Evaluation of the Relationships between Intestinal Regional Lymph Nodes and Immune Responses in Viral Infections in Children
Abstract
:1. Introduction
2. Results
2.1. Comparisons between Clinical Reference Values and Values Obtained from Autopsies
2.1.1. Blood Total IgA and sIgA Levels by Infection Category
2.1.2. Blood IFNβ Levels by Infection Category
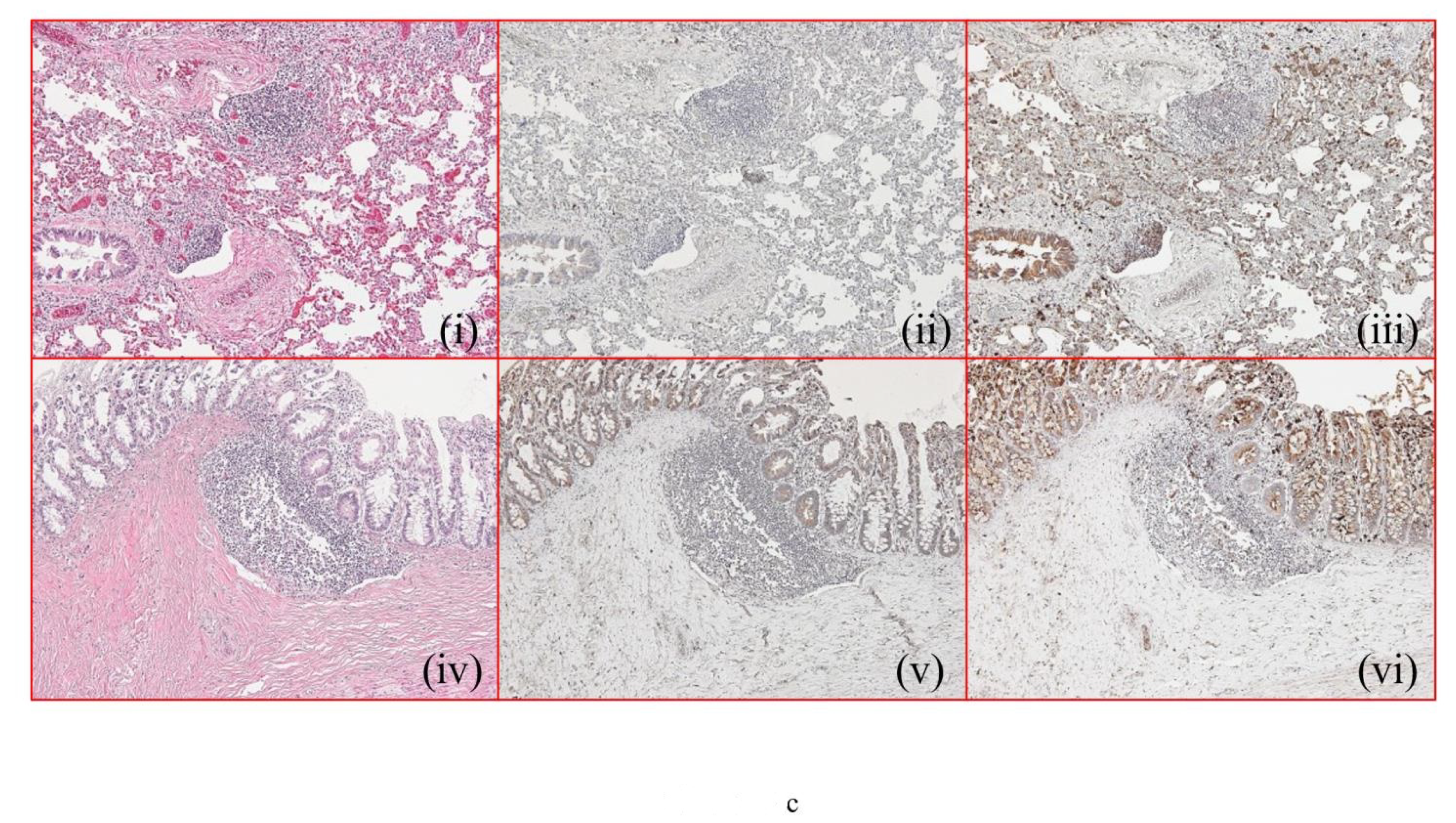
2.2. Histopathology and Immunohistochemistry Findings
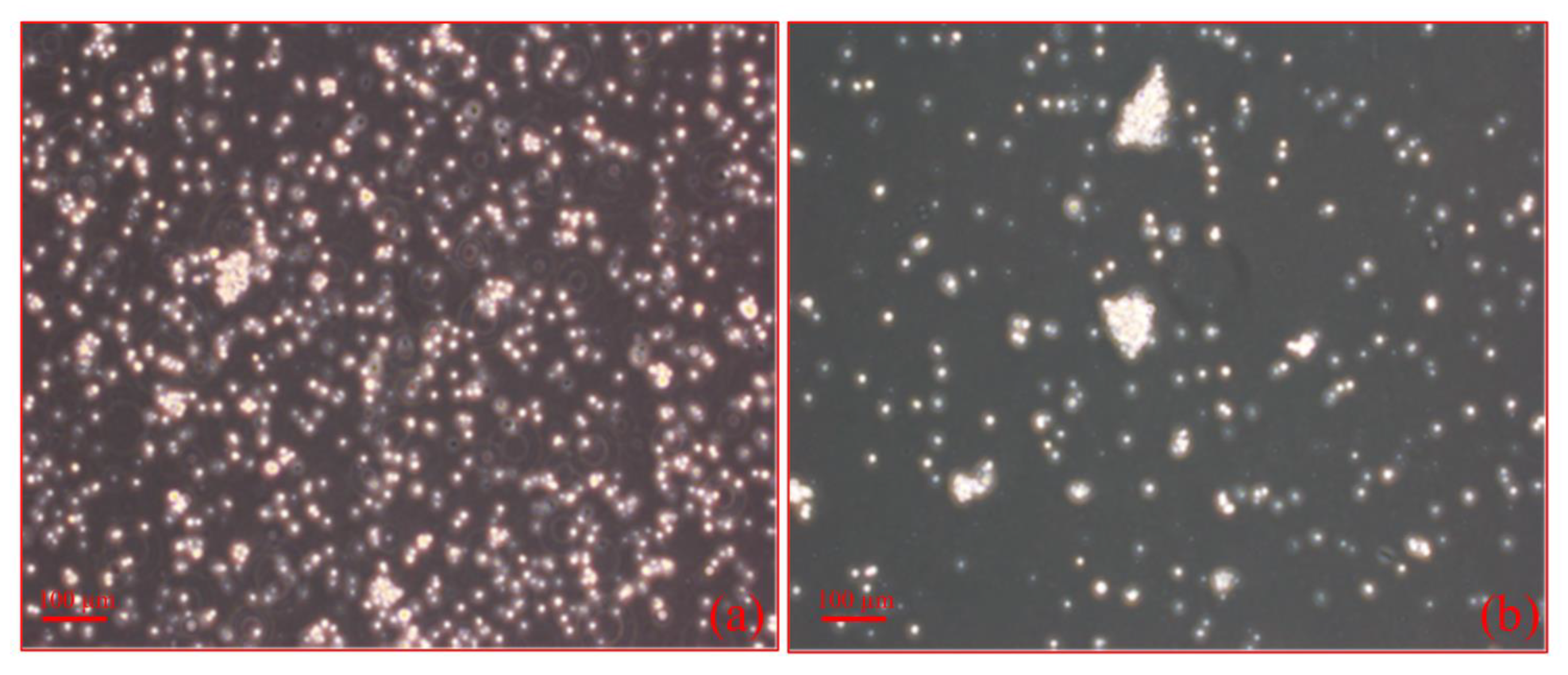
2.3. Cell Culture and Cell Stimulation
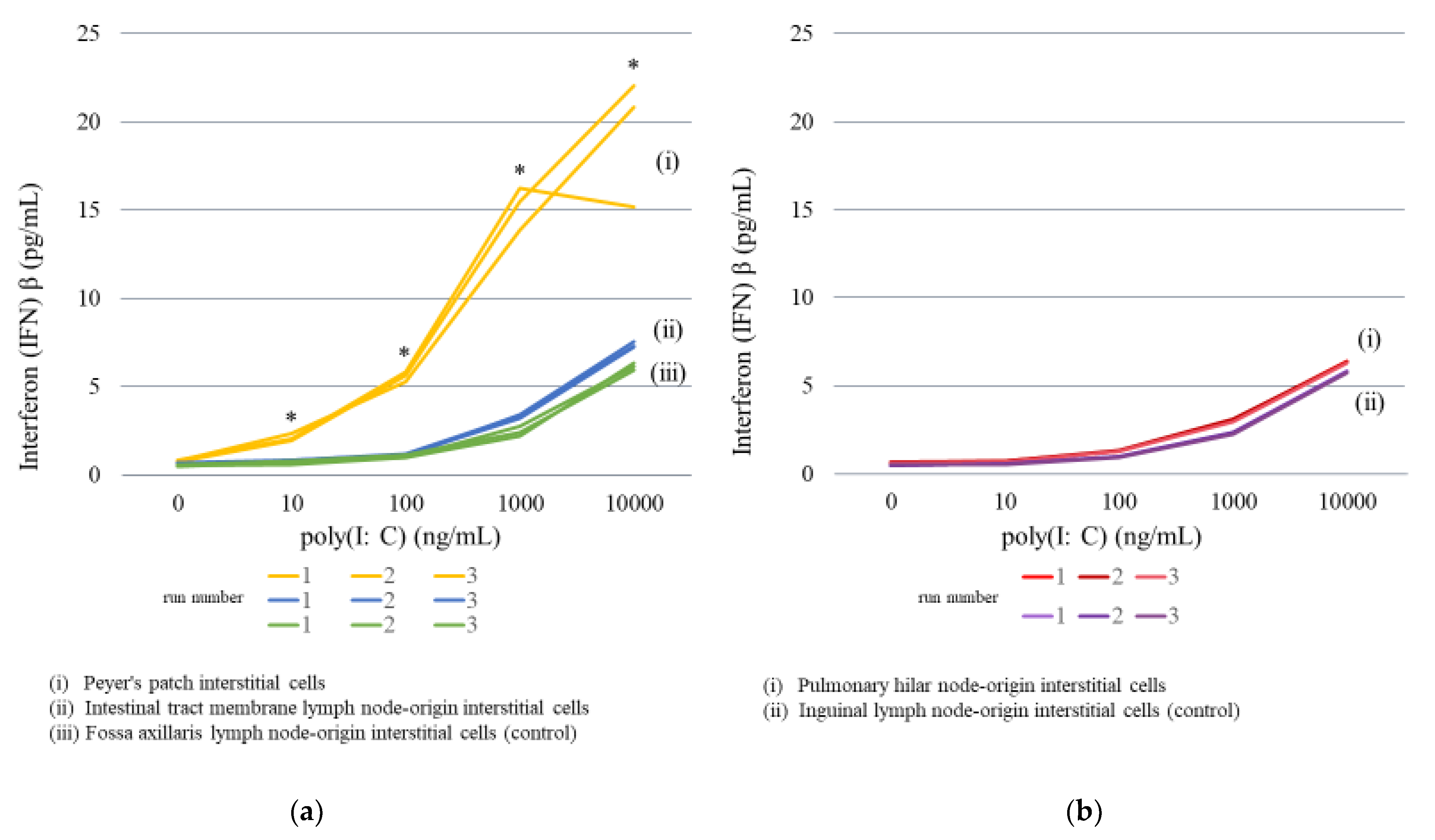
2.3.1. Lymph Node Interstitial Cell Stimulation
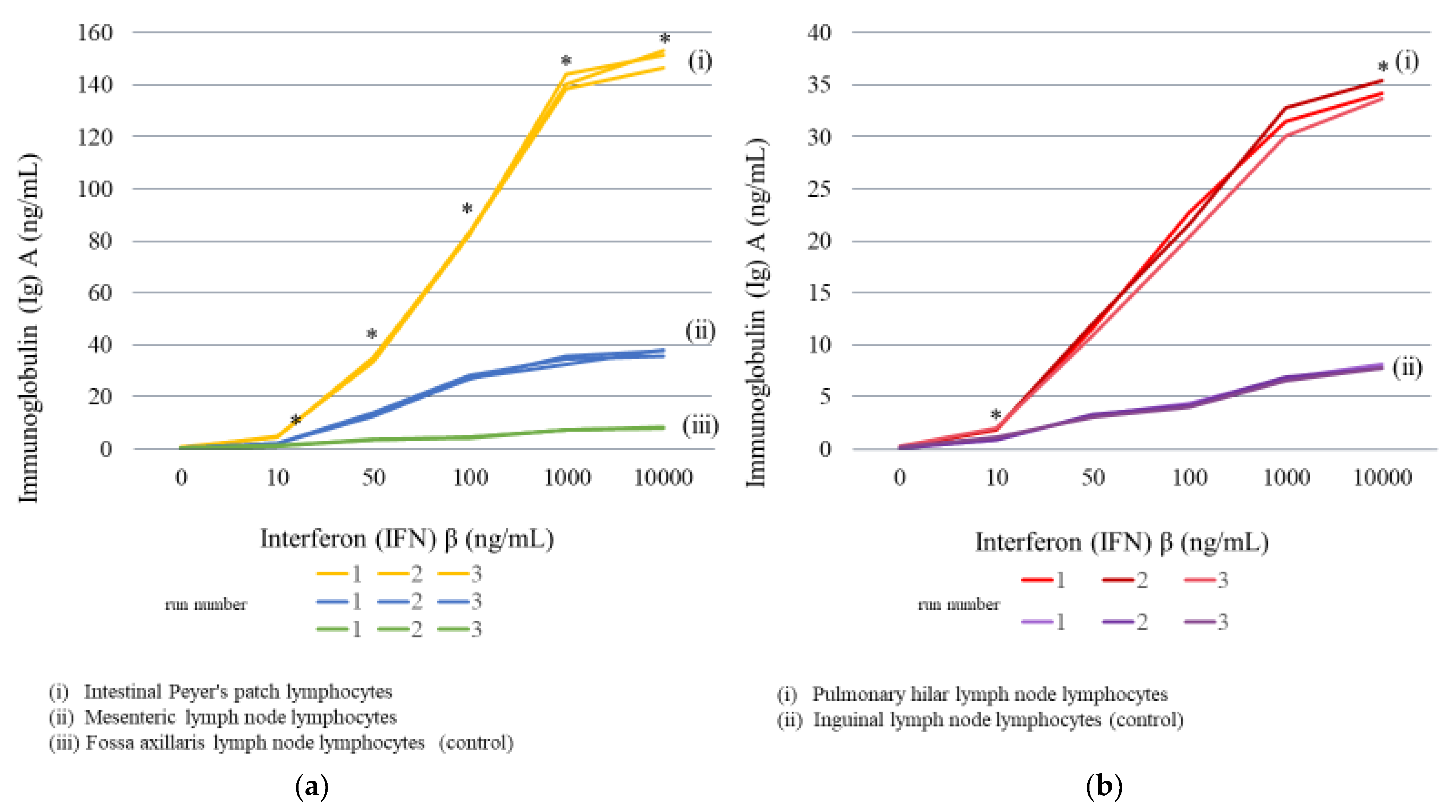
2.3.2. Lymph Node Lymphocyte Stimulation
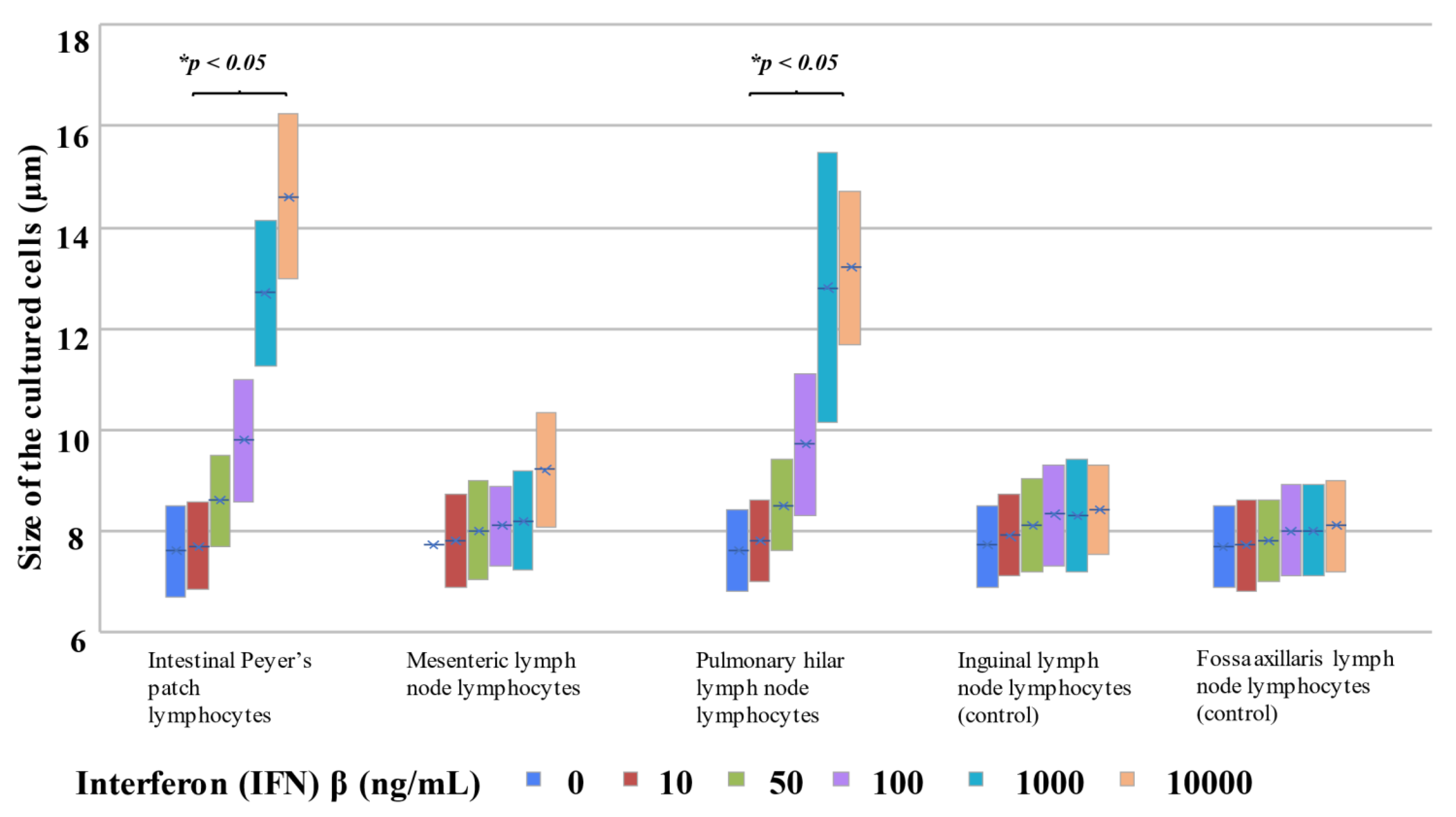
2.3.3. Morphological Changes upon Addition of IFNβ to Each Cultured Cell Type
3. Discussion
4. Materials and Methods
4.1. Autopsy Samples
4.2. Measurement of Blood IgA and IFNβ Levels in Autopsy Cases
4.2.1. Measurement of Blood Total IgA and sIgA Levels
4.2.2. Measurement of Blood IFNβ Levels
4.3. Histopathology and Immunohistochemistry
4.4. Cell Culture and Cell Stimulation
4.4.1. Cell Culture System
4.4.2. Lymph Node Interstitial Cell Stimulation Experiment
4.4.3. Lymphocyte Stimulation Experiment
4.4.4. Measurement of IFNβ and IgA Levels in Culture Media
4.5. Statistical Analyses
5. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Sevelsted, A.; Stokholm, J.; Bønnelykke, K.; Bisgaard, H. Cesarean section and chronic immune disorders. Pediatrics 2015, 135, e92–e98. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Cheung, D.S.; Grayson, M.H. Role of viruses in the development of atopic disease in pediatric patients. Curr. Allergy Asthma Rep. 2012, 12, 613–620. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ebisawa, M.; Nishima, S.; Ohnishi, H.; Kondo, N. Pediatric allergy and immunology in Japan. Pediatr. Allergy Immunol. 2013, 24, 704–714. [Google Scholar] [CrossRef] [PubMed]
- Tantilipikorn, P.; Auewarakul, P. Airway allergy and viral infection. Asian Pac. J. Allergy Immunol. 2011, 29, 113–119. [Google Scholar] [PubMed]
- Castillo, J.R.; Peters, S.P.; Busse, W.W. Asthma exacerbations: Pathogenesis, prevention, and treatment. J. Allergy Immunol. Pract. 2017, 5, 918–927. [Google Scholar] [CrossRef]
- Oh, J.H.; Kim, W.J. Interaction between allergy and middle ear infection. Curr. Allergy Asthma Rep. 2016, 16, 66. [Google Scholar] [CrossRef] [PubMed]
- Cox, L.S.; Sanchez-Borges, M.; Lockey, R.F. World Allergy Organization systemic allergic reaction grading system: Is a modification needed? J. Allergy Clin. Immunol. Pract. 2017, 5, 58–62.e5. [Google Scholar] [CrossRef]
- Lomidze, N.; Gotua, T.; Gotua, M. IgE-mediated food allergy–current problems and future perspectives. Georgian Med. News 2015, 238, 73–78. [Google Scholar]
- Tezuka, H.; Abe, Y.; Asano, J.; Sato, T.; Liu, J.; Iwata, M.; Ohteki, T. Prominent role for plasmacytoid dendritic cells in mucosal T cell-independent IgA induction. Immunity 2011, 34, 247–257. [Google Scholar] [CrossRef] [Green Version]
- Kawamoto, S.; Tran, T.H.; Maruya, M.; Suzuki, K.; Doi, Y.; Tsutsui, Y.; Kato, L.M.; Fagarasan, S. The inhibitory receptor PD-1 regulates IgA selection and bacterial composition in the gut. Science 2012, 336, 485–489. [Google Scholar] [CrossRef]
- Gommerman, J.L.; Rojas, O.L.; Fritz, J.H. Re-thinking the functions of IgA(+) plasma cells. Gut Microbes 2014, 5, 652–662. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tezuka, H.; Ohteki, T. Regulation of IgA production by intestinal dendritic cells and related cells. Front. Immunol. 2019, 10, 1891. [Google Scholar] [CrossRef]
- Binsker, U.; Lees, J.A.; Hammond, A.J.; Weiser, J.N. Immune exclusion by naturally-acquired secretory IgA to the pneumococcal pilus-1. J. Clin. Investig. 2020, 130, 927–941. [Google Scholar] [CrossRef] [Green Version]
- Gautier, C.; Charpin, D. Environmental triggers and avoidance in the management of asthma. J. Asthma Allergy 2017, 10, 47–56. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fousteri, G.; Jhatakia, A.D. Viral infections and autoimmune disease: Roles of LCMV in delineating mechanisms of immune tolerance. Viruses 2019, 11, 885. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stephens, R.; Randolph, D.A.; Huang, G.; Holtzman, M.J.; Chaplin, D.D. Antigen-nonspecific recruitment of Th2 cells to the lung as a mechanism for viral infection-induced allergic asthma. J. Immunol. 2002, 169, 5458–5467. [Google Scholar] [CrossRef]
- Orga, P.L.; Mestecky, J.; Lamm, M.E.; Strober, W.; McGhee, J.R.; Bienenstock, J. (Eds.) Handbook of Mucosal Immunology, 1st ed.; Academic Press, Inc.: Cambridge, UK, 1994. [Google Scholar]
- Sangster, M.Y.; Riberdy, J.M.; Gonzalez, M.; Topham, D.J.; Baumgarth, N.; Doherty, P.C. An early CD4+ T cell-dependent immunoglobulin A response to influenza infection in the absence of key cognate T-B interactions. J. Exp. Med. 2003, 198, 1011–1021. [Google Scholar] [CrossRef]
- Guy, B.; Fourage, S.; Hessler, C.; Sanchez, V.; Millet, M.J. Effects of the nature of adjuvant and site of parenteral immunization on the serum and mucosal immune responses induced by a nasal boost with a vaccine alone. Clin. Diagn. Lab. Immunol. 1998, 5, 732–736. [Google Scholar] [CrossRef] [Green Version]
- Blutt, S.E.; Miller, A.D.; Salmon, S.L.; Metzger, D.W.; Conner, M.E. IgA is important for clearance and critical for protection from rotavirus infection. Mucosal Immunol. 2012, 5, 712–719. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Jiang, Y.; Jia, S.; Zheng, D.; Li, F.; Wang, S.; Wang, L.; Qiao, X.; Cui, W.; Tang, L.; Xu, Y.; et al. Protective immunity against canine distemper virus in dogs induced by intranasal immunization with a recombinant probiotic expressing the viral H protein. Vaccines 2019, 7, 213. [Google Scholar] [CrossRef] [Green Version]
- Frossard, C.P.; Hauser, C.; Eigenmann, P.A. Antigen-specific secretory IgA antibodies in the gut are decreased in a mouse model of food allergy. J. Allergy Clin. Immunol. 2004, 114, 377–382. [Google Scholar] [CrossRef]
- Santiago, A.F.; Fernandes, R.M.; Santos, B.P.; Assis, F.A.; Oliveira, R.P.; Carvalho, C.R.; Faria, A.M. Role of mesenteric lymph nodes and aging in secretory IgA production in mice. Cell Immunol. 2008, 253, 5–10. [Google Scholar] [CrossRef] [PubMed]
- Lin, S.; Sun, Q.; Mao, W.; Chen, Y. Serum Immunoglobulin A (IgA) Level Is a Potential Biomarker Indicating Cirrhosis during Chronic Hepatitis B Infection. Gastroenterol. Res. Pract. 2016, 2016, 2495073. [Google Scholar] [CrossRef] [Green Version]
- Coppo, R. Corticosteroids in IgA Nephropathy: Lessons from Recent Studies. J. Am. Soc. Nephrol. 2017, 28, 25–33. [Google Scholar] [CrossRef]
- Moon, I.Y.; Kim, J.W. Methylation profile of hepatitis B virus is not influenced by interferon α in human liver cancer cells. Mol. Med. Rep. 2021, 24, 715. [Google Scholar] [CrossRef] [PubMed]
- Fenton, T.M.; Jørgensen, P.B.; Niss, K.; Rubin, S.J.S.; Mörbe, U.M.; Riis, L.B.; Silva, C.D.; Plumb, A.; Vandamme, J.; Jakobsen, H.L.; et al. Immune Profiling of Human Gut-Associated Lymphoid Tissue Identifies a Role for Isolated Lymphoid Follicles in Priming of Region-Specific Immunity. Immunity 2020, 52, 557–570.e6. [Google Scholar] [CrossRef]
- Kusunoki, M.; Sato, D.; Nakamura, T.; Oshida, Y.; Tsutsui, H.; Natsume, Y.; Tsutsumi, K.; Miyata, T. DPP-4 inhibitor teneligliptin improves insulin resistance and serum lipid profile in Japanese patients with type 2 diabetes. Drug Res. 2015, 65, 532–534. [Google Scholar] [CrossRef] [PubMed]
- Franco, A.; Parrella, R.; Murru, F.; Ames, P.R.J.; Martucci, F.; Rotiroti, G.; Manfroni, P.V.; Cioffi, D.; Tommasino, C.; Esposito, V. Lack of association between IgA deficiency and respiratory atopy in young male adults. In Vivo 2011, 25, 829–832. [Google Scholar]
- Murakami, I.; Sakuragi, T.; Uemura, H.; Menda, H.; Shindo, M.; Tanaka, H. Significant effect of a pre-exercise high-fat meal after a 3-day high-carbohydrate diet on endurance performance. Nutrients 2012, 4, 625–637. [Google Scholar] [CrossRef] [Green Version]
- Kabeerdoss, J.; Devi, R.S.; Mary, R.R.; Prabhavathi, D.; Vidya, R.; Mechenro, J.; Mahendri, N.V.; Pugazhendhi, S.; Ramakrishna, B.S. Effect of yoghurt containing Bifidobacterium lactis Bb12® on faecal excretion of secretory immunoglobulin A and human beta-defensin 2 in healthy adult volunteers. Nutr. J. 2011, 10, 138. [Google Scholar] [CrossRef] [Green Version]
- Senol, A.; Işler, M.; Karahan, A.G.; Kiliç, G.B.; Kuleaşan, H.; Gören, I.; Saritaş, U.; Kaya, S.; Ciriş, M.; Aktürk, O.; et al. Effect of probiotics on aspirin-induced gastric mucosal lesions. Turk. J. Gastroenterol. 2011, 22, 18–26. [Google Scholar] [CrossRef] [Green Version]
- Kaur, R.; Kim, T.; Casey, J.R.; Pichichero, M.E. Antibody in middle ear fluid of children originates predominantly from sera and nasopharyngeal secretions. Clin. Vaccine Immunol. 2012, 19, 1593–1596. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zavala, K.; Gottlieb, C.A.; Teles, R.M.; Adams, J.S.; Hewison, M.; Modlin, R.L.; Liu, P.T. Intrinsic activation of the vitamin D antimicrobial pathway by M. leprae infection is inhibited by type I IFN. PLoS Negl. Trop. Dis. 2018, 12, e0006815. [Google Scholar] [CrossRef]
- Feng, T.; Deng, L.; Lu, X.; Pan, W.; Wu, Q.; Dai, J. Ubiquitin-conjugating enzyme UBE2J1 negatively modulates interferon pathway and promotes RNA virus infection. Virol. J. 2018, 15, 132. [Google Scholar] [CrossRef]
- Ji, Z.; Li, F.; Xia, Z.; Guo, X.; Gao, M.; Sun, F.; Cheng, Y.; Wu, Y.; Li, W.; Ali, S.A.; et al. The scorpion venom peptide Smp76 inhibits viral infection by regulating type-I interferon response. Virol. Sin. 2018, 33, 545–556. [Google Scholar] [CrossRef] [PubMed]
- Ikeda, T.; Tani, N.; Aoki, Y.; Shida, A.; Morioka, F.; Ishikawa, T. Immunohistochemical detection of respiratory syncytial virus at autopsy. Pediatr. Int. 2019, 61, 781–791. [Google Scholar] [CrossRef]
- Kannaki, T.R.; Priyanka, E.; Abhilash, M.; Haunshi, S. Co-administration of toll-like receptor (TLR)-3 agonist Poly I:C with different infectious bursal disease (IBD) vaccines improves IBD specific immune response in chicken. Veter Res. Commun. 2021, 45, 285–292. [Google Scholar] [CrossRef]
- Yoon, J.Y.; Lee, Y.; Yu, S.L.; Yoon, H.K.; Park, H.Y.; Joung, C.I.; Park, S.R.; Kwon, M.; Kang, J. Aberrant expression of interleukin-10 and activation-induced cytidine deaminase in B cells from patients with Behçet’s disease. Biomed. Rep. 2017, 7, 520–526. [Google Scholar] [CrossRef] [PubMed]
- Zhou, Z.Q.; Tong, D.N.; Guan, J.; Tan, H.W.; Zhao, L.D.; Zhu, Y.; Yao, J.; Yang, J.; Zhang, Z.Y. Follicular helper T cell exhaustion induced by PD-L1 expression in hepatocellular carcinoma results in impaired cytokine expression and B cell help, and is associated with advanced tumor stages. Am. J. Transl. Res. 2016, 8, 2926–2936. [Google Scholar]
- Göpfert, N.C.; von Heydendorff, S.C.; Dreßing, H.; Bailer, J. Applying Corrigan’s progressive model of self-stigma to people with depression. PLoS ONE 2019, 14, e0224418. [Google Scholar] [CrossRef] [Green Version]
- O’Toole, S.; Osnes, C.; Bartlett, D.; Keeling, A. Investigation into the accuracy and measurement methods of sequential 3D dental scan alignment. Dent. Mater. 2019, 35, 495–500. [Google Scholar] [CrossRef] [PubMed]
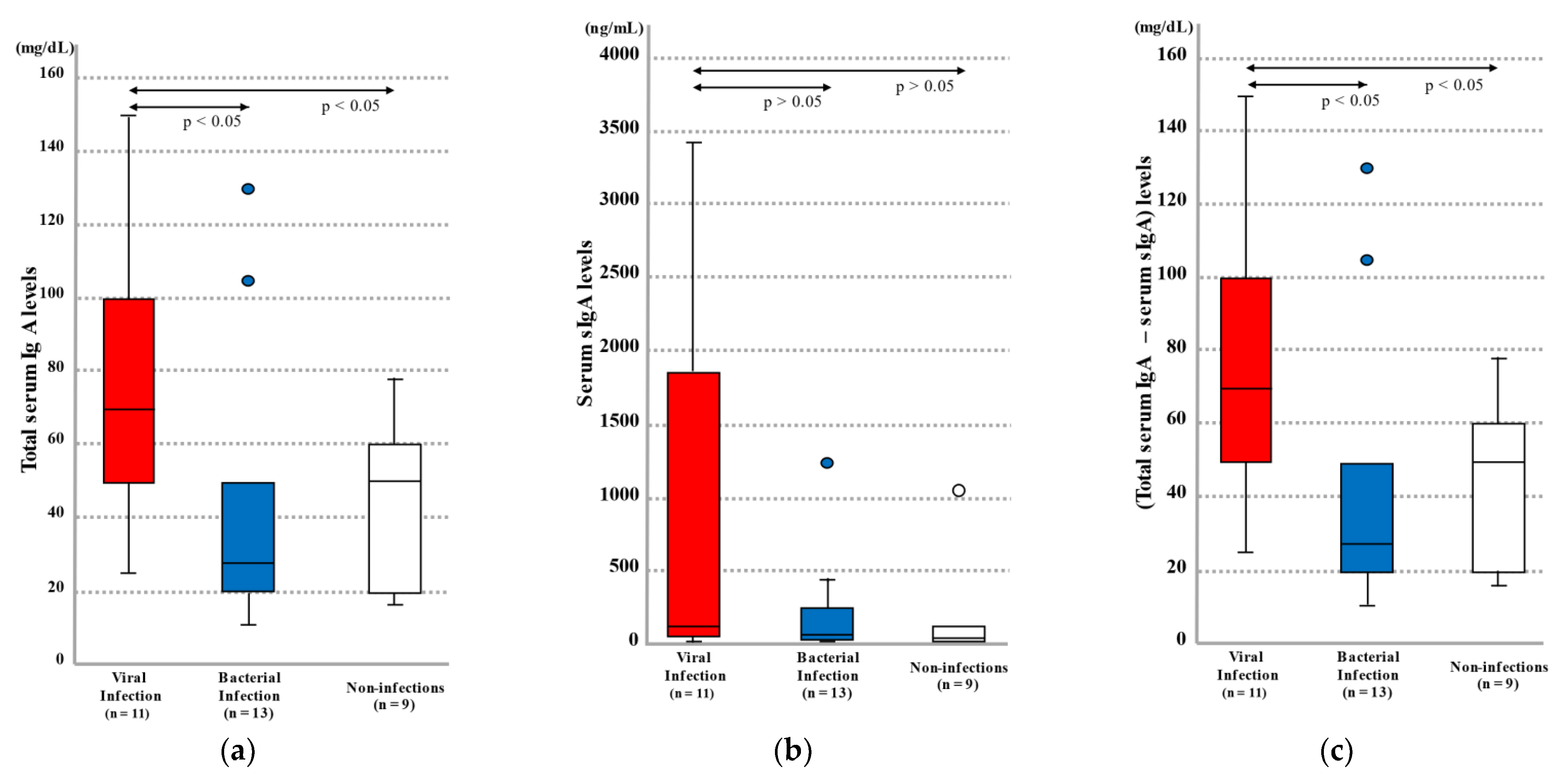

| Case No. | Age (Years) | Age (Months) | Sex | Cause of Death | Clinical Reference Value for Total Serum IgA (mg/dL) | Total IgA (mg/dL) (Right Heart Blood) | sIgA (ng/mL)(Right Heart Blood) | Total IgA–sIgA (mg/dL) (Right Heart Blood) | ||
|---|---|---|---|---|---|---|---|---|---|---|
| V1 | 0 | 1 | Male | Viral infection |  | 7–37 | 100 | ↑ | 2294.0 | 99.8 |
| V2 | 0 | 1 | Female | Viral infection | 50 | ↑ | 3428.0 | 49.7 | ||
| V3 | 0 | 2 | Female | Viral infection | 100 | ↑ | 1866.4 | 99.8 | ||
| V4 | 0 | 3 | Male | Viral infection | 50 | ↑ | 52.9 | 50.0 | ||
| V5 | 0 | 4 | Male | Viral infection | 25 | → | 50.0 | 25.0 | ||
| V6 | 0 | 5 | Male | Viral infection | 16–50 | 150 | ↑ | 709.8 | 149.9 | |
| V7 | 0 | 10 | Female | Viral infection | 27–66 | 80 | ↑ | 53.7 | 80.0 | |
| V8 | 1 | 1 | Male | Viral infection | 36–79 | 50 | → | 141.3 | 50.0 | |
| V9 | 3 | 2 | Female | Viral infection | 27–246 | 64 | → | 419.3 | 64.0 | |
| V10 | 4 | 3 | Female | Viral infection | 29–256 | 70 | → | 17.1 | 70.0 | |
| V11 | 11 | 8 | Female | Viral infection | 42–295 | 94 | → | 14.0 | 94.0 | |
| B1 | 0 | 1 | Male | Bacterial infection |  | 7–37 | 105 | ↑ | 116.3 | 105.0 |
| B2 | 0 | 2 | Male | Bacterial infection | 20 | → | 118.6 | 20.0 | ||
| B3 | 0 | 3 | Male | Bacterial infection | 40 | ↑ | 35.3 | 40.0 | ||
| B4 | 0 | 3 | Male | Bacterial infection | 11 | → | 447.7 | 11.0 | ||
| B5 | 0 | 4 | Female | Bacterial infection | 20 | → | 77.9 | 20.0 | ||
| B6 | 0 | 4 | Female | Bacterial infection | 20 | → | 66.9 | 20.0 | ||
| B7 | 0 | 5 | Female | Bacterial infection |  | 16–50 | 50 | → | 30.2 | 50.0 |
| B8 | 0 | 7 | Male | Bacterial infection | 130 | ↑ | 96.7 | 130.0 | ||
| B9 | 0 | 8 | Female | Bacterial infection | 25 | → | 48.2 | 25.0 | ||
| B10 | 0 | 10 | Female | Bacterial infection |  | 27–66 | 28 | → | 404.2 | 28.0 |
| B11 | 0 | 10 | Male | Bacterial infection | 18 | ↓ | 1240.6 | 17.9 | ||
| B12 | 1 | 3 | Female | Bacterial infection |  | 36–79 | 50 | → | 3.6 | 50.0 |
| B13 | 1 | 5 | Male | Bacterial infection | 35 | ↓ | 54.5 | 35.0 | ||
| O1 | 0 | 1 | Male | Non-infection |  | 7–37 | 50 | ↑ | 1051.0 | 49.9 |
| O2 | 0 | 2 | Female | Non-infection | 20 | → | 130.7 | 20.0 | ||
| O3 | 0 | 3 | Female | Non-infection | 20 | → | 84.4 | 20.0 | ||
| O4 | 0 | 4 | Male | Non-infection | 50 | ↑ | 158.5 | 50.0 | ||
| O5 | 0 | 7 | Female | Non-infection | 16–50 | 50 | → | 45.1 | 50.0 | |
| O6 | 0 | 12 | Male | Non-infection | 27–66 | 50 | → | 41.4 | 50.0 | |
| O7 | 2 | 2 | Female | Non-infection | 27–246 | 70 | → | 27.4 | 70.0 | |
| O8 | 5 | 1 | Male | Non-infection | 29–256 | 78 | → | 26.3 | 78.0 | |
| O9 | 11 | 9 | Female | Non-infection | 42–295 | 16 | ↓ | 62.0 | 16.0 | |
| Case No. | Age (Years) | Age (Months) | Sex | Cause of Death | IFNβ (pg/mL) |
|---|---|---|---|---|---|
| (Right Heart Blood) | |||||
| (Cutoff Level < 1.2 pg/mL) | |||||
| V1 | 0 | 1 | Male | Viral infection | <1.2 |
| V2 | 0 | 1 | Female | Viral infection | <1.2 |
| V3 | 0 | 2 | Female | Viral infection | 4.24 |
| V4 | 0 | 3 | Male | Viral infection | <1.2 |
| V5 | 0 | 4 | Male | Viral infection | <1.2 |
| V6 | 0 | 5 | Male | Viral infection | <1.2 |
| V7 | 0 | 10 | Female | Viral infection | <1.2 |
| V8 | 1 | 1 | Male | Viral infection | <1.2 |
| V9 | 3 | 2 | Female | Viral infection | 3.90 |
| V10 | 4 | 3 | Female | Viral infection | <1.2 |
| V11 | 11 | 8 | Female | Viral infection | <1.2 |
| B1 | 0 | 1 | Male | Bacterial infection | <1.2 |
| B2 | 0 | 2 | Male | Bacterial infection | 3.32 |
| B3 | 0 | 3 | Male | Bacterial infection | <1.2 |
| B4 | 0 | 3 | Male | Bacterial infection | <1.2 |
| B5 | 0 | 4 | Female | Bacterial infection | 1.36 |
| B6 | 0 | 4 | Female | Bacterial infection | 6.37 |
| B7 | 0 | 5 | Female | Bacterial infection | <1.2 |
| B8 | 0 | 7 | Male | Bacterial infection | <1.2 |
| B9 | 0 | 8 | Female | Bacterial infection | <1.2 |
| B10 | 0 | 10 | Female | Bacterial infection | 3.21 |
| B11 | 0 | 10 | Male | Bacterial infection | 7.30 |
| B12 | 1 | 3 | Female | Bacterial infection | 4.40 |
| B13 | 1 | 5 | Male | Bacterial infection | 2.17 |
| O1 | 0 | 1 | Male | Non-infection | <1.2 |
| O2 | 0 | 2 | Female | Non-infection | 2.20 |
| O3 | 0 | 3 | Female | Non-infection | 2.27 |
| O4 | 0 | 4 | Male | Non-infection | <1.2 |
| O5 | 0 | 7 | Female | Non-infection | <1.2 |
| O6 | 0 | 12 | Male | Non-infection | <1.2 |
| O7 | 2 | 2 | Female | Non-infection | <1.2 |
| O8 | 5 | 1 | Male | Non-infection | <1.2 |
| O9 | 11 | 9 | Female | Non-infection | <1.2 |
| Case No. | Cause of Death | Lung | Gastrointestinal Tract | Lung | GASTROINTESTINAL TRACT | ||
|---|---|---|---|---|---|---|---|
| (Pulmonary Hilar | (Peyer’s Patch) | (Pulmonary Hilar | (Peyer’s Patch) | ||||
| Lymph Node) | Lymph Node) | ||||||
| Congestion/Edema | Immunohistochemistry | Immunohistochemistry | |||||
| IgA | IFNβ | IgA | IFNβ | ||||
| V1 | Viral infection | +++/++ | +/− | 1 | 1 | 2 | 3 |
| V2 | Viral infection | +++/++ | +/− | 3 | 1 | 3 | 1 |
| V3 | Viral infection | +++/+++ | −/+ | 2 | 1 | 2 | 1 |
| V4 | Viral infection | +++/++ | −/+ | 2 | 1 | 2 | 1 |
| V5 | Viral infection | +++/++ | −/+ | 2 | 2 | 3 | 2 |
| V6 | Viral infection | +++/++ | −/+ | 2 | 1 | 3 | 2 |
| V7 | Viral infection | ++/++ | +/− | 2 | 1 | 3 | 2 |
| V8 | Viral infection | +++/++ | −/− | 2 | 1 | 3 | 2 |
| V9 | Viral infection | +++/++ | +/− | 2 | 1 | 3 | 2 |
| V10 | Viral infection | +++/++ | +/− | 3 | 1 | 3 | 2 |
| V11 | Viral infection | +++/++ | +/− | 2 | 2 | 2 | 2 |
| B1 | Bacterial infection | +/+ | −/− | 1 | 1 | 2 | 1 |
| B2 | Bacterial infection | +++/+++ | +/++ | 2 | 2 | 1 | 1 |
| B3 | Bacterial infection | +/+ | −/+ | 1 | 1 | 1 | 1 |
| B4 | Bacterial infection | +++/++ | −/+ | 1 | 1 | 1 | 1 |
| B5 | Bacterial infection | +++/+++ | −/− | 2 | 1 | 2 | 1 |
| B6 | Bacterial infection | ++/+++ | +/+ | 1 | 2 | 2 | 1 |
| B7 | Bacterial infection | +/++ | ++/+ | 1 | 1 | 2 | 1 |
| B8 | Bacterial infection | ++/+++ | −/+ | 1 | 1 | 2 | 1 |
| B9 | Bacterial infection | ++/+++ | −/− | 1 | 1 | 2 | 1 |
| B10 | Bacterial infection | +/++ | −/+ | 1 | 1 | 1 | 2 |
| B11 | Bacterial infection | +++/++ | −/+ | 1 | 1 | 2 | 1 |
| B12 | Bacterial infection | +/+ | −/− | 1 | 1 | 1 | 1 |
| B13 | Bacterial infection | +++/+++ | −/− | 1 | 1 | 2 | 1 |
| O1 | Non-infection | ++/+ | −/− | 1 | 1 | 1 | 1 |
| O2 | Non-infection | +++/+ | +/+ | 1 | 1 | 1 | 1 |
| O3 | Non-infection | +++/− | +/+ | 2 | 2 | 1 | 2 |
| O4 | Non-infection | ++/+ | −/− | 1 | 2 | 1 | 1 |
| O5 | Non-infection | +/− | −/− | 1 | 2 | 1 | 1 |
| O6 | Non-infection | +/− | −/+ | 1 | 1 | 1 | 1 |
| O7 | Non-infection | +++/+ | −/− | 1 | 1 | 1 | 1 |
| O8 | Non-infection | ++/+ | −/− | 1 | 2 | 1 | 1 |
| O9 | Non-infection | +/− | −/− | 1 | 1 | 1 | 1 |
| Concentration of Poly(I:C) | |||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (ng/mL) | 10 (ng/mL) | 100 (ng/mL) | 1000 (ng/mL) | 10,000 (ng/mL) | |||||||
| Secretion of IFNβ (pg/mL) | Peyer’s patch | 0.74 | 1.97 | 5.66 | 15.49 | 22.06 | |||||
| interstitial cells | 0.79 | 2.35 | 5.27 | 13.85 | 20.84 | ||||||
| 0.85 | 2.06 | 5.85 | 16.23 | 15.18 | |||||||
| average | 0.79 | average | 2.13 | average | 5.59 | average | 15.19 | average | 19.36 | ||
| Intestinal tract membrane | 0.68 | 0.86 | 1.17 | 3.22 | 7.26 | ||||||
| lymph node-origin | 0.72 | 0.75 | 1.06 | 3.42 | 7.56 | ||||||
| interstitial cells | 0.54 | 0.83 | 1.21 | 3.39 | 7.33 | ||||||
| average | 0.65 | average | 0.81 | average | 1.15 | average | 3.34 | average | 7.38 | ||
| Fossa axillaris lymph | 0.56 | 0.62 | 1.02 | 2.78 | 6.14 | ||||||
| node-origin interstitial | 0.51 | 0.73 | 0.98 | 2.23 | 6.33 | ||||||
| cells (control) | 0.58 | 0.78 | 1.16 | 2.41 | 5.94 | ||||||
| average | 0.55 | average | 0.71 | average | 1.05 | average | 2.47 | average | 6.14 | ||
| Pulmonary hilar | 0.64 | 0.73 | 1.27 | 3.05 | 6.41 | ||||||
| node-origin | 0.68 | 0.70 | 1.33 | 3.11 | 6.34 | ||||||
| interstitial cells | 0.62 | 0.69 | 1.28 | 2.96 | 6.27 | ||||||
| average | 0.65 | average | 0.71 | average | 1.29 | average | 3.04 | average | 6.34 | ||
| Inguinal lymph | 0.48 | 0.55 | 0.97 | 2.33 | 5.86 | ||||||
| node-origin | 0.48 | 0.57 | 0.98 | 2.35 | 5.78 | ||||||
| interstitial cells (control) | 0.50 | 0.52 | 0.95 | 2.26 | 5.72 | ||||||
| average | 0.49 | average | 0.55 | average | 0.97 | average | 2.31 | average | 5.79 | ||
| Concentration of IFNβ (ng/mL) | |||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|---|
| 0 (ng/mL) | 10 (ng/mL) | 50 (ng/mL) | 100 (ng/mL) | 1000 (ng/mL) | 10,000 (ng/mL) | ||||||||
| Secretion of IgA (ng/mL) | Intestinal | 0.5 | 4.8 | 33.5 | 82.6 | 144.2 | 151.6 | ||||||
| Peyer’s patch | 0.6 | 4.5 | 34.2 | 83.6 | 140.2 | 153.3 | |||||||
| lymphocytes | 0.5 | 4.7 | 35.5 | 82.9 | 138.6 | 146.6 | |||||||
| average | 0.5 | average | 4.7 | average | 34.4 | average | 83.0 | average | 141.0 | average | 150.5 | ||
| Mesenteric | 0.3 | 2.2 | 13.4 | 26.8 | 35.8 | 37.7 | |||||||
| lymph node | 0.3 | 2.1 | 12.6 | 27.2 | 32.6 | 38.3 | |||||||
| lymphocytes | 0.4 | 2.2 | 13.9 | 28.4 | 34.8 | 35.7 | |||||||
| average | 0.3 | average | 2.2 | average | 13.3 | average | 27.5 | average | 34.4 | average | 37.2 | ||
| Fossa axillaris lymph | 0.2 | 1.1 | 3.4 | 4.6 | 7.3 | 8.4 | |||||||
| node lymphocytes | 0.2 | 1.3 | 3.6 | 4.4 | 7.2 | 8.1 | |||||||
| (control) | 0.2 | 1.2 | 3.8 | 4.2 | 7.4 | 8.2 | |||||||
| average | 0.2 | average | 1.2 | average | 3.6 | average | 4.4 | average | 7.3 | average | 8.2 | ||
| Pulmonary hilar | 0.2 | 1.8 | 11.6 | 22.7 | 31.5 | 34.2 | |||||||
| lymph node | 0.2 | 1.9 | 12.1 | 21.6 | 32.8 | 35.4 | |||||||
| lymphocytes | 0.3 | 2.0 | 10.9 | 20.4 | 30.1 | 33.7 | |||||||
| average | 0.2 | average | 1.9 | average | 11.5 | average | 21.6 | average | 31.5 | average | 34.4 | ||
| Inguinal lymph node | 0.1 | 0.9 | 3.2 | 4.4 | 6.8 | 8.1 | |||||||
| lymphocytes | 0.1 | 0.9 | 3.3 | 4.2 | 6.9 | 7.8 | |||||||
| (control) | 0.2 | 1.1 | 3.1 | 4.0 | 6.6 | 7.8 | |||||||
| average | 0.1 | average | 1.0 | average | 3.2 | average | 4.2 | average | 6.8 | average | 7.9 | ||
| Case | Animal | Age (Week) | Sex | Preparation | Materials | Methods | Object | Results | Publication |
|---|---|---|---|---|---|---|---|---|---|
| 1 | mouse | 8~12 | female | intranasally infection of influenza virus | nasal wash | ELISA | IgA | ↑ | Sangster et al. 2003 [18] |
| 2 | mouse | 6~8 | female | subcutaneous immunizationof influenza virus | serum | ELISA | IgA | ↑ | Guy et al. 1998 [19] |
| ↓ | |||||||||
| 3 | mouse | 6~ | male and female | oral inoculation and challenge of rotavirus | fecal pellet | ELISA | IgA | ↑ | Blutt et al. 2012 [20] |
| 4 | mouse | 6 | unidentified | canine distemper virus vaccination via intranasal route | intestinal mucus | ELISA | IgA | ↑ | Jiang et al. 2019 [21] |
| genital tract wash | ↑ | ||||||||
| nasal wash | ↑ | ||||||||
| lungs | ↑ | ||||||||
| fecal pellet | ↑ | ||||||||
| canine distemper virus vaccination via oral route | intestinal mucus | ELISA | IgA | ↑ | |||||
| genital tract wash | ↑ | ||||||||
| nasal wash | ↑ | ||||||||
| lungs | ↑ | ||||||||
| fecal pellet | ↑ | ||||||||
| 5 | mouse | 4~5 | female | specific antigen administration | serum | Enzyme-linkedimmunoassay | IgA | ↑ | Frossard et al. 2004 [22] |
| fecal pellet | → | ||||||||
| 6 | mouse | 8~72 | female | OVA administration and challenge | serum | ELISA | sIgA | ↑ | Santiago et al. 2008 [23] |
| (a) Viral Infection | (b) Bacterial Infection | (c) Other Causes of Death | |||
|---|---|---|---|---|---|
| (Non-Infection Cases) | |||||
| Case No. | Case No. | Case No. | |||
| V1 | Respiratory syncytial virus | B1 | Staphylococcus aureus | O1 | Heat stroke |
| Influenza virus | Acinetobacter sp. | O2 | Asphyxia | ||
| V2 | Respiratory syncytial virus | Citrobacter freundii | O3 | Acute circulation | |
| V3 | Respiratory syncytial virus | B2 | Mycoplasma pneumoniae | failure | |
| V4 | Unidentified | B3 | Staphylococcus aureus | O4 | Drowning |
| V5 | Respiratory syncytial virus | Enterococcus sp. | O5 | Fire fatality | |
| V6 | Cytomegalovirus | Mycoplasma pneumoniae | O6 | Heat stroke | |
| V7 | Adenoviridae | Atopobium parvulum | O7 | Blunt force injury | |
| V8 | Cytomegalovirus | Candida zeylanoides | (contusion of the lung) | ||
| Adenoviridae | Enterococcus sp. | O8 | Drowning | ||
| V9 | Respiratory syncytial virus | Enterococcus durans | O9 | Fire fatality | |
| Adenoviridae | Pseudomonas putida | ||||
| V10 | Adenoviridae | Rhodotorula mucilaginosa | |||
| V11 | Respiratory syncytial virus | Trichosporon sp. | |||
| B4 | Acinetobacter sp. | ||||
| Escherichia coli | |||||
| Enterococcus faecalis | |||||
| Micrococcus sp. | |||||
| B5 | Mycoplasma pneumoniae | ||||
| B6 | Mycoplasma pneumoniae | ||||
| Methicillin-resistant | |||||
| Staphylococcus aureus (MRSA) | |||||
| Escherichia coli | |||||
| Klebsiella pneumoniae | |||||
| B7 | Streptococcus sp. | ||||
| Klebsiella pneumoniae | |||||
| Enterobacter cloacae | |||||
| Bacillus cereus | |||||
| Morganella morganii | |||||
| Peptostreptococcus sp. | |||||
| Porphyromonas asaccharolytica | |||||
| Veillonella sp. | |||||
| B8 | Staphylococcus aureus | ||||
| Group B Streptococcus | |||||
| Pseudomonas aeruginosa | |||||
| B9 | Staphylococcus aureus | ||||
| Streptococcus sp. | |||||
| Acinetobacter sp. | |||||
| Escherichia coli | |||||
| Klebsiella pneumoniae | |||||
| Citrobacter freundii | |||||
| Candida sp. | |||||
| Enterococcus faecalis | |||||
| B10 | Staphylococcus aureus | ||||
| Streptococcus sp. | |||||
| Escherichia coli | |||||
| B11 | Methicillin-resistant | ||||
| Staphylococcus aureus (MRSA) | |||||
| Bacillus cereus | |||||
| B12 | Klebsiella pneumoniae | ||||
| B13 | Streptococcus sp. | ||||
| Escherichia coli | |||||
| Mycoplasma pneumoniae | |||||
| Staphylococcus aureus | |||||
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Aoki, Y.; Ikeda, T.; Tani, N.; Watanabe, M.; Ishikawa, T. Evaluation of the Relationships between Intestinal Regional Lymph Nodes and Immune Responses in Viral Infections in Children. Int. J. Mol. Sci. 2022, 23, 318. https://doi.org/10.3390/ijms23010318
Aoki Y, Ikeda T, Tani N, Watanabe M, Ishikawa T. Evaluation of the Relationships between Intestinal Regional Lymph Nodes and Immune Responses in Viral Infections in Children. International Journal of Molecular Sciences. 2022; 23(1):318. https://doi.org/10.3390/ijms23010318
Chicago/Turabian StyleAoki, Yayoi, Tomoya Ikeda, Naoto Tani, Miho Watanabe, and Takaki Ishikawa. 2022. "Evaluation of the Relationships between Intestinal Regional Lymph Nodes and Immune Responses in Viral Infections in Children" International Journal of Molecular Sciences 23, no. 1: 318. https://doi.org/10.3390/ijms23010318






