New Proteins Contributing to Immune Cell Infiltration and Pannus Formation of Synovial Membrane from Arthritis Diseases
Abstract
1. Introduction
2. Results
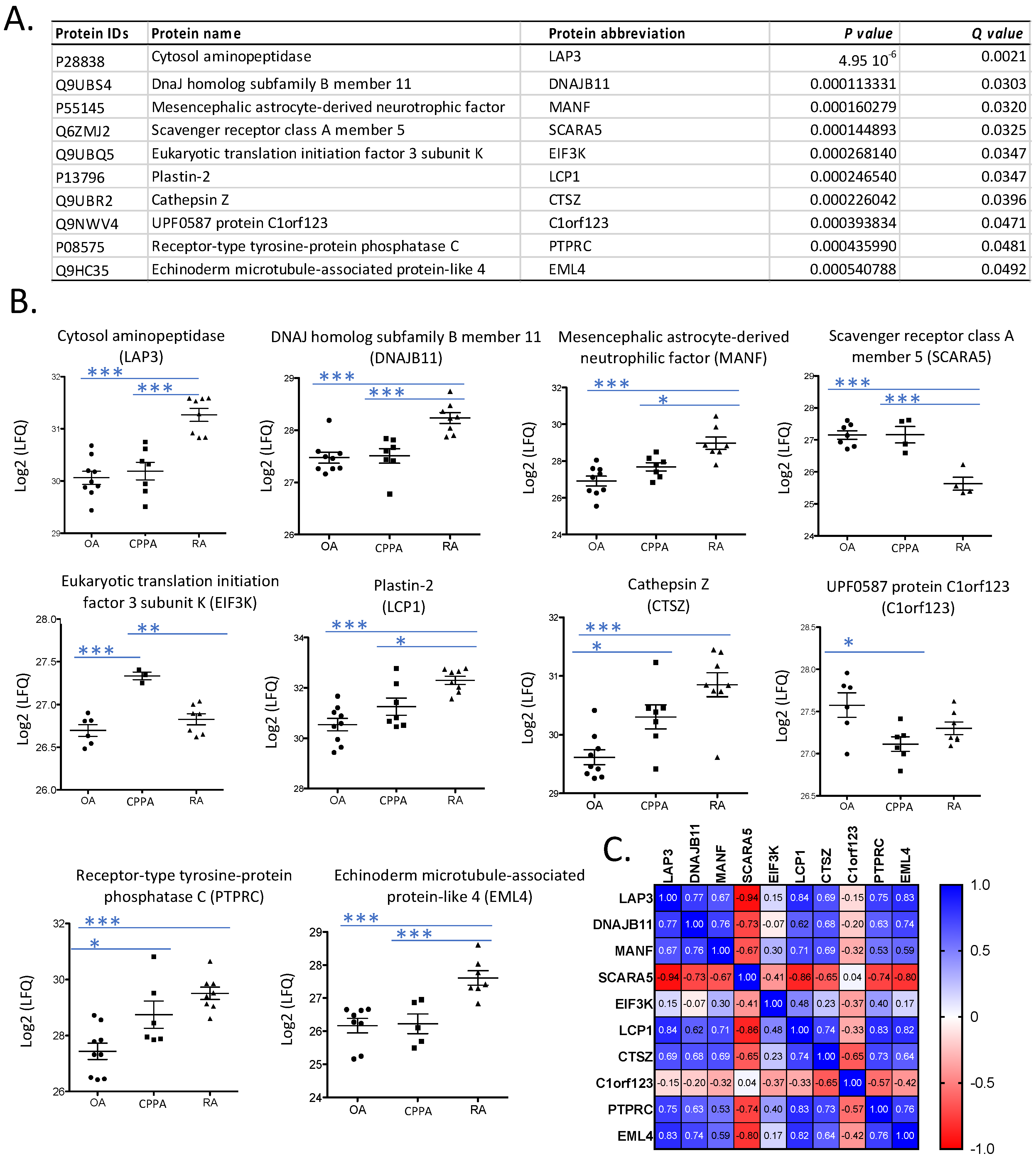
2.1. Proteomic Analysis
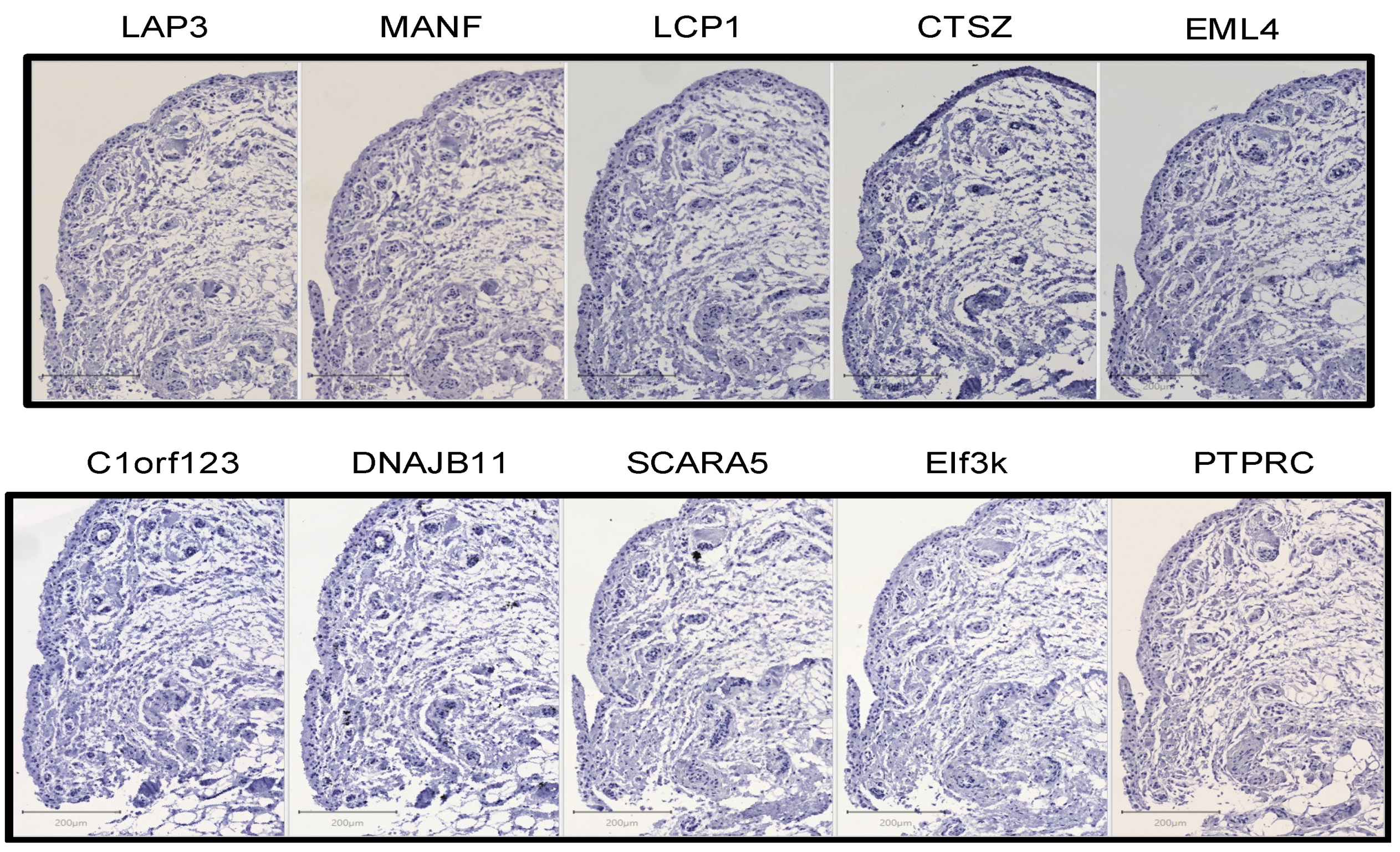
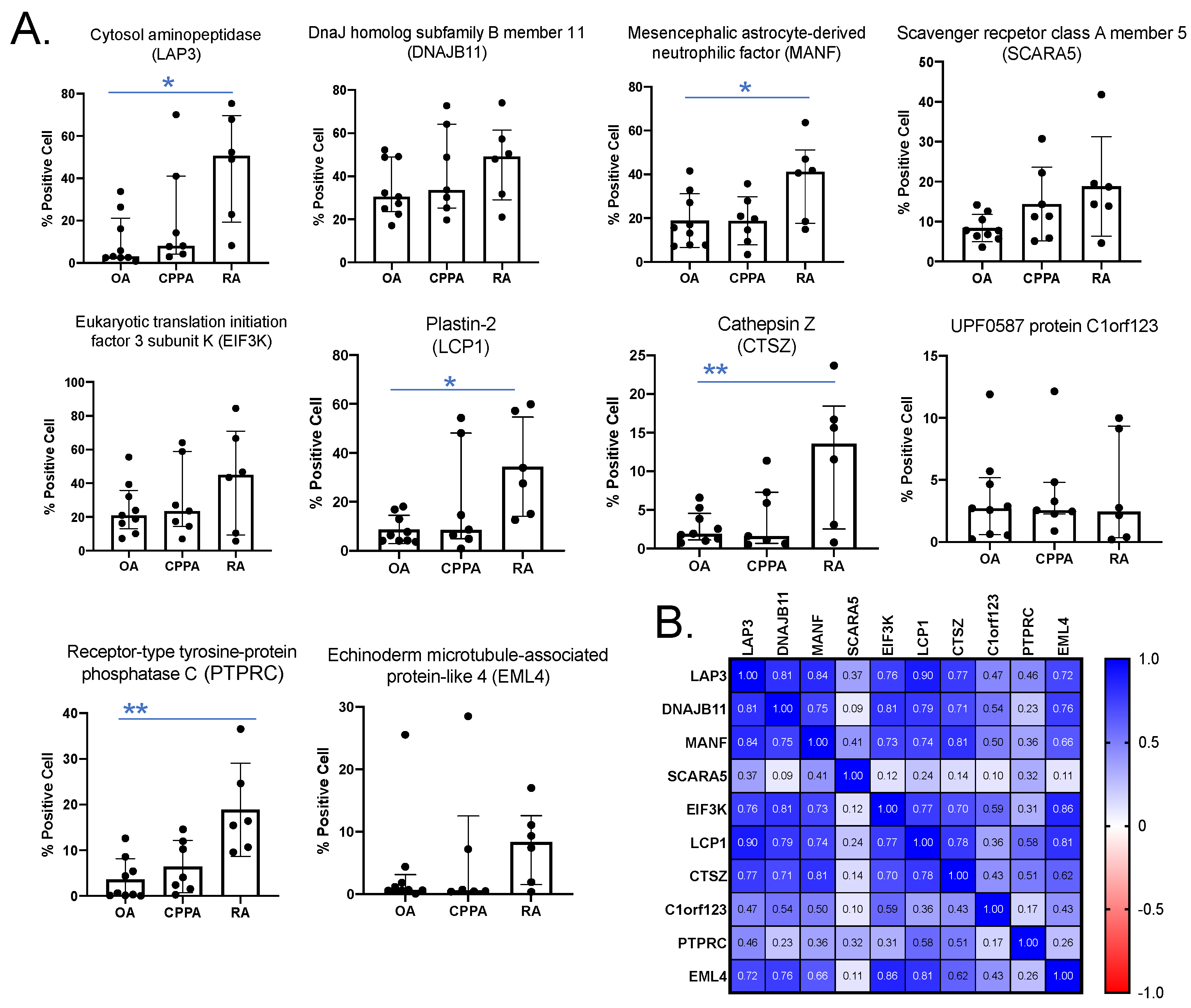
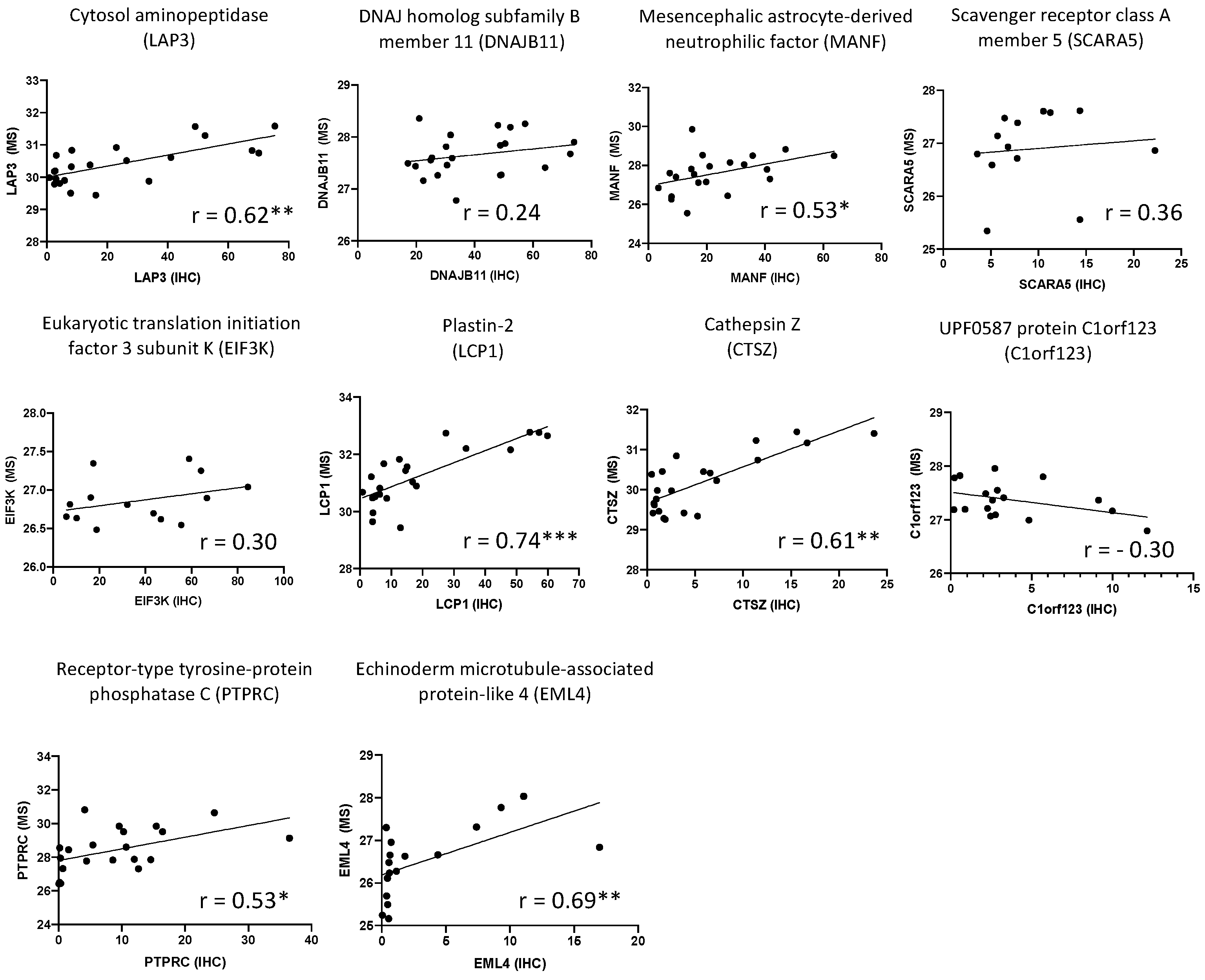
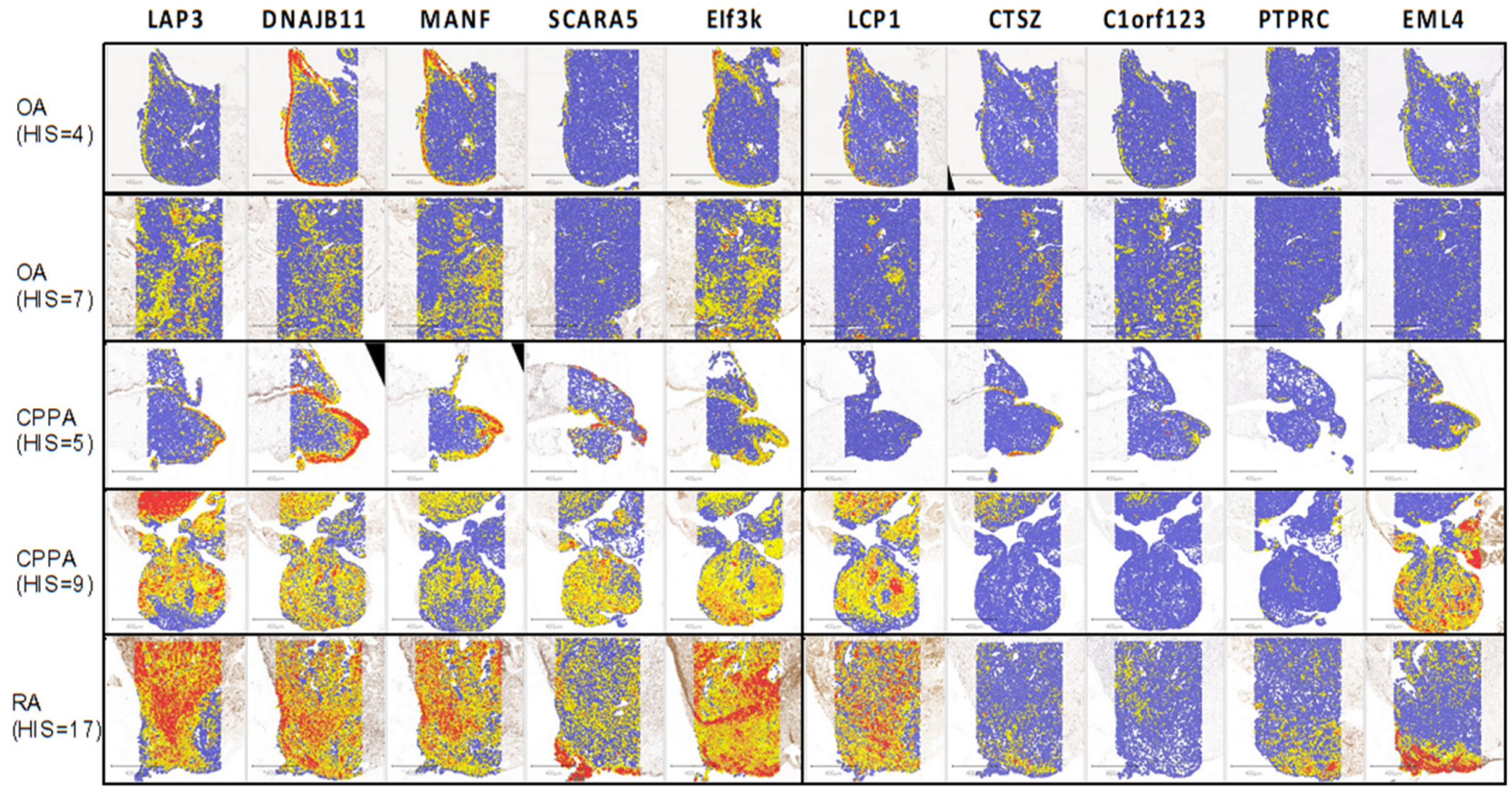
2.2. Immunohistochemistry
3. Discussion
4. Materials and Methods
4.1. Patients and Synovial Tissue
4.2. D-nano-UPLC-ESI-Q-Orbitrap for Proteomic Analysis
4.3. Immunohistochemistry
4.4. Quantification of IHC Using QuPath
4.5. Data Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
Appendix A
References
- Smith, M.D. The Normal Synovium. Open Rheumatol. J. 2011, 5, 100–106. [Google Scholar] [CrossRef]
- Scanzello, C.R.; Goldring, S.R. The Role of Synovitis in Osteoarthritis Pathogenesis. Bone 2012, 51, 249–257. [Google Scholar] [CrossRef]
- Croft, A.P.; Campos, J.; Jansen, K.; Turner, J.D.; Marshall, J.; Attar, M.; Savary, L.; Wehmeyer, C.; Naylor, A.J.; Kemble, S.; et al. Distinct Fibroblast Subsets Drive Inflammation and Damage in Arthritis. Nature 2019, 570, 246–251. [Google Scholar] [CrossRef]
- Chou, C.-H.; Jain, V.; Gibson, J.; Attarian, D.E.; Haraden, C.A.; Yohn, C.B.; Laberge, R.-M.; Gregory, S.; Kraus, V.B. Synovial Cell Cross-Talk with Cartilage Plays a Major Role in the Pathogenesis of Osteoarthritis. Sci. Rep. 2020, 10, 10868. [Google Scholar] [CrossRef]
- Zhang, F.; Wei, K.; Slowikowski, K.; Fonseka, C.Y.; Rao, D.A.; Kelly, S.; Goodman, S.M.; Tabechian, D.; Hughes, L.B.; Salomon-Escoto, K.; et al. Defining Inflammatory Cell States in Rheumatoid Arthritis Joint Synovial Tissues by Integrating Single-Cell Transcriptomics and Mass Cytometry. Nat. Immunol. 2019, 20, 928–942. [Google Scholar] [CrossRef]
- Alivernini, S.; MacDonald, L.; Elmesmari, A.; Finlay, S.; Tolusso, B.; Gigante, M.R.; Petricca, L.; Di Mario, C.; Bui, L.; Perniola, S.; et al. Distinct Synovial Tissue Macrophage Subsets Regulate Inflammation and Remission in Rheumatoid Arthritis. Nat. Med. 2020, 26, 1295–1306. [Google Scholar] [CrossRef]
- Culemann, S.; Grüneboom, A.; Nicolás-Ávila, J.Á.; Weidner, D.; Lämmle, K.F.; Rothe, T.; Quintana, J.A.; Kirchner, P.; Krljanac, B.; Eberhardt, M.; et al. Locally Renewing Resident Synovial Macrophages Provide a Protective Barrier for the Joint. Nature 2019, 572, 670–675. [Google Scholar] [CrossRef]
- Firestein, G.S.; McInnes, I.B. Immunopathogenesis of Rheumatoid Arthritis. Immunity 2017, 46, 183–196. [Google Scholar] [CrossRef]
- Smolen, J.S.; Aletaha, D.; Barton, A.; Burmester, G.R.; Emery, P.; Firestein, G.S.; Kavanaugh, A.; McInnes, I.B.; Solomon, D.H.; Strand, V.; et al. Rheumatoid Arthritis. Nat. Rev. Dis. Prim. 2018, 4, 18001. [Google Scholar] [CrossRef]
- Udalova, I.A.; Mantovani, A.; Feldmann, M. Macrophage Heterogeneity in the Context of Rheumatoid Arthritis. Nat. Rev. Rheumatol. 2016, 12, 472–485. [Google Scholar] [CrossRef]
- Filer, A. The Fibroblast as a Therapeutic Target in Rheumatoid Arthritis. Curr. Opin. Pharmacol. 2013, 13, 413–419. [Google Scholar] [CrossRef]
- Roemer, F.W.; Kassim Javaid, M.; Guermazi, A.; Thomas, M.; Kiran, A.; Keen, R.; King, L.; Arden, N.K. Anatomical Distribution of Synovitis in Knee Osteoarthritis and Its Association with Joint Effusion Assessed on Non-Enhanced and Contrast-Enhanced MRI. Osteoarthr. Cartil. 2010, 18, 1269–1274. [Google Scholar] [CrossRef]
- Sellam, J.; Berenbaum, F. The Role of Synovitis in Pathophysiology and Clinical Symptoms of Osteoarthritis. Nat. Rev. Rheumatol. 2010, 6, 625–635. [Google Scholar] [CrossRef]
- Griffin, T.M.; Scanzello, C.R. Innate Inflammation and Synovial Macrophages in Osteoarthritis Pathophysiology. Clin. Exp. Rheumatol. 2019, 37 (Suppl. 1), 57–63. [Google Scholar]
- de Seny, D.; Bianchi, E.; Baiwir, D.; Cobraiville, G.; Collin, C.; Deliège, M.; Kaiser, M.-J.; Mazzucchelli, G.; Hauzeur, J.-P.; Delvenne, P.; et al. Proteins Involved in the Endoplasmic Reticulum Stress Are Modulated in Synovitis of Osteoarthritis, Chronic Pyrophosphate Arthropathy and Rheumatoid Arthritis, and Correlate with the Histological Inflammatory Score. Sci. Rep. 2020, 10, 14159. [Google Scholar] [CrossRef]
- Hayashi, J.; Kihara, M.; Kato, H.; Nishimura, T. A Proteomic Profile of Synoviocyte Lesions Microdissected from Formalin-Fixed Paraffin-Embedded Synovial Tissues of Rheumatoid Arthritis. Clin. Proteom. 2015, 12, 20. [Google Scholar] [CrossRef]
- Sehnert, B.; Cavcic, A.; Böhm, B.; Kalden, J.R.; Nandakumar, K.S.; Holmdahl, R.; Burkhardt, H. Antileukoproteinase: Modulation of Neutrophil Function and Therapeutic Effects on Anti-Type II Collagen Antibody-Induced Arthritis. Arthritis Rheum. 2004, 50, 2347–2359. [Google Scholar] [CrossRef]
- Kamijo, S.; Nakajima, A.; Kamata, K.; Kurosawa, H.; Yagita, H.; Okumura, K. Involvement of TWEAK/Fn14 Interaction in the Synovial Inflammation of RA. Rheumatology 2008, 47, 442–450. [Google Scholar] [CrossRef]
- Yang, Q.; Roehrl, M.H.; Wang, J.Y. Proteomic Profiling of Antibody-Inducing Immunogens in Tumor Tissue Identifies PSMA1, LAP3, ANXA3, and Maspin as Colon Cancer Markers. Oncotarget 2018, 9, 3996–4019. [Google Scholar] [CrossRef]
- Tian, S.-Y.; Chen, S.-H.; Shao, B.-F.; Cai, H.-Y.; Zhou, Y.; Zhou, Y.-L.; Xu, A.-B. Expression of Leucine Aminopeptidase 3 (LAP3) Correlates with Prognosis and Malignant Development of Human Hepatocellular Carcinoma (HCC). Int. J. Clin. Exp. Pathol. 2014, 7, 3752–3762. [Google Scholar]
- Wang, X.; Shi, L.; Deng, Y.; Qu, M.; Mao, S.; Xu, L.; Xu, W.; Fang, C. Inhibition of Leucine Aminopeptidase 3 Suppresses Invasion of Ovarian Cancer Cells through Down-Regulation of Fascin and MMP-2/9. Eur. J. Pharmacol. 2015, 768, 116–122. [Google Scholar] [CrossRef]
- Zhang, S.; Yang, X.; Shi, H.; Li, M.; Xue, Q.; Ren, H.; Yao, L.; Chen, X.; Zhang, J.; Wang, H. Overexpression of Leucine Aminopeptidase 3 Contributes to Malignant Development of Human Esophageal Squamous Cell Carcinoma. J. Mol. Histol. 2014, 45, 283–292. [Google Scholar] [CrossRef]
- Fang, C.; Zhang, J.; Yang, H.; Peng, L.; Wang, K.; Wang, Y.; Zhao, X.; Liu, H.; Dou, C.; Shi, L.; et al. Leucine Aminopeptidase 3 Promotes Migration and Invasion of Breast Cancer Cells through Upregulation of Fascin and Matrix Metalloproteinases-2/9 Expression. J. Cell. Biochem. 2019, 120, 3611–3620. [Google Scholar] [CrossRef]
- He, X.; Huang, Q.; Qiu, X.; Liu, X.; Sun, G.; Guo, J.; Ding, Z.; Yang, L.; Ban, N.; Tao, T.; et al. LAP3 Promotes Glioma Progression by Regulating Proliferation, Migration and Invasion of Glioma Cells. Int. J. Biol. Macromol. 2015, 72, 1081–1089. [Google Scholar] [CrossRef]
- Koide, N.; Kasamatsu, A.; Endo-Sakamoto, Y.; Ishida, S.; Shimizu, T.; Kimura, Y.; Miyamoto, I.; Yoshimura, S.; Shiiba, M.; Tanzawa, H.; et al. Evidence for Critical Role of Lymphocyte Cytosolic Protein 1 in Oral Cancer. Sci. Rep. 2017, 7, 43379. [Google Scholar] [CrossRef]
- Chen, C.; Cai, Q.; He, W.; Lam, T.B.; Lin, J.; Zhao, Y.; Chen, X.; Gu, P.; Huang, H.; Xue, M.; et al. AP4 Modulated by the PI3K/AKT Pathway Promotes Prostate Cancer Proliferation and Metastasis of Prostate Cancer via Upregulating L-Plastin. Cell Death Dis. 2017, 8, e3060. [Google Scholar] [CrossRef]
- Ge, X.; Liu, W.; Zhao, W.; Feng, S.; Duan, A.; Ji, C.; Shen, K.; Liu, W.; Zhou, J.; Jiang, D.; et al. Exosomal Transfer of LCP1 Promotes Osteosarcoma Cell Tumorigenesis and Metastasis by Activating the JAK2/STAT3 Signaling Pathway. Mol. Ther. Nucleic Acids 2020, 21, 900–915. [Google Scholar] [CrossRef]
- Wabnitz, G.; Balta, E.; Samstag, Y. L-Plastin Regulates the Stability of the Immune Synapse of Naive and Effector T-Cells. Adv. Biol. Regul. 2017, 63, 107–114. [Google Scholar] [CrossRef]
- Morley, S.C. The Actin-Bundling Protein L-Plastin Supports T-Cell Motility and Activation. Immunol. Rev. 2013, 256, 48–62. [Google Scholar] [CrossRef]
- Zhou, J.Y.; Szasz, T.P.; Stewart-Hutchinson, P.J.; Sivapalan, J.; Todd, E.M.; Deady, L.E.; Cooper, J.A.; Onken, M.D.; Morley, S.C. L-Plastin Promotes Podosome Longevity and Supports Macrophage Motility. Mol. Immunol. 2016, 78, 79–88. [Google Scholar] [CrossRef]
- Chellaiah, M.A.; Ma, T.; Majumdar, S. L-Plastin Phosphorylation Regulates the Early Phase of Sealing Ring Formation by Actin Bundling Process in Mouse Osteoclasts. Exp. Cell Res. 2018, 372, 73–82. [Google Scholar] [CrossRef]
- Chellaiah, M.A.; Majumdar, S.; Aljohani, H. Peptidomimetic Inhibitors of L-Plastin Reduce the Resorptive Activity of Osteoclast but Not the Bone Forming Activity of Osteoblasts In Vitro. PLoS ONE 2018, 13, e0204209. [Google Scholar] [CrossRef]
- Kos, J.; Jevnikar, Z.; Obermajer, N. The Role of Cathepsin X in Cell Signaling. Cell Adh. Migr. 2009, 3, 164–166. [Google Scholar] [CrossRef]
- Obermajer, N.; Svajger, U.; Bogyo, M.; Jeras, M.; Kos, J. Maturation of Dendritic Cells Depends on Proteolytic Cleavage by Cathepsin, X.J. Leukoc. Biol. 2008, 84, 1306–1315. [Google Scholar] [CrossRef]
- Lechner, A.M.; Assfalg-Machleidt, I.; Zahler, S.; Stoeckelhuber, M.; Machleidt, W.; Jochum, M.; Nägler, D.K. RGD-Dependent Binding of Procathepsin X to Integrin Alphavbeta3 Mediates Cell-Adhesive Properties. J. Biol. Chem. 2006, 281, 39588–39597. [Google Scholar] [CrossRef]
- Akkari, L.; Gocheva, V.; Kester, J.C.; Hunter, K.E.; Quick, M.L.; Sevenich, L.; Wang, H.-W.; Peters, C.; Tang, L.H.; Klimstra, D.S.; et al. Distinct Functions of Macrophage-Derived and Cancer Cell-Derived Cathepsin Z Combine to Promote Tumor Malignancy via Interactions with the Extracellular Matrix. Genes Dev. 2014, 28, 2134–2150. [Google Scholar] [CrossRef]
- Jakoš, T.; Pišlar, A.; Jewett, A.; Kos, J. Cysteine Cathepsins in Tumor-Associated Immune Cells. Front. Immunol. 2019, 10, 2037. [Google Scholar] [CrossRef]
- Vidak, E.; Javoršek, U.; Vizovišek, M.; Turk, B. Cysteine Cathepsins and Their Extracellular Roles: Shaping the Microenvironment. Cells 2019, 8, 264. [Google Scholar] [CrossRef]
- Kelley, J.L.; Ozment, T.R.; Li, C.; Schweitzer, J.B.; Williams, D.L. Scavenger Receptor-A (CD204): A Two-Edged Sword in Health and Disease. Crit. Rev. Immunol. 2014, 34, 241–261. [Google Scholar] [CrossRef]
- Guo, D.; Cao, C.; Zhang, X.; Xiang, L.; Shao, J. Scavenger Receptor SCARA5 Acts as an HMGB1 Recognition Molecule Negatively Involved in HMGB1-Mediated Inflammation in Fish Models. J. Immunol. 2016, 197, 3198–3213. [Google Scholar] [CrossRef]
- Ojala, J.R.M.; Pikkarainen, T.; Elmberger, G.; Tryggvason, K. Progressive Reactive Lymphoid Connective Tissue Disease and Development of Autoantibodies in Scavenger Receptor A5-Deficient Mice. Am. J. Pathol. 2013, 182, 1681–1695. [Google Scholar] [CrossRef]
- Zhang, H.; Liu, C.; Wang, X.; Wang, Y.; Zheng, J. SCARA5 Inhibits Gastric Cancer Progression via Epithelial-Mesenchymal Transition Suppression. J. Cancer 2021, 12, 2412–2421. [Google Scholar] [CrossRef]
- Huang, J.; Zheng, D.-L.; Qin, F.-S.; Cheng, N.; Chen, H.; Wan, B.-B.; Wang, Y.-P.; Xiao, H.-S.; Han, Z.-G. Genetic and Epigenetic Silencing of SCARA5 May Contribute to Human Hepatocellular Carcinoma by Activating FAK Signaling. J. Clin. Investig. 2010, 120, 223–241. [Google Scholar] [CrossRef]
- Wen, X.; Wang, N.; Zhang, F.; Dong, C. Overexpression of SCARA5 Inhibits Tumor Proliferation and Invasion in Osteosarcoma via Suppression of the FAK Signaling Pathway. Mol. Med. Rep. 2016, 13, 2885–2891. [Google Scholar] [CrossRef][Green Version]
- You, K.; Su, F.; Liu, L.; Lv, X.; Zhang, J.; Zhang, Y.; Liu, B. SCARA5 Plays a Critical Role in the Progression and Metastasis of Breast Cancer by Inactivating the ERK1/2, STAT3, and AKT Signaling Pathways. Mol. Cell. Biochem. 2017, 435, 47–58. [Google Scholar] [CrossRef]
- Liu, J.; Hu, G.; Chen, D.; Gong, A.-Y.; Soori, G.S.; Dobleman, T.J.; Chen, X.-M. Suppression of SCARA5 by Snail1 Is Essential for EMT-Associated Cell Migration of A549 Cells. Oncogenesis 2013, 2, e73. [Google Scholar] [CrossRef]
- Lee, H.; Lee, Y.J.; Choi, H.; Seok, J.W.; Yoon, B.K.; Kim, D.; Han, J.Y.; Lee, Y.; Kim, H.J.; Kim, J.-W. SCARA5 Plays a Critical Role in the Commitment of Mesenchymal Stem Cells to Adipogenesis. Sci. Rep. 2017, 7, 14833. [Google Scholar] [CrossRef]
- Zhao, J.; Jian, L.; Zhang, L.; Ding, T.; Li, X.; Cheng, D.; Niu, S.; Sun, L.; Li, E.; Liu, S.; et al. Knockdown of SCARA5 Inhibits PDGF-BB-Induced Vascular Smooth Muscle Cell Proliferation and Migration through Suppression of the PDGF Signaling Pathway. Mol. Med. Rep. 2016, 13, 4455–4460. [Google Scholar] [CrossRef]
- Neves, J.; Zhu, J.; Sousa-Victor, P.; Konjikusic, M.; Riley, R.; Chew, S.; Qi, Y.; Jasper, H.; Lamba, D.A. Immune Modulation by MANF Promotes Tissue Repair and Regenerative Success in the Retina. Science 2016, 353, aaf3646. [Google Scholar] [CrossRef]
- Chen, L.; Feng, L.; Wang, X.; Du, J.; Chen, Y.; Yang, W.; Zhou, C.; Cheng, L.; Shen, Y.; Fang, S.; et al. Mesencephalic Astrocyte-Derived Neurotrophic Factor Is Involved in Inflammation by Negatively Regulating the NF-ΚB Pathway. Sci. Rep. 2015, 5, 8133. [Google Scholar] [CrossRef]
- Hakonen, E.; Chandra, V.; Fogarty, C.L.; Yu, N.Y.-L.; Ustinov, J.; Katayama, S.; Galli, E.; Danilova, T.; Lindholm, P.; Vartiainen, A.; et al. MANF Protects Human Pancreatic Beta Cells against Stress-Induced Cell Death. Diabetologia 2018, 61, 2202–2214. [Google Scholar] [CrossRef] [PubMed]
- Hou, C.; Mei, Q.; Song, X.; Bao, Q.; Li, X.; Wang, D.; Shen, Y. Mono-Macrophage-Derived MANF Protects Against Lipopolysaccharide-Induced Acute Kidney Injury via Inhibiting Inflammation and Renal M1 Macrophages. Inflammation 2021, 44, 693–703. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; Neves, J.; Cedron-Craft, W.; Ventura, P.B.; Liao, C.-Y.; Riley, R.R.; Soifer, I.; van Bruggen, N.; Kolumam, G.A.; Villeda, S.A.; et al. MANF Regulates Metabolic and Immune Homeostasis in Ageing and Protects against Liver Damage. Nat. Metab. 2019, 1, 276–290. [Google Scholar] [CrossRef] [PubMed]
- Sousa-Victor, P.; Jasper, H.; Neves, J. Trophic Factors in Inflammation and Regeneration: The Role of ManF and CDNF. Front. Physiol. 2018, 9, 1629. [Google Scholar] [CrossRef] [PubMed]
- Guo, F.; Snapp, E.L. ERdj3 Regulates BiP Occupancy in Living Cells. J. Cell Sci. 2013, 126 Pt 6, 1429–1439. [Google Scholar] [CrossRef]
- Genereux, J.C.; Qu, S.; Zhou, M.; Ryno, L.M.; Wang, S.; Shoulders, M.D.; Kaufman, R.J.; Lasmézas, C.I.; Kelly, J.W.; Wiseman, R.L. Unfolded Protein Response-induced ER Dj3 Secretion Links ER Stress to Extracellular Proteostasis. EMBO J. 2015, 34, 4–19. [Google Scholar] [CrossRef]
- Rheinländer, A.; Schraven, B.; Bommhardt, U. CD45 in Human Physiology and Clinical Medicine. Immunol. Lett. 2018, 196, 22–32. [Google Scholar] [CrossRef]
- Cao, Z.; Gao, Q.; Fu, M.; Ni, N.; Pei, Y.; Ou, W.-B. Anaplastic Lymphoma Kinase Fusions: Roles in Cancer and Therapeutic Perspectives. Oncol. Lett. 2019, 17, 2020–2030. [Google Scholar] [CrossRef]
- Soda, M.; Choi, Y.L.; Enomoto, M.; Takada, S.; Yamashita, Y.; Ishikawa, S.; Fujiwara, S.; Watanabe, H.; Kurashina, K.; Hatanaka, H.; et al. Identification of the Transforming EML4-ALK Fusion Gene in Non-Small-Cell Lung Cancer. Nature 2007, 448, 561–566. [Google Scholar] [CrossRef]
- Ji, J.H.; Oh, Y.L.; Hong, M.; Yun, J.W.; Lee, H.-W.; Kim, D.; Ji, Y.; Kim, D.-H.; Park, W.-Y.; Shin, H.-T.; et al. Identification of Driving ALK Fusion Genes and Genomic Landscape of Medullary Thyroid Cancer. PLoS Genet. 2015, 11, e1005467. [Google Scholar] [CrossRef]
- Yin, Y.; Long, J.; Sun, Y.; Li, H.; Jiang, E.; Zeng, C.; Zhu, W. The Function and Clinical Significance of EIF3 in Cancer. Gene 2018, 673, 130–133. [Google Scholar] [CrossRef] [PubMed]
- Lin, Y.-M.; Chen, Y.-R.; Lin, J.-R.; Wang, W.-J.; Inoko, A.; Inagaki, M.; Wu, Y.-C.; Chen, R.-H. EIF3k Regulates Apoptosis in Epithelial Cells by Releasing Caspase 3 from Keratin-Containing Inclusions. J. Cell Sci. 2008, 121 Pt 14, 2382–2393. [Google Scholar] [CrossRef] [PubMed]
- Restelli, L.; Codrea, M.C.; Savoini, G.; Ceciliani, F.; Bendixen, E. LC-MS/MS Analysis of Visceral and Subcutaneous Adipose Tissue Proteomes in Young Goats with Focus on Innate Immunity and Inflammation Related Proteins. J. Proteom. 2014, 108, 295–305. [Google Scholar] [CrossRef]
- Deng, R.-P.; He, X.; Guo, S.-J.; Liu, W.-F.; Tao, Y.; Tao, S.-C. Global Identification of O-GlcNAc Transferase (OGT) Interactors by a Human Proteome Microarray and the Construction of an OGT Interactome. Proteomics 2014, 14, 1020–1030. [Google Scholar] [CrossRef] [PubMed]
- Furukawa, Y.; Lim, C.; Tosha, T.; Yoshida, K.; Hagai, T.; Akiyama, S.; Watanabe, S.; Nakagome, K.; Shiro, Y. Identification of a Novel Zinc-Binding Protein, C1orf123, as an Interactor with a Heavy Metal-Associated Domain. PLoS ONE 2018, 13, e0204355. [Google Scholar] [CrossRef] [PubMed]
- Rahaman, S.N.A.; Mat Yusop, J.; Mohamed-Hussein, Z.-A.; Aizat, W.M.; Ho, K.L.; Teh, A.-H.; Waterman, J.; Tan, B.K.; Tan, H.L.; Li, A.Y.; et al. Crystal Structure and Functional Analysis of Human C1ORF123. PeerJ 2018, 6, e5377. [Google Scholar] [CrossRef]
- Dennis, G.; Holweg, C.T.J.; Kummerfeld, S.K.; Choy, D.F.; Setiadi, A.F.; Hackney, J.A.; Haverty, P.M.; Gilbert, H.; Lin, W.Y.; Diehl, L.; et al. Synovial Phenotypes in Rheumatoid Arthritis Correlate with Response to Biologic Therapeutics. Arthritis Res. Ther. 2014, 16, R90. [Google Scholar] [CrossRef] [PubMed]
- Floudas, A.; Canavan, M.; McGarry, T.; Mullan, R.; Nagpal, S.; Veale, D.J.; Fearon, U. ACPA Status Correlates with Differential Immune Profile in Patients with Rheumatoid Arthritis. Cells 2021, 10, 647. [Google Scholar] [CrossRef]
- Gupta, A.; Kaushik, R.; Kaushik, R.M.; Saini, M.; Kakkar, R. Association of Anti-Cyclic Citrullinated Peptide Antibodies with Clinical and Radiological Disease Severity in Rheumatoid Arthritis. Curr. Rheumatol. Rev. 2014, 10, 136–143. [Google Scholar] [CrossRef]
- Kellgren, J.H.; Lawrence, J.S. Radiological Assessment of Osteo-Arthrosis. Ann. Rheum. Dis. 1957, 16, 494–502. [Google Scholar] [CrossRef]
- Tak, P.P.; Thurkow, E.W.; Daha, M.R.; Kluin, P.M.; Smeets, T.J.M.; Meinders, A.E.; Breedveld, F.C. Expression of Adhesion Molecules in Early Rheumatoid Synovial Tissue. Clin. Immunol. Immunopathol. 1995, 77, 236–242. [Google Scholar] [CrossRef]
- Costanza, B.; Turtoi, A.; Bellahcène, A.; Hirano, T.; Peulen, O.; Blomme, A.; Hennequière, V.; Mutijima, E.; Boniver, J.; Meuwis, M.-A.; et al. Innovative Methodology for the Identification of Soluble Biomarkers in Fresh Tissues. Oncotarget 2018, 9, 10665–10680. [Google Scholar] [CrossRef] [PubMed]
- Bankhead, P.; Loughrey, M.B.; Fernández, J.A.; Dombrowski, Y.; McArt, D.G.; Dunne, P.D.; McQuaid, S.; Gray, R.T.; Murray, L.J.; Coleman, H.G.; et al. QuPath: Open Source Software for Digital Pathology Image Analysis. Sci. Rep. 2017, 7, 16878. [Google Scholar] [CrossRef] [PubMed]
- Cox, J.; Hein, M.Y.; Luber, C.A.; Paron, I.; Nagaraj, N.; Mann, M. Accurate Proteome-Wide Label-Free Quantification by Delayed Normalization and Maximal Peptide Ratio Extraction, Termed MaxLFQ. Mol. Cell. Proteom. 2014, 13, 2513–2526. [Google Scholar] [CrossRef] [PubMed]
| Mass Spectrometry | ||||||||||||
|---|---|---|---|---|---|---|---|---|---|---|---|---|
| MS | HIS (0–18) | hs (0–4) | ly (0–4) | pl (0–4) | PMN (0–3) | MΦ (0–3) | ||||||
| LAP3 | 0.77 | *** | 0.38 | 0.78 | *** | 0.67 | *** | 0.63 | *** | 0.61 | ** | |
| DNAJB11 | 0.77 | *** | 0.43 | 0.71 | *** | 0.81 | *** | 0.58 | ** | 0.48 | ||
| MANF | 0.79 | *** | 0.59 | ** | 0.75 | *** | 0.80 | *** | 0.54 | ** | 0.41 | |
| SCARA5 | −0.85 | *** | −0.33 | −0.90 | *** | −0.62 | −0.80 | *** | −0.65 | ** | ||
| EIF3K | 0.19 | 0.13 | 0.33 | −0.03 | 0.06 | 0.37 | ||||||
| LCP1 | 0.74 | *** | 0.30 | 0.73 | *** | 0.59 | ** | 0.61 | ** | 0.74 | *** | |
| CTSZ | 0.69 | *** | 0.39 | 0.62 | ** | 0.54 | ** | 0.63 | ** | 0.63 | ** | |
| C1orf123 | −0.37 | −0.36 | −0.34 | −0.25 | -0.16 | −0.42 | ||||||
| PTPRC | 0.68 | *** | 0.22 | 0.68 | *** | 0.52 | 0.54 | ** | 0.75 | *** | ||
| EML4 | 0.78 | *** | 0.58 | ** | 0.76 | *** | 0.66 | ** | 0.55 | 0.67 | ** | |
| Immunohistochemistry | ||||||||||||
| IHC | HIS (0–18) | hs (0–4) | ly (0–4) | pl (0–4) | PMN (0–3) | MΦ(0–3) | ||||||
| LAP3 | 0.83 | *** | 0.48 | 0.78 | *** | 0.62 | ** | 0.62 | ** | 0.68 | *** | |
| DNAJB11 | 0.54 | ** | 0.30 | 0.49 | 0.33 | 0.32 | 0.45 | |||||
| MANF | 0.70 | *** | 0.50 | 0.68 | *** | 0.52 | 0.55 | ** | 0.51 | |||
| SCARA5 | 0.40 | 0.30 | 0.46 | 0.47 | 0.22 | 0.20 | ||||||
| EIF3K | 0.47 | 0.21 | 0.41 | 0.34 | 0.46 | 0.47 | ||||||
| LCP1 | 0.82 | *** | 0.48 | 0.71 | *** | 0.70 | *** | 0.63 | ** | 0.69 | *** | |
| CTSZ | 0.60 | ** | 0.38 | 0.57 | ** | 0.39 | 0.64 | ** | 0.55 | ** | ||
| C1orf123 | 0.07 | −0.17 | 0.02 | 0.10 | 0.02 | −0.05 | ||||||
| PTPRC | 0.48 | 0.23 | 0.40 | 0.58 | ** | 0.67 | *** | 0.41 | ||||
| EML4 | 0.56 | ** | 0.31 | 0.48 | 0.39 | 0.31 | 0.56 | ** | ||||
| Method | Method | Correlation | Correlation | Correlation | Localization by IHC | ||
|---|---|---|---|---|---|---|---|
| MS/MS | IHC | MS/MS vs IHC | MS/MS vs HIS | IHC vs HIS | |||
| Increased expression in RA | LAP3 | V | V | V | V | V | Lining in OA/CPPA with low HIS; Stroma of OA/CPPA/RA with high HIS |
| DNAJB11 | V | X | X | V | V | Lining in OA/CPPA with low HIS; Stroma of OA/CPPA/RA with high HIS | |
| MANF | V | V | V | V | V | Lining in OA/CPPA with low HIS; Stroma of OA/CPPA/RA with high HIS | |
| LCP1 | V | V | V | V | V | Stroma of CPPA/RA with high HIS | |
| CTSZ | V | V | V | V | V | Stroma of RA with high HIS | |
| PTPRC | V | V | V | V | X | Stroma of RA with high HIS | |
| EML4 | V | (V) * | V | V | V | Stroma of CPPA/RA with high HIS | |
| Decreased expression in RA | SCARA5 | V | X | X | V | X | Stroma of CPPA/RA with high HIS |
| Increased expression in CPPA | EIF3K | V | X | X | X | X | Lining in OA/CPPA with low HIS; Stroma of OA/CPPA/RA with high HIS |
| Increased expression in OA | C1orf123 | V | X | X | X | X | Not a high percentage of positive cells |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2021 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
de Seny, D.; Baiwir, D.; Bianchi, E.; Cobraiville, G.; Deroyer, C.; Poulet, C.; Malaise, O.; Paulissen, G.; Kaiser, M.-J.; Hauzeur, J.-P.; et al. New Proteins Contributing to Immune Cell Infiltration and Pannus Formation of Synovial Membrane from Arthritis Diseases. Int. J. Mol. Sci. 2022, 23, 434. https://doi.org/10.3390/ijms23010434
de Seny D, Baiwir D, Bianchi E, Cobraiville G, Deroyer C, Poulet C, Malaise O, Paulissen G, Kaiser M-J, Hauzeur J-P, et al. New Proteins Contributing to Immune Cell Infiltration and Pannus Formation of Synovial Membrane from Arthritis Diseases. International Journal of Molecular Sciences. 2022; 23(1):434. https://doi.org/10.3390/ijms23010434
Chicago/Turabian Stylede Seny, Dominique, Dominique Baiwir, Elettra Bianchi, Gaël Cobraiville, Céline Deroyer, Christophe Poulet, Olivier Malaise, Geneviève Paulissen, Marie-Joëlle Kaiser, Jean-Philippe Hauzeur, and et al. 2022. "New Proteins Contributing to Immune Cell Infiltration and Pannus Formation of Synovial Membrane from Arthritis Diseases" International Journal of Molecular Sciences 23, no. 1: 434. https://doi.org/10.3390/ijms23010434
APA Stylede Seny, D., Baiwir, D., Bianchi, E., Cobraiville, G., Deroyer, C., Poulet, C., Malaise, O., Paulissen, G., Kaiser, M.-J., Hauzeur, J.-P., Mazzucchelli, G., Delvenne, P., & Malaise, M. (2022). New Proteins Contributing to Immune Cell Infiltration and Pannus Formation of Synovial Membrane from Arthritis Diseases. International Journal of Molecular Sciences, 23(1), 434. https://doi.org/10.3390/ijms23010434






