Multivariate Optimization of the FLC-dc-APGD-Based Reaction-Discharge System for Continuous Production of a Plasma-Activated Liquid of Defined Physicochemical and Anti-Phytopathogenic Properties
Abstract
:1. Introduction
2. Results and Discussion
2.1. Response Surface Regression Models Describing the Effect of the Operating Parameters of the FLC-dc-APGD System on Electrical Conductivity of the Resultant NH4NO3-Based PAL
2.2. Selection of the Optimal Experimental Conditions for Production of the NH4NO3-Based PALs of the Highest Electrical Conductivity
2.3. Antibacterial Action Against Plant Pathogens of 0.5% NH4NO3 Treated in the Optimized FLC-dc-APGD System
2.4. Examination of Interactions and Processes Leading to Acquisition of NH4NO3-Based PAL of the Defined Physicochemical and Antibacterial Properties
3. Materials and Methods
3.1. Reagents and Solutions
3.2. Production of the Plasma-Treated Liquid by FLC-dc-APGD
3.3. Multiparameter Optimization of the Operating Parameters of FLC-dc-APGD System
3.4. Assessment of Antibacterial Properties of 0.5% NH4NO3 Activated in the Optimized FLC-dc-APGD System
3.5. Plasma Reactive Species Responsible for the Antibacterial Action of the PAL
4. Conclusions
5. Patents
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Acknowledgments
Conflicts of Interest
Abbreviations
| ANOVA | analysis of variance |
| BBD | Box-Behnken Design |
| D | composite desirability |
| DBD | dielectric-barrier discharge |
| dc-APGD | direct current atmospheric pressure glow discharge |
| DOE | design of experiments |
| FLC | flowing liquid cathode |
| GRAS | generally regarded as safe |
| MBC | minimal bactericidal concentration |
| McF | McFarland |
| MIC | minimal inhibitory concentration |
| NTAP | non-thermal atmospheric pressure plasma |
| OES | optical emission spectrometry |
| OFAT | one-factor-at-time |
| PAL | plasma-activated liquid |
| PAW | plasma-activated water |
| RONS | reactive oxygen and nitrogen species |
| ROS | reactive oxygen species |
| RNS | reactive nitrogen species |
| RSM | response surface methodology |
| SRP | soft rot Pectobacteriaceae |
| TSA | Trypticase Soy Agar |
| TSB | Trypticase Soy Broth |
References
- Kannan, V.R.; Bastas, K.K.; Antony, R. Plant pathogenic bacteria. An overview. In Sustainable Approaches to Controlling Plant Pathogenic Bacteria; Kannan, V.R., Bastas, K.K., Eds.; CRC Press: Boca Raton, FL, USA, 2015; pp. 1–16. ISBN 978-1-4822-4054-2. [Google Scholar]
- Adeolu, M.; Alnajar, S.; Naushad, S.; Gupta, R.S. Genome-based phylogeny and taxonomy of the ‘Enterobacteriales’: Proposal for Enterobacterales ord. nov. divided into the families Enterobacteriaceae, Erwiniaceae fam. nov., Pectobacteriaceae fam. nov., Yersiniaceae fam. nov., Hafniaceae fam. nov., Morganellaceae fam. nov., and Budviciaceae fam. nov. Int. J. Syst. Evol. Microbiol. 2016, 66, 5575–5599. [Google Scholar] [CrossRef] [PubMed]
- Mansfield, J.; Genin, S.; Magori, S.; Citovsky, V.; Sriariyanum, M.; Ronald, P.; Dow, M.; Verdier, V.; Beer, S.V.; Machado, M.A.; et al. Top 10 plant pathogenic bacteria in molecular plant pathology. Mol. Plant Pathol. 2012, 13, 614–629. [Google Scholar] [CrossRef] [Green Version]
- Motyka, A.; Zoledowska, S.; Sledz, W.; Lojkowska, E. Molecular methods as tools to control plant diseases caused by Dickeya and Pectobacterium spp: A minireview. N. Biotechnol. 2017, 39, 181–189. [Google Scholar] [CrossRef] [PubMed]
- Zoledowska, S.; Motyka, A.; Zukowska, D.; Sledz, W.; Lojkowska, E. Population structure and biodiversity of Pectobacterium parmentieri isolated from potato fields in temperate climate. Plant Dis. 2018, 102, 154–164. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Potrykus, M.; Golanowska, M.; Sledz, W.; Zoledowska, S.; Motyka, A.; Kolodziejska, A.; Butrymowicz, J.; Lojkowska, E. Biodiversity of Dickeya spp. isolated from potato plants and water sources in temperate climate. Plant Dis. 2016, 100, 408–417. [Google Scholar] [CrossRef] [Green Version]
- Toth, I.K.; van der Wolf, J.M.; Saddler, G.; Lojkowska, E.; Helias, V.; Pirhonen, M.; Tsror (Lahkim), L.; Elphinstone, J.G. Dickeya species: An emerging problem for potato production in Europe. Plant Pathol. 2011, 60, 385–399. [Google Scholar] [CrossRef]
- Perombelon, M.C.M. Potato diseases caused by soft rot erwinias: An overview of pathogenesis. Plant Pathol. 2002, 51, 1–12. [Google Scholar] [CrossRef]
- Perombelon, M.C.M.; Kelman, A. Ecology of the soft rot Erwinias. Annu. Rev. Phytopathol. 1980, 18, 361–387. [Google Scholar] [CrossRef]
- Van Gijsegem, F.; Toth, I.K.; van der Wolf, J.M. Outlook—Challenges and perspectives for management of diseases caused by Pectobacterium and Dickeya species. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Van Gijsegem, F., van der Wolf, J.M., Toth, I.K., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 283–289. ISBN 978-3-030-61459-1. [Google Scholar]
- Van der Wolf, J.M.; Acuña, I.; De Boer, S.H.; Brurberg, M.B.; Cahill, G.; Charkowski, A.O.; Coutinho, T.; Davey, T.; Dees, M.W.; Degefu, Y.; et al. Diseases caused by Pectobacterium and Dickeya species around the world. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Van Gijsegem, F., van der Wolf, J.M., Toth, I.K., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 215–262. ISBN 978-3-030-61458-4. [Google Scholar]
- Dupuis, B.; Nkuriyingoma, P.; Van Gijsegem, F. Economic impact of Pectobacterium and Dickeya species on potato crops: A review and case study. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Van Gijsegem, F., van der Wolf, J.M., Toth, I.K., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 263–282. ISBN 978-3-030-61458-4. [Google Scholar]
- Czajkowski, R.; Pérombelon, M.C.M.; van Veen, J.A.; van der Wolf, J.M. Control of blackleg and tuber soft rot of potato caused by Pectobacterium and Dickeya species: A review. Plant Pathol. 2011, 60, 999–1013. [Google Scholar] [CrossRef]
- Van der Wolf, J.M.; De Boer, S.H.; Czajkowski, R.; Cahill, G.; Van Gijsegem, F.; Davey, T.; Dupuis, B.; Ellicott, J.; Jafra, S.; Kooman, M.; et al. Management of diseases caused by Pectobacterium and Dickeya species. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Van Gijsegem, F., van der Wolf, J.M., Toth, I.K., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 175–214. ISBN 978-3-030-61458-4. [Google Scholar]
- Braný, D.; Dvorská, D.; Halašová, E.; Škovierová, H. Cold Atmospheric Plasma: A Powerful Tool for Modern Medicine. Int. J. Mol. Sci. 2020, 21, 2932. [Google Scholar] [CrossRef] [Green Version]
- Lackmann, J.W.; Bandow, J.E. Inactivation of microbes and macromolecules by atmospheric-pressure plasma jets. Appl. Microbiol. Biotechnol. 2014, 98, 6205–6213. [Google Scholar] [CrossRef] [PubMed]
- Thirumdas, R.; Kothakota, A.; Annapure, U.; Siliveru, K.; Blundell, R.; Gatt, R.; Valdramidis, V.P. Plasma activated water (PAW): Chemistry, physico-chemical properties, applications in food and agriculture. Trends Food Sci. Technol. 2018, 77, 21–31. [Google Scholar] [CrossRef]
- Gorbanev, Y.; Privat-Maldonado, A.; Bogaerts, A. Analysis of Short-Lived Reactive Species in Plasma-Air-Water Systems: The Dos and the Do Nots. Anal. Chem. 2018, 90, 13151–13158. [Google Scholar] [CrossRef] [Green Version]
- Zhang, Q.; Liang, Y.; Feng, H.; Ma, R.; Tian, Y.; Zhang, J.; Fang, J. A study of oxidative stress induced by non-thermal plasma-activated water for bacterial damage. Appl. Phys. Lett. 2013, 102, 203701. [Google Scholar] [CrossRef]
- Traylor, M.J.; Pavlovich, M.J.; Karim, S.; Hait, P.; Sakiyama, Y.; Clark, D.S.; Graves, D.B. Long-term antibacterial efficacy of air plasma-activated water. J. Phys. D. Appl. Phys. 2011, 44, 472001. [Google Scholar] [CrossRef] [Green Version]
- Chen, T.-P.; Su, T.-L.; Liang, J. Plasma-Activated Solutions for Bacteria and Biofilm Inactivation. Curr. Bioact. Compd. 2017, 13, 59–65. [Google Scholar] [CrossRef]
- Bălan, G.G.; Roşca, I.; Ursu, E.L.; Doroftei, F.; Bostănaru, A.C.; Hnatiuc, E.; Năstasă, V.; Şandru, V.; Ştefănescu, G.; Trifan, A.; et al. Plasma-activated water: A new and effective alternative for duodenoscope reprocessing. Infect. Drug Resist. 2018, 11, 727–733. [Google Scholar] [CrossRef] [Green Version]
- Ma, R.; Wang, G.; Tian, Y.; Wang, K.; Zhang, J.; Fang, J. Non-thermal plasma-activated water inactivation of food-borne pathogen on fresh produce. J. Hazard. Mater. 2015, 300, 643–651. [Google Scholar] [CrossRef] [PubMed]
- Bai, Y.; Idris Muhammad, A.; Hu, Y.; Koseki, S.; Liao, X.; Chen, S.; Ye, X.; Liu, D.; Ding, T. Inactivation kinetics of Bacillus cereus spores by Plasma activated water (PAW). Food Res. Int. 2020, 131, 109041. [Google Scholar] [CrossRef]
- Pan, J.; Li, Y.L.; Liu, C.M.; Tian, Y.; Yu, S.; Wang, K.L.; Zhang, J.; Fang, J. Investigation of Cold Atmospheric Plasma-Activated Water for the Dental Unit Waterline System Contamination and Safety Evaluation in Vitro. Plasma Chem. Plasma Process. 2017, 37, 1091–1103. [Google Scholar] [CrossRef]
- Smet, C.; Govaert, M.; Kyrylenko, A.; Easdani, M.; Walsh, J.L.; Van Impe, J.F. Inactivation of Single Strains of Listeria monocytogenes and Salmonella Typhimurium Planktonic Cells Biofilms with Plasma Activated Liquids. Front. Microbiol. 2019, 10, 1539. [Google Scholar] [CrossRef] [PubMed]
- Kamgang-Youbi, G.; Herry, J.-M.; Meylheuc, T.; Brisset, J.-L.; Bellon-Fontaine, M.-N.; Doubla, A.; Naïtali, M. Microbial inactivation using plasma-activated water obtained by gliding electric discharges. Lett. Appl. Microbiol. 2009, 48, 13–18. [Google Scholar] [CrossRef] [PubMed]
- Mai-Prochnow, A.; Zhou, R.; Zhang, T.; Ostrikov, K.K.; Mugunthan, S.; Rice, S.A.; Cullen, P.J. Interactions of plasma-activated water with biofilms: Inactivation, dispersal effects and mechanisms of action. NPJ Biofilms Microbiomes 2021, 7, 1–12. [Google Scholar] [CrossRef] [PubMed]
- Xu, Y.; Tian, Y.; Ma, R.; Liu, Q.; Zhang, J. Effect of plasma activated water on the postharvest quality of button mushrooms, Agaricus bisporus. Food Chem. 2016, 197, 436–444. [Google Scholar] [CrossRef]
- Xiang, Q.; Zhang, R.; Fan, L.; Ma, Y.; Wu, D.; Li, K. Microbial inactivation and quality of grapes treated by plasma-activated water combined with mild heat. LWT 2020, 126, 109336. [Google Scholar] [CrossRef]
- Chen, C.; Liu, C.; Jiang, A.; Guan, Q.; Sun, X.; Liu, S.; Hao, K.; Hu, W. The Effects of Cold Plasma-Activated Water Treatment on the Microbial Growth and Antioxidant Properties of Fresh-Cut Pears. Food Bioprocess Technol. 2019, 12, 1842–1851. [Google Scholar] [CrossRef]
- Xiang, Q.; Liu, X.; Liu, S.; Ma, Y.; Xu, C.; Bai, Y. Effect of plasma-activated water on microbial quality and physicochemical characteristics of mung bean sprouts. Innov. Food Sci. Emerg. Technol. 2019, 52, 49–56. [Google Scholar] [CrossRef]
- Ma, R.; Yu, S.; Tian, Y.; Wang, K.; Sun, C.; Li, X.; Zhang, J.; Chen, K.; Fang, J. Effect of Non-Thermal Plasma-Activated Water on Fruit Decay and Quality in Postharvest Chinese Bayberries. Food Bioprocess Technol. 2016, 9, 1825–1834. [Google Scholar] [CrossRef]
- Adhikari, B.; Pangomm, K.; Veerana, M.; Mitra, S.; Park, G. Plant Disease Control by Non-Thermal Atmospheric-Pressure Plasma. Front. Plant Sci. 2020, 11, 77. [Google Scholar] [CrossRef]
- Wu, M.C.; Liu, C.T.; Chiang, C.Y.; Lin, Y.J.; Lin, Y.H.; Chang, Y.W.; Wu, J.S. Inactivation Effect of Colletotrichum Gloeosporioides by Long-Lived Chemical Species Using Atmospheric-Pressure Corona Plasma-Activated Water. IEEE Trans. Plasma Sci. 2019, 47, 1100–1104. [Google Scholar] [CrossRef]
- Perez, S.M.; Biondi, E.; Laurita, R.; Proto, M.; Sarti, F.; Gherardi, M.; Bertaccini, A.; Colombo, V. Plasma activated water as resistance inducer against bacterial leaf spot of tomato. PLoS ONE 2019, 14, e0217788. [Google Scholar] [CrossRef]
- Siddique, S.S.; Hardy GE, S.J.; Bayliss, K.L. Plasma-activated water inhibits in vitro conidial germination of Colletotrichum alienum, a postharvest pathogen of avocado. Plant Pathol. 2021, 70, 367–376. [Google Scholar] [CrossRef]
- Guo, J.; Qin, D.; Li, W.; Wu, F.; Li, L.; Liu, X. Inactivation of Penicillium italicum on kumquat via plasma-activated water and its effects on quality attributes. Int. J. Food Microbiol. 2021, 343, 109090. [Google Scholar] [CrossRef] [PubMed]
- Moreau, M.; Feuilloley, M.G.J.; Orange, N.; Brisset, J.-L. Lethal effect of the gliding arc discharges on Erwinia spp. J. Appl. Microbiol. 2005, 98, 1039–1046. [Google Scholar] [CrossRef]
- Motyka, A.; Dzimitrowicz, A.; Jamroz, P.; Lojkowska, E.; Sledz, W.; Pohl, P. Rapid eradication of bacterial phytopathogens by atmospheric pressure glow discharge generated in contact with a flowing liquid cathode. Biotechnol. Bioeng. 2018, 115, 1581–1593. [Google Scholar] [CrossRef] [PubMed]
- Xiang, Q.; Fan, L.; Li, Y.; Dong, S.; Li, K.; Bai, Y. A review on recent advances in plasma-activated water for food safety: Current applications and future trends. Crit. Rev. Food Sci. Nutr. 2020, 1–20. [Google Scholar] [CrossRef]
- Graves, D.; Bakken, L.; Jensen, M.; Ingels, R. Plasma activated organic fertilizer. Plasma Chem. Plasma Process. 2019, 39, 1–19. [Google Scholar] [CrossRef]
- Zalewski, A. Zmiany na rynku nawozów azotowych w Polsce w latach 2000–2010. J. Agribus. Rural Dev. 2013, 4, 257–267. [Google Scholar]
- Zhao, Y.M.; Ojha, S.; Burgess, C.M.; Sun, D.W.; Tiwari, B.K. Inactivation efficacy and mechanisms of plasma activated water on bacteria in planktonic state. J. Appl. Microbiol. 2020, 129, 1248–1260. [Google Scholar] [CrossRef]
- Kučerová, K.; Machala, Z.; Hensel, K. Transient Spark Discharge Generated in Various N2/O2 Gas Mixtures: Reactive Species in the Gas and Water and Their Antibacterial Effects. Plasma Chem. Plasma Process. 2020, 40, 749–773. [Google Scholar] [CrossRef]
- Machala, Z.; Tarabová, B.; Sersenová, D.; Janda, M.; Hensel, K. Chemical and antibacterial effects of plasma activated water: Correlation with gaseous and aqueous reactive oxygen and nitrogen species, plasma sources and air flow conditions. J. Phys. D. Appl. Phys. 2019, 52, 034002. [Google Scholar] [CrossRef]
- Jamróz, P.; Gręda, K.; Pohl, P.; Żyrnicki, W. Atmospheric pressure glow discharges generated in contact with flowing liquid cathode: Production of active species and application in wastewater purification processes. Plasma Chem. Plasma Process. 2014, 34, 25–37. [Google Scholar] [CrossRef] [Green Version]
- Jamróz, P.; Zyrnicki, W.; Pohl, P. The effect of a miniature argon flow rate on the spectral characteristics of a direct current atmospheric pressure glow micro-discharge between an argon microjet and a small sized flowing liquid cathode. Spectrochim. Acta Part B At. Spectrosc. 2012, 73, 26–34. [Google Scholar] [CrossRef]
- Jamroz, P.; Greda, K.; Pohl, P. Development of direct-current, atmospheric-pressure, glow discharges generated in contact with flowing electrolyte solutions for elemental analysis by optical emission spectrometry. TrAC Trends Anal. Chem. 2012, 41, 105–121. [Google Scholar] [CrossRef]
- Machala, Z.; Tarabova, B.; Hensel, K.; Spetlikova, E.; Sikurova, L.; Lukes, P. Formation of ROS and RNS in Water Electro-Sprayed through Transient Spark Discharge in Air and their Bactericidal Effects. Plasma Process. Polym. 2013, 10, 649–659. [Google Scholar] [CrossRef]
- Wang, J.; He, M.; Zheng, P.; Chen, Y.; Mao, X. Comparison of the Plasma Temperature and Electron Number Density of the Pulsed Electrolyte Cathode Atmospheric Pressure Discharge and the Direct Current Solution Cathode Glow Discharge. Anal. Lett. 2019, 52, 697–712. [Google Scholar] [CrossRef]
- Śledź, W.; Jafra, S.; Waleron, M.; Lojkowska, E. Genetic diversity of Erwinia carotovora strains isolated from infected plants grown in Poland. EPPO Bull. 2000, 30, 403–407. [Google Scholar] [CrossRef]
- Waleron, M.; Waleron, K.; Podhajska, A.J.; Łojkowska, E. Genotyping of bacteria belonging to the former Erwinia genus by PCR-RFLP analysis of a recA gene fragment. Microbiology 2002, 148, 583–595. [Google Scholar] [CrossRef] [Green Version]
- Golanowska, M.; Potrykus, M.; Motyka-Pomagruk, A.; Kabza, M.; Bacci, G.; Galardini, M.; Bazzicalupo, M.; Makalowska, I.; Smalla, K.; Mengoni, A.; et al. Comparison of highly and weakly virulent Dickeya solani strains, with a view on the pangenome and panregulon of this species. Front. Microbiol. 2018, 9, 1940. [Google Scholar] [CrossRef] [Green Version]
- Motyka-Pomagruk, A.; Zoledowska, S.; Misztak, A.E.; Sledz, W.; Mengoni, A.; Lojkowska, E. Comparative genomics and pangenome-oriented studies reveal high homogeneity of the agronomically relevant enterobacterial plant pathogen Dickeya solani. BMC Genomics 2020, 21, 449. [Google Scholar] [CrossRef]
- Van Gijsegem, F.; Toth, I.K.; van der Wolf, J.M. Soft Rot Pectobacteriaceae: A brief overview. In Plant Diseases Caused by Dickeya and Pectobacterium Species; Van Gijsegem, F., van der Wolf, J.M., Toth, I.K., Eds.; Springer Nature: Cham, Switzerland, 2021; pp. 1–12. ISBN 978-3-030-61458-4. [Google Scholar]
- Lerouge, S.; Wertheimer, M.R.; Yahia, L. Plasma sterilization: A review of parameters, mechanisms, and limitations. Plasmas Polym. 2001, 6, 175–188. [Google Scholar] [CrossRef]
- Schnabel, U.; Niquet, R.; Schmidt, C.; Stachowiak, J.; Schlüter, O.; Andrasch, M.; Ehlbeck, J. Antimicrobial efficiency of non-thermal atmospheric pressure plasma processed water (PPW) against agricultural relevant bacteria suspensions. Int. J. Environ. Agric. Res. 2016, 2, 212–2014. [Google Scholar]
- Uchida, G.; Nakajima, A.; Ito, T.; Takenaka, K.; Kawasaki, T.; Koga, K.; Shiratani, M.; Setsuhara, Y. Effects of nonthermal plasma jet irradiation on the selective production of H2O2 and NO2- in liquid water. J. Appl. Phys. 2016, 120, 203302. [Google Scholar] [CrossRef]
- Kawasaki, T.; Sato, A.; Kusumegi, S.; Kudo, A.; Sakanoshita, T.; Tsurumaru, T.; Uchida, G.; Koga, K.; Shiratani, M. Two-dimensional concentration distribution of reactive oxygen species transported through a tissue phantom by atmospheric-pressure plasma-jet irradiation. Appl. Phys. Express 2016, 9, 076202. [Google Scholar] [CrossRef]
- Busco, G.; Omran, A.V.; Ridou, L.; Pouvesle, J.M.; Robert, E.; Grillon, C. Cold atmospheric plasma-induced acidification of tissue surface: Visualization and quantification using agarose gel models. J. Phys. D Appl. Phys. 2019, 52, 24LT01. [Google Scholar] [CrossRef]
- Kaushik, N.K.; Ghimire, B.; Li, Y.; Adhikari, M.; Veerana, M.; Kaushik, N.; Jha, N.; Adhikari, B.; Lee, S.J.; Masur, K.; et al. Biological and medical applications of plasma-activated media, water and solutions. Biol. Chem. 2018, 400, 39–62. [Google Scholar] [CrossRef] [PubMed]
- Dzimitrowicz, A.; Greda, K.; Lesniewicz, T.; Jamroz, P.; Nyk, M.; Pohl, P. Size-controlled synthesis of gold nanoparticles by a novel atmospheric pressure glow discharge system with a metallic pin electrode and a flowing liquid electrode. RSC Adv. 2016, 6, 80773–80783. [Google Scholar] [CrossRef] [Green Version]
- Slawiak, M.; Łojkowska, E.; van der Wolf, J.M. First report of bacterial soft rot on potato caused by Dickeya sp. (syn. Erwinia chrysanthemi) in Poland. Plant Pathol. 2009, 58, 794. [Google Scholar] [CrossRef]
- Potrykus, M.; Sledz, W.; Golanowska, M.; Slawiak, M.; Binek, A.; Motyka, A.; Zoledowska, S.; Czajkowski, R.; Lojkowska, E. Simultaneous detection of major blackleg and soft rot bacterial pathogens in potato by multiplex polymerase chain reaction. Ann. Appl. Biol. 2014, 165, 474–487. [Google Scholar] [CrossRef]
- Cockerill, F.R.; Wikler, M.A.; Alder, J.; Dudley, M.N.; Eliopoulos, G.M.; Ferraro, M.J.; Hardy, D.J.; Hecht, D.W.; Hindler, J.A.; Patel, J.B.; et al. Methods for Dilution Antimicrobial Susceptibility Tests for Bacteria That Grow Aerobically; Approved Standard, 9th ed.; Clinical and Laboratory Standards Institute: Wayne, PA, USA, 2012. [Google Scholar]
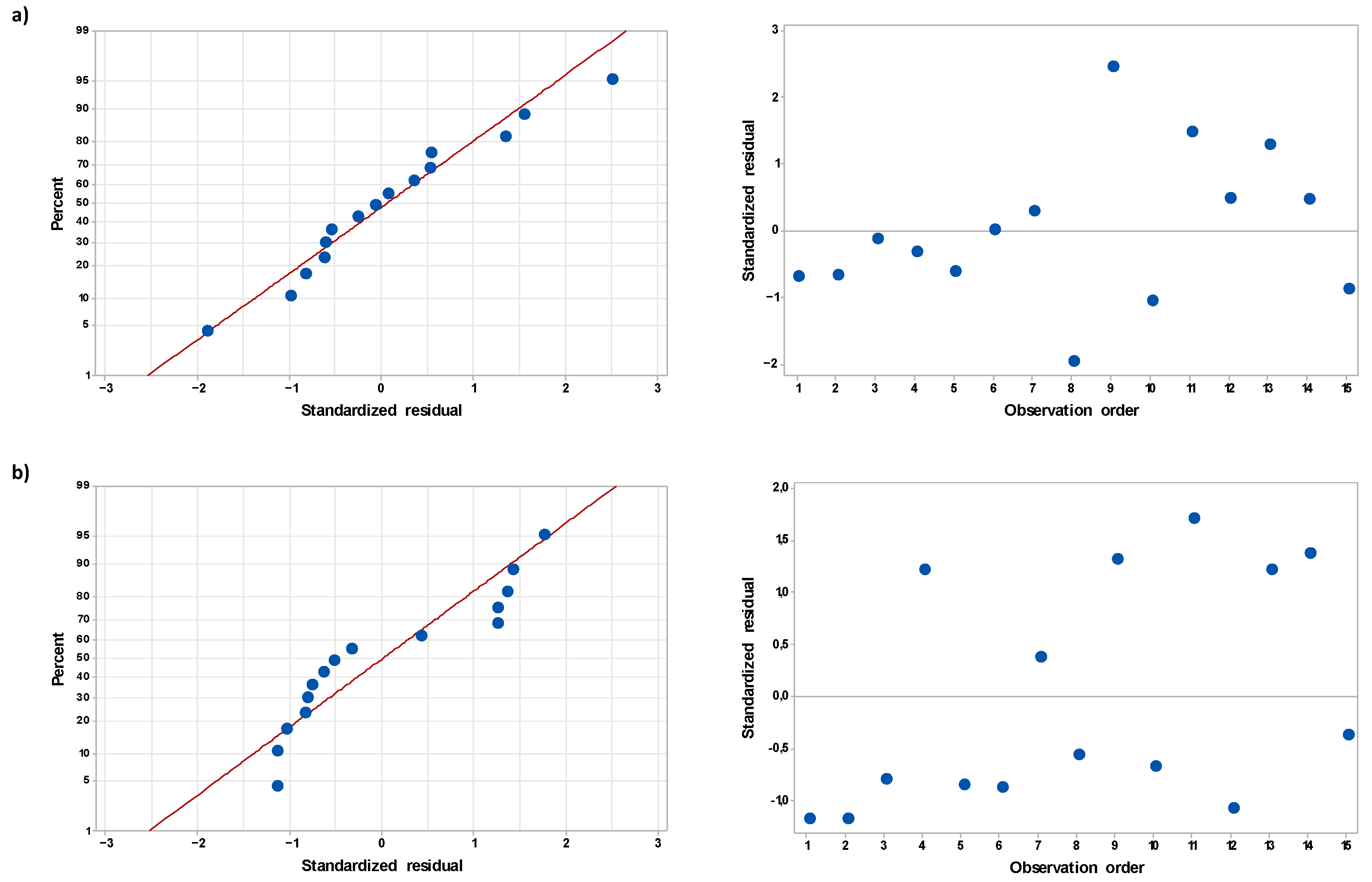
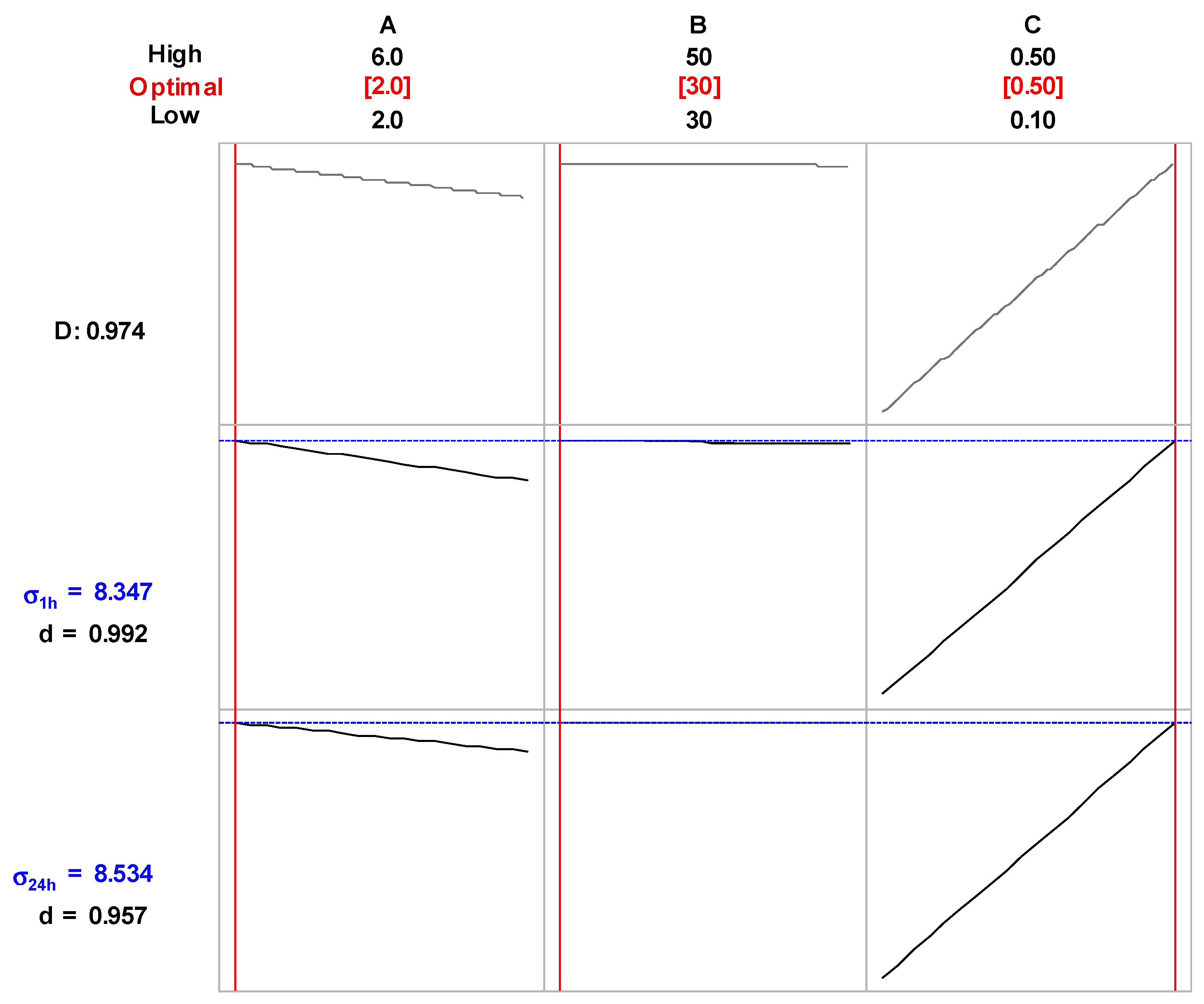
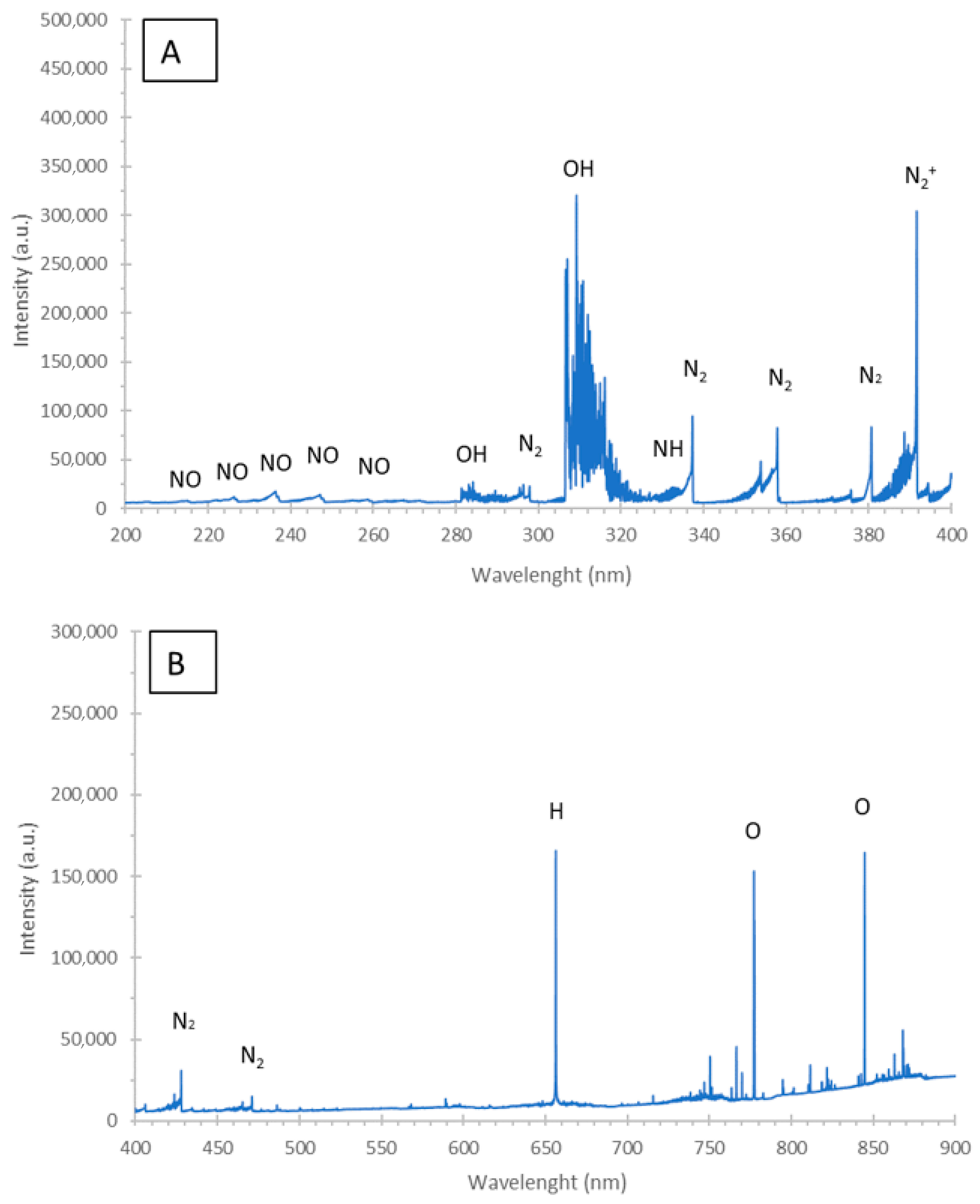

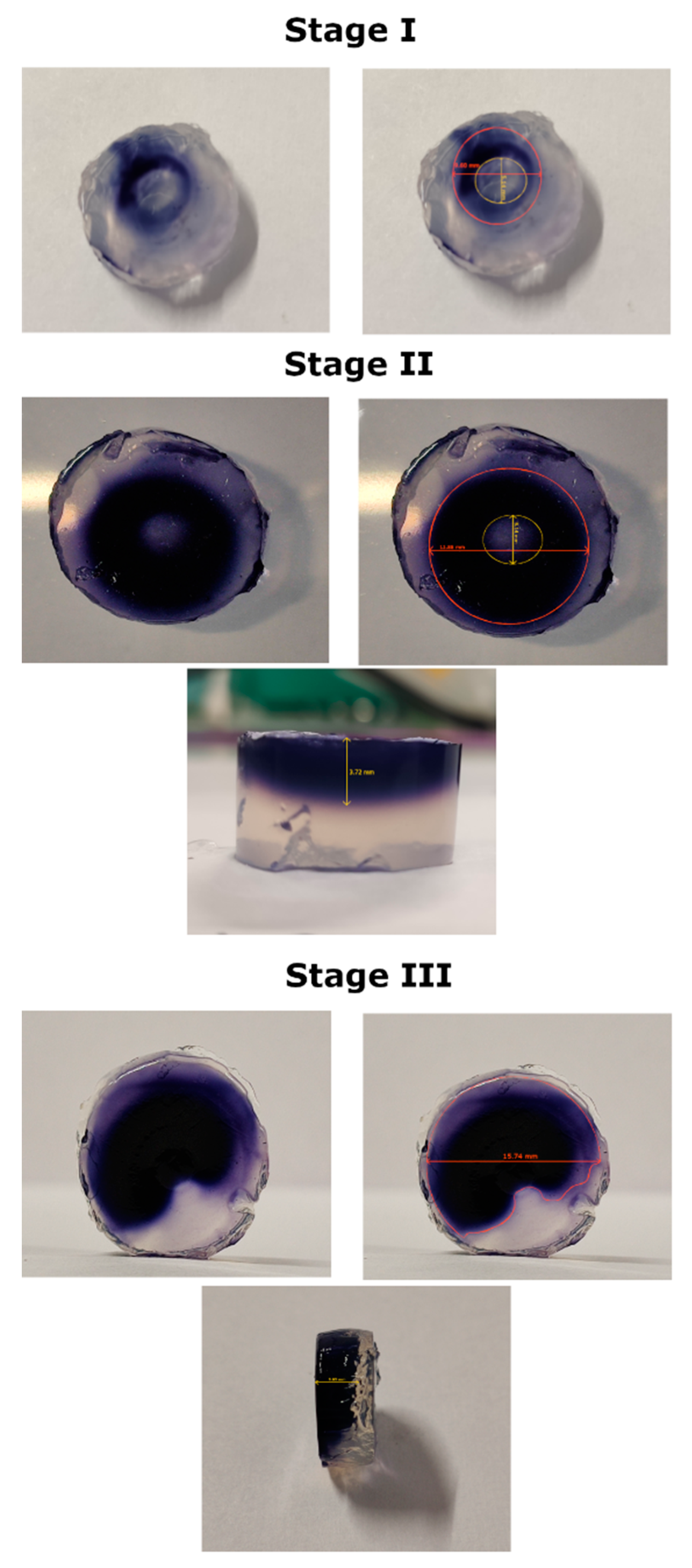

| Order | A, mL min−1 | B, mA | C, % | σ 1h, mS cm−1 | σ 24h, mS cm−1 | |
|---|---|---|---|---|---|---|
| Standard | Run | |||||
| 11 | 1 | 6.0 (+1) | 40 (0) | 0.1 (−1) | 2.230 | 2.310 |
| 5 | 2 | 2.0 (−1) | 30 (−1) | 0.3 (0) | 5.180 | 5.230 |
| 13 | 3 a | 4.0 (0) | 40 (0) | 0.3 (0) | 5.000 | 5.200 |
| 7 | 4 | 6.0 (+1) | 30 (−1) | 0.3 (0) | 4.810 | 5.060 |
| 4 | 5 | 4.0 (0) | 50 (+1) | 0.5 (+1) | 7.960 | 8.090 |
| 2 | 6 | 4.0 (0) | 50 (+1) | 0.1 (−1) | 2.240 | 2.260 |
| 15 | 7 a | 4.0 (0) | 40 (0) | 0.3 (0) | 5.130 | 5.270 |
| 3 | 8 | 4.0 (0) | 30 (−1) | 0.5 (+1) | 7.800 | 7.880 |
| 10 | 9 | 2.0 (−1) | 40 (0) | 0.5 (+1) | 8.400 | 8.820 |
| 6 | 10 | 2.0 (−1) | 50 (+1) | 0.3 (0) | 5.120 | 5.170 |
| 8 | 11 | 6.0 (+1) | 50 (+1) | 0.3 (0) | 5.320 | 5.340 |
| 12 | 12 | 6.0 (+1) | 40 (0) | 0.5 (+1) | 7.510 | 7.880 |
| 9 | 13 | 2.0 (−1) | 40 (0) | 0.1 (−1) | 2.230 | 2.270 |
| 14 | 14 a | 4.0 (0) | 40 (0) | 0.3 (0) | 5.240 | 5.300 |
| 1 | 15 | 4.0 (0) | 30 (−1) | 0.1 (−1) | 2.100 | 2.120 |
| p-Values | R2, % | R2 Adjusted, % | R2 Predicted, % | S | |||||
|---|---|---|---|---|---|---|---|---|---|
| Model | Linear | Square | Two-Way Interactions | Lack-of-Fit | |||||
| σ1h | 0.000 | 0.000 (A, C) | - | 0.016 (A × C) | 0.070 | 99.5 | 99.4 | 98.7 | 0.173 |
| σ24h | 0.000 | 0.000 (A, B, C) | - | 0.004 (A × B, A × C) | 0.626 | 99.8 | 99.7 | 99.3 | 0.114 |
| Regression equations modelling the effect of examined parameters (A, B, and C) a | |||||||||
| σ1h | 0.258−0.128 × A + 17.269 × C−0.613 × A × C | ||||||||
| σ24h | 1.159−0.184 × A−0.019 × B + 16.519 × C + 0.007 × A × B + 0.556 × A × C | ||||||||
| Source of Data | DF | Adjusted SS | Adjusted MS | F-Value a | p-Value |
|---|---|---|---|---|---|
| Electrical conductivity measured after 1 h (σ1h) | |||||
| Model | 3 | 70.612 | 23.537 | 785.36 | 0.000 < 0.05 |
| Linear | 2 | 65.172 | 32.586 | 1087.29 | 0.000 < 0.05 |
| A | 1 | 0.101 | 0.101 | 3.28 | 0.093 |
| C | 1 | 65.071 | 65.071 | 2171.20 | 0.000 < 0.05 |
| Two-way interactions | 1 | 0.240 | 0.240 | 8.01 | 0.016 < 0.05 |
| A×C | 1 | 0.240 | 0.240 | 8.01 | 0.016 < 0.05 |
| Error | 11 | 0.330 | 0.030 | ||
| Lack-of-fit | 9 | 0.324 | 0.036 | 13.69 | 0.070 > 0.05 |
| Pure error | 2 | 0.005 | 0.003 | ||
| Total | 14 | 70.942 | |||
| Electrical conductivity measured after 24 h (σ24h) | |||||
| Model | 5 | 65.870 | 13.174 | 1006.94 | 0.000 < 0.05 |
| Linear | 3 | 60.683 | 20.228 | 1546.08 | 0.000 < 0.05 |
| A | 1 | 0.221 | 0.221 | 16.92 | 0.003 < 0.05 |
| B | 1 | 0.024 | 0.024 | 1.84 | 0.208 |
| C | 1 | 60.399 | 60.399 | 4616.53 | 0.000 < 0.05 |
| Two-way interactions | 2 | 0.279 | 0.140 | 10.67 | 0.004 < 0.05 |
| A×B | 1 | 0.081 | 0.081 | 6.21 | 0.034 < 0.05 |
| A×C | 1 | 0.198 | 0.198 | 15.14 | 0.004 < 0.05 |
| Error | 9 | 0.118 | 0.013 | ||
| Lack-of-fit | 7 | 0.089 | 0.013 | 0.88 | 0.625 > 0.05 |
| Pure error | 2 | 0.029 | 0.014 | ||
| Total | 14 | 65.987 | |||
| Bacterial Strain | Assay | Concentration of PAL | |||
|---|---|---|---|---|---|
| 1% | 10% | 25% | 50% | ||
| Dickeya solani IFB0099 | MIC | + | +/− | - | - |
| MBC | + | +/− | - | - | |
| Pectobacterium atrosepticum IFB5103 | MIC | + | +/− | - | - |
| MBC | + | +/− | - | - | |
| Bacterial Species | Strain Nos a | Disease Caused | Host | Year of Isolation | Country of Isolation | Reference |
|---|---|---|---|---|---|---|
| Dickeya solani | IFB0099, IPO2276, LMG28824. | Blackleg and soft rot | Solanum tuberosum | 2005 | Poland | Slawiak et al. [64] |
| Pectobacterium atrosepticum | IFB5103, SCRI1086. | 1985 | Canada | SCRI collection [65] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Dzimitrowicz, A.; Jamroz, P.; Pohl, P.; Babinska, W.; Terefinko, D.; Sledz, W.; Motyka-Pomagruk, A. Multivariate Optimization of the FLC-dc-APGD-Based Reaction-Discharge System for Continuous Production of a Plasma-Activated Liquid of Defined Physicochemical and Anti-Phytopathogenic Properties. Int. J. Mol. Sci. 2021, 22, 4813. https://doi.org/10.3390/ijms22094813
Dzimitrowicz A, Jamroz P, Pohl P, Babinska W, Terefinko D, Sledz W, Motyka-Pomagruk A. Multivariate Optimization of the FLC-dc-APGD-Based Reaction-Discharge System for Continuous Production of a Plasma-Activated Liquid of Defined Physicochemical and Anti-Phytopathogenic Properties. International Journal of Molecular Sciences. 2021; 22(9):4813. https://doi.org/10.3390/ijms22094813
Chicago/Turabian StyleDzimitrowicz, Anna, Piotr Jamroz, Pawel Pohl, Weronika Babinska, Dominik Terefinko, Wojciech Sledz, and Agata Motyka-Pomagruk. 2021. "Multivariate Optimization of the FLC-dc-APGD-Based Reaction-Discharge System for Continuous Production of a Plasma-Activated Liquid of Defined Physicochemical and Anti-Phytopathogenic Properties" International Journal of Molecular Sciences 22, no. 9: 4813. https://doi.org/10.3390/ijms22094813
APA StyleDzimitrowicz, A., Jamroz, P., Pohl, P., Babinska, W., Terefinko, D., Sledz, W., & Motyka-Pomagruk, A. (2021). Multivariate Optimization of the FLC-dc-APGD-Based Reaction-Discharge System for Continuous Production of a Plasma-Activated Liquid of Defined Physicochemical and Anti-Phytopathogenic Properties. International Journal of Molecular Sciences, 22(9), 4813. https://doi.org/10.3390/ijms22094813








