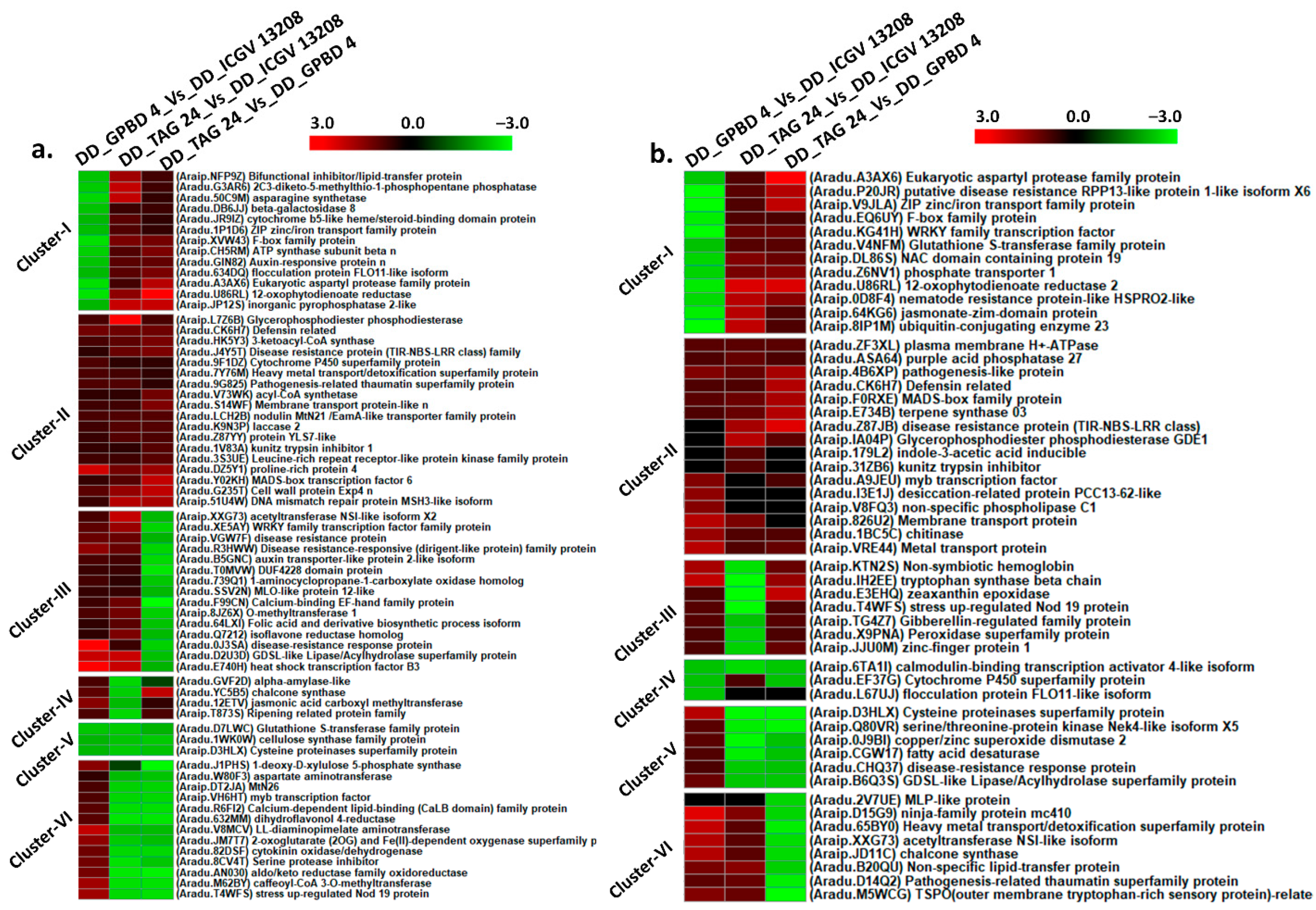
2.4. Differentially Expressed Genes between Resistant and Susceptible Genotypes at Disease Development (DD) Stage
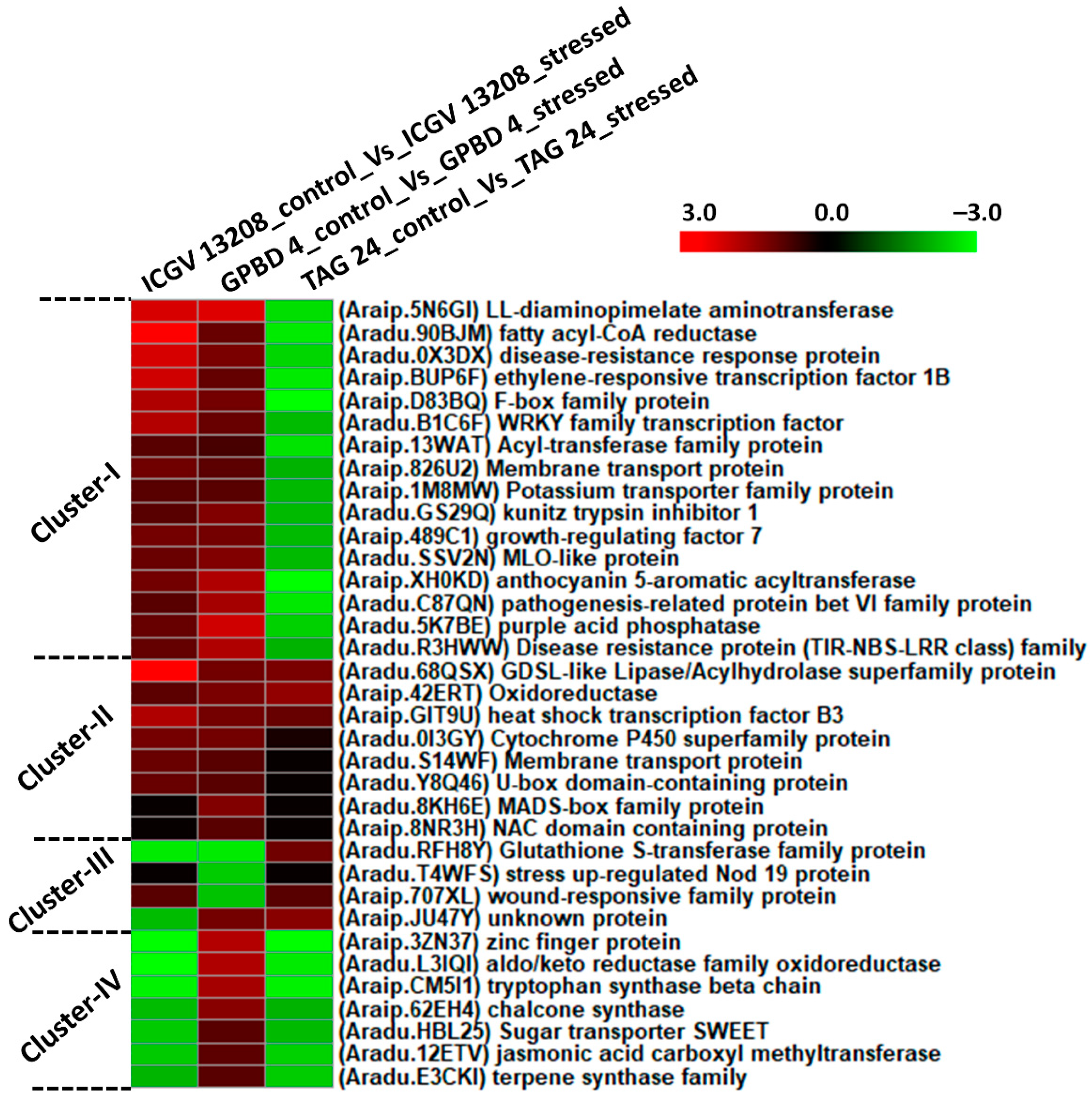
2.5. Differentially Expressed Genes between Resistant and Susceptible Genotypes at Symptom Development (SD) Stage
2.6. Differentially Expressed Genes from Previously Reported QTL Regions for LLS Resistance
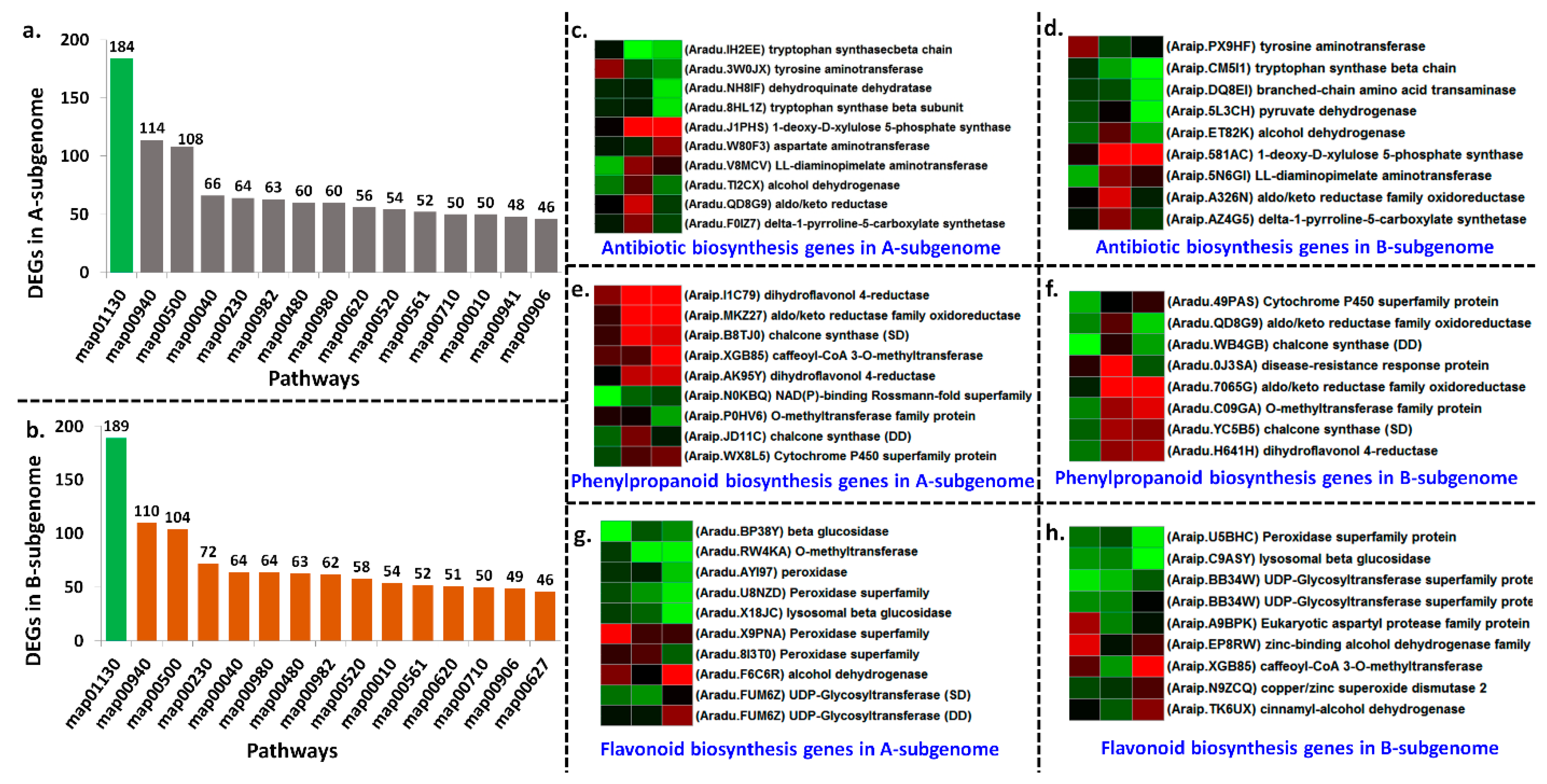
2.7. Gene Annotations, GO (Gene Ontology) Term, and Pathway Analysis
2.8. Joint Pathways Triggered by Homologous Chromosomes in Both Subgenomes upon LLS Infection
2.9. Validation of Differentially Expressed Genes Using qRT-PCR
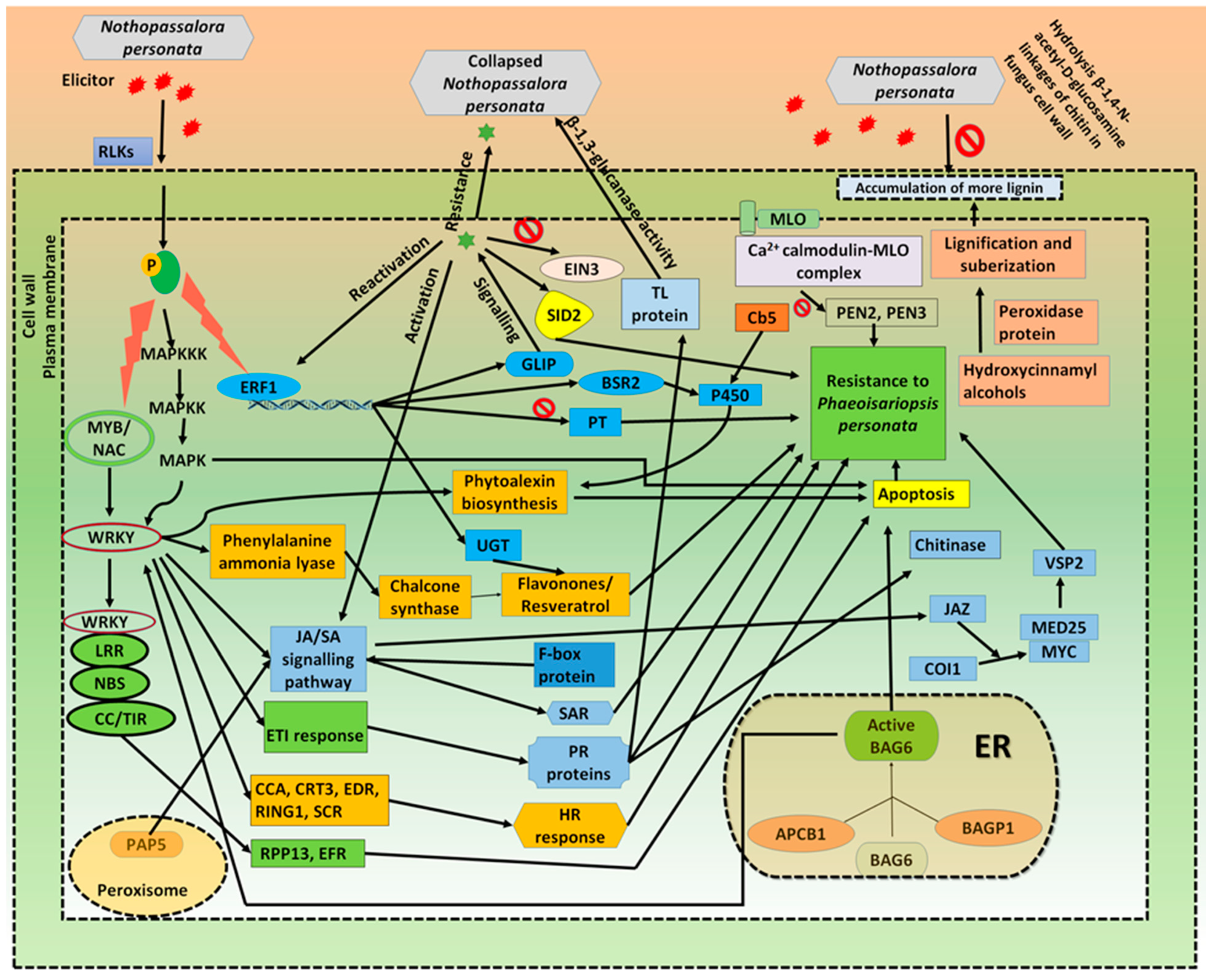
3. Discussion
4. Materials and Methods
4.1. Plant Material and Experimental Conditions
4.2. Inoculation with Spores of N. personata and Sample Collection
4.3. RNA Isolation and Sequencing
4.4. Read Alignment, Transcript Abundance and Gene Expression Analysis
4.5. Clustering and Principle Component Analysis (PCA)
4.6. Quantitative PCR (qPCR) Analysis
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Abbreviations
References
- FAOSTAT. 2019. Available online: http://www.fao.org/faostat/en (accessed on 10 March 2021).
- Bertioli, D.J.; Jenkins, J.; Clevenger, J.; Dudchenko, O.; Gao, D.; Seijo, G.; Leal Bertioli, S.C.; Ren, L.; Farmer, A.D.; Pandey, M.K.; et al. The genome sequence of peanut (Arachis hypogaea), a segmental allotetraploid. Nat. Genet. 2019, 51, 877–884. [Google Scholar] [CrossRef] [Green Version]
- Chen, X.; Lu, Q.; Liu, H.; Zhang, J.; Hong, Y.; Lan, H.; Li, H.; Wang, J.; Liu, H.; Li, S.; et al. Sequencing of cultivated peanut, Arachis hypogaea, yields insights into genome evolution and oil improvement. Mol. Plant. 2019, 12, 920–934. [Google Scholar] [CrossRef] [Green Version]
- Zhuang, W.; Chen, H.; Yang, M.; Wang, J.; Pandey, M.K.; Zhang, C.; Chang, W.C.; Zhang, L.; Zhang, X.; Tang, R.; et al. The genome of cultivated peanut provides insight into legume karyotypes, polyploid evolution and crop domestication. Nat. Genet. 2019, 51, 865–876. [Google Scholar] [CrossRef]
- Arya, S.S.; Salve, A.R.; Chauhan, S. Peanuts as functional food: A review. J. Food Sci. Technol. 2016, 53, 31–41. [Google Scholar] [CrossRef] [Green Version]
- Varshney, R.K.; Mohan, S.M.; Gaur, P.M.; Gangarao, N.V.; Pandey, M.K.; Bohra, A.; Sawargaonkar, S.L.; Chitikineni, A.; Kimurto, P.K.; Janila, P.; et al. Achievements and prospects of genomics-assisted breeding in three legume crops of the semi-arid tropics. Biotechnol. Adv. 2013, 31, 1120–1134. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.K.; Khan, A.W.; Singh, V.K.; Vishwakarma, M.K.; Shasidhar, Y.; Kumar, V.; Garg, V.; Bhat, R.S.; Chitikineni, A.; Janila, P.; et al. QTL-seq approach identified genomic regions and diagnostic markers for rust and late leaf spot resistance in groundnut (Arachis hypogaea L.). Plant Biotechnol. J. 2017, 15, 927–941. [Google Scholar] [CrossRef] [Green Version]
- Gangurde, S.S.; Kumar, R.; Pandey, A.K.; Burow, M.; Laza, H.E.; Nayak, S.N.; Guo, B.; Liao, B.; Bhat, R.S.; Madhuri, N.; et al. Climate-smart groundnuts for achieving high productivity and improved quality: Current status, challenges and opportunities. In Genomic Designing of Climate-Smart Oilseed Crops; Springer: Cham, Switzerland, 2019; pp. 133–172. [Google Scholar]
- Shasidhar, Y.; Variath, M.T.; Vishwakarma, M.K.; Manohar, S.S.; Gangurde, S.S.; Sriswathi, M.; Sudini, H.K.; Dobariya, K.L.; Bera, S.K.; Radhakrishnan, T.; et al. Improvement of three popular Indian groundnut varieties for foliar disease resistance and high oleic acid using SSR markers and SNP array in marker-assisted backcrossing. Crop J. 2020, 8, 1–15. [Google Scholar] [CrossRef]
- Mallikarjuna, N.; Srikanth, S.; Vellanki, R.K.; Jadhav, D.R.; Das, K.; Upadhyaya, H.D. Meiotic analysis of the hybrids between cultivated and synthetic tetraploid groundnuts. Plant Breed. 2012, 131, 135–138. [Google Scholar] [CrossRef] [Green Version]
- Subrahmanyam, P.; Rao, V.R.; McDonald, D.; Moss, J.; Gibbons, R. Origins of resistances to rust and late leaf spot in peanut (Arachis hypogaea, Fabaceae). Econ. Bot. 1989, 43, 444–455. [Google Scholar] [CrossRef] [Green Version]
- Waliyar, F. Evaluation of yield losses due to groundnut leaf diseases in West Africa. In Proceedings of the Second ICRISAT Regional Groundnut Meeting for West Africa, Niamey, Niger, 11–14 September 1990; p. 1114. [Google Scholar]
- Nath, B.C.; Singh, J.P.; Srivastava, S.; Singh, R.B. Management of late leaf spot of groundnut by different fungicides and their impact on yield. J. Plant Pathol. 2013, 12, 85–91. [Google Scholar]
- Cycoń, M.; Piotrowska-Seget, Z.; Kaczyńska, A.; Kozdrój, J. Microbiological characteristics of a sandy loam soil exposed to tebuconazole and λ-cyhalothrin under laboratory conditions. Ecotoxicology 2006, 15, 639–646. [Google Scholar] [CrossRef] [PubMed]
- Muñoz-Leoz, B.; Ruiz-Romera, E.; Antigüedad, I.; Garbisu, C. Tebuconazole application decreases soil microbial biomass and activity. Soil Biol. Biochem. 2011, 43, 2176–2183. [Google Scholar] [CrossRef]
- Gowda, M.; Motagi, B.; Naidu, G.; Diddimani, S.; Sheshagiri, R. GPBD 4: A Spanish bunch groundnut genotype resistant to rust and late leaf spot. Int. Arachis Newsltr. 2002, 22, 29–32. [Google Scholar]
- Subrahmanyam, P.; McDonald, D.; Gibbons, R.; Nigam, S.; Nevill, D. Resistance to rust and late leafspot diseases in some genotypes of Arachis hypogaea. Peanut Sci. 1982, 9, 6–10. [Google Scholar] [CrossRef] [Green Version]
- Sharma, S.; Pandey, M.K.; Sudini, H.K.; Upadhyaya, H.D.; Varshney, R.K. Harnessing genetic diversity of wild Arachis species for genetic enhancement of cultivated peanut. Crop Sci. 2017, 57, 1121–1131. [Google Scholar] [CrossRef] [Green Version]
- Khedikar, Y.; Gowda, M.; Sarvamangala, C.; Patgar, K.; Upadhyaya, H.; Varshney, R.K. A QTL study on late leaf spot and rust revealed one major QTL for molecular breeding for rust resistance in groundnut (Arachis hypogaea L.). Theor. Appl. Genet. 2010, 121, 971–984. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sujay, V.; Gowda, M.V.; Pandey, M.K.; Bhat, R.S.; Khedikar, Y.P.; Nadaf, H.L.; Gautami, B.; Sarvamangala, C.; Lingaraju, S.; Radhakrishan, T.; et al. Quantitative trait locus analysis and construction of consensus genetic map for foliar disease resistance based on two recombinant inbred line populations in cultivated groundnut (Arachis hypogaea L.). Mol. Breed. 2012, 30, 773–788. [Google Scholar] [CrossRef] [Green Version]
- Pandey, M.K.; Agarwal, G.; Kale, S.M.; Clevenger, J.; Nayak, S.N.; Sriswathi, M.; Chitikineni, A.; Chavarro, C.; Chen, X.; Upadhyaya, H.D.; et al. Development and evaluation of a high density genotyping ‘Axiom_Arachis’ array with 58 k SNPs for accelerating genetics and breeding in groundnut. Sci. Rep. 2017, 7, 1–10. [Google Scholar] [CrossRef]
- Shirasawa, K.; Bhat, R.S.; Khedikar, Y.P.; Sujay, V.; Kolekar, R.M.; Yeri, S.B.; Sukruth, M.; Cholin, S.; Asha, B.; Pandey, M.K. Sequencing analysis of genetic loci for resistance for late leaf spot and rust in peanut (Arachis hypogaea L.). Front. Plant Sci. 2018, 9, 1727. [Google Scholar] [CrossRef] [Green Version]
- Agarwal, G.; Clevenger, J.; Pandey, M.K.; Wang, H.; Shasidhar, Y.; Chu, Y.; Fountain, J.C.; Choudhary, D.; Culbreath, A.K.; Liu, X.; et al. High-density genetic map using whole-genome resequencing for fine mapping and candidate gene discovery for disease resistance in peanut. Plant Biotechnol. J. 2018, 16, 1954–1967. [Google Scholar] [CrossRef] [Green Version]
- Kolekar, R.M.; Sukruth, M.; Shirasawa, K.; Nadaf, H.L.; Motagi, B.N.; Lingaraju, S.; Patil, P.V.; Bhat, R.S. Marker-assisted backcrossing to develop foliar disease-resistant genotypes in TMV 2 variety of peanut (Arachis hypogaea L.). Plant Breed. 2017, 136, 948–953. [Google Scholar] [CrossRef]
- Yeri, S.B.; Bhat, R.S. Development of late leaf spot and rust resistant backcross lines in JL 24 variety of groundnut (Arachis hypogaea L.). Electron. J. Plant Breed. 2016, 7, 37–41. [Google Scholar] [CrossRef]
- Deshmukh, D.; Marathi, B.; Sudini, H.K.; Variath, M.T.; Chaudhari, S.; Manohar, S.S.; Rani, C.V.D.; Pandey, M.K.; Pasupuleti, J. Combining high oleic acid trait and resistance to late leaf spot and rust diseases in groundnut (Arachis hypogaea L.). Front. Genet. 2020, 11, 514. [Google Scholar] [CrossRef]
- Kudapa, H.; Garg, V.; Chitikineni, A.; Varshney, R.K. The RNA-Seq-based high resolution gene expression atlas of chickpea (Cicer arietinum L.) reveals dynamic spatio-temporal changes associated with growth and development. Plant Cell Environ. 2018, 41, 2209–2225. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rathod, V.; Hamid, R.; Tomar, R.S.; Patel, R.; Padhiyar, S.; Kheni, J.; Thirumalaisamy, P.P.; Munshi, N.S. Comparative RNA-Seq profiling of a resistant and susceptible peanut (Arachis hypogaea) genotypes in response to leaf rust infection caused by Puccinia arachidis. 3 Biotech 2020, 10, 1–15. [Google Scholar] [CrossRef] [PubMed]
- Rathod, V.; Hamid, R.; Tomar, R.S.; Padhiyar, S.; Kheni, J.; Thirumalaisamy, P.; Munshi, N.S. Peanut (Arachis hypogaea) transcriptome revealed the molecular interactions of the defense mechanism in response to early leaf spot fungi (Cercospora arachidicola). Plant Gene 2020, 23, 100243. [Google Scholar] [CrossRef]
- Varshney, R.K.; Pandey, M.K.; Janila, P.; Nigam, S.N.; Sudini, H.; Gowda, M.; Sriswathi, M.; Radhakrishnan, T.; Manohar, S.S.; Nagesh, P. Marker-assisted introgression of a QTL region to improve rust resistance in three elite and popular varieties of peanut (Arachis hypogaea L.). Theor. Appl. Genet. 2014, 127, 1771–1781. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Shoba, D.; Manivannan, N.; Vindhiyavarman, P.; Nigam, S. SSR markers associated for late leaf spot disease resistance by bulked segregant analysis in groundnut (Arachis hypogaea L.). Euphytica 2012, 188, 265–272. [Google Scholar] [CrossRef] [Green Version]
- Clevenger, J.; Chu, Y.; Chavarro, C.; Botton, S.; Culbreath, A.; Isleib, T.G.; Holbrook, C.C.; Ozias-Akins, P. Mapping late leaf spot resistance in peanut (Arachis hypogaea) using QTL-seq reveals markers for marker-assisted selection. Front. Plant Sci. 2018, 9, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chen, X.; Li, H.; Pandey, M.K.; Yang, Q.; Wang, X.; Garg, V.; Li, H.; Chi, X.; Doddamani, D.; Hong, Y.; et al. Draft genome of the peanut A-genome progenitor (Arachis duranensis) provides insights into geocarpy, oil biosynthesis, and allergens. Proc. Natl. Acad. Sci. USA 2016, 113, 6785–6790. [Google Scholar] [CrossRef] [Green Version]
- Bertioli, D.J.; Cannon, S.B.; Froenicke, L.; Huang, G.; Farmer, A.D.; Cannon, E.K.; Liu, X.; Gao, D.; Clevenger, J.; Dash, S.; et al. The genome sequences of Arachis duranensis and Arachis ipaensis, the diploid ancestors of cultivated peanut. Nat. Genet. 2016, 48, 438–446. [Google Scholar] [CrossRef] [PubMed]
- Damicone, J.; Melouk, H.A. Foliar Diseases of Peanut; Oklahoma State University: Stillwater, OK, USA, 2010. [Google Scholar]
- Power, I.L.; Tillman, B.L.; Brenneman, T.B.; Kemerait, R.C.; Stevenson, K.L.; Culbreath, A.K. Field evaluation and components of peanut rust resistance of newly developed breeding lines. Peanut Sci. 2019, 46, 22–36. [Google Scholar] [CrossRef]
- Koyama, T. The roles of ethylene and transcription factors in the regulation of onset of leaf senescence. Front. Plant Sci. 2014, 5, 650. [Google Scholar] [CrossRef] [PubMed]
- Koyama, T.; Nii, H.; Mitsuda, N.; Ohta, M.; Kitajima, S.; Ohme-Takagi, M.; Ato, F. A regulatory cascade involving class II Ethylene Response Factor transcriptional repressors operates in the progression of leaf senescence. Plant Physiol. 2013, 162, 991–1005. [Google Scholar] [CrossRef] [Green Version]
- Kao, C.H.; Yang, S.F. Role of ethylene in the senescence of detached rice leaves. Plant Physiol. 1983, 73, 881–885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- John, I.; Drake, R.; Farrell, A.; Cooper, W.; Lee, P.; Horton, P.; Grierson, D. Delayed leaf senescence in ethylene-deficient ACC-oxidase antisense tomato plants: Molecular and physiological analysis. Plant J. 1995, 7, 483–490. [Google Scholar] [CrossRef]
- Bi, G.; Zhou, Z.; Wang, W.; Li, L.; Rao, S.; Wu, Y.; Zhang, X.; Menke, F.L.; Chen, S.; Zhou, J.M. Receptor-like cytoplasmic kinases directly link diverse pattern recognition receptors to the activation of mitogen-activated protein kinase cascades in Arabidopsis. Plant Cell 2018, 30, 1543–1561. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meng, X.; Zhang, S. MAPK cascades in plant disease resistance signalling. Annu. Rev. Phytopathol. 2013, 51, 245–266. [Google Scholar] [CrossRef]
- Li, J.; Brader, G.; Kariola, T.; Tapio, P.E. WRKY70 modulates the selection of signaling pathways in plant defense. Plant J. 2006, 46, 477–491. [Google Scholar] [CrossRef] [PubMed]
- Zou, B.; Jia, Z.; Tian, S.; Wang, X.; Gou, Z.; Lü, B.; Dong, H. AtMYB44 positively modulates disease resistance to Pseudomonas syringae through the salicylic acid signalling pathway in Arabidopsis. Funct. Plant Biol. 2013, 40, 304–313. [Google Scholar] [CrossRef]
- Shen, Q.H.; Saijo, Y.; Mauch, S.; Biskup, C.; Bieri, S.; Keller, B.; Seki, H.; Ülker, B.; Somssich, I.E.; Schulze-Lefert, P. Nuclear activity of MLA immune receptors links isolate-specific and basal disease-resistance responses. Sci. J. 2007, 315, 1098–1103. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Grant, M.; Lamb, C. Systemic immunity. Curr. Opin. Plant Biol. 2006, 9, 414–420. [Google Scholar] [CrossRef]
- Kim, H.G.; Kwon, S.J.; Jang, Y.J.; Chung, J.H.; Nam, M.H.; Park, O.K. GDSL lipase 1 regulates ethylene signalling and ethylene-associated systemic immunity in Arabidopsis. FEBS Lett. 2014, 588, 1652–1658. [Google Scholar] [CrossRef] [Green Version]
- Yang, J.; Duan, G.; Li, C.; Liu, L.; Han, G.; Zhang, Y.; Wang, C. The crosstalks between jasmonic acid and other plant hormone signaling highlight the involvement of jasmonic acid as a core component in plant response to biotic and abiotic stresses. Front. Plant Sci. 2019, 10, 1349. [Google Scholar] [CrossRef] [Green Version]
- Maeda, S.; Dubouzet, J.G.; Kondou, Y.; Yusuke, J.; Shigemi, S.; Kenji, O.; Minami, M.; Hirohiko, H.; Masaki, M. The rice CYP78A gene BSR2 confers resistance to Rhizoctonia solani and affects seed size and growth in Arabidopsis and rice. Sci. Rep. 2019, 9, 1–14. [Google Scholar] [CrossRef] [Green Version]
- Caputi, L.; Malnoy, M.; Goremykin, V.; Nikiforova, S.; Martens, S. A genome-wide phylogenetic reconstruction of family 1 UDP-glycosyltransferases revealed the expansion of the family during the adaptation of plants to life on land. Plant J. 2012, 69, 1030–1042. [Google Scholar] [CrossRef] [PubMed]
- Cao, Y.; Yang, Y.; Zhang, H.; Li, D.; Zheng, Z.; Song, F. Overexpression of a rice defense-related F-box protein gene OsDRF1 in tobacco improves disease resistance through potentiation of defense gene expression. Physiol. Plant. 2008, 134, 440–452. [Google Scholar] [CrossRef]
- Lipka, V.; Dittgen, J.; Bednarek, P.; Bhat, R.; Wiermer, M.; Stein, M.; Landtag, J.; Brandt, W.; Rosahl, S.; Scheel, D.; et al. Pre- and post-invasion defenses both contribute to nonhost resistance in Arabidopsis. J. Sci. 2005, 310, 1180–1183. [Google Scholar]
- Stein, M.; Dittgen, J.; Sánchez-Rodríguez, C.; Hou, B.H.; Molina, A.; Schulze-Lefert, P.; Lipka, V.; Somerville, S. Arabidopsis PEN3/PDR8, an ATP binding cassette transporter, contributes to nonhost resistance to inappropriate pathogens that enter by direct penetration. Plant Cell 2006, 18, 731–746. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Chittoor, J.M.; Leach, J.E.; White, F.F. Induction of peroxidase during defense against pathogens. In Pathogenesis-Related Proteins in Plants; Datta, S.K., Muthukrishnan, S., Eds.; CRC Press: Boca Raton, FL, USA, 1999; p. 291. [Google Scholar]
- Chamnongpol, S.; Willekens, H.; Moeder, W.; Langebartels, C.; Sandermann, H.; Van Montagu, A.; Inze, D.; Van Camp, W. Defense activation and enhanced pathogen tolerance induced by H2O2 in transgenic tobacco. Proc. Natl. Acad. Sci. USA 1998, 95, 5818–5823. [Google Scholar] [CrossRef] [Green Version]
- Li, Y.; Kabbage, M.; Liu, W.; Dickman, M.B. Aspartyl protease-mediated cleavage of BAG6 is necessary for autophagy and fungal resistance in plants. Plant Cell 2016, 28, 233–247. [Google Scholar] [CrossRef] [Green Version]
- Grenier, J.; Potvin, C.; Trudel, J.; Asselin, A. Some thaumatin-like proteins hydrolyse polymeric β-1,3-glucans. Plant J. 1999, 19, 473–480. [Google Scholar] [CrossRef]
- Luo, Z.; Cui, R.; Chavarro, C.; Tseng, Y.C.; Zhou, H.; Peng, Z.; Chu, Y.; Yang, X.; Lopez, Y.; Tillman, B.; et al. Mapping quantitative trait loci (QTLs) and estimating the epistasis controlling stem rot resistance in cultivated peanut (Arachis hypogaea). Theor. Appl. Genet. 2020, 133, 1201–1212. [Google Scholar] [CrossRef] [PubMed]
- Katta, M.A.; Khan, A.W.; Doddamani, D.; Thudi, M.; Varshney, R.K. NGS-QCbox and raspberry for parallel, automated and rapid quality control analysis of large-scale next generation sequencing (Illumina) data. PLoS ONE 2015, 10, e0139868. [Google Scholar] [CrossRef] [Green Version]
- Trapnell, C.; Pachter, L.; Salzberg, S.L. TopHat: Discovering splice junctions with RNA-Seq. J. Bioinform. 2009, 25, 1105–1111. [Google Scholar] [CrossRef]
- Trapnell, C.; Williams, B.A.; Pertea, G.; Mortazavi, A.; Kwan, G.; van Baren, M.J.; Salzberg, S.L.; Wold, B.J.; Pachter, L. Transcript assembly and quantification by RNA-Seq reveals unannotated transcripts and isoform switching during cell differentiation. Nat. Biotechnol. 2010, 28, 511–515. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Trapnell, C.; Roberts, A.; Goff, L.; Pertea, G.; Kim, D.; Kelley, D.R.; Pimentel, H.; Salzberg, S.L.; Rinn, J.L.; Pachter, L. Differential gene and transcript expression analysis of RNA-seq experiments with TopHat and Cufflinks. Nat. Protoc. 2012, 7, 562–578. [Google Scholar] [CrossRef] [Green Version]
- Benjamini, Y.; Hochberg, Y. Controlling the false discovery rate: A practical and powerful approach to multiple testing. J. R. Stat. Soc. Ser. B 1995, 57, 289–300. [Google Scholar] [CrossRef]
- Kolde, R. Pheatmap: Pretty Heatmaps; R Package Version; R Foundation for Statistical Computing: Vienna, Austria, 2012; Volume 1, p. 2. [Google Scholar]
- Brand, Y.; Hovav, R. Identification of suitable internal control genes for quantitative real-time PCR expression analyses in peanut (Arachis hypogaea). Peanut Sci. 2010, 37, 12–19. [Google Scholar] [CrossRef]
- Livak, K.J.; Schmittgen, T. Analysis of relative gene expression data using real-time quantitative PCR and the 2-[Delta][Delta] CT method. Methods 2001, 25, 402–408. [Google Scholar] [CrossRef]




Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Gangurde, S.S.; Nayak, S.N.; Joshi, P.; Purohit, S.; Sudini, H.K.; Chitikineni, A.; Hong, Y.; Guo, B.; Chen, X.; Pandey, M.K.; et al. Comparative Transcriptome Analysis Identified Candidate Genes for Late Leaf Spot Resistance and Cause of Defoliation in Groundnut. Int. J. Mol. Sci. 2021, 22, 4491. https://doi.org/10.3390/ijms22094491
Gangurde SS, Nayak SN, Joshi P, Purohit S, Sudini HK, Chitikineni A, Hong Y, Guo B, Chen X, Pandey MK, et al. Comparative Transcriptome Analysis Identified Candidate Genes for Late Leaf Spot Resistance and Cause of Defoliation in Groundnut. International Journal of Molecular Sciences. 2021; 22(9):4491. https://doi.org/10.3390/ijms22094491
Chicago/Turabian StyleGangurde, Sunil S., Spurthi N. Nayak, Pushpesh Joshi, Shilp Purohit, Hari K. Sudini, Annapurna Chitikineni, Yanbin Hong, Baozhu Guo, Xiaoping Chen, Manish K. Pandey, and et al. 2021. "Comparative Transcriptome Analysis Identified Candidate Genes for Late Leaf Spot Resistance and Cause of Defoliation in Groundnut" International Journal of Molecular Sciences 22, no. 9: 4491. https://doi.org/10.3390/ijms22094491








