Structural and Functional Deviations of the Hippocampus in Schizophrenia and Schizophrenia Animal Models
Abstract
:1. Introduction
2. Structural Abnormalities of the Hippocampus in Schizophrenia Patients
3. Functional Aberrations of the Hippocampus in Schizophrenia Patients
4. Genetic and Environmental Risk Factors Induce Hippocampal Deviations
5. Inflammatory Processes Contribute to Hippocampal Abnormalities
6. Structural and Functional Hippocampus Deviations in Animal Models for Schizophrenia
7. Interneuron-Abnormalities in the Hippocampus of Schizophrenic Patients and Schizophrenia Animal Models
8. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Adityanjee; Aderibigbe, Y.A.; Theodoridis, D.; Vieweg, V.R. Dementia praecox to schizophrenia: The first 100 years. Psychiatry Clin. Neurosci. 1999, 53, 437–448. [Google Scholar] [CrossRef] [PubMed]
- Moskowitz, A.; Heim, G. Eugen Bleuler’s Dementia praecox or the group of schizophrenias (1911): A centenary appreciation and reconsideration. Schizophr. Bull. 2011, 37, 471–479. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Saha, S.; Chant, D.; Welham, J.; McGrath, J. A systematic review of the prevalence of schizophrenia. PLoS Med. 2005, 2, e141. [Google Scholar] [CrossRef] [PubMed]
- Hjorthøj, C.; Stürup, A.E.; McGrath, J.J.; Nordentoft, M. Years of potential life lost and life expectancy in schizophrenia: A systematic review and meta-analysis. Lancet Psychiatry 2017, 4, 295–301. [Google Scholar] [CrossRef]
- McCutcheon, R.A.; Reis Marques, T.; Howes, O.D. Schizophrenia-An Overview. JAMA Psychiatry 2020, 77, 201–210. [Google Scholar] [CrossRef]
- Murray, R.M.; Lewis, S.W. Is schizophrenia a neurodevelopmental disorder? Br. Med. J. 1988, 296, 63. [Google Scholar] [CrossRef] [Green Version]
- Erlenmeyer-Kimling, L.; Rock, D.; Roberts, S.A.; Janal, M.; Kestenbaum, C.; Cornblatt, B.; Adamo, U.H.; Gottesman, I.I. Attention, memory, and motor skills as childhood predictors of schizophrenia-related psychoses: The New York High-Risk Project. Am. J. Psychiatry 2000, 157, 1416–1422. [Google Scholar] [CrossRef]
- Picchioni, M.M.; Murray, R.M. Schizophrenia. BMJ 2007, 335, 91–95. [Google Scholar] [CrossRef]
- Correll, C.U.; Schooler, N.R. Negative Symptoms in Schizophrenia: A Review and Clinical Guide for Recognition, Assessment, and Treatment. Neuropsychiatr. Dis. Treat. 2020, 16, 519–534. [Google Scholar] [CrossRef] [Green Version]
- Bowie, C.R.; Harvey, P.D. Cognitive deficits and functional outcome in schizophrenia. Neuropsychiatr. Dis. Treat. 2006, 2, 531–536. [Google Scholar] [CrossRef] [Green Version]
- Van Haren, N.E.M.; Schnack, H.G.; Cahn, W.; van den Heuvel, M.P.; Lepage, C.; Collins, L.; Evans, A.C.; Hulshoff Pol, H.E.; Kahn, R.S. Changes in cortical thickness during the course of illness in schizophrenia. Arch. Gen. Psychiatry 2011, 68, 871–880. [Google Scholar] [CrossRef] [PubMed]
- Nesvåg, R.; Lawyer, G.; Varnäs, K.; Fjell, A.M.; Walhovd, K.B.; Frigessi, A.; Jönsson, E.G.; Agartz, I. Regional thinning of the cerebral cortex in schizophrenia: Effects of diagnosis, age and antipsychotic medication. Schizophr. Res. 2008, 98, 16–28. [Google Scholar] [CrossRef] [PubMed]
- Gaser, C.; Nenadic, I.; Buchsbaum, B.R.; Hazlett, E.A.; Buchsbaum, M.S. Ventricular enlargement in schizophrenia related to volume reduction of the thalamus, striatum, and superior temporal cortex. Am. J. Psychiatry 2004, 161, 154–156. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Andreasen, N.C.; Olsen, S.A.; Dennert, J.W.; Smith, M.R. Ventricular enlargement in schizophrenia: Relationship to positive and negative symptoms. Am. J. Psychiatry 1982, 139, 297–302. [Google Scholar] [CrossRef] [PubMed]
- Horga, G.; Bernacer, J.; Dusi, N.; Entis, J.; Chu, K.; Hazlett, E.A.; Haznedar, M.M.; Kemether, E.; Byne, W.; Buchsbaum, M.S. Correlations between ventricular enlargement and gray and white matter volumes of cortex, thalamus, striatum, and internal capsule in schizophrenia. Eur. Arch. Psychiatry Clin. Neurosci. 2011, 261, 467–476. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Narr, K.L.; Bilder, R.M.; Woods, R.P.; Thompson, P.M.; Szeszko, P.; Robinson, D.; Ballmaier, M.; Messenger, B.; Wang, Y.; Toga, A.W. Regional specificity of cerebrospinal fluid abnormalities in first episode schizophrenia. Psychiatry Res. 2006, 146, 21–33. [Google Scholar] [CrossRef]
- Bird, C.M.; Burgess, N. The hippocampus and memory: Insights from spatial processing. Nat. Rev. Neurosci. 2008, 9, 182–194. [Google Scholar] [CrossRef]
- Bogerts, B.; Falkai, P.; Haupts, M.; Greve, B.; Ernst, S.; Tapernon-Franz, U.; Heinzmann, U. Post-mortem volume measurements of limbic system and basal ganglia structures in chronic schizophrenics. Initial results from a new brain collection. Schizophr. Res. 1990, 3, 295–301. [Google Scholar] [CrossRef]
- Bogerts, B.; Lieberman, J.A.; Ashtari, M.; Bilder, R.M.; Degreef, G.; Lerner, G.; Johns, C.; Masiar, S. Hippocampus-amygdala volumes and psychopathology in chronic schizophrenia. Biol. Psychiatry 1993, 33, 236–246. [Google Scholar] [CrossRef]
- Szeszko, P.R.; Goldberg, E.; Gunduz-Bruce, H.; Ashtari, M.; Robinson, D.; Malhotra, A.K.; Lencz, T.; Bates, J.; Crandall, D.T.; Kane, J.M.; et al. Smaller anterior hippocampal formation volume in antipsychotic-naive patients with first-episode schizophrenia. Am. J. Psychiatry 2003, 160, 2190–2197. [Google Scholar] [CrossRef]
- Velakoulis, D.; Pantelis, C.; McGorry, P.D.; Dudgeon, P.; Brewer, W.; Cook, M.; Desmond, P.; Bridle, N.; Tierney, P.; Murrie, V.; et al. Hippocampal volume in first-episode psychoses and chronic schizophrenia: A high-resolution magnetic resonance imaging study. Arch. Gen. Psychiatry 1999, 56, 133–141. [Google Scholar] [CrossRef] [PubMed]
- Walter, A.; Studerus, E.; Smieskova, R.; Kuster, P.; Aston, J.; Lang, U.E.; Radue, E.-W.; Riecher-Rössler, A.; Borgwardt, S. Hippocampal volume in subjects at high risk of psychosis: A longitudinal MRI study. Schizophr. Res. 2012, 142, 217–222. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lawrie, S.M.; Whalley, H.; Kestelman, J.N.; Abukmeil, S.S.; Byrne, M.; Hodges, A.; Rimmington, J.E.; Best, J.J.; Owens, D.G.; Johnstone, E.C. Magnetic resonance imaging of brain in people at high risk of developing schizophrenia. Lancet 1999, 353, 30–33. [Google Scholar] [CrossRef]
- Shenton, M.E.; Dickey, C.C.; Frumin, M.; McCarley, R.W. A review of MRI findings in schizophrenia. Schizophr. Res. 2001, 49, 1–52. [Google Scholar] [CrossRef] [Green Version]
- Roeske, M.J.; Konradi, C.; Heckers, S.; Lewis, A.S. Hippocampal volume and hippocampal neuron density, number and size in schizophrenia: A systematic review and meta-analysis of postmortem studies. Mol. Psychiatry 2021, 26, 3524–3535. [Google Scholar] [CrossRef] [PubMed]
- Matosin, N.; Fernandez-Enright, F.; Lum, J.S.; Engel, M.; Andrews, J.L.; Gassen, N.C.; Wagner, K.V.; Schmidt, M.V.; Newell, K.A. Molecular evidence of synaptic pathology in the CA1 region in schizophrenia. NPJ Schizophr. 2016, 2, 16022. [Google Scholar] [CrossRef] [Green Version]
- Li, W.; Ghose, S.; Gleason, K.; Begovic, A.; Perez, J.; Bartko, J.; Russo, S.; Wagner, A.D.; Selemon, L.; Tamminga, C.A. Synaptic proteins in the hippocampus indicative of increased neuronal activity in CA3 in schizophrenia. Am. J. Psychiatry 2015, 172, 373–382. [Google Scholar] [CrossRef] [Green Version]
- Toro, C.; Deakin, J.F.W. NMDA receptor subunit NRI and postsynaptic protein PSD-95 in hippocampus and orbitofrontal cortex in schizophrenia and mood disorder. Schizophr. Res. 2005, 80, 323–330. [Google Scholar] [CrossRef]
- Avery, S.N.; Rogers, B.P.; Heckers, S. Hippocampal Network Modularity Is Associated with Relational Memory Dysfunction in Schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2018, 3, 423–432. [Google Scholar] [CrossRef]
- Du, Y.; Hao, H.; Wang, S.; Pearlson, G.D.; Calhoun, V.D. Identifying commonality and specificity across psychosis sub-groups via classification based on features from dynamic connectivity analysis. Neuroimage Clin. 2020, 27, 102284. [Google Scholar] [CrossRef]
- Edmiston, E.K.; Song, Y.; Chang, M.; Yin, Z.; Zhou, Q.; Zhou, Y.; Jiang, X.; Wei, S.; Xu, K.; Tang, Y.; et al. Hippocampal Resting State Functional Connectivity in Patients with Schizophrenia and Unaffected Family Members. Front. Psychiatry 2020, 11, 278. [Google Scholar] [CrossRef] [PubMed]
- Nelson, E.A.; Kraguljac, N.V.; Maximo, J.O.; Briend, F.; Armstrong, W.; Hoef, L.W.V.; Johnson, V.; Lahti, A.C. Hippocampal Dysconnectivity and Altered Glutamatergic Modulation of the Default Mode Network: A Combined Resting-State Connectivity and Magnetic Resonance Spectroscopy Study in Schizophrenia. Biol. Psychiatry Cogn. Neurosci. Neuroimaging 2022, 7, 108–118. [Google Scholar] [CrossRef] [PubMed]
- Harrisberger, F.; Buechler, R.; Smieskova, R.; Lenz, C.; Walter, A.; Egloff, L.; Bendfeldt, K.; Simon, A.E.; Wotruba, D.; Theodoridou, A.; et al. Alterations in the hippocampus and thalamus in individuals at high risk for psychosis. NPJ Schizophr. 2016, 2, 16033. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, S.W.; Murray, R.M. Obstetric complications, neurodevelopmental deviance, and risk of schizophrenia. J. Psychiatr. Res. 1987, 21, 413–421. [Google Scholar] [CrossRef]
- Waddington, J.L.; Torrey, E.F.; Crow, T.J.; Hirsch, S.R. Schizophrenia, neurodevelopment, and disease. The Fifth Biannual Winter Workshop on Schizophrenia, Badgastein, Austria, January 28 to February 3, 1990. Arch. Gen. Psychiatry 1991, 48, 271–273. [Google Scholar] [CrossRef]
- Rapoport, J.L.; Addington, A.M.; Frangou, S.; Psych, M.R.C. The neurodevelopmental model of schizophrenia: Update 2005. Mol. Psychiatry 2005, 10, 434–449. [Google Scholar] [CrossRef]
- Fatemi, S.H.; Folsom, T.D. The neurodevelopmental hypothesis of schizophrenia, revisited. Schizophr. Bull. 2009, 35, 528–548. [Google Scholar] [CrossRef]
- Roeske, M.J.; McHugo, M.; Vandekar, S.; Blackford, J.U.; Woodward, N.D.; Heckers, S. Incomplete hippocampal inversion in schizophrenia: Prevalence, severity, and impact on hippocampal structure. Mol. Psychiatry 2021, 26, 5407–5416. [Google Scholar] [CrossRef]
- Cury, C.; Toro, R.; Cohen, F.; Fischer, C.; Mhaya, A.; Samper-González, J.; Hasboun, D.; Mangin, J.-F.; Banaschewski, T.; Bokde, A.L.W.; et al. Incomplete Hippocampal Inversion: A Comprehensive MRI Study of Over 2000 Subjects. Front. Neuroanat. 2015, 9, 160. [Google Scholar] [CrossRef]
- Bajic, D.; Wang, C.; Kumlien, E.; Mattsson, P.; Lundberg, S.; Eeg-Olofsson, O.; Raininko, R. Incomplete inversion of the hippocampus--a common developmental anomaly. Eur. Radiol. 2008, 18, 138–142. [Google Scholar] [CrossRef]
- Bajic, D.; Kumlien, E.; Mattsson, P.; Lundberg, S.; Wang, C.; Raininko, R. Incomplete hippocampal inversion-is there a relation to epilepsy? Eur. Radiol. 2009, 19, 2544–2550. [Google Scholar] [CrossRef] [PubMed]
- Cachia, A.; Cury, C.; Brunelin, J.; Plaze, M.; Delmaire, C.; Oppenheim, C.; Medjkane, F.; Thomas, P.; Jardri, R. Deviations in early hippocampus development contribute to visual hallucinations in schizophrenia. Transl. Psychiatry 2020, 10, 102. [Google Scholar] [CrossRef] [PubMed]
- Arnold, S.E.; Trojanowski, J.Q. Human fetal hippocampal development: I. Cytoarchitecture, myeloarchitecture, and neuronal morphologic features. J. Comp. Neurol. 1996, 367, 274–292. [Google Scholar] [CrossRef]
- Kier, E.L.; Kim, J.H.; Fulbright, R.K.; Bronen, R.A. Embryology of the human fetal hippocampus: MR imaging, anatomy, and histology. AJNR Am. J. Neuroradiol. 1997, 18, 525–532. [Google Scholar] [PubMed]
- Bajic, D.; Ewald, U.; Raininko, R. Hippocampal development at gestation weeks 23 to 36. An ultrasound study on preterm neonates. Neuroradiology 2010, 52, 489–494. [Google Scholar] [CrossRef]
- Cury, C.; Scelsi, M.A.; Toro, R.; Frouin, V.; Artiges, E.; Grigis, A.; Heinz, A.; Lemaître, H.; Martinot, J.-L.; Poline, J.-B.; et al. Genome wide association study of incomplete hippocampal inversion in adolescents. PLoS ONE 2020, 15, e0227355. [Google Scholar] [CrossRef] [Green Version]
- Allswede, D.M.; Cannon, T.D. Prenatal inflammation and risk for schizophrenia: A role for immune proteins in neurodevelopment. Dev. Psychopathol. 2018, 30, 1157–1178. [Google Scholar] [CrossRef]
- Buss, C.; Davis, E.P.; Muftuler, L.T.; Head, K.; Sandman, C.A. High pregnancy anxiety during mid-gestation is associated with decreased gray matter density in 6–9-year-old children. Psychoneuroendocrinology 2010, 35, 141–153. [Google Scholar] [CrossRef] [Green Version]
- Qiu, A.; Rifkin-Graboi, A.; Chen, H.; Chong, Y.-S.; Kwek, K.; Gluckman, P.D.; Fortier, M.V.; Meaney, M.J. Maternal anxiety and infants’ hippocampal development: Timing matters. Transl. Psychiatry 2013, 3, e306. [Google Scholar] [CrossRef] [Green Version]
- Thompson, D.K.; Adamson, C.; Roberts, G.; Faggian, N.; Wood, S.J.; Warfield, S.K.; Doyle, L.W.; Anderson, P.J.; Egan, G.F.; Inder, T.E. Hippocampal shape variations at term equivalent age in very preterm infants compared with term controls: Perinatal predictors and functional significance at age 7. Neuroimage 2013, 70, 278–287. [Google Scholar] [CrossRef] [Green Version]
- Nosarti, C.; Reichenberg, A.; Murray, R.M.; Cnattingius, S.; Lambe, M.P.; Yin, L.; MacCabe, J.; Rifkin, L.; Hultman, C.M. Preterm birth and psychiatric disorders in young adult life. Arch. Gen. Psychiatry 2012, 69, 610–617. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Vanes, L.D.; Murray, R.M.; Nosarti, C. Adult outcome of preterm birth: Implications for neurodevelopmental theories of psychosis. Schizophr. Res. 2021. [Google Scholar] [CrossRef] [PubMed]
- Rifkin, L.; Lewis, S.; Jones, P.; Toone, B.; Murray, R. Low birth weight and schizophrenia. Br. J. Psychiatry 1994, 165, 357–362. [Google Scholar] [CrossRef] [PubMed]
- Foerster, A.; Lewis, S.W.; Owen, M.J.; Murray, R.M. Low birth weight and a family history of schizophrenia predict poor premorbid functioning in psychosis. Schizophr. Res. 1991, 5, 13–20. [Google Scholar] [CrossRef]
- Narr, K.L.; Thompson, P.M.; Szeszko, P.; Robinson, D.; Jang, S.; Woods, R.P.; Kim, S.; Hayashi, K.M.; Asunction, D.; Toga, A.W.; et al. Regional specificity of hippocampal volume reductions in first-episode schizophrenia. Neuroimage 2004, 21, 1563–1575. [Google Scholar] [CrossRef]
- Nugent, T.F.; Herman, D.H.; Ordonez, A.; Greenstein, D.; Hayashi, K.M.; Lenane, M.; Clasen, L.; Jung, D.; Toga, A.W.; Giedd, J.N.; et al. Dynamic mapping of hippocampal development in childhood onset schizophrenia. Schizophr. Res. 2007, 90, 62–70. [Google Scholar] [CrossRef]
- Johnson, S.L.M.; Wang, L.; Alpert, K.I.; Greenstein, D.; Clasen, L.; Lalonde, F.; Miller, R.; Rapoport, J.; Gogtay, N. Hippocampal shape abnormalities of patients with childhood-onset schizophrenia and their unaffected siblings. J. Am. Acad. Child Adolesc. Psychiatry 2013, 52, 527–536.e2. [Google Scholar] [CrossRef] [Green Version]
- Hinney, B.; Walter, A.; Aghlmandi, S.; Andreou, C.; Borgwardt, S. Does Hippocampal Volume Predict Transition to Psychosis in a High-Risk Group? A Meta-Analysis. Front. Psychiatry 2020, 11, 614659. [Google Scholar] [CrossRef]
- Walter, A.; Suenderhauf, C.; Harrisberger, F.; Lenz, C.; Smieskova, R.; Chung, Y.; Cannon, T.D.; Bearden, C.E.; Rapp, C.; Bendfeldt, K.; et al. Hippocampal volume in subjects at clinical high-risk for psychosis: A systematic review and meta-analysis. Neurosci. Biobehav. Rev. 2016, 71, 680–690. [Google Scholar] [CrossRef]
- Radhakrishnan, R.; Skosnik, P.D.; Ranganathan, M.; Naganawa, M.; Toyonaga, T.; Finnema, S.; Hillmer, A.T.; Esterlis, I.; Huang, Y.; Nabulsi, N.; et al. In vivo evidence of lower synaptic vesicle density in schizophrenia. Mol. Psychiatry 2021, 26, 7690–7698. [Google Scholar] [CrossRef]
- Osimo, E.F.; Beck, K.; Reis Marques, T.; Howes, O.D. Synaptic loss in schizophrenia: A meta-analysis and systematic review of synaptic protein and mRNA measures. Mol. Psychiatry 2019, 24, 549–561. [Google Scholar] [CrossRef] [PubMed]
- Li, W.; Lv, L.; Luo, X.-J. In vivo study sheds new light on the dendritic spine pathology hypothesis of schizophrenia. Mol. Psychiatry 2022, 1–3. [Google Scholar] [CrossRef] [PubMed]
- Van Berdenis Berlekom, A.; Muflihah, C.H.; Snijders, G.J.L.J.; MacGillavry, H.D.; Middeldorp, J.; Hol, E.M.; Kahn, R.S.; de Witte, L.D. Synapse Pathology in Schizophrenia: A Meta-analysis of Postsynaptic Elements in Postmortem Brain Studies. Schizophr. Bull. 2020, 46, 374–386. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Garey, L.J.; Ong, W.Y.; Patel, T.S.; Kanani, M.; Davis, A.; Mortimer, A.M.; Barnes, T.R.; Hirsch, S.R. Reduced dendritic spine density on cerebral cortical pyramidal neurons in schizophrenia. J. Neurol. Neurosurg. Psychiatry 1998, 65, 446–453. [Google Scholar] [CrossRef] [PubMed]
- Glantz, L.A.; Lewis, D.A. Decreased dendritic spine density on prefrontal cortical pyramidal neurons in schizophrenia. Arch. Gen. Psychiatry 2000, 57, 65–73. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Konopaske, G.T.; Lange, N.; Coyle, J.T.; Benes, F.M. Prefrontal cortical dendritic spine pathology in schizophrenia and bipolar disorder. JAMA Psychiatry 2014, 71, 1323–1331. [Google Scholar] [CrossRef] [Green Version]
- Kolluri, N.; Sun, Z.; Sampson, A.R.; Lewis, D.A. Lamina-specific reductions in dendritic spine density in the prefrontal cortex of subjects with schizophrenia. Am. J. Psychiatry 2005, 162, 1200–1202. [Google Scholar] [CrossRef]
- Toyooka, K.; Iritani, S.; Makifuchi, T.; Shirakawa, O.; Kitamura, N.; Maeda, K.; Nakamura, R.; Niizato, K.; Watanabe, M.; Kakita, A.; et al. Selective reduction of a PDZ protein, SAP-97, in the prefrontal cortex of patients with chronic schizophrenia. J. Neurochem. 2002, 83, 797–806. [Google Scholar] [CrossRef]
- Provenzano, F.A.; Guo, J.; Wall, M.M.; Feng, X.; Sigmon, H.C.; Brucato, G.; First, M.B.; Rothman, D.L.; Girgis, R.R.; Lieberman, J.A.; et al. Hippocampal Pathology in Clinical High-Risk Patients and the Onset of Schizophrenia. Biol. Psychiatry 2020, 87, 234–242. [Google Scholar] [CrossRef]
- Tregellas, J.R.; Smucny, J.; Harris, J.G.; Olincy, A.; Maharajh, K.; Kronberg, E.; Eichman, L.C.; Lyons, E.; Freedman, R. Intrinsic hippocampal activity as a biomarker for cognition and symptoms in schizophrenia. Am. J. Psychiatry 2014, 171, 549–556. [Google Scholar] [CrossRef]
- Tregellas, J.R.; Ellis, J.; Shatti, S.; Du, Y.P.; Rojas, D.C. Increased hippocampal, thalamic, and prefrontal hemodynamic response to an urban noise stimulus in schizophrenia. Am. J. Psychiatry 2009, 166, 354–360. [Google Scholar] [CrossRef] [Green Version]
- Schobel, S.A.; Chaudhury, N.H.; Khan, U.A.; Paniagua, B.; Styner, M.A.; Asllani, I.; Inbar, B.P.; Corcoran, C.M.; Lieberman, J.A.; Moore, H.; et al. Imaging patients with psychosis and a mouse model establishes a spreading pattern of hippocampal dysfunction and implicates glutamate as a driver. Neuron 2013, 78, 81–93. [Google Scholar] [CrossRef] [Green Version]
- Schobel, S.A.; Lewandowski, N.M.; Corcoran, C.M.; Moore, H.; Brown, T.; Malaspina, D.; Small, S.A. Differential targeting of the CA1 subfield of the hippocampal formation by schizophrenia and related psychotic disorders. Arch. Gen. Psychiatry 2009, 66, 938–946. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tregellas, J.R.; Davalos, D.B.; Rojas, D.C.; Waldo, M.C.; Gibson, L.; Wylie, K.; Du, Y.P.; Freedman, R. Increased hemodynamic response in the hippocampus, thalamus and prefrontal cortex during abnormal sensory gating in schizophrenia. Schizophr. Res. 2007, 92, 262–272. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tregellas, J. Connecting brain structure and function in schizophrenia. Am. J. Psychiatry 2009, 166, 134–136. [Google Scholar] [CrossRef] [PubMed]
- Weiss, A.P.; Schacter, D.L.; Goff, D.C.; Rauch, S.L.; Alpert, N.M.; Fischman, A.J.; Heckers, S. Impaired hippocampal recruitment during normal modulation of memory performance in schizophrenia. Biol. Psychiatry 2003, 53, 48–55. [Google Scholar] [CrossRef]
- Ongür, D.; Cullen, T.J.; Wolf, D.H.; Rohan, M.; Barreira, P.; Zalesak, M.; Heckers, S. The neural basis of relational memory deficits in schizophrenia. Arch. Gen. Psychiatry 2006, 63, 356–365. [Google Scholar] [CrossRef]
- Williams, L.E.; Blackford, J.U.; Luksik, A.; Gauthier, I.; Heckers, S. Reduced habituation in patients with schizophrenia. Schizophr. Res. 2013, 151, 124–132. [Google Scholar] [CrossRef] [Green Version]
- Holt, D.J.; Kunkel, L.; Weiss, A.P.; Goff, D.C.; Wright, C.I.; Shin, L.M.; Rauch, S.L.; Hootnick, J.; Heckers, S. Increased medial temporal lobe activation during the passive viewing of emotional and neutral facial expressions in schizophrenia. Schizophr. Res. 2006, 82, 153–162. [Google Scholar] [CrossRef]
- Talati, P.; Rane, S.; Kose, S.; Blackford, J.U.; Gore, J.; Donahue, M.J.; Heckers, S. Increased hippocampal CA1 cerebral blood volume in schizophrenia. Neuroimage Clin. 2014, 5, 359–364. [Google Scholar] [CrossRef] [Green Version]
- Allen, P.; Chaddock, C.A.; Egerton, A.; Howes, O.D.; Bonoldi, I.; Zelaya, F.; Bhattacharyya, S.; Murray, R.; McGuire, P. Resting Hyperperfusion of the Hippocampus, Midbrain, and Basal Ganglia in People at High Risk for Psychosis. Am. J. Psychiatry 2016, 173, 392–399. [Google Scholar] [CrossRef] [PubMed]
- Ghoshal, A.; Conn, P.J. The hippocampo-prefrontal pathway: A possible therapeutic target for negative and cognitive symptoms of schizophrenia. Future Neurol. 2015, 10, 115–128. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Blessing, E.M.; Murty, V.P.; Zeng, B.; Wang, J.; Davachi, L.; Goff, D.C. Anterior Hippocampal-Cortical Functional Connectivity Distinguishes Antipsychotic Naïve First-Episode Psychosis Patients from Controls and May Predict Response to Second-Generation Antipsychotic Treatment. Schizophr. Bull. 2020, 46, 680–689. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, Y.; Shu, N.; Liu, Y.; Song, M.; Hao, Y.; Liu, H.; Yu, C.; Liu, Z.; Jiang, T. Altered resting-state functional connectivity and anatomical connectivity of hippocampus in schizophrenia. Schizophr. Res. 2008, 100, 120–132. [Google Scholar] [CrossRef] [PubMed]
- Xi, Y.-B.; Li, C.; Cui, L.-B.; Liu, J.; Guo, F.; Li, L.; Liu, T.-T.; Liu, K.; Chen, G.; Xi, M.; et al. Anterior Cingulate Cortico-Hippocampal Dysconnectivity in Unaffected Relatives of Schizophrenia Patients: A Stochastic Dynamic Causal Modeling Study. Front. Hum. Neurosci. 2016, 10, 383. [Google Scholar] [CrossRef] [Green Version]
- Sullivan, P.F.; Kendler, K.S.; Neale, M.C. Schizophrenia as a complex trait: Evidence from a meta-analysis of twin studies. Arch. Gen. Psychiatry 2003, 60, 1187–1192. [Google Scholar] [CrossRef] [Green Version]
- Cardno, A.G.; Gottesman, I.I. Twin studies of schizophrenia: From bow-and-arrow concordances to star wars Mx and functional genomics. Am. J. Med. Genet. 2000, 97, 12–17. [Google Scholar] [CrossRef]
- Perez, J.M.; Berto, S.; Gleason, K.; Ghose, S.; Tan, C.; Kim, T.-K.; Konopka, G.; Tamminga, C.A. Hippocampal subfield transcriptome analysis in schizophrenia psychosis. Mol. Psychiatry 2020, 26, 2577–2589. [Google Scholar] [CrossRef]
- Jaffe, A.E.; Hoeppner, D.J.; Saito, T.; Blanpain, L.; Ukaigwe, J.; Burke, E.E.; Collado-Torres, L.; Tao, R.; Tajinda, K.; Maynard, K.R.; et al. Profiling gene expression in the human dentate gyrus granule cell layer reveals insights into schizophrenia and its genetic risk. Nat. Neurosci. 2020, 23, 510–519. [Google Scholar] [CrossRef]
- Etemadikhah, M.; Niazi, A.; Wetterberg, L.; Feuk, L. Transcriptome analysis of fibroblasts from schizophrenia patients reveals differential expression of schizophrenia-related genes. Sci. Rep. 2020, 10, 630. [Google Scholar] [CrossRef] [Green Version]
- Hall, L.S.; Medway, C.W.; Pain, O.; Pardiñas, A.F.; Rees, E.G.; Escott-Price, V.; Pocklington, A.; Bray, N.J.; Holmans, P.A.; Walters, J.T.R.; et al. A transcriptome-wide association study implicates specific pre- and post-synaptic abnormalities in schizophrenia. Hum. Mol. Genet. 2020, 29, 159–167. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gusev, A.; Mancuso, N.; Won, H.; Kousi, M.; Finucane, H.K.; Reshef, Y.; Song, L.; Safi, A.; McCarroll, S.; Neale, B.M.; et al. Transcriptome-wide association study of schizophrenia and chromatin activity yields mechanistic disease insights. Nat. Genet. 2018, 50, 538–548. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Frajman, A.; Maggio, N.; Muler, I.; Haroutunian, V.; Katsel, P.; Yitzhaky, A.; Weiser, M.; Hertzberg, L. Gene expression meta-analysis reveals the down-regulation of three GABA receptor subunits in the superior temporal gyrus of patients with schizophrenia. Schizophr. Res. 2020, 220, 29–37. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Chen, J.; Yu, H.; He, L.; Xu, Y.; Zhang, D.; Yi, Q.; Li, C.; Li, X.; Shen, J.; et al. Genome-wide association analysis identifies 30 new susceptibility loci for schizophrenia. Nat. Genet. 2017, 49, 1576–1583. [Google Scholar] [CrossRef] [PubMed]
- Read, J.; van Os, J.; Morrison, A.P.; Ross, C.A. Childhood trauma, psychosis and schizophrenia: A literature review with theoretical and clinical implications. Acta Psychiatr. Scand. 2005, 112, 330–350. [Google Scholar] [CrossRef]
- Hoy, K.; Barrett, S.; Shannon, C.; Campbell, C.; Watson, D.; Rushe, T.; Shevlin, M.; Bai, F.; Cooper, S.; Mulholland, C. Childhood trauma and hippocampal and amygdalar volumes in first-episode psychosis. Schizophr. Bull. 2012, 38, 1162–1169. [Google Scholar] [CrossRef] [Green Version]
- Du Plessis, S.; Scheffler, F.; Luckhoff, H.; Asmal, L.; Kilian, S.; Phahladira, L.; Emsley, R. Childhood trauma and hippocampal subfield volumes in first-episode schizophrenia and healthy controls. Schizophr. Res. 2020, 215, 308–313. [Google Scholar] [CrossRef]
- Vythilingam, M.; Heim, C.; Newport, J.; Miller, A.H.; Anderson, E.; Bronen, R.; Brummer, M.; Staib, L.; Vermetten, E.; Charney, D.S.; et al. Childhood trauma associated with smaller hippocampal volume in women with major depression. Am. J. Psychiatry 2002, 159, 2072–2080. [Google Scholar] [CrossRef]
- Frodl, T.; Reinhold, E.; Koutsouleris, N.; Reiser, M.; Meisenzahl, E.M. Interaction of childhood stress with hippocampus and prefrontal cortex volume reduction in major depression. J. Psychiatr. Res. 2010, 44, 799–807. [Google Scholar] [CrossRef]
- Bremner, J.D.; Vythilingam, M.; Vermetten, E.; Southwick, S.M.; McGlashan, T.; Nazeer, A.; Khan, S.; Vaccarino, L.V.; Soufer, R.; Garg, P.K.; et al. MRI and PET study of deficits in hippocampal structure and function in women with childhood sexual abuse and posttraumatic stress disorder. Am. J. Psychiatry 2003, 160, 924–932. [Google Scholar] [CrossRef] [Green Version]
- Teicher, M.H.; Anderson, C.M.; Polcari, A. Childhood maltreatment is associated with reduced volume in the hippocampal subfields CA3, dentate gyrus, and subiculum. Proc. Natl. Acad. Sci. USA 2012, 109, E563–E572. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gould, E.; Tanapat, P. Stress and hippocampal neurogenesis. Biol. Psychiatry 1999, 46, 1472–1479. [Google Scholar] [CrossRef]
- Herzog, J.I.; Thome, J.; Demirakca, T.; Koppe, G.; Ende, G.; Lis, S.; Rausch, S.; Priebe, K.; Müller-Engelmann, M.; Steil, R.; et al. Influence of Severity of Type and Timing of Retrospectively Reported Childhood Maltreatment on Female Amygdala and Hippocampal Volume. Sci. Rep. 2020, 10, 1903. [Google Scholar] [CrossRef]
- Li, C.-X.; Li, Z.; Hu, X.; Zhang, X.; Bachevalier, J. Altered hippocampal-prefrontal functional network integrity in adult macaque monkeys with neonatal hippocampal lesions. Neuroimage 2021, 227, 117645. [Google Scholar] [CrossRef] [PubMed]
- Beauregard, M.; Bachevalier, J. Neonatal insult to the hippocampal region and schizophrenia: A review and a putative animal model. Can. J. Psychiatry 1996, 41, 446–456. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Scheinost, D.; Spann, M.N.; McDonough, L.; Peterson, B.S.; Monk, C. Associations between different dimensions of prenatal distress, neonatal hippocampal connectivity, and infant memory. Neuropsychopharmacology 2020, 45, 1272–1279. [Google Scholar] [CrossRef] [Green Version]
- Coe, C.L.; Kramer, M.; Czéh, B.; Gould, E.; Reeves, A.J.; Kirschbaum, C.; Fuchs, E. Prenatal stress diminishes neurogenesis in the dentate gyrus of juvenile rhesus monkeys. Biol. Psychiatry 2003, 54, 1025–1034. [Google Scholar] [CrossRef]
- Lesh, T.A.; Careaga, M.; Rose, D.R.; McAllister, A.K.; van de Water, J.; Carter, C.S.; Ashwood, P. Cytokine alterations in first-episode schizophrenia and bipolar disorder: Relationships to brain structure and symptoms. J. Neuroinflammation 2018, 15, 165. [Google Scholar] [CrossRef] [Green Version]
- Lanz, T.A.; Reinhart, V.; Sheehan, M.J.; Rizzo, S.J.S.; Bove, S.E.; James, L.C.; Volfson, D.; Lewis, D.A.; Kleiman, R.J. Postmortem transcriptional profiling reveals widespread increase in inflammation in schizophrenia: A comparison of prefrontal cortex, striatum, and hippocampus among matched tetrads of controls with subjects diagnosed with schizophrenia, bipolar or major depressive disorder. Transl. Psychiatry 2019, 9, 151. [Google Scholar] [CrossRef] [Green Version]
- Hwang, Y.; Kim, J.; Shin, J.Y.; Kim, J., II; Seo, J.S.; Webster, M.J.; Lee, D.; Kim, S. Gene expression profiling by mRNA sequencing reveals increased expression of immune/inflammation-related genes in the hippocampus of individuals with schizophrenia. Transl. Psychiatry 2013, 3, e321. [Google Scholar] [CrossRef] [Green Version]
- Monji, A.; Kato, T.; Kanba, S. Cytokines and schizophrenia: Microglia hypothesis of schizophrenia. Psychiatry Clin. Neurosci. 2009, 63, 257–265. [Google Scholar] [CrossRef] [PubMed]
- Sierra, A.; de Castro, F.; Del Río-Hortega, J.; Rafael Iglesias-Rozas, J.; Garrosa, M.; Kettenmann, H. The “Big-Bang” for modern glial biology: Translation and comments on Pío del Río-Hortega 1919 series of papers on microglia. Glia 2016, 64, 1801–1840. [Google Scholar] [CrossRef] [PubMed]
- Prinz, M.; Priller, J. Microglia and brain macrophages in the molecular age: From origin to neuropsychiatric disease. Nat. Rev. Neurosci. 2014, 15, 300–312. [Google Scholar] [CrossRef] [PubMed]
- Kierdorf, K.; Erny, D.; Goldmann, T.; Sander, V.; Schulz, C.; Perdiguero, E.G.; Wieghofer, P.; Heinrich, A.; Riemke, P.; Hölscher, C.; et al. Microglia emerge from erythromyeloid precursors via Pu.1- and Irf8-dependent pathways. Nat. Neurosci. 2013, 16, 273–280. [Google Scholar] [CrossRef]
- Ginhoux, F.; Greter, M.; Leboeuf, M.; Nandi, S.; See, P.; Gokhan, S.; Mehler, M.F.; Conway, S.J.; Ng, L.G.; Stanley, E.R.; et al. Fate mapping analysis reveals that adult microglia derive from primitive macrophages. Science 2010, 330, 841–845. [Google Scholar] [CrossRef] [Green Version]
- Paolicelli, R.C.; Bolasco, G.; Pagani, F.; Maggi, L.; Scianni, M.; Panzanelli, P.; Giustetto, M.; Ferreira, T.A.; Guiducci, E.; Dumas, L.; et al. Synaptic pruning by microglia is necessary for normal brain development. Science 2011, 333, 1456–1458. [Google Scholar] [CrossRef] [Green Version]
- Zhan, Y.; Paolicelli, R.C.; Sforazzini, F.; Weinhard, L.; Bolasco, G.; Pagani, F.; Vyssotski, A.L.; Bifone, A.; Gozzi, A.; Ragozzino, D.; et al. Deficient neuron-microglia signaling results in impaired functional brain connectivity and social behavior. Nat. Neurosci. 2014, 17, 400–406. [Google Scholar] [CrossRef]
- Ji, K.; Akgul, G.; Wollmuth, L.P.; Tsirka, S.E. Microglia actively regulate the number of functional synapses. PLoS ONE 2013, 8, e56293. [Google Scholar] [CrossRef] [Green Version]
- Weinhard, L.; Di Bartolomei, G.; Bolasco, G.; Machado, P.; Schieber, N.L.; Neniskyte, U.; Exiga, M.; Vadisiute, A.; Raggioli, A.; Schertel, A.; et al. Microglia remodel synapses by presynaptic trogocytosis and spine head filopodia induction. Nat. Commun. 2018, 9, 1228. [Google Scholar] [CrossRef] [Green Version]
- Hattori, Y.; Naito, Y.; Tsugawa, Y.; Nonaka, S.; Wake, H.; Nagasawa, T.; Kawaguchi, A.; Miyata, T. Transient microglial absence assists postmigratory cortical neurons in proper differentiation. Nat. Commun. 2020, 11, 1631. [Google Scholar] [CrossRef]
- Tan, Y.-L.; Yuan, Y.; Tian, L. Microglial regional heterogeneity and its role in the brain. Mol. Psychiatry 2020, 25, 351–367. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Pocock, J.M.; Kettenmann, H. Neurotransmitter receptors on microglia. Trends Neurosci. 2007, 30, 527–535. [Google Scholar] [CrossRef] [PubMed]
- York, E.M.; Bernier, L.-P.; MacVicar, B.A. Microglial modulation of neuronal activity in the healthy brain. Dev. Neurobiol. 2018, 78, 593–603. [Google Scholar] [CrossRef] [PubMed]
- Kettenmann, H.; Hanisch, U.-K.; Noda, M.; Verkhratsky, A. Physiology of microglia. Physiol. Rev. 2011, 91, 461–553. [Google Scholar] [CrossRef] [PubMed]
- Viviani, B.; Bartesaghi, S.; Gardoni, F.; Vezzani, A.; Behrens, M.M.; Bartfai, T.; Binaglia, M.; Corsini, E.; Di Luca, M.; Galli, C.L.; et al. Interleukin-1beta enhances NMDA receptor-mediated intracellular calcium increase through activation of the Src family of kinases. J. Neurosci. 2003, 23, 8692–8700. [Google Scholar] [CrossRef]
- Vezzani, A.; Viviani, B. Neuromodulatory properties of inflammatory cytokines and their impact on neuronal excitability. Neuropharmacology 2015, 96, 70–82. [Google Scholar] [CrossRef]
- Gardoni, F.; Boraso, M.; Zianni, E.; Corsini, E.; Galli, C.L.; Cattabeni, F.; Marinovich, M.; Di Luca, M.; Viviani, B. Distribution of interleukin-1 receptor complex at the synaptic membrane driven by interleukin-1β and NMDA stimulation. J. Neuroinflammation 2011, 8, 14. [Google Scholar] [CrossRef] [Green Version]
- Stellwagen, D.; Beattie, E.C.; Seo, J.Y.; Malenka, R.C. Differential regulation of AMPA receptor and GABA receptor trafficking by tumor necrosis factor-alpha. J. Neurosci. 2005, 25, 3219–3228. [Google Scholar] [CrossRef] [Green Version]
- Szepesi, Z.; Manouchehrian, O.; Bachiller, S.; Deierborg, T. Bidirectional Microglia-Neuron Communication in Health and Disease. Front. Cell. Neurosci. 2018, 12, 323. [Google Scholar] [CrossRef]
- Badimon, A.; Strasburger, H.J.; Ayata, P.; Chen, X.; Nair, A.; Ikegami, A.; Hwang, P.; Chan, A.T.; Graves, S.M.; Uweru, J.O.; et al. Negative feedback control of neuronal activity by microglia. Nature 2020, 586, 417–423. [Google Scholar] [CrossRef]
- Bayer, T.A.; Buslei, R.; Havas, L.; Falkai, P. Evidence for activation of microglia in patients with psychiatric illnesses. Neurosci. Lett. 1999, 271, 126–128. [Google Scholar] [CrossRef]
- Busse, S.; Busse, M.; Schiltz, K.; Bielau, H.; Gos, T.; Brisch, R.; Mawrin, C.; Schmitt, A.; Jordan, W.; Müller, U.J.; et al. Different distribution patterns of lymphocytes and microglia in the hippocampus of patients with residual versus paranoid schizophrenia: Further evidence for disease course-related immune alterations? Brain Behav. Immun. 2012, 26, 1273–1279. [Google Scholar] [CrossRef] [PubMed]
- Sellgren, C.M.; Gracias, J.; Watmuff, B.; Biag, J.D.; Thanos, J.M.; Whittredge, P.B.; Fu, T.; Worringer, K.; Brown, H.E.; Wang, J.; et al. Increased synapse elimination by microglia in schizophrenia patient-derived models of synaptic pruning. Nat. Neurosci. 2019, 22, 374–385. [Google Scholar] [CrossRef] [PubMed]
- Schizophrenia Working Group of the Psychiatric Genomics Consortium. Biological insights from 108 schizophrenia-associated genetic loci. Nature 2014, 511, 421–427. [Google Scholar] [CrossRef] [Green Version]
- Yilmaz, M.; Yalcin, E.; Presumey, J.; Aw, E.; Ma, M.; Whelan, C.W.; Stevens, B.; McCarroll, S.A.; Carroll, M.C. Overexpression of schizophrenia susceptibility factor human complement C4A promotes excessive synaptic loss and behavioral changes in mice. Nat. Neurosci. 2021, 24, 214–224. [Google Scholar] [CrossRef]
- Comer, A.L.; Jinadasa, T.; Sriram, B.; Phadke, R.A.; Kretsge, L.N.; Nguyen, T.P.H.; Antognetti, G.; Gilbert, J.P.; Lee, J.; Newmark, E.R.; et al. Increased expression of schizophrenia-associated gene C4 leads to hypoconnectivity of prefrontal cortex and reduced social interaction. PLoS Biol. 2020, 18, e3000604. [Google Scholar] [CrossRef] [Green Version]
- Sekar, A.; Bialas, A.R.; de Rivera, H.; Davis, A.; Hammond, T.R.; Kamitaki, N.; Tooley, K.; Presumey, J.; Baum, M.; van Doren, V.; et al. Schizophrenia risk from complex variation of complement component 4. Nature 2016, 530, 177–183. [Google Scholar] [CrossRef] [Green Version]
- Conen, S.; Gregory, C.J.; Hinz, R.; Smallman, R.; Corsi-Zuelli, F.; Deakin, B.; Talbot, P.S. Neuroinflammation as measured by positron emission tomography in patients with recent onset and established schizophrenia: Implications for immune pathogenesis. Mol. Psychiatry 2020, 26, 5398–5406. [Google Scholar] [CrossRef]
- Di Biase, M.A.; Zalesky, A.; O’keefe, G.; Laskaris, L.; Baune, B.T.; Weickert, C.S.; Olver, J.; McGorry, P.D.; Amminger, G.P.; Nelson, B.; et al. PET imaging of putative microglial activation in individuals at ultra-high risk for psychosis, recently diagnosed and chronically ill with schizophrenia. Transl. Psychiatry 2017, 7, e1225. [Google Scholar] [CrossRef] [Green Version]
- Marques, T.R.; Ashok, A.H.; Pillinger, T.; Veronese, M.; Turkheimer, F.E.; Dazzan, P.; Sommer, I.E.C.; Howes, O.D. Neuroinflammation in schizophrenia: Meta-analysis of in vivo microglial imaging studies. Psychol. Med. 2019, 49, 2186–2196. [Google Scholar] [CrossRef] [Green Version]
- Holmes, S.E.; Hinz, R.; Drake, R.J.; Gregory, C.J.; Conen, S.; Matthews, J.C.; Anton-Rodriguez, J.M.; Gerhard, A.; Talbot, P.S. In vivo imaging of brain microglial activity in antipsychotic-free and medicated schizophrenia: A 11C(R)-PK11195 positron emission tomography study. Mol. Psychiatry 2016, 21, 1672–1679. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sneeboer, M.A.M.; van der Doef, T.; Litjens, M.; Psy, N.B.B.; Melief, J.; Hol, E.M.; Kahn, R.S.; de Witte, L.D. Microglial activation in schizophrenia: Is translocator 18 kDa protein (TSPO) the right marker? Schizophr. Res. 2020, 215, 167–172. [Google Scholar] [CrossRef] [PubMed]
- Van Berckel, B.N.; Bossong, M.G.; Boellaard, R.; Kloet, R.; Schuitemaker, A.; Caspers, E.; Luurtsema, G.; Windhorst, A.D.; Cahn, W.; Lammertsma, A.A.; et al. Microglia activation in recent-onset schizophrenia: A quantitative (R)-11CPK11195 positron emission tomography study. Biol. Psychiatry 2008, 64, 820–822. [Google Scholar] [CrossRef]
- Bloomfield, P.S.; Selvaraj, S.; Veronese, M.; Rizzo, G.; Bertoldo, A.; Owen, D.R.; Bloomfield, M.A.; Bonoldi, I.; Kalk, N.; Turkheimer, F.; et al. Microglial Activity in People at Ultra High Risk of Psychosis and in Schizophrenia: An (11)CPBR28 PET Brain Imaging Study. Am. J. Psychiatry 2016, 173, 44–52. [Google Scholar] [CrossRef] [Green Version]
- Zhang, L.; Zheng, H.; Wu, R.; Kosten, T.R.; Zhang, X.-Y.; Zhao, J. The effect of minocycline on amelioration of cognitive deficits and pro-inflammatory cytokines levels in patients with schizophrenia. Schizophr. Res. 2019, 212, 92–98. [Google Scholar] [CrossRef]
- Levkovitz, Y.; Mendlovich, S.; Riwkes, S.; Braw, Y.; Levkovitch-Verbin, H.; Gal, G.; Fennig, S.; Treves, I.; Kron, S. A double-blind, randomized study of minocycline for the treatment of negative and cognitive symptoms in early-phase schizophrenia. J. Clin. Psychiatry 2010, 71, 138–149. [Google Scholar] [CrossRef]
- Miyaoka, T.; Yasukawa, R.; Yasuda, H.; Hayashida, M.; Inagaki, T.; Horiguchi, J. Minocycline as adjunctive therapy for schizophrenia: An open-label study. Clin. Neuropharmacol. 2008, 31, 287–292. [Google Scholar] [CrossRef]
- Chaudhry, I.B.; Hallak, J.; Husain, N.; Minhas, F.; Stirling, J.; Richardson, P.; Dursun, S.; Dunn, G.; Deakin, B. Minocycline benefits negative symptoms in early schizophrenia: A randomised double-blind placebo-controlled clinical trial in patients on standard treatment. J. Psychopharmacol. 2012, 26, 1185–1193. [Google Scholar] [CrossRef]
- Solmi, M.; Veronese, N.; Thapa, N.; Facchini, S.; Stubbs, B.; Fornaro, M.; Carvalho, A.F.; Correll, C.U. Systematic review and meta-analysis of the efficacy and safety of minocycline in schizophrenia. CNS Spectr. 2017, 22, 415–426. [Google Scholar] [CrossRef] [Green Version]
- Xiang, Y.-Q.; Zheng, W.; Wang, S.-B.; Yang, X.-H.; Cai, D.-B.; Ng, C.H.; Ungvari, G.S.; Kelly, D.L.; Xu, W.-Y.; Xiang, Y.-T. Adjunctive minocycline for schizophrenia: A meta-analysis of randomized controlled trials. Eur. Neuropsychopharmacol. 2017, 27, 8–18. [Google Scholar] [CrossRef]
- Zheng, W.; Zhu, X.-M.; Zhang, Q.-E.; Cheng, G.; Cai, D.-B.; He, J.; Ng, C.H.; Ungvari, G.S.; Peng, X.-J.; Ning, Y.-P.; et al. Adjunctive minocycline for major mental disorders: A systematic review. J. Psychopharmacol. 2019, 33, 1215–1226. [Google Scholar] [CrossRef] [PubMed]
- Deakin, B.; Suckling, J.; Barnes, T.R.E.; Byrne, K.; Chaudhry, I.B.; Dazzan, P.; Drake, R.J.; Giordano, A.; Husain, N.; Jones, P.B.; et al. The benefit of minocycline on negative symptoms of schizophrenia in patients with recent-onset psychosis (BeneMin): A randomised, double-blind, placebo-controlled trial. Lancet Psychiatry 2018, 5, 885–894. [Google Scholar] [CrossRef] [Green Version]
- Weiser, M.; Levi, L.; Burshtein, S.; Chiriță, R.; Cirjaliu, D.; Gonen, I.; Yolken, R.; Davidson, M.; Zamora, D.; Davis, J.M. The effect of minocycline on symptoms in schizophrenia: Results from a randomized controlled trial. Schizophr. Res. 2019, 206, 325–332. [Google Scholar] [CrossRef]
- Kishimoto, T.; Horigome, T.; Takamiya, A. Minocycline as a treatment for schizophrenia: Is the discussion truly finished? Lancet Psychiatry 2018, 5, 856–857. [Google Scholar] [CrossRef] [Green Version]
- Tarasov, V.V.; Svistunov, A.A.; Chubarev, V.N.; Sologova, S.S.; Mukhortova, P.; Levushkin, D.; Somasundaram, S.G.; Kirkland, C.E.; Bachurin, S.O.; Aliev, G. Alterations of Astrocytes in the Context of Schizophrenic Dementia. Front. Pharmacol. 2019, 10, 1612. [Google Scholar] [CrossRef] [Green Version]
- Kim, R.; Healey, K.L.; Sepulveda-Orengo, M.T.; Reissner, K.J. Astroglial correlates of neuropsychiatric disease: From astrocytopathy to astrogliosis. Prog. Neuropsychopharmacol. Biol. Psychiatry 2018, 87, 126–146. [Google Scholar] [CrossRef]
- Notter, T. Astrocytes in schizophrenia. Brain Neurosci. Adv. 2021, 5, 1–8. [Google Scholar] [CrossRef]
- Goudriaan, A.; de Leeuw, C.; Ripke, S.; Hultman, C.M.; Sklar, P.; Sullivan, P.F.; Smit, A.B.; Posthuma, D.; Verheijen, M.H.G. Specific glial functions contribute to schizophrenia susceptibility. Schizophr. Bull. 2014, 40, 925–935. [Google Scholar] [CrossRef] [Green Version]
- Schmitt, A.; Steyskal, C.; Bernstein, H.-G.; Schneider-Axmann, T.; Parlapani, E.; Schaeffer, E.L.; Gattaz, W.F.; Bogerts, B.; Schmitz, C.; Falkai, P. Stereologic investigation of the posterior part of the hippocampus in schizophrenia. Acta Neuropathol. 2009, 117, 395–407. [Google Scholar] [CrossRef] [Green Version]
- Webster, M.J.; Knable, M.B.; Johnston-Wilson, N.; Nagata, K.; Inagaki, M.; Yolken, R.H. Immunohistochemical localization of phosphorylated glial fibrillary acidic protein in the prefrontal cortex and hippocampus from patients with schizophrenia, bipolar disorder, and depression. Brain Behav. Immun. 2001, 15, 388–400. [Google Scholar] [CrossRef] [Green Version]
- Toker, L.; Mancarci, B.O.; Tripathy, S.; Pavlidis, P. Transcriptomic Evidence for Alterations in Astrocytes and Parvalbumin Interneurons in Subjects with Bipolar Disorder and Schizophrenia. Biol. Psychiatry 2018, 84, 787–796. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Terrillion, C.E.; Abazyan, B.; Yang, Z.; Crawford, J.; Shevelkin, A.V.; Jouroukhin, Y.; Yoo, K.H.; Cho, C.H.; Roychaudhuri, R.; Snyder, S.H.; et al. DISC1 in Astrocytes Influences Adult Neurogenesis and Hippocampus-Dependent Behaviors in Mice. Neuropsychopharmacology 2017, 42, 2242–2251. [Google Scholar] [CrossRef] [PubMed]
- Swanson, L.W.; Hahn, J.D. A qualitative solution with quantitative potential for the mouse hippocampal cortex flatmap problem. Proc. Natl. Acad. Sci. USA 2020, 117, 3220–3231. [Google Scholar] [CrossRef]
- Bjerke, I.E.; Yates, S.C.; Laja, A.; Witter, M.P.; Puchades, M.A.; Bjaalie, J.G.; Leergaard, T.B. Densities and numbers of calbindin and parvalbumin positive neurons across the rat and mouse brain. iScience 2021, 24, 101906. [Google Scholar] [CrossRef] [PubMed]
- Snyder, J.S.; Choe, J.S.; Clifford, M.A.; Jeurling, S.I.; Hurley, P.; Brown, A.; Kamhi, J.F.; Cameron, H.A. Adult-born hippocampal neurons are more numerous, faster maturing, and more involved in behavior in rats than in mice. J. Neurosci. 2009, 29, 14484–14495. [Google Scholar] [CrossRef] [Green Version]
- Buzsáki, G.; Buhl, D.L.; Harris, K.D.; Csicsvari, J.; Czéh, B.; Morozov, A. Hippocampal network patterns of activity in the mouse. Neuroscience 2003, 116, 201–211. [Google Scholar] [CrossRef]
- Mou, X.; Cheng, J.; Yu, Y.S.W.; Kee, S.E.; Ji, D. Comparing Mouse and Rat Hippocampal Place Cell Activities and Firing Sequences in the Same Environments. Front. Cell. Neurosci. 2018, 12, 332. [Google Scholar] [CrossRef] [Green Version]
- Langova, V.; Vales, K.; Horka, P.; Horacek, J. The Role of Zebrafish and Laboratory Rodents in Schizophrenia Research. Front. Psychiatry 2020, 11, 703. [Google Scholar] [CrossRef]
- Mueller, T.; Dong, Z.; Berberoglu, M.A.; Guo, S. The dorsal pallium in zebrafish, Danio rerio (Cyprinidae, Teleostei). Brain Res. 2011, 1381, 95–105. [Google Scholar] [CrossRef] [Green Version]
- Thyme, S.B.; Pieper, L.M.; Li, E.H.; Pandey, S.; Wang, Y.; Morris, N.S.; Sha, C.; Choi, J.W.; Herrera, K.J.; Soucy, E.R.; et al. Phenotypic Landscape of Schizophrenia-Associated Genes Defines Candidates and Their Shared Functions. Cell 2019, 177, 478–491.e20. [Google Scholar] [CrossRef] [Green Version]
- Mednick, S.A.; Machon, R.A.; Huttunen, M.O.; Bonett, D. Adult schizophrenia following prenatal exposure to an influenza epidemic. Arch. Gen. Psychiatry 1988, 45, 189–192. [Google Scholar] [CrossRef] [PubMed]
- McGrath, J.J.; Pemberton, M.R.; Welham, J.L.; Murray, R.M. Schizophrenia and the influenza epidemics of 1954, 1957 and 1959: A southern hemisphere study. Schizophr. Res. 1994, 14, 1–8. [Google Scholar] [CrossRef]
- O’Callaghan, E.; Sham, P.; Takei, N.; Glover, G.; Murray, R.M. Schizophrenia after prenatal exposure to 1957 A2 influenza epidemic. Lancet 1991, 337, 1248–1250. [Google Scholar] [CrossRef]
- Adams, W.; Kendell, R.E.; Hare, E.H.; Munk-Jørgensen, P. Epidemiological evidence that maternal influenza contributes to the aetiology of schizophrenia. An analysis of Scottish, English, and Danish data. Br. J. Psychiatry 1993, 163, 522–534. [Google Scholar] [CrossRef] [PubMed]
- Limosin, F.; Rouillon, F.; Payan, C.; Cohen, J.-M.; Strub, N. Prenatal exposure to influenza as a risk factor for adult schizophrenia. Acta Psychiatr. Scand. 2003, 107, 331–335. [Google Scholar] [CrossRef] [PubMed]
- Brown, A.S.; Derkits, E.J. Prenatal infection and schizophrenia: A review of epidemiologic and translational studies. Am. J. Psychiatry 2010, 167, 261–280. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Fatemi, S.H.; Reutiman, T.J.; Folsom, T.D.; Huang, H.; Oishi, K.; Mori, S.; Smee, D.F.; Pearce, D.A.; Winter, C.; Sohr, R.; et al. Maternal infection leads to abnormal gene regulation and brain atrophy in mouse offspring: Implications for genesis of neurodevelopmental disorders. Schizophr. Res. 2008, 99, 56–70. [Google Scholar] [CrossRef] [Green Version]
- Brown, A.S.; Schaefer, C.A.; Quesenberry, C.P.; Liu, L.; Babulas, V.P.; Susser, E.S. Maternal exposure to toxoplasmosis and risk of schizophrenia in adult offspring. Am. J. Psychiatry 2005, 162, 767–773. [Google Scholar] [CrossRef]
- Brown, A.S.; Cohen, P.; Harkavy-Friedman, J.; Babulas, V.; Malaspina, D.; Gorman, J.M.; Susser, E.S. Prenatal rubella, premorbid abnormalities, and adult schizophrenia. Biol. Psychiatry 2001, 49, 473–486. [Google Scholar] [CrossRef]
- Buka, S.L.; Tsuang, M.T.; Torrey, E.F.; Klebanoff, M.A.; Bernstein, D.; Yolken, R.H. Maternal infections and subsequent psychosis among offspring. Arch. Gen. Psychiatry 2001, 58, 1032–1037. [Google Scholar] [CrossRef] [Green Version]
- Buka, S.L.; Cannon, T.D.; Torrey, E.F.; Yolken, R.H. Maternal exposure to herpes simplex virus and risk of psychosis among adult offspring. Biol. Psychiatry 2008, 63, 809–815. [Google Scholar] [CrossRef] [PubMed]
- Mortensen, P.B.; Nørgaard-Pedersen, B.; Waltoft, B.L.; Sørensen, T.L.; Hougaard, D.; Torrey, E.F.; Yolken, R.H. Toxoplasma gondii as a risk factor for early-onset schizophrenia: Analysis of filter paper blood samples obtained at birth. Biol. Psychiatry 2007, 61, 688–693. [Google Scholar] [CrossRef] [PubMed]
- Suvisaari, J.; Haukka, J.; Tanskanen, A.; Hovi, T.; Lönnqvist, J. Association between prenatal exposure to poliovirus infection and adult schizophrenia. Am. J. Psychiatry 1999, 156, 1100–1102. [Google Scholar] [CrossRef] [PubMed]
- Meyer, U.; Nyffeler, M.; Engler, A.; Urwyler, A.; Schedlowski, M.; Knuesel, I.; Yee, B.K.; Feldon, J. The time of prenatal immune challenge determines the specificity of inflammation-mediated brain and behavioral pathology. J. Neurosci. 2006, 26, 4752–4762. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Meyer, U.; Yee, B.K.; Feldon, J. The neurodevelopmental impact of prenatal infections at different times of pregnancy: The earlier the worse? Neuroscientist 2007, 13, 241–256. [Google Scholar] [CrossRef]
- Meyer, U. Developmental neuroinflammation and schizophrenia. Prog. Neuropsychopharmacol. Biol. Psychiatry 2013, 42, 20–34. [Google Scholar] [CrossRef]
- Ashdown, H.; Dumont, Y.; Ng, M.; Poole, S.; Boksa, P.; Luheshi, G.N. The role of cytokines in mediating effects of prenatal infection on the fetus: Implications for schizophrenia. Mol. Psychiatry 2006, 11, 47–55. [Google Scholar] [CrossRef]
- Gilmore, J.H.; Fredrik Jarskog, L.; Vadlamudi, S.; Lauder, J.M. Prenatal infection and risk for schizophrenia: IL-1beta, IL-6, and TNFalpha inhibit cortical neuron dendrite development. Neuropsychopharmacology 2004, 29, 1221–1229. [Google Scholar] [CrossRef]
- Patterson, P.H. Immune involvement in schizophrenia and autism: Etiology, pathology and animal models. Behav. Brain Res. 2009, 204, 313–321. [Google Scholar] [CrossRef]
- Goldstein, J.M.; Cherkerzian, S.; Seidman, L.J.; Donatelli, J.-A.L.; Remington, A.G.; Tsuang, M.T.; Hornig, M.; Buka, S.L. Prenatal maternal immune disruption and sex-dependent risk for psychoses. Psychol. Med. 2014, 44, 3249–3261. [Google Scholar] [CrossRef] [Green Version]
- Mattei, D.; Ivanov, A.; Ferrai, C.; Jordan, P.; Guneykaya, D.; Buonfiglioli, A.; Schaafsma, W.; Przanowski, P.; Deuther-Conrad, W.; Brust, P.; et al. Maternal immune activation results in complex microglial transcriptome signature in the adult offspring that is reversed by minocycline treatment. Transl. Psychiatry 2017, 7, e1120. [Google Scholar] [CrossRef] [PubMed]
- Luchicchi, A.; Lecca, S.; Melis, M.; de Felice, M.; Cadeddu, F.; Frau, R.; Muntoni, A.L.; Fadda, P.; Devoto, P.; Pistis, M. Maternal Immune Activation Disrupts Dopamine System in the Offspring. Int. J. Neuropsychopharmacol. 2016, 19, pyw007. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hui, C.W.; St-Pierre, A.; El Hajj, H.; Remy, Y.; Hébert, S.S.; Luheshi, G.N.; Srivastava, L.K.; Tremblay, M.-È. Prenatal Immune Challenge in Mice Leads to Partly Sex-Dependent Behavioral, Microglial, and Molecular Abnormalities Associated with Schizophrenia. Front. Mol. Neurosci. 2018, 11, 13. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Ding, S.; Hu, Y.; Luo, B.; Cai, Y.; Hao, K.; Yang, Y.; Zhang, Y.; Wang, X.; Ding, M.; Zhang, H.; et al. Age-related changes in neuroinflammation and prepulse inhibition in offspring of rats treated with Poly I:C in early gestation. Behav. Brain Funct. 2019, 15, 3. [Google Scholar] [CrossRef]
- Smith, S.E.P.; Li, J.; Garbett, K.; Mirnics, K.; Patterson, P.H. Maternal immune activation alters fetal brain development through interleukin-6. J. Neurosci. 2007, 27, 10695–10702. [Google Scholar] [CrossRef] [Green Version]
- Wolff, A.R.; Bilkey, D.K. Immune activation during mid-gestation disrupts sensorimotor gating in rat offspring. Behav. Brain Res. 2008, 190, 156–159. [Google Scholar] [CrossRef]
- Eßlinger, M.; Wachholz, S.; Manitz, M.-P.; Plümper, J.; Sommer, R.; Juckel, G.; Friebe, A. Schizophrenia associated sensory gating deficits develop after adolescent microglia activation. Brain Behav. Immun. 2016, 58, 99–106. [Google Scholar] [CrossRef]
- Zuckerman, L.; Rehavi, M.; Nachman, R.; Weiner, I. Immune activation during pregnancy in rats leads to a postpubertal emergence of disrupted latent inhibition, dopaminergic hyperfunction, and altered limbic morphology in the offspring: A novel neurodevelopmental model of schizophrenia. Neuropsychopharmacology 2003, 28, 1778–1789. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Qi, F.; Song, D.; He, Z.; Zuo, Z.; Yang, Y.; Liu, Q.; Hu, S.; Wang, X.; Zheng, X.; et al. Prenatal influenza vaccination rescues impairments of social behavior and lamination in a mouse model of autism. J. Neuroinflammation 2018, 15, 228. [Google Scholar] [CrossRef]
- Fernández de Cossío, L.; Guzmán, A.; van der Veldt, S.; Luheshi, G.N. Prenatal infection leads to ASD-like behavior and altered synaptic pruning in the mouse offspring. Brain Behav. Immun. 2017, 63, 88–98. [Google Scholar] [CrossRef]
- Bauman, M.D.; Iosif, A.-M.; Smith, S.E.P.; Bregere, C.; Amaral, D.G.; Patterson, P.H. Activation of the maternal immune system during pregnancy alters behavioral development of rhesus monkey offspring. Biol. Psychiatry 2014, 75, 332–341. [Google Scholar] [CrossRef] [Green Version]
- Weir, R.K.; Forghany, R.; Smith, S.E.P.; Patterson, P.H.; McAllister, A.K.; Schumann, C.M.; Bauman, M.D. Preliminary evidence of neuropathology in nonhuman primates prenatally exposed to maternal immune activation. Brain Behav. Immun. 2015, 48, 139–146. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; van Praag, H. Maternal immune activation differentially impacts mature and adult-born hippocampal neurons in male mice. Brain Behav. Immun. 2015, 45, 60–70. [Google Scholar] [CrossRef] [PubMed]
- Winter, C.; Djodari-Irani, A.; Sohr, R.; Morgenstern, R.; Feldon, J.; Juckel, G.; Meyer, U. Prenatal immune activation leads to multiple changes in basal neurotransmitter levels in the adult brain: Implications for brain disorders of neurodevelopmental origin such as schizophrenia. Int. J. Neuropsychopharmacol. 2009, 12, 513–524. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lowe, G.C.; Luheshi, G.N.; Williams, S. Maternal infection and fever during late gestation are associated with altered synaptic transmission in the hippocampus of juvenile offspring rats. Am. J. Physiol. Regul. Integr. Comp. Physiol. 2008, 295, R1563–R1571. [Google Scholar] [CrossRef] [Green Version]
- Ito, H.T.; Smith, S.E.P.; Hsiao, E.; Patterson, P.H. Maternal immune activation alters nonspatial information processing in the hippocampus of the adult offspring. Brain Behav. Immun. 2010, 24, 930–941. [Google Scholar] [CrossRef] [Green Version]
- Wegrzyn, D.; Manitz, M.-P.; Kostka, M.; Freund, N.; Juckel, G.; Faissner, A. Poly I:C-induced maternal immune challenge reduces perineuronal net area and raises spontaneous network activity of hippocampal neurons in vitro. Eur. J. Neurosci. 2020, 53, 3920–3941. [Google Scholar] [CrossRef]
- Celio, M.R.; Blümcke, I. Perineuronal nets—A specialized form of extracellular matrix in the adult nervous system. Brain Res. Brain Res. Rev. 1994, 19, 128–145. [Google Scholar] [CrossRef]
- Baghel, M.S.; Singh, B.; Dhuriya, Y.K.; Shukla, R.K.; Patro, N.; Khanna, V.K.; Patro, I.K.; Thakur, M.K. Postnatal exposure to poly (I:C) impairs learning and memory through changes in synaptic plasticity gene expression in developing rat brain. Neurobiol. Learn. Mem. 2018, 155, 379–389. [Google Scholar] [CrossRef]
- Mattei, D.; Djodari-Irani, A.; Hadar, R.; Pelz, A.; de Cossío, L.F.; Goetz, T.; Matyash, M.; Kettenmann, H.; Winter, C.; Wolf, S.A. Minocycline rescues decrease in neurogenesis, increase in microglia cytokines and deficits in sensorimotor gating in an animal model of schizophrenia. Brain Behav. Immun. 2014, 38, 175–184. [Google Scholar] [CrossRef] [Green Version]
- Zhu, F.; Zheng, Y.; Liu, Y.; Zhang, X.; Zhao, J. Minocycline alleviates behavioral deficits and inhibits microglial activation in the offspring of pregnant mice after administration of polyriboinosinic-polyribocytidilic acid. Psychiatry Res. 2014, 219, 680–686. [Google Scholar] [CrossRef]
- Reisinger, S.; Khan, D.; Kong, E.; Berger, A.; Pollak, A.; Pollak, D.D. The poly(I:C)-induced maternal immune activation model in preclinical neuropsychiatric drug discovery. Pharmacol. Ther. 2015, 149, 213–226. [Google Scholar] [CrossRef] [Green Version]
- Juckel, G.; Manitz, M.P.; Brüne, M.; Friebe, A.; Heneka, M.T.; Wolf, R.J. Microglial activation in a neuroinflammational animal model of schizophrenia--a pilot study. Schizophr. Res. 2011, 131, 96–100. [Google Scholar] [CrossRef]
- Miller, B.J.; Herzig, K.-H.; Jokelainen, J.; Karhu, T.; Keinänen-Kiukaanniemi, S.; Järvelin, M.-R.; Veijola, J.; Viinamäki, H.; Päivikki, T.; Jääskeläinen, E.; et al. Inflammation, hippocampal volume, and cognition in schizophrenia: Results from the Northern Finland Birth Cohort 1966. Eur. Arch. Psychiatry Clin. Neurosci. 2020, 271, 609–622. [Google Scholar] [CrossRef]
- Dahlgren, J.; Samuelsson, A.-M.; Jansson, T.; Holmäng, A. Interleukin-6 in the maternal circulation reaches the rat fetus in mid-gestation. Pediatr. Res. 2006, 60, 147–151. [Google Scholar] [CrossRef] [Green Version]
- Zhao, X.; Mohammed, R.; Tran, H.; Erickson, M.; Kentner, A.C. Poly (I:C)-induced maternal immune activation modifies ventral hippocampal regulation of stress reactivity: Prevention by environmental enrichment. Brain Behav. Immun. 2021, 95, 203–215. [Google Scholar] [CrossRef]
- Noonan, L.R.; Caldwell, J.D.; Li, L.; Walker, C.H.; Pedersen, C.A.; Mason, G.A. Neonatal stress transiently alters the development of hippocampal oxytocin receptors. Brain Res. Dev. Brain Res. 1994, 80, 115–120. [Google Scholar] [CrossRef]
- Lodge, D.J. The MAM rodent model of schizophrenia. Curr. Protoc. Neurosci. 2013, 63, 9.43.1–9.43.7. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Featherstone, R.E.; Rizos, Z.; Nobrega, J.N.; Kapur, S.; Fletcher, P.J. Gestational methylazoxymethanol acetate treatment impairs select cognitive functions: Parallels to schizophrenia. Neuropsychopharmacology 2007, 32, 483–492. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Moore, H.; Jentsch, J.D.; Ghajarnia, M.; Geyer, M.A.; Grace, A.A. A neurobehavioral systems analysis of adult rats exposed to methylazoxymethanol acetate on E17: Implications for the neuropathology of schizophrenia. Biol. Psychiatry 2006, 60, 253–264. [Google Scholar] [CrossRef] [Green Version]
- Hradetzky, E.; Sanderson, T.M.; Tsang, T.M.; Sherwood, J.L.; Fitzjohn, S.M.; Lakics, V.; Malik, N.; Schoeffmann, S.; O’Neill, M.J.; Cheng, T.M.; et al. The methylazoxymethanol acetate (MAM-E17) rat model: Molecular and functional effects in the hippocampus. Neuropsychopharmacology 2012, 37, 364–377. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodge, D.J.; Grace, A.A. Aberrant hippocampal activity underlies the dopamine dysregulation in an animal model of schizophrenia. J. Neurosci. 2007, 27, 11424–11430. [Google Scholar] [CrossRef] [PubMed]
- Lodge, D.J.; Grace, A.A. Gestational methylazoxymethanol acetate administration alters proteomic and metabolomic markers of hippocampal glutamatergic transmission. Neuropsychopharmacology 2012, 37, 319–320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lodge, D.J.; Behrens, M.M.; Grace, A.A. A loss of parvalbumin-containing interneurons is associated with diminished oscillatory activity in an animal model of schizophrenia. J. Neurosci. 2009, 29, 2344–2354. [Google Scholar] [CrossRef]
- Chalkiadaki, K.; Velli, A.; Kyriazidis, E.; Stavroulaki, V.; Vouvoutsis, V.; Chatzaki, E.; Aivaliotis, M.; Sidiropoulou, K. Development of the MAM model of schizophrenia in mice: Sex similarities and differences of hippocampal and prefrontal cortical function. Neuropharmacology 2019, 144, 193–207. [Google Scholar] [CrossRef]
- Du, Y.; Grace, A.A. Loss of Parvalbumin in the Hippocampus of MAM Schizophrenia Model Rats Is Attenuated by Peripubertal Diazepam. Int. J. Neuropsychopharmacol. 2016, 19, pyw065. [Google Scholar] [CrossRef] [Green Version]
- Du, Y.; Grace, A.A. Peripubertal diazepam administration prevents the emergence of dopamine system hyperresponsivity in the MAM developmental disruption model of schizophrenia. Neuropsychopharmacology 2013, 38, 1881–1888. [Google Scholar] [CrossRef]
- Zhu, X.; Grace, A.A. Prepubertal Environmental Enrichment Prevents Dopamine Dysregulation and Hippocampal Hyperactivity in MAM Schizophrenia Model Rats. Biol. Psychiatry 2021, 89, 298–307. [Google Scholar] [CrossRef]
- Lee, G.; Zhou, Y. NMDAR Hypofunction Animal Models of Schizophrenia. Front. Mol. Neurosci. 2019, 12, 185. [Google Scholar] [CrossRef] [Green Version]
- Bondi, C.; Matthews, M.; Moghaddam, B. Glutamatergic animal models of schizophrenia. Curr. Pharm. Des. 2012, 18, 1593–1604. [Google Scholar] [CrossRef]
- Frohlich, J.; van Horn, J.D. Reviewing the ketamine model for schizophrenia. J. Psychopharmacol. 2014, 28, 287–302. [Google Scholar] [CrossRef] [PubMed]
- Kayser, M.S.; Dalmau, J. Anti-NMDA Receptor Encephalitis, Autoimmunity, and Psychosis. Focus 2016, 14, 510–515. [Google Scholar] [CrossRef] [PubMed]
- Omdal, R.; Brokstad, K.; Waterloo, K.; Koldingsnes, W.; Jonsson, R.; Mellgren, S.I. Neuropsychiatric disturbances in SLE are associated with antibodies against NMDA receptors. Eur. J. Neurol. 2005, 12, 392–398. [Google Scholar] [CrossRef] [PubMed]
- Javitt, D.C.; Zukin, S.R. Recent advances in the phencyclidine model of schizophrenia. Am. J. Psychiatry 1991, 148, 1301–1308. [Google Scholar] [CrossRef] [PubMed]
- Carlsson, M.; Carlsson, A. The NMDA antagonist MK-801 causes marked locomotor stimulation in monoamine-depleted mice. J. Neural Transm. 1989, 75, 221–226. [Google Scholar] [CrossRef] [PubMed]
- Lopes-Aguiar, C.; Ruggiero, R.N.; Rossignoli, M.T.; Esteves, I.d.M.; Peixoto-Santos, J.E.; Romcy-Pereira, R.N.; Leite, J.P. Long-term potentiation prevents ketamine-induced aberrant neurophysiological dynamics in the hippocampus-prefrontal cortex pathway in vivo. Sci. Rep. 2020, 10, 7167. [Google Scholar] [CrossRef]
- Fujikawa, R.; Yamada, J.; Jinno, S. Subclass imbalance of parvalbumin-expressing GABAergic neurons in the hippocampus of a mouse ketamine model for schizophrenia, with reference to perineuronal nets. Schizophr. Res. 2020, 229, 80–93. [Google Scholar] [CrossRef]
- Kaushik, R.; Lipachev, N.; Matuszko, G.; Kochneva, A.; Dvoeglazova, A.; Becker, A.; Paveliev, M.; Dityatev, A. Fine structure analysis of perineuronal nets in the ketamine model of schizophrenia. Eur. J. Neurosci. 2020, 53, 3988–4004. [Google Scholar] [CrossRef]
- Bast, T.; Zhang, W.N.; Heidbreder, C.; Feldon, J. Hyperactivity and disruption of prepulse inhibition induced by N-methyl-D-aspartate stimulation of the ventral hippocampus and the effects of pretreatment with haloperidol and clozapine. Neuroscience 2001, 103, 325–335. [Google Scholar] [CrossRef]
- Howland, J.G.; MacKenzie, E.M.; Yim, T.T.; Taepavarapruk, P.; Phillips, A.G. Electrical stimulation of the hippocampus disrupts prepulse inhibition in rats: Frequency- and site-dependent effects. Behav. Brain Res. 2004, 152, 187–197. [Google Scholar] [CrossRef]
- San-Martin, R.; Castro, L.A.; Menezes, P.R.; Fraga, F.J.; Simões, P.W.; Salum, C. Meta-Analysis of Sensorimotor Gating Deficits in Patients With Schizophrenia Evaluated by Prepulse Inhibition Test. Schizophr. Bull. 2020, 46, 1482–1497. [Google Scholar] [CrossRef] [PubMed]
- Swerdlow, N.R.; Shoemaker, J.M.; Noh, H.R.; Ma, L.; Gaudet, I.; Munson, M.; Crain, S.; Auerbach, P.P. The ventral hippocampal regulation of prepulse inhibition and its disruption by apomorphine in rats are not mediated via the fornix. Neuroscience 2004, 123, 675–685. [Google Scholar] [CrossRef] [PubMed]
- Segev, A.; Yanagi, M.; Scott, D.; Southcott, S.A.; Lister, J.M.; Tan, C.; Li, W.; Birnbaum, S.G.; Kourrich, S.; Tamminga, C.A. Reduced GluN1 in mouse dentate gyrus is associated with CA3 hyperactivity and psychosis-like behaviors. Mol. Psychiatry 2020, 25, 2832–2843. [Google Scholar] [CrossRef]
- Alvarez, R.J.; Pafundo, D.E.; Zold, C.L.; Belforte, J.E. Interneuron NMDA Receptor Ablation Induces Hippocampus-Prefrontal Cortex Functional Hypoconnectivity after Adolescence in a Mouse Model of Schizophrenia. J. Neurosci. 2020, 40, 3304–3317. [Google Scholar] [CrossRef] [PubMed]
- Grimm, C.M.; Aksamaz, S.; Schulz, S.; Teutsch, J.; Sicinski, P.; Liss, B.; Kätzel, D. Schizophrenia-related cognitive dysfunction in the Cyclin-D2 knockout mouse model of ventral hippocampal hyperactivity. Transl. Psychiatry 2018, 8, 212. [Google Scholar] [CrossRef] [Green Version]
- Peled, A. Optogenetic neuronal control in schizophrenia. Med. Hypotheses 2011, 76, 914–921. [Google Scholar] [CrossRef]
- Wolff, A.R.; Bygrave, A.M.; Sanderson, D.J.; Boyden, E.S.; Bannerman, D.M.; Kullmann, D.M.; Kätzel, D. Optogenetic induction of the schizophrenia-related endophenotype of ventral hippocampal hyperactivity causes rodent correlates of positive and cognitive symptoms. Sci. Rep. 2018, 8, 12871. [Google Scholar] [CrossRef]
- Fan, Z.-L.; Wu, B.; Wu, G.-Y.; Yao, J.; Li, X.; Hu, K.-H.; Zhou, Z.-H.; Sui, J.-F. Optogenetic inhibition of ventral hippocampal neurons alleviates associative motor learning dysfunction in a rodent model of schizophrenia. PLoS ONE 2019, 14, e0227200. [Google Scholar] [CrossRef]
- Bauer, J.P.; Rader, S.L.; Joffe, M.E.; Kwon, W.; Quay, J.; Seanez, L.; Zhou, C.; Conn, P.J.; Lewis, A.S. Modeling intrahippocampal effects of anterior hippocampal hyperactivity relevant to schizophrenia using chemogenetic excitation of long axis-projecting mossy cells in the mouse dentate gyrus. Biol. Psychiatry Glob. Open Sci. 2021, 1, 101–111. [Google Scholar] [CrossRef]
- Gonzalez-Burgos, G.; Lewis, D.A. GABA neurons and the mechanisms of network oscillations: Implications for understanding cortical dysfunction in schizophrenia. Schizophr. Bull. 2008, 34, 944–961. [Google Scholar] [CrossRef] [Green Version]
- Konradi, C.; Yang, C.K.; Zimmerman, E.I.; Lohmann, K.M.; Gresch, P.; Pantazopoulos, H.; Berretta, S.; Heckers, S. Hippocampal interneurons are abnormal in schizophrenia. Schizophr. Res. 2011, 131, 165–173. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhang, Z.; Sun, J.; Reynolds, G.P. A selective reduction in the relative density of parvalbumin-immunoreactive neurons in the hippocampus in schizophrenia patients. Chin. Med. J. 2002, 115, 819–823. [Google Scholar] [PubMed]
- Knable, M.B.; Barci, B.M.; Webster, M.J.; Meador-Woodruff, J.; Torrey, E.F. Molecular abnormalities of the hippocampus in severe psychiatric illness: Postmortem findings from the Stanley Neuropathology Consortium. Mol. Psychiatry 2004, 9, 609–620; 544. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Antonoudiou, P.; Tan, Y.L.; Kontou, G.; Upton, A.L.; Mann, E.O. Parvalbumin and Somatostatin Interneurons Contribute to the Generation of Hippocampal Gamma Oscillations. J. Neurosci. 2020, 40, 7668–7687. [Google Scholar] [CrossRef]
- Craig, M.T.; McBain, C.J. Fast gamma oscillations are generated intrinsically in CA1 without the involvement of fast-spiking basket cells. J. Neurosci. 2015, 35, 3616–3624. [Google Scholar] [CrossRef] [Green Version]
- Cardin, J.A.; Carlén, M.; Meletis, K.; Knoblich, U.; Zhang, F.; Deisseroth, K.; Tsai, L.-H.; Moore, C.I. Driving fast-spiking cells induces gamma rhythm and controls sensory responses. Nature 2009, 459, 663–667. [Google Scholar] [CrossRef] [Green Version]
- Sohal, V.S.; Zhang, F.; Yizhar, O.; Deisseroth, K. Parvalbumin neurons and gamma rhythms enhance cortical circuit performance. Nature 2009, 459, 698–702. [Google Scholar] [CrossRef] [Green Version]
- Spencer, K.M.; Niznikiewicz, M.A.; Shenton, M.E.; McCarley, R.W. Sensory-evoked gamma oscillations in chronic schizophrenia. Biol. Psychiatry 2008, 63, 744–747. [Google Scholar] [CrossRef] [Green Version]
- Ferrarelli, F.; Massimini, M.; Peterson, M.J.; Riedner, B.A.; Lazar, M.; Murphy, M.J.; Huber, R.; Rosanova, M.; Alexander, A.L.; Kalin, N.; et al. Reduced evoked gamma oscillations in the frontal cortex in schizophrenia patients: A TMS/EEG study. Am. J. Psychiatry 2008, 165, 996–1005. [Google Scholar] [CrossRef]
- Gallinat, J.; Winterer, G.; Herrmann, C.S.; Senkowski, D. Reduced oscillatory gamma-band responses in unmedicated schizophrenic patients indicate impaired frontal network processing. Clin. Neurophysiol. 2004, 115, 1863–1874. [Google Scholar] [CrossRef]
- Farzan, F.; Barr, M.S.; Levinson, A.J.; Chen, R.; Wong, W.; Fitzgerald, P.B.; Daskalakis, Z.J. Evidence for gamma inhibition deficits in the dorsolateral prefrontal cortex of patients with schizophrenia. Brain 2010, 133, 1505–1514. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lewis, D.A.; Curley, A.A.; Glausier, J.R.; Volk, D.W. Cortical parvalbumin interneurons and cognitive dysfunction in schizophrenia. Trends Neurosci. 2012, 35, 57–67. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Nguyen, R.; Morrissey, M.D.; Mahadevan, V.; Cajanding, J.D.; Woodin, M.A.; Yeomans, J.S.; Takehara-Nishiuchi, K.; Kim, J.C. Parvalbumin and GAD65 interneuron inhibition in the ventral hippocampus induces distinct behavioral deficits relevant to schizophrenia. J. Neurosci. 2014, 34, 14948–14960. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Molina, J.L.; Thomas, M.L.; Joshi, Y.B.; Hochberger, W.C.; Koshiyama, D.; Nungaray, J.A.; Cardoso, L.; Sprock, J.; Braff, D.L.; Swerdlow, N.R.; et al. Gamma oscillations predict pro-cognitive and clinical response to auditory-based cognitive training in schizophrenia. Transl. Psychiatry 2020, 10, 405. [Google Scholar] [CrossRef]
- Yi, Y.; Song, Y.; Lu, Y. Parvalbumin Interneuron Activation-Dependent Adult Hippocampal Neurogenesis Is Required for Treadmill Running to Reverse Schizophrenia-Like Phenotypes. Front. Cell Dev. Biol. 2020, 8, 24. [Google Scholar] [CrossRef] [Green Version]
- Donegan, J.J.; Tyson, J.A.; Branch, S.Y.; Beckstead, M.J.; Anderson, S.A.; Lodge, D.J. Stem cell-derived interneuron transplants as a treatment for schizophrenia: Preclinical validation in a rodent model. Mol. Psychiatry 2017, 22, 1492–1501. [Google Scholar] [CrossRef] [Green Version]
- Perez, S.M.; Lodge, D.J. Hippocampal interneuron transplants reverse aberrant dopamine system function and behavior in a rodent model of schizophrenia. Mol. Psychiatry 2013, 18, 1193–1198. [Google Scholar] [CrossRef] [Green Version]
- Gilani, A.I.; Chohan, M.O.; Inan, M.; Schobel, S.A.; Chaudhury, N.H.; Paskewitz, S.; Chuhma, N.; Glickstein, S.; Merker, R.J.; Xu, Q.; et al. Interneuron precursor transplants in adult hippocampus reverse psychosis-relevant features in a mouse model of hippocampal disinhibition. Proc. Natl. Acad. Sci. USA 2014, 111, 7450–7455. [Google Scholar] [CrossRef] [Green Version]
- Southwell, D.G.; Seifikar, H.; Malik, R.; Lavi, K.; Vogt, D.; Rubenstein, J.L.; Sohal, V.S. Interneuron Transplantation Rescues Social Behavior Deficits without Restoring Wild-Type Physiology in a Mouse Model of Autism with Excessive Synaptic Inhibition. J. Neurosci. 2020, 40, 2215–2227. [Google Scholar] [CrossRef]
- Xia, Y.; Zhang, Z.; Lin, W.; Yan, J.; Zhu, C.; Yin, D.; He, S.; Su, Y.; Xu, N.; Caldwell, R.W.; et al. Modulating microglia activation prevents maternal immune activation induced schizophrenia-relevant behavior phenotypes via arginase 1 in the dentate gyrus. Neuropsychopharmacology 2020, 45, 1896–1908. [Google Scholar] [CrossRef]
- Carstens, K.E.; Phillips, M.L.; Pozzo-Miller, L.; Weinberg, R.J.; Dudek, S.M. Perineuronal Nets Suppress Plasticity of Excitatory Synapses on CA2 Pyramidal Neurons. J. Neurosci. 2016, 36, 6312–6320. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- O’Connor, A.M.; Burton, T.J.; Mansuri, H.; Hand, G.R.; Leamey, C.A.; Sawatari, A. Environmental Enrichment From Birth Impacts Parvalbumin Expressing Cells and Wisteria Floribunda Agglutinin Labelled Peri-Neuronal Nets Within the Developing Murine Striatum. Front. Neuroanat. 2019, 13, 90. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Celio, M.R.; Spreafico, R.; de Biasi, S.; Vitellaro-Zuccarello, L. Perineuronal nets: Past and present. Trends Neurosci. 1998, 21, 510–515. [Google Scholar] [CrossRef]
- Pizzorusso, T.; Medini, P.; Berardi, N.; Chierzi, S.; Fawcett, J.W.; Maffei, L. Reactivation of ocular dominance plasticity in the adult visual cortex. Science 2002, 298, 1248–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gogolla, N.; Caroni, P.; Lüthi, A.; Herry, C. Perineuronal nets protect fear memories from erasure. Science 2009, 325, 1258–1261. [Google Scholar] [CrossRef] [Green Version]
- Frischknecht, R.; Heine, M.; Perrais, D.; Seidenbecher, C.I.; Choquet, D.; Gundelfinger, E.D. Brain extracellular matrix affects AMPA receptor lateral mobility and short-term synaptic plasticity. Nat. Neurosci. 2009, 12, 897–904. [Google Scholar] [CrossRef]
- Gottschling, C.; Wegrzyn, D.; Denecke, B.; Faissner, A. Elimination of the four extracellular matrix molecules tenascin-C, tenascin-R, brevican and neurocan alters the ratio of excitatory and inhibitory synapses. Sci. Rep. 2019, 9, 13939. [Google Scholar] [CrossRef] [Green Version]
- Lensjø, K.K.; Lepperød, M.E.; Dick, G.; Hafting, T.; Fyhn, M. Removal of Perineuronal Nets Unlocks Juvenile Plasticity Through Network Mechanisms of Decreased Inhibition and Increased Gamma Activity. J. Neurosci. 2017, 37, 1269–1283. [Google Scholar] [CrossRef] [Green Version]
- Pizzorusso, T.; Medini, P.; Landi, S.; Baldini, S.; Berardi, N.; Maffei, L. Structural and functional recovery from early monocular deprivation in adult rats. Proc. Natl. Acad. Sci. USA 2006, 103, 8517–8522. [Google Scholar] [CrossRef] [Green Version]
- Cabungcal, J.-H.; Steullet, P.; Morishita, H.; Kraftsik, R.; Cuenod, M.; Hensch, T.K.; Do, K.Q. Perineuronal nets protect fast-spiking interneurons against oxidative stress. Proc. Natl. Acad. Sci. USA 2013, 110, 9130–9135. [Google Scholar] [CrossRef] [Green Version]
- Härtig, W.; Derouiche, A.; Welt, K.; Brauer, K.; Grosche, J.; Mäder, M.; Reichenbach, A.; Brückner, G. Cortical neurons immunoreactive for the potassium channel Kv3.1b subunit are predominantly surrounded by perineuronal nets presumed as a buffering system for cations. Brain Res. 1999, 842, 15–29. [Google Scholar] [CrossRef]
- Morawski, M.; Brückner, M.K.; Riederer, P.; Brückner, G.; Arendt, T. Perineuronal nets potentially protect against oxidative stress. Exp. Neurol. 2004, 188, 309–315. [Google Scholar] [CrossRef] [PubMed]
- Morawski, M.; Reinert, T.; Meyer-Klaucke, W.; Wagner, F.E.; Tröger, W.; Reinert, A.; Jäger, C.; Brückner, G.; Arendt, T. Ion exchanger in the brain: Quantitative analysis of perineuronally fixed anionic binding sites suggests diffusion barriers with ion sorting properties. Sci. Rep. 2015, 5, 16471. [Google Scholar] [CrossRef] [PubMed]
- Enwright, J.F.; Sanapala, S.; Foglio, A.; Berry, R.; Fish, K.N.; Lewis, D.A. Reduced Labeling of Parvalbumin Neurons and Perineuronal Nets in the Dorsolateral Prefrontal Cortex of Subjects with Schizophrenia. Neuropsychopharmacology 2016, 41, 2206–2214. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Mauney, S.A.; Athanas, K.M.; Pantazopoulos, H.; Shaskan, N.; Passeri, E.; Berretta, S.; Woo, T.-U.W. Developmental pattern of perineuronal nets in the human prefrontal cortex and their deficit in schizophrenia. Biol. Psychiatry 2013, 74, 427–435. [Google Scholar] [CrossRef] [Green Version]
- Pantazopoulos, H.; Woo, T.-U.W.; Lim, M.P.; Lange, N.; Berretta, S. Extracellular matrix-glial abnormalities in the amygdala and entorhinal cortex of subjects diagnosed with schizophrenia. Arch. Gen. Psychiatry 2010, 67, 155–166. [Google Scholar] [CrossRef] [Green Version]
- Pantazopoulos, H.; Markota, M.; Jaquet, F.; Ghosh, D.; Wallin, A.; Santos, A.; Caterson, B.; Berretta, S. Aggrecan and chondroitin-6-sulfate abnormalities in schizophrenia and bipolar disorder: A postmortem study on the amygdala. Transl. Psychiatry 2015, 5, e496. [Google Scholar] [CrossRef] [Green Version]
- Kilonzo, V.W.; Sweet, R.A.; Glausier, J.R.; Pitts, M.W. Deficits in Glutamic Acid Decarboxylase 67 Immunoreactivity, Parvalbumin Interneurons, and Perineuronal Nets in the Inferior Colliculus of Subjects with Schizophrenia. Schizophr. Bull. 2020, 46, 1053–1059. [Google Scholar] [CrossRef]
- Alcaide, J.; Guirado, R.; Crespo, C.; Blasco-Ibáñez, J.M.; Varea, E.; Sanjuan, J.; Nacher, J. Alterations of perineuronal nets in the dorsolateral prefrontal cortex of neuropsychiatric patients. Int. J. Bipolar Disord. 2019, 7, 24. [Google Scholar] [CrossRef]
- Wen, T.H.; Binder, D.K.; Ethell, I.M.; Razak, K.A. The Perineuronal ‘Safety’ Net? Perineuronal Net Abnormalities in Neurological Disorders. Front. Mol. Neurosci. 2018, 11, 270. [Google Scholar] [CrossRef]
- Berretta, S.; Pantazopoulos, H.; Markota, M.; Brown, C.; Batzianouli, E.T. Losing the sugar coating: Potential impact of perineuronal net abnormalities on interneurons in schizophrenia. Schizophr. Res. 2015, 167, 18–27. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitanihirwe, B.K.Y.; Woo, T.-U.W. Perineuronal nets and schizophrenia: The importance of neuronal coatings. Neurosci. Biobehav. Rev. 2014, 45, 85–99. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Bitanihirwe, B.K.Y.; Mauney, S.A.; Woo, T.-U.W. Weaving a Net of Neurobiological Mechanisms in Schizophrenia and Unraveling the Underlying Pathophysiology. Biol. Psychiatry 2016, 80, 589–598. [Google Scholar] [CrossRef] [Green Version]
- Sorg, B.A.; Berretta, S.; Blacktop, J.M.; Fawcett, J.W.; Kitagawa, H.; Kwok, J.C.F.; Miquel, M. Casting a Wide Net: Role of Perineuronal Nets in Neural Plasticity. J. Neurosci. 2016, 36, 11459–11468. [Google Scholar] [CrossRef] [Green Version]
- Klimczak, P.; Rizzo, A.; Castillo-Gómez, E.; Perez-Rando, M.; Gramuntell, Y.; Beltran, M.; Nacher, J. Parvalbumin Interneurons and Perineuronal Nets in the Hippocampus and Retrosplenial Cortex of Adult Male Mice After Early Social Isolation Stress and Perinatal NMDA Receptor Antagonist Treatment. Front. Synaptic Neurosci. 2021, 13, 733989. [Google Scholar] [CrossRef]
- Cope, E.C.; Zych, A.D.; Katchur, N.J.; Waters, R.C.; Laham, B.J.; Diethorn, E.J.; Park, C.Y.; Meara, W.R.; Gould, E. Atypical perineuronal nets in the CA2 region interfere with social memory in a mouse model of social dysfunction. Mol. Psychiatry 2021, 1–5. [Google Scholar] [CrossRef]
- Christensen, A.C.; Lensjø, K.K.; Lepperød, M.E.; Dragly, S.-A.; Sutterud, H.; Blackstad, J.S.; Fyhn, M.; Hafting, T. Perineuronal nets stabilize the grid cell network. Nat. Commun. 2021, 12, 253. [Google Scholar] [CrossRef]
- Romberg, C.; Yang, S.; Melani, R.; Andrews, M.R.; Horner, A.E.; Spillantini, M.G.; Bussey, T.J.; Fawcett, J.W.; Pizzorusso, T.; Saksida, L.M. Depletion of perineuronal nets enhances recognition memory and long-term depression in the perirhinal cortex. J. Neurosci. 2013, 33, 7057–7065. [Google Scholar] [CrossRef] [Green Version]
- Yang, S.; Cacquevel, M.; Saksida, L.M.; Bussey, T.J.; Schneider, B.L.; Aebischer, P.; Melani, R.; Pizzorusso, T.; Fawcett, J.W.; Spillantini, M.G. Perineuronal net digestion with chondroitinase restores memory in mice with tau pathology. Exp. Neurol. 2015, 265, 48–58. [Google Scholar] [CrossRef] [Green Version]
- Xue, Y.-X.; Xue, L.-F.; Liu, J.-F.; He, J.; Deng, J.-H.; Sun, S.-C.; Han, H.-B.; Luo, Y.-X.; Xu, L.-Z.; Wu, P.; et al. Depletion of perineuronal nets in the amygdala to enhance the erasure of drug memories. J. Neurosci. 2014, 34, 6647–6658. [Google Scholar] [CrossRef] [Green Version]
- Shah, A.; Lodge, D.J. A loss of hippocampal perineuronal nets produces deficits in dopamine system function: Relevance to the positive symptoms of schizophrenia. Transl. Psychiatry 2013, 3, e215. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Sun, Z.Y.; Bozzelli, P.L.; Caccavano, A.; Allen, M.; Balmuth, J.; Vicini, S.; Wu, J.-Y.; Conant, K. Disruption of perineuronal nets increases the frequency of sharp wave ripple events. Hippocampus 2018, 28, 42–52. [Google Scholar] [CrossRef] [PubMed]
- Hayani, H.; Song, I.; Dityatev, A. Increased Excitability and Reduced Excitatory Synaptic Input into Fast-Spiking CA2 Interneurons After Enzymatic Attenuation of Extracellular Matrix. Front. Cell. Neurosci. 2018, 12, 149. [Google Scholar] [CrossRef] [Green Version]
- Shi, W.; Wei, X.; Wang, X.; Du, S.; Liu, W.; Song, J.; Wang, Y. Perineuronal nets protect long-term memory by limiting activity-dependent inhibition from parvalbumin interneurons. Proc. Natl. Acad. Sci. USA 2019, 116, 27063–27073. [Google Scholar] [CrossRef] [PubMed]
- Wegrzyn, D.; Freund, N.; Faissner, A.; Juckel, G. Poly I:C Activated Microglia Disrupt Perineuronal Nets and Modulate Synaptic Balance in Primary Hippocampal Neurons in vitro. Front. Synaptic Neurosci. 2021, 13, 637549. [Google Scholar] [CrossRef] [PubMed]
- Pyka, M.; Wetzel, C.; Aguado, A.; Geissler, M.; Hatt, H.; Faissner, A. Chondroitin sulfate proteoglycans regulate astrocyte-dependent synaptogenesis and modulate synaptic activity in primary embryonic hippocampal neurons. Eur. J. Neurosci. 2011, 33, 2187–2202. [Google Scholar] [CrossRef]
- Crapser, J.D.; Arreola, M.A.; Tsourmas, K.I.; Green, K.N. Microglia as hackers of the matrix: Sculpting synapses and the extracellular space. Cell. Mol. Immunol. 2021, 18, 2472–2488. [Google Scholar] [CrossRef]
- Crapser, J.D.; Ochaba, J.; Soni, N.; Reidling, J.C.; Thompson, L.M.; Green, K.N. Microglial depletion prevents extracellular matrix changes and striatal volume reduction in a model of Huntington’s disease. Brain 2020, 143, 266–288. [Google Scholar] [CrossRef]
- Crapser, J.D.; Spangenberg, E.E.; Barahona, R.A.; Arreola, M.A.; Hohsfield, L.A.; Green, K.N. Microglia facilitate loss of perineuronal nets in the Alzheimer’s disease brain. EBioMedicine 2020, 58, 102919. [Google Scholar] [CrossRef]
- Strackeljan, L.; Baczynska, E.; Cangalaya, C.; Baidoe-Ansah, D.; Wlodarczyk, J.; Kaushik, R.; Dityatev, A. Microglia Depletion-Induced Remodeling of Extracellular Matrix and Excitatory Synapses in the Hippocampus of Adult Mice. Cells 2021, 10, 1862. [Google Scholar] [CrossRef]
- Dubisova, J.; Burianova, J.S.; Svobodova, L.; Makovicky, P.; Martinez-Varea, N.; Cimpean, A.; Fawcett, J.W.; Kwok, J.C.F.; Kubinova, S. Oral treatment of 4-methylumbelliferone reduced perineuronal nets and improved recognition memory in mice. Brain Res. Bull. 2022, 181, 144–156. [Google Scholar] [CrossRef] [PubMed]
- Slaker, M.; Barnes, J.; Sorg, B.A.; Grimm, J.W. Impact of Environmental Enrichment on Perineuronal Nets in the Prefrontal Cortex following Early and Late Abstinence from Sucrose Self-Administration in Rats. PLoS ONE 2016, 11, e0168256. [Google Scholar] [CrossRef] [PubMed] [Green Version]
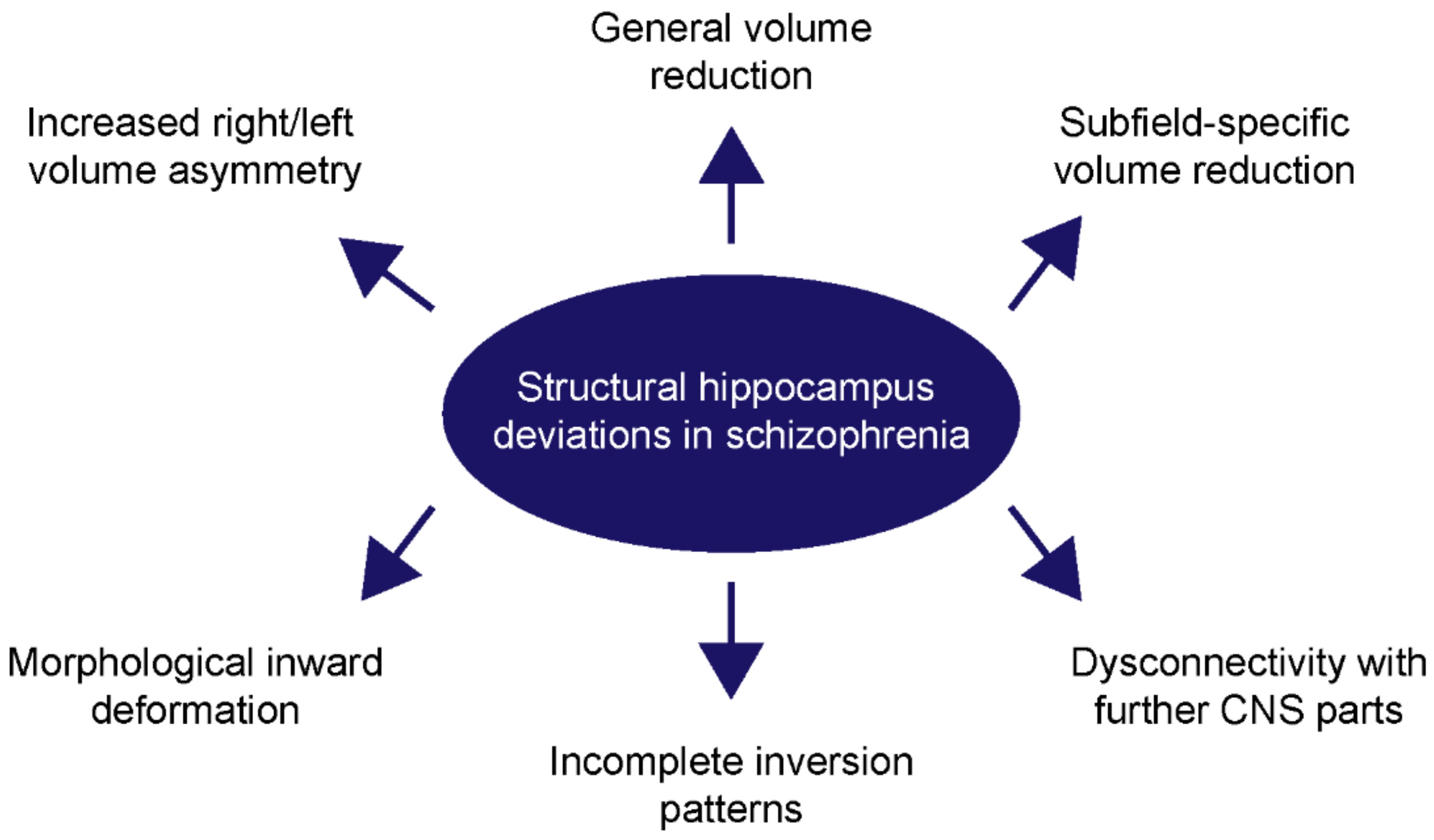



| Type of Structural Deviation | Experimental Approach | Reference |
|---|---|---|
| general volume reduction of the hippocampus | postmortem morphometric measurement | [18] |
| general volume reduction of the hippocampus | Coronal MRI/ high-resolution MRI | [19,21,22,55,56] |
| volume reduction of the left amygdala/hippocampal complex (AHC) | Coronal MRI/ high-resolution MRI | [23] |
| volume reduction of hippocampal subfields in the left hippocampus | Meta-analysis of postmortem studies | [25] |
| higher rate of incomplete hippocampal inversions with a reduced hippocampal volume | Coronal MRI/ high-resolution MRI | [38,39] |
| association of visual hallucinations with specific inversion patterns | Coronal MRI/ high-resolution MRI | [42] |
| volume reduction of anterior and midbody CA1- and CA2- regions with increased peri-hippocampal CSF levels | Coronal MRI/ high-resolution MRI | [20,55] |
| bilateral inward deformation of the anterior hippocampus | Coronal MRI/ high-resolution MRI | [57] |
| reduction of SV2A-positive synaptic vesicles in the hippocampus | PET-scan analysis | [60] |
| unchanged density of postsynaptic elements in the hippocampus | Meta-analysis of postmortem studies | [63] |
| reduced PSD-95 levels in the CA1-region/dentate molecular layer | Immunoblot-analysis of postmortem samples/ immuno-autoradiography | [26,28] |
| reduced SAP-102 levels in the hippocampus | Western blot analysis of postmortem samples | [68] |
| increased spine density and PSD-95 levels on CA3 pyramidal cell dendrites | Western blot analysis of postmortem samples/ Golgi staining | [27] |
| abnormal resting-state cortico-hippocampal network coherence | Functional connectivity analysis | [29] |
| reduced connectivity between the hippocampus and the striatum | Functional MRI analysis | [31] |
| functional hypoconnectivity to regions of the default mode network and hyperconnectivity to the lateral occipital cortex | Connectivity and Magnetic Resonance Spectroscopy Study | [32] |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Wegrzyn, D.; Juckel, G.; Faissner, A. Structural and Functional Deviations of the Hippocampus in Schizophrenia and Schizophrenia Animal Models. Int. J. Mol. Sci. 2022, 23, 5482. https://doi.org/10.3390/ijms23105482
Wegrzyn D, Juckel G, Faissner A. Structural and Functional Deviations of the Hippocampus in Schizophrenia and Schizophrenia Animal Models. International Journal of Molecular Sciences. 2022; 23(10):5482. https://doi.org/10.3390/ijms23105482
Chicago/Turabian StyleWegrzyn, David, Georg Juckel, and Andreas Faissner. 2022. "Structural and Functional Deviations of the Hippocampus in Schizophrenia and Schizophrenia Animal Models" International Journal of Molecular Sciences 23, no. 10: 5482. https://doi.org/10.3390/ijms23105482
APA StyleWegrzyn, D., Juckel, G., & Faissner, A. (2022). Structural and Functional Deviations of the Hippocampus in Schizophrenia and Schizophrenia Animal Models. International Journal of Molecular Sciences, 23(10), 5482. https://doi.org/10.3390/ijms23105482






