In Vitro Investigation of Binding Interactions between Albumin–Gliclazide Model and Typical Hypotensive Drugs
Abstract
:1. Introduction
2. Results and Discussion
2.1. Fluorescence Quenching
2.2. Thermodynamic Analysis
2.3. α-Helical Content of BSA Molecule
3. Materials and Methods
3.1. Binding Experiment
3.2. Fluorescence Quenching
3.3. Calculation of Standard Thermodynamic Parameters
3.4. α-Helical Content of BSA Molecule
3.5. Statistical Analysis
4. Conclusions
Supplementary Materials
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Cavallari, I.; Bhatt, D.L.; Steg, P.G.; Leiter, L.A.; McGuire, D.K.; Mosenzon, O.; Im, K.; Raz, I.; Braunwald, E.; Scirica, B.M. Causes and Risk Factors for Death in Diabetes: A Competing-Risk Analysis from the SAVOR-TIMI 53 Trial. J. Am. Coll. Cardiol. 2021, 77, 1837–1840. [Google Scholar] [CrossRef] [PubMed]
- Einarson, T.R.; Acs, A.; Ludwig, C.; Panton, U.H. Prevalence of cardiovascular disease in type 2 diabetes: A systematic literature review of scientific evidence from across the world in 2007–2017. Cardiovasc. Diabetol. 2018, 17, 83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Petrie, J.R.; Guzik, T.J.; Touyz, R.M. Diabetes, Hypertension, and Cardiovascular Disease: Clinical Insights and Vascular Mechanisms. Can. J. Cardiol. 2018, 34, 575–584. [Google Scholar] [CrossRef] [Green Version]
- De Boer, I.H.; Bangalore, S.; Benetos, A.; Davis, A.M.; Michos, E.D.; Muntner, P.; Rossing, P.; Zoungas, S.; Bakris, G. Diabetes and Hypertension: A Position Statement by the American Diabetes Association. Diabetes Care 2017, 40, 1273–1284. [Google Scholar] [CrossRef] [Green Version]
- Landman, G.W.; De Bock, G.H.; van Hateren, K.J.; van Dijk, P.R.; Groenier, K.H.; Gans, R.O.; Houweling, S.T.; Bilo, H.J.; Kleefstra, N. Safety and efficacy of gliclazide as treatment for type 2 diabetes: A systematic review and meta-analysis of randomized trials. PLoS ONE 2014, 9, e82880. [Google Scholar] [CrossRef] [PubMed]
- Khunti, K.; Hassanein, M.; Lee, M.K.; Mohan, V.; Amod, A. Role of Gliclazide MR in the Management of Type 2 Diabetes: Report of a Symposium on Real-World Evidence and New Perspectives. Diabetes Ther. 2020, 11, 33–48. [Google Scholar] [CrossRef]
- Colagiuri, S.; Matthews, D.; Leiter, L.A.; Chan, S.P.; Sesti, G.; Marre, M. The place of gliclazide MR in the evolving type 2 diabetes landscape: A comparison with other sulfonylureas and newer oral antihyperglycemic agents. Diabetes Res. Clin. Pract. 2018, 143, 1–14. [Google Scholar] [CrossRef]
- Sarkar, A.; Tiwari, A.; Bhasin, P.S.; Mitra, M. Pharmacological and Pharmaceutical Profile of Gliclazide: A Review. J. Appl. Pharm. Sci. 2011, 1, 11–19. [Google Scholar]
- Seedher, N.; Kanojia, M. Mechanism of interaction of hypoglycemic agents glimepiride and glipizide with human serum albumin. Cent. Eur. J. Chem. 2009, 7, 96–104. [Google Scholar] [CrossRef]
- Peters, T., Jr. All about Albumin: Biochemistry, Genetics, and Medical Applications; Academic press: San Diego, CA, USA, 1995. [Google Scholar]
- Ràfols, C.; Amézqueta, S.; Fuguet, E.; Bosch, E. Molecular interactions between warfarin and human (HSA) or bovine (BSA) serum albumin evaluated by isothermal titration calorimetry (ITC), fluorescence spectrometry (FS) and frontal analysis capillary electrophoresis (FA/CE). J. Pharm. Biomed. Anal. 2018, 150, 452–459. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Keserü, G.M.; Swinney, D.C. Thermodynamics and Kinetics of Drug Binding; Wiley-VCH Verlag GmbH & Co. KGaA: Weinheim, Germany, 2015; Chapters 1–2; pp. 1–35. [Google Scholar]
- Perera, R.P.; Liyanage, V. Spectroscopic Methods in Ligand Protein Interactions: New Insight into Drug-Protein Binding Mechanisms; Lap Lambert Academic Publishing: Sunnyvale, CA, USA, 2010. [Google Scholar]
- Quilan, G.J.; Martin, G.S.; Evans, T.W. Albumin: Biochemical properties and therapeutic potential. Hepatology 2005, 41, 1211–1219. [Google Scholar] [CrossRef] [PubMed]
- Carter, D.C.; Ho, J.X. Structure of serum albumin. Adv. Protein Chem. 1994, 45, 153–203. [Google Scholar]
- Qin, X.; Yang, X.; Du, L.; Minyong, L. Polarity-based fluorescence probes: Properties and applications. RSC Med. Chem. 2021, 12, 1826–1838. [Google Scholar] [CrossRef]
- Lakowicz, J.R. Quenching of Fluorescence. In Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; Chapter 8; pp. 277–330. [Google Scholar]
- Ahmed, A.; Mam, B.; Sowdhamini, R. DEELIG: A Deep Learning Approach to Predict Protein-Ligand Binding Affinity. Bioinform. Biol. Insights 2021, 15, 11779322211030364. [Google Scholar] [CrossRef] [PubMed]
- Liu, S. Bioprocess Engineering: Kinetics, Sustainability, and Reactor Design, 3rd ed.; Elsevier: New York, NY, USA, 2020; Chapter 9; pp. 351–399. [Google Scholar]
- Triplitt, C. Drug Interactions of Medications Commonly Used in Diabetes. Diabetes Spectr. 2006, 19, 202–211. [Google Scholar] [CrossRef] [Green Version]
- Miccoli, R.; Penno, G.; Del Prato, S. Multidrug Treatment of Type 2 Diabetes A challenge for compliance. Diabetes Care 2011, 34, S231–S235. [Google Scholar] [CrossRef] [Green Version]
- Palleria, C.; Di Paolo, A.; Giofrè, C.; Caglioti, C.; Leuzzi, G.; Siniscalchi, A.; De Sarro, G.; Gallelli, L. Pharmacokinetic drug-drug interaction and their implication in clinical management. J. Res. Med. Sci. 2013, 18, 601–610. [Google Scholar]
- Seedher, N.; Kanojia, M. Effect of competitive binding of hypoglycemic agents to human serum albumin on drug pharmacology. Int. J. Pharm. 2015, 5, 1261–1271. [Google Scholar]
- Żurawska-Płaksej, E.; Rorbach-Dolata, A.; Wiglusz, K.; Piwowar, A. The effect of glycation on bovine serum albumin conformation and ligand binding properties with regard to gliclazide. Spectrochim. Acta Part A Mol. Biomol. Spectrosc. 2018, 189, 625–633. [Google Scholar] [CrossRef]
- Pawar, S.K.; Jaldappagari, S. Interaction of repaglinide with bovine serum albumin: Spectroscopic and molecular docking approaches. J. Pharm. Anal. 2019, 9, 274–283. [Google Scholar] [CrossRef] [PubMed]
- Cao, S.; Liu, B.; Li, Z.; Chong, B. A fluorescence spectroscopic study of the interaction between Glipizide and bovine serum albumin and its analytical application. J. Lumin. 2014, 145, 94–99. [Google Scholar] [CrossRef]
- Bojko, B.; Sułkowska, A.; Maciazek-Jurczyk, M.; Równicka, J.; Sułkowski, W.W. Influence of myristic acid on furosemide binding to bovine serum albumin. Comparison with furosemide-human serum albumin complex. Spectrochim. Acta Mol. Biomol. Spectrosc. 2010, 76, 6–11. [Google Scholar] [CrossRef] [PubMed]
- Hammad, M.A.; Tangiisuran, B.; Kharshid, A.M.; Abdul-Aziz, N.; Hassan, Y.; Aziz, N.A.; Elsayed, T.M. Drug-drug Interaction-related Uncontrolled Glycemia. J. Pharm. Bioallied. Sci. 2017, 9, 221–228. [Google Scholar] [PubMed]
- Siddiqui, N.; Husain, A.; Chaudhry, L.; Alam, M.S.; Mitra, M.; Bhasin, P.S. Pharmacological and Pharmaceutical Profile of Valsartan: A Review. J. Appl. Pharm. Sci. 2011, 1, 12–19. [Google Scholar]
- Shareful Islam, S.M.; Hossain, M.K.; Kabir, S. Binding of Valsartan to Bovine Serum Albumin: An in vitro Study. Bangladesh Pharm. J. 2012, 15, 39–41. [Google Scholar]
- Zsila, F. Subdomain IB Is the Third Major Drug Binding Region of Human Serum Albumin: Toward the Three-Sites Model. Mol. Pharm. 2013, 10, 1668–1682. [Google Scholar] [CrossRef] [PubMed]
- Rama Narsimha Reddy, A.; Vijay Kumar, B. Effect of Valsartan on Pharmacokinetics and Pharmacodynamics of Gliclazide in Diabetic Rats. Curr. Res. Cardiovas. Pharmacol. 2017, 6, 22–28. [Google Scholar] [CrossRef] [Green Version]
- Khan, M.S.; Gupta, A.K. Hypoglycaemic effect and interactions of angiotensin II type I receptor blocker (Telmisartan) with oral hypoglycaemic agents instreptozocin-induced diabetic rats. World J. Pharm. Pharmaceut. Sci. 2015, 4, 1505–1515. [Google Scholar]
- Borchard, U. Pharmacological properties of β-adrenoceptor blocking drugs. J. Clin. Bas. Cardiol. 1998, 1, 5–9. [Google Scholar]
- Fitzgerald, J.D.; Ruffin, R.; Smedstad, K.G.; Roberts, R.; McAinsh, J. Studies on the pharmacokinetics and pharmacodynamics of atenolol in man. Eur. J. Clin. Pharmacol. 1978, 13, 81–89. [Google Scholar] [CrossRef]
- Tsujimoto, T.; Sugiyama, T.; Shapiro, M.F.; Noda, M.; Kajio, H. Risk of Cardiovascular Events in Patients with Diabetes Mellitus on β-Blockers. Hypertension 2017, 70, 103–110. [Google Scholar] [CrossRef]
- May, M.; Schindler, C. Clinically and pharmacologically relevant interactions of antidiabetic drugs. Ther. Adv. Endocrinol. Metab. 2016, 7, 69–83. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Alam, M.A.; Awal, M.A.; Subhan, N.; Mostofa, M. In-vitro relationship between protein-binding and free drug concentrations of a water-soluble selective beta-adrenoreceptor antagonist (atenolol) and its interaction with arsenic. J. Health Popul. Nutr. 2009, 27, 20–30. [Google Scholar] [CrossRef]
- Jiang, T.Y.; Zhou, K.L.; Lou, Y.Y.; Pan, D.Q.; Shi, J.H. Probing the behavior of bovine serum albumin upon binding to atenolol: Insights from spectroscopic and molecular docking approaches. J. Biomol. Struct. Dyn. 2018, 36, 1095–1107. [Google Scholar] [CrossRef]
- Wang, Q.; Huang, C.R.; Jiang, M.; Zhu, Y.Y.; Wang, J.; Chen, J.; Shi, J.H. Binding interaction of atorvastatin with bovine serum albumin: Spectroscopic methods and molecular docking. Spectrochim. Acta Mol. Biomol. Spectrosc. 2016, 156, 155–163. [Google Scholar] [CrossRef]
- Shi, J.; Pan, D.; Jiang, M.; Liu, T.T.; Wang, Q. In vitro study on binding interaction of quinapril with bovine serum albumin (BSA) using multispectroscopic and molecular docking methods. J. Biomol. Struc Dyn. 2017, 35, 2211–2223. [Google Scholar] [CrossRef] [PubMed]
- Amlodipine Combined with Gliclazide. Available online: http://drug-data.com/combinations/A/P/amlodipine-gliclazide.html (accessed on 25 November 2021).
- Ross, P.D.; Subramanian, S. Thermodynamics of protein association reactions: Forces contributing to stability. Biochemistry 1981, 20, 3096–3102. [Google Scholar] [CrossRef]
- Du, X.; Li, Y.; Xia, Y.-L.; Ai, S.-M.; Liang, J.; Sang, P.; Ji, X.-L.; Liu, S.-Q. Insights into Protein–Ligand Interactions: Mechanisms, Models, and Methods. Int. J. Mol. Sci. 2016, 17, 144. [Google Scholar] [CrossRef]
- He, Y.; Wang, Y.; Tang, L.; Liu, H.; Chen, W.; Zheng, Z.; Zou, G. Binding of puerarin to human serum albumin: A spectroscopic analysis and molecular docking. J. Fluoresc. 2008, 18, 433–442. [Google Scholar] [CrossRef]
- Wiglusz, K.; Żurawska-Płaksej, E.; Rorbach-Dolata, A.; Piwowar, A. How Does Glycation Affect Binding Parameters of the Albumin-Gliclazide System in the Presence of Drugs Commonly Used in Diabetes? In Vitro Spectroscopic Study. Molecules 2021, 26, 3869. [Google Scholar] [CrossRef] [PubMed]
- Protein Data Bank. Crystal Structure of Bovine Serum Albumin. Available online: https://www.rcsb.org/structure/4F5S (accessed on 25 November 2021).
- Lakowicz, J.R. Instrumentation for Fluorescence Spectroscopy. In Principles of Fluorescence Spectroscopy, 3rd ed.; Springer: New York, NY, USA, 2006; Chapter 2; pp. 55–57. [Google Scholar]


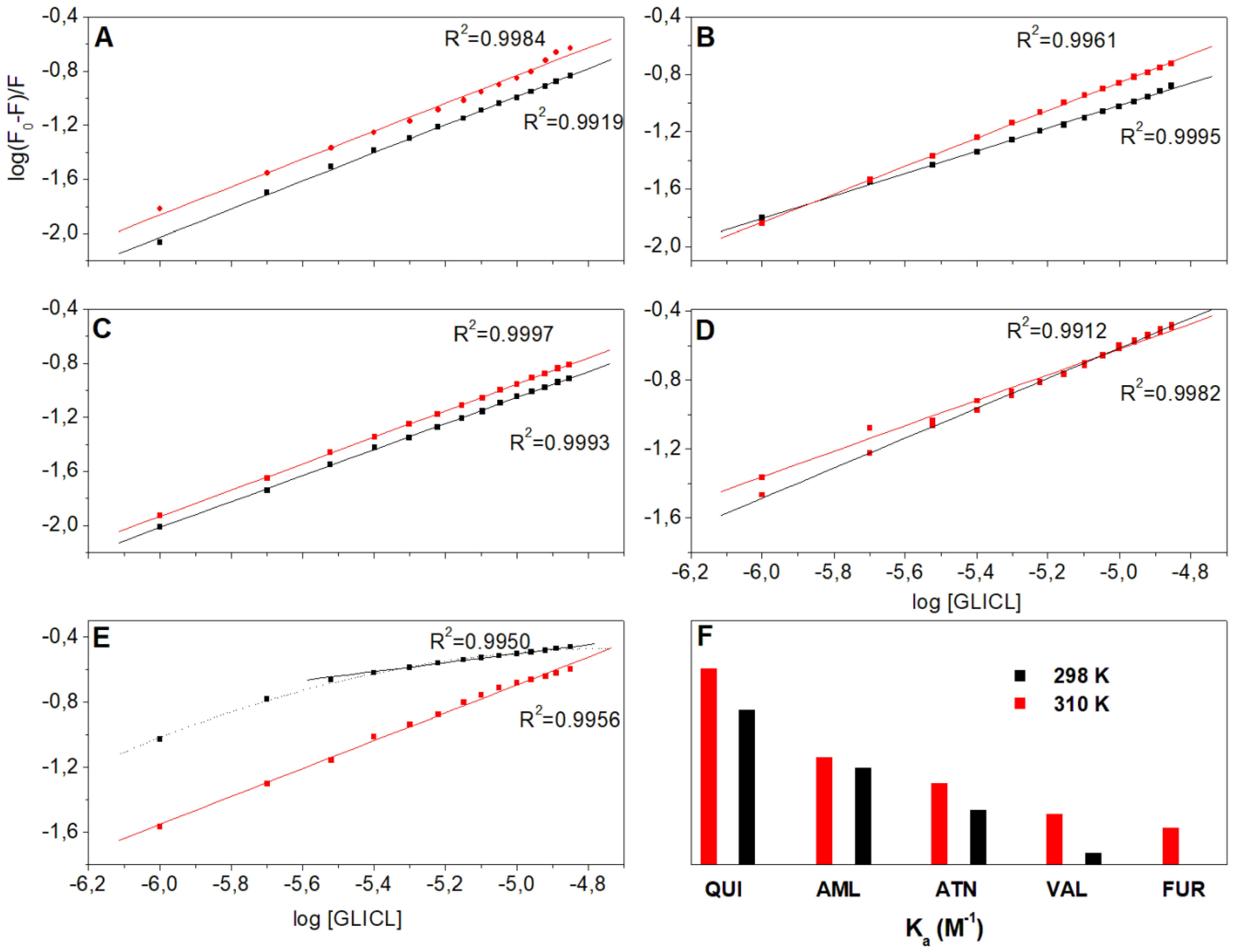
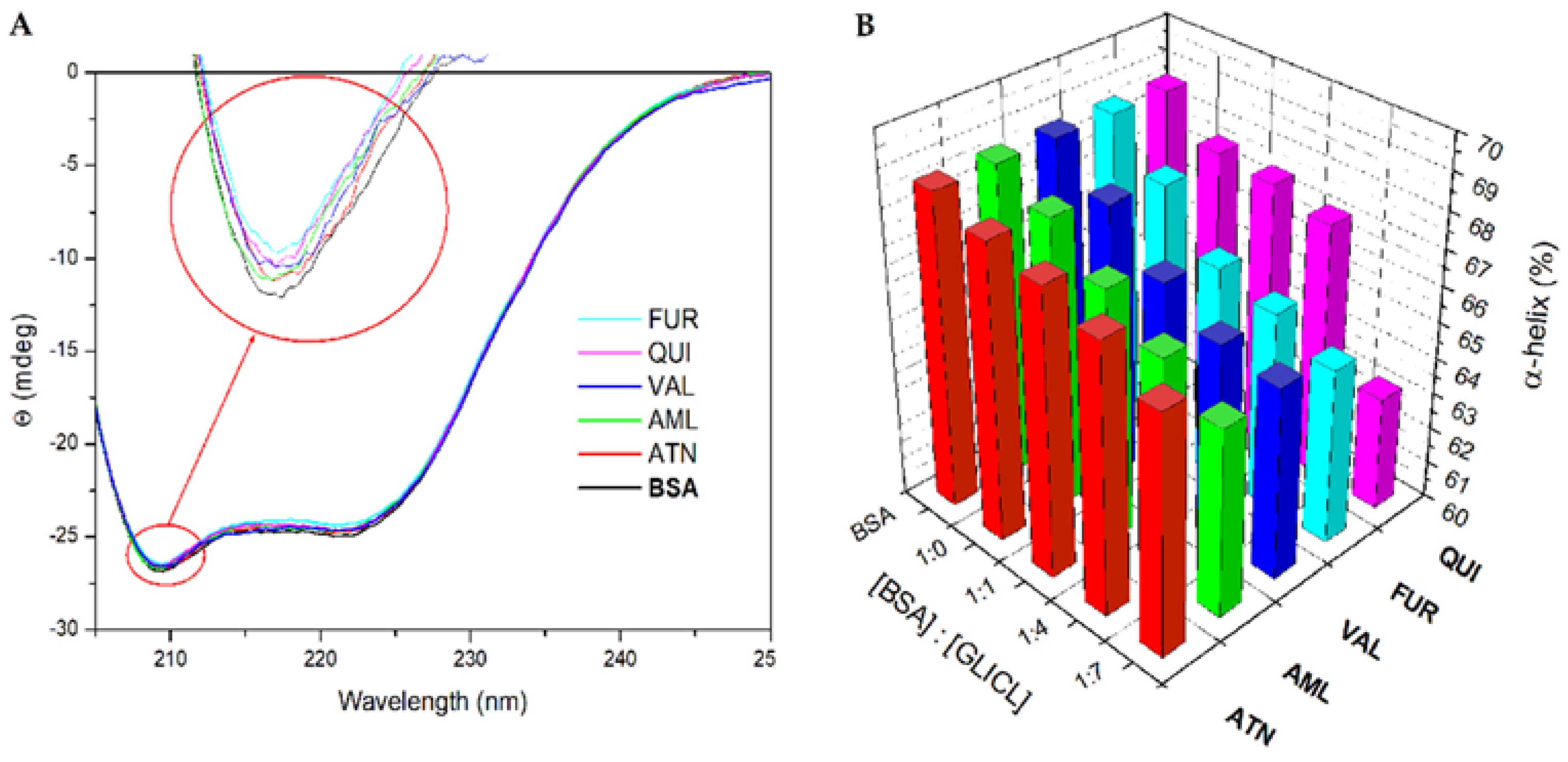
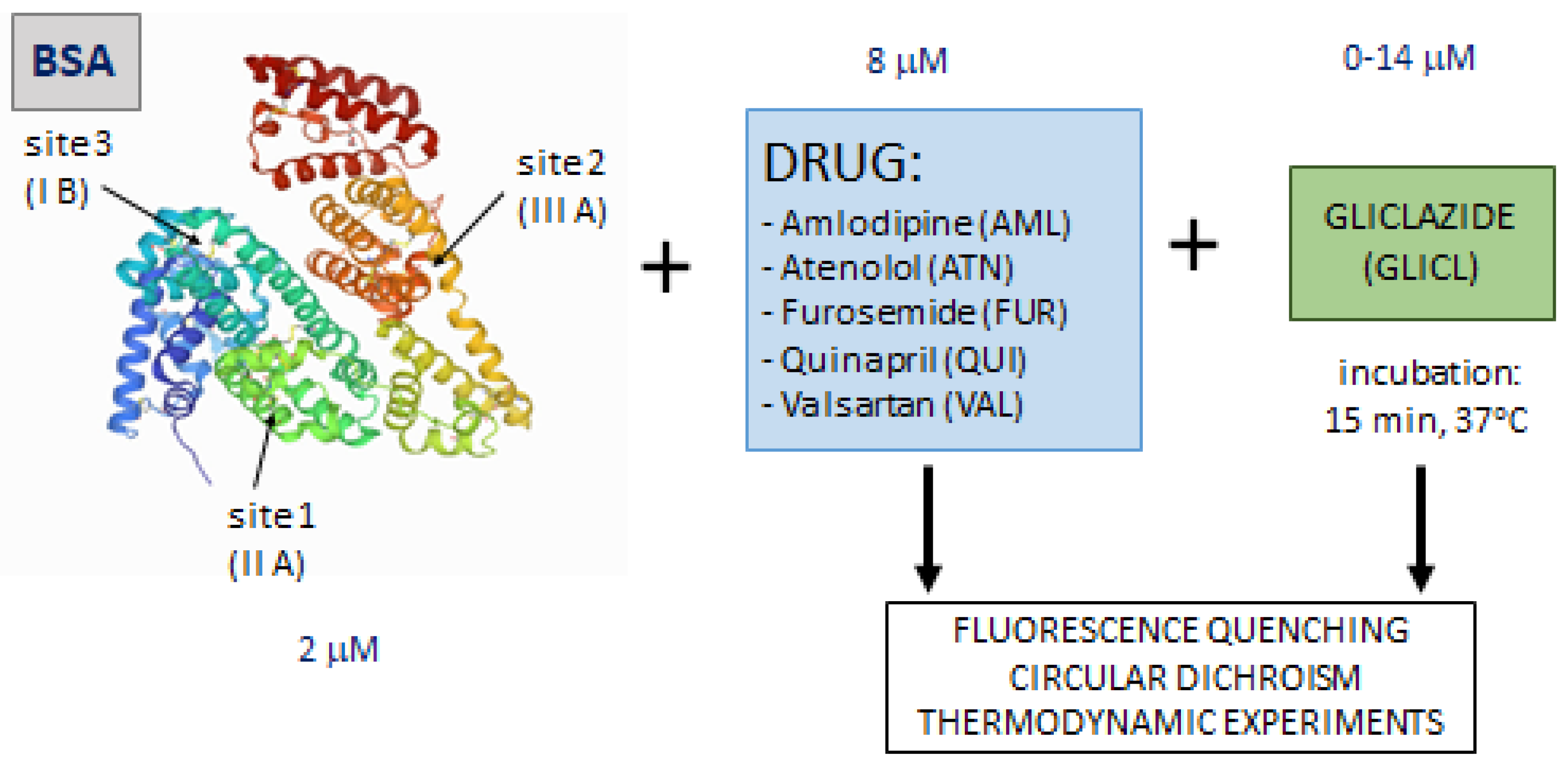
| Parameters | T [K] | AML | ATN | FUR | QUI | VAL |
|---|---|---|---|---|---|---|
| KSV [104 M−1] | 298 | 8.51 ± 0.15 | 1.32 ± 0.13 | 2.80 ± 0.01 | 2.23 ± 0.01 | 11.10 ± 0.02 |
| 310 | 0.30 ± 0.01 | 0.90 ± 0.10 | 3.93 ± 0.09 | 0.96 ± 0.16 | 1.19 ± 0.06 | |
| R2 | 298 | 0.9999 | 0.9993 | 0.9940 | 0.9967 | 0.9973 |
| 310 | 0.9934 | 0.9989 | 0.9968 | 0.9947 | 0.9931 | |
| Ka [104 M−1] | 298 | 1.03 ± 0.02 | 0.58 ± 0.06 | - | 1.65 ± 0.02 | 0.12 ± 0.04 |
| 310 | 1.15 ± 0.01 | 0.87 ± 0.05 | 0.39 ± 0.10 | 2.09 ± 0.07 | 0.54 ± 0.08 | |
| n | 298 | 0.97 ± 0.01 | 0.96 ± 0.01 | - | 1.04 ± 0.01 | 0.74 ± 0.02 |
| 310 | 0.93 ± 0.01 | 0.98 ± 0.01 | 0.86 ± 0.01 | 1.03 ± 0.03 | 0.87 ± 0.01 | |
| R2 | 298 | 0.9995 | 0.9993 | - | 0.9984 | 0.9912 |
| 310 | 0.9961 | 0.9997 | 0.9956 | 0.9919 | 0.9982 |
| Parameters | T [K] | AML | ATN | FUR | QUI | VAL |
|---|---|---|---|---|---|---|
| ΔH (kJ/mol) | 298 303 310 | 6.99 | 25.94 | 380.01 | 15.08 | 100.10 |
| ΔS (J/mol K) | 298 303 310 | 100.32 | 159.06 | 1310.00 | 131.36 | 393.25 |
| ΔG (kJ/mol) | 298 | −22.90 | −21.46 | −10.38 | −24.07 | −17.08 |
| 303 | −23.40 | −22.25 | −16.93 | −24.72 | −19.05 | |
| 310 | −24.10 | −23.37 | −26.10 | −25.64 | −21.80 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Zurawska-Plaksej, E.; Wiglusz, R.; Piwowar, A.; Wiglusz, K. In Vitro Investigation of Binding Interactions between Albumin–Gliclazide Model and Typical Hypotensive Drugs. Int. J. Mol. Sci. 2022, 23, 286. https://doi.org/10.3390/ijms23010286
Zurawska-Plaksej E, Wiglusz R, Piwowar A, Wiglusz K. In Vitro Investigation of Binding Interactions between Albumin–Gliclazide Model and Typical Hypotensive Drugs. International Journal of Molecular Sciences. 2022; 23(1):286. https://doi.org/10.3390/ijms23010286
Chicago/Turabian StyleZurawska-Plaksej, Ewa, Rafal Wiglusz, Agnieszka Piwowar, and Katarzyna Wiglusz. 2022. "In Vitro Investigation of Binding Interactions between Albumin–Gliclazide Model and Typical Hypotensive Drugs" International Journal of Molecular Sciences 23, no. 1: 286. https://doi.org/10.3390/ijms23010286







