Improvement of Glycaemia and Endothelial Function by a New Low-Dose Curcuminoid in an Animal Model of Type 2 Diabetes
Abstract
:1. Introduction
2. Results
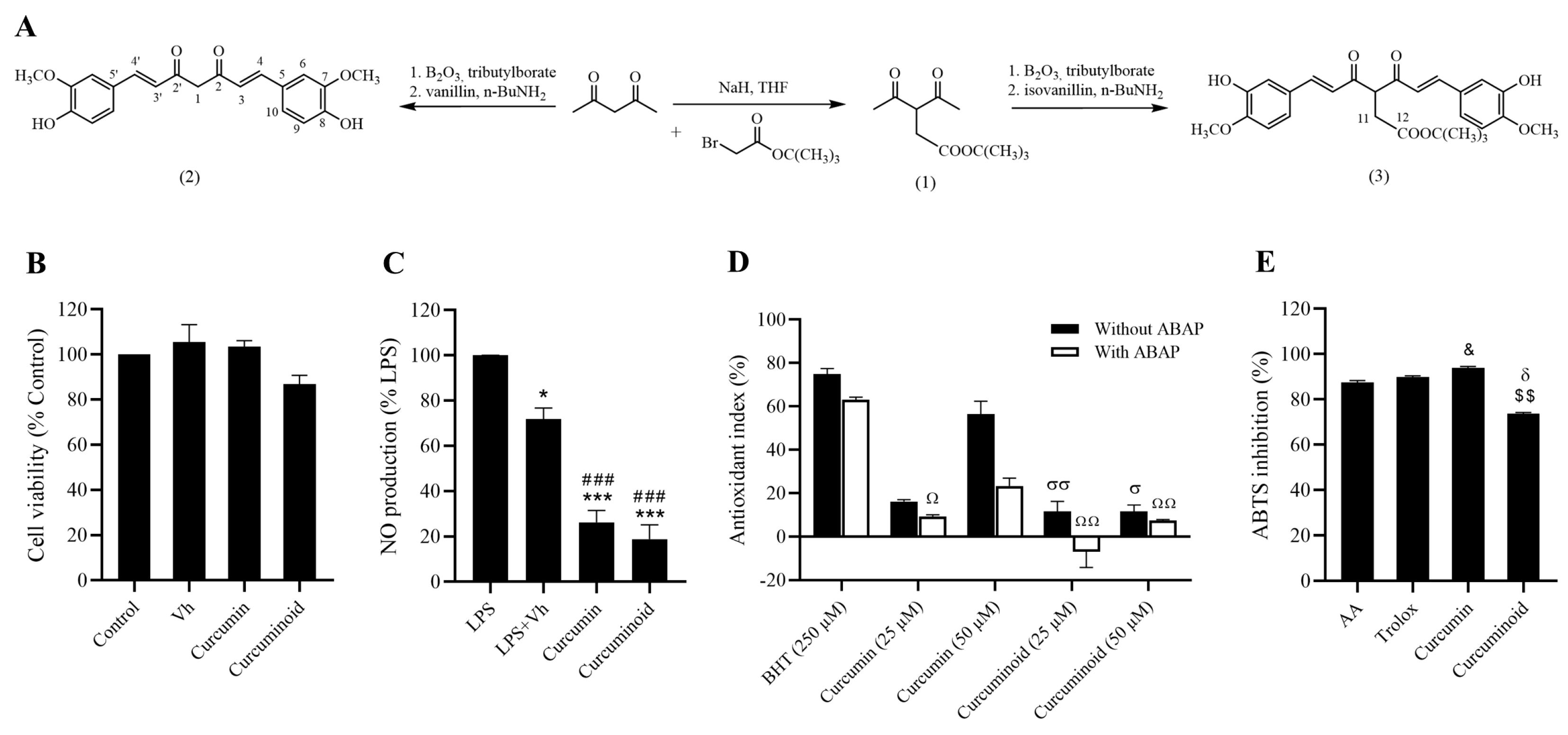
2.1. In Vitro Antioxidant and Anti-Inflammatory Effects of the Curcuminoid
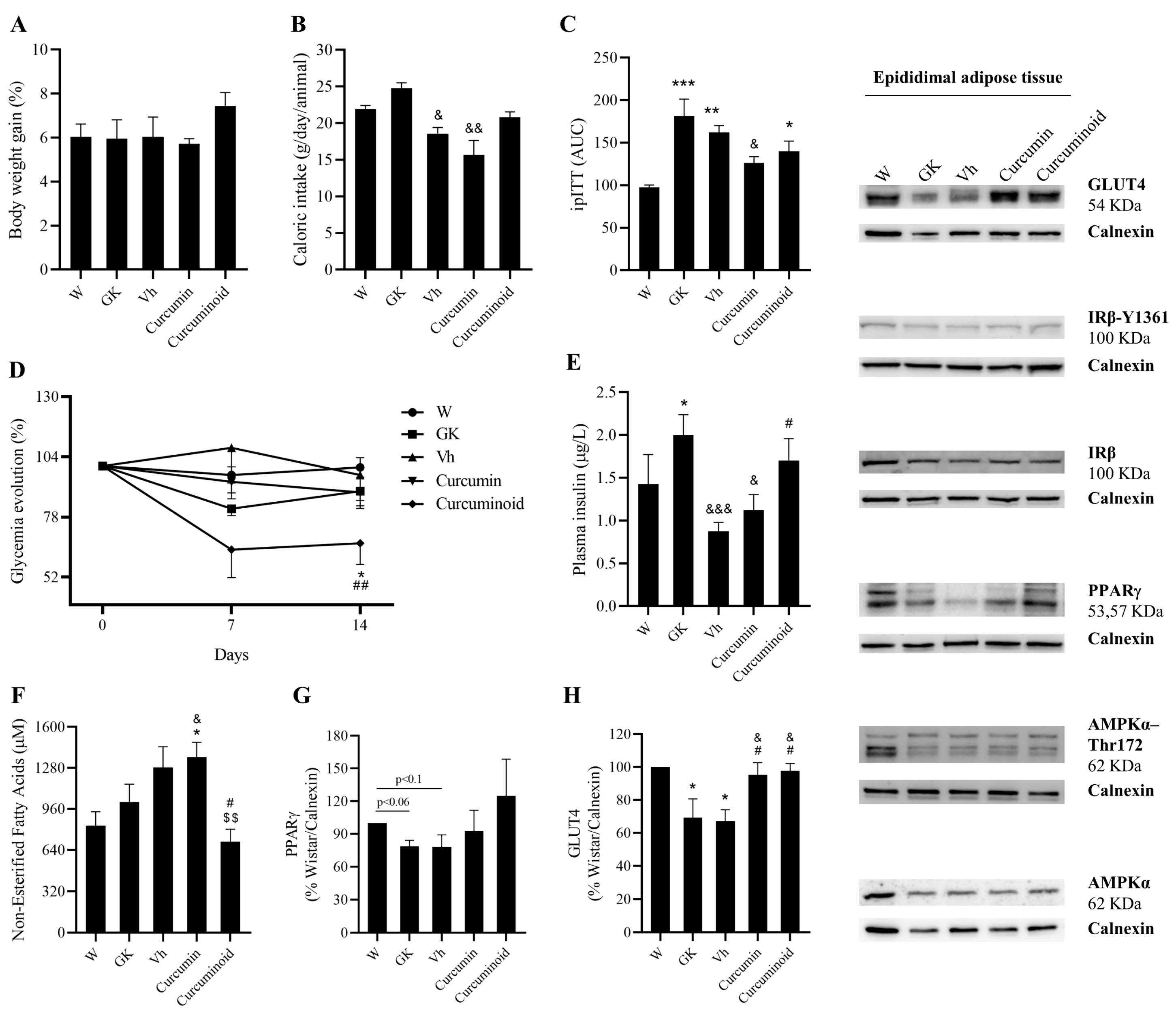
2.2. The Curcuminoid Has Marked Hypoglycaemic Effects
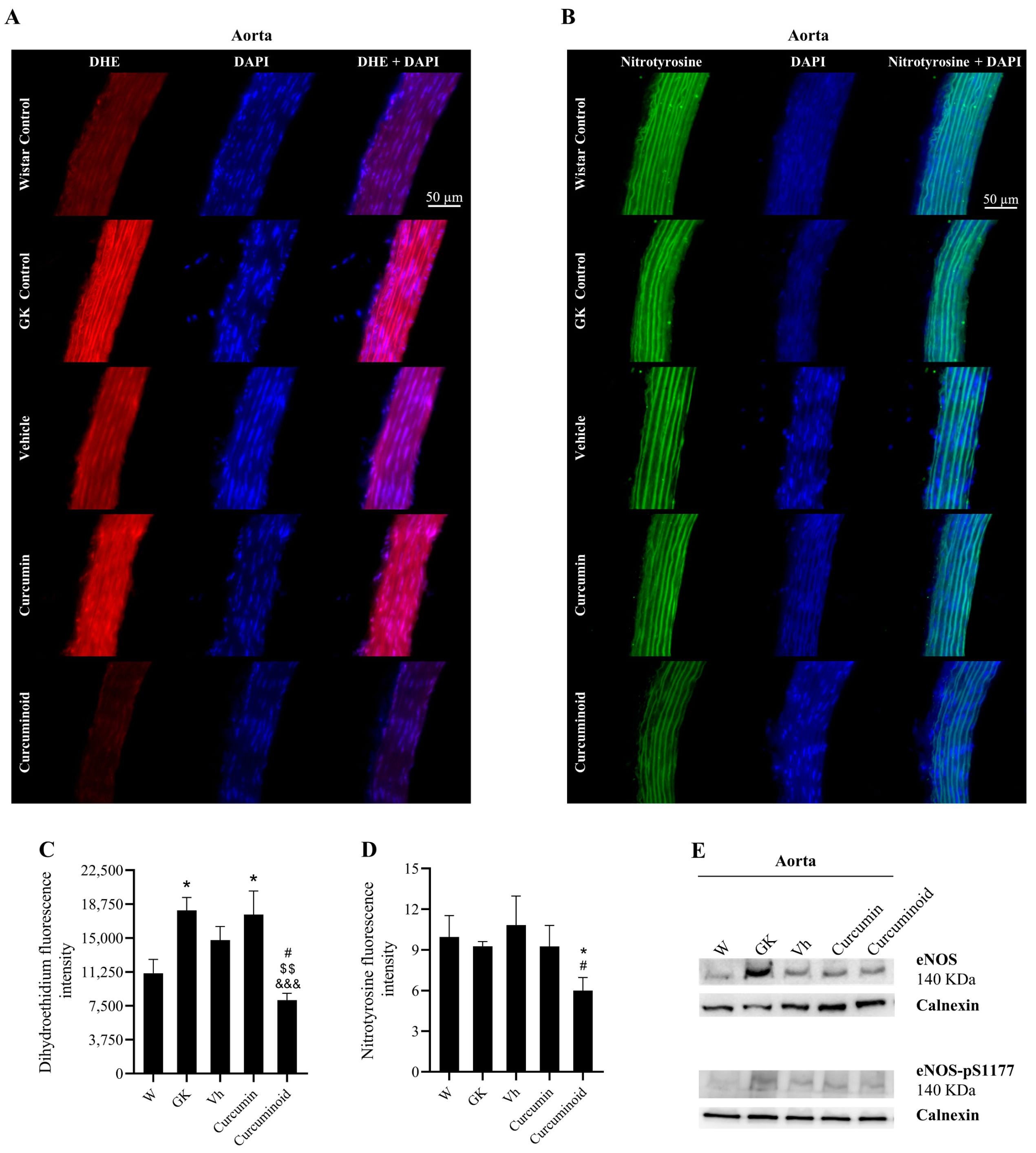
2.3. The Curcuminoid Improves Endothelial Function and Oxidative Stress in Type 2 Diabetes
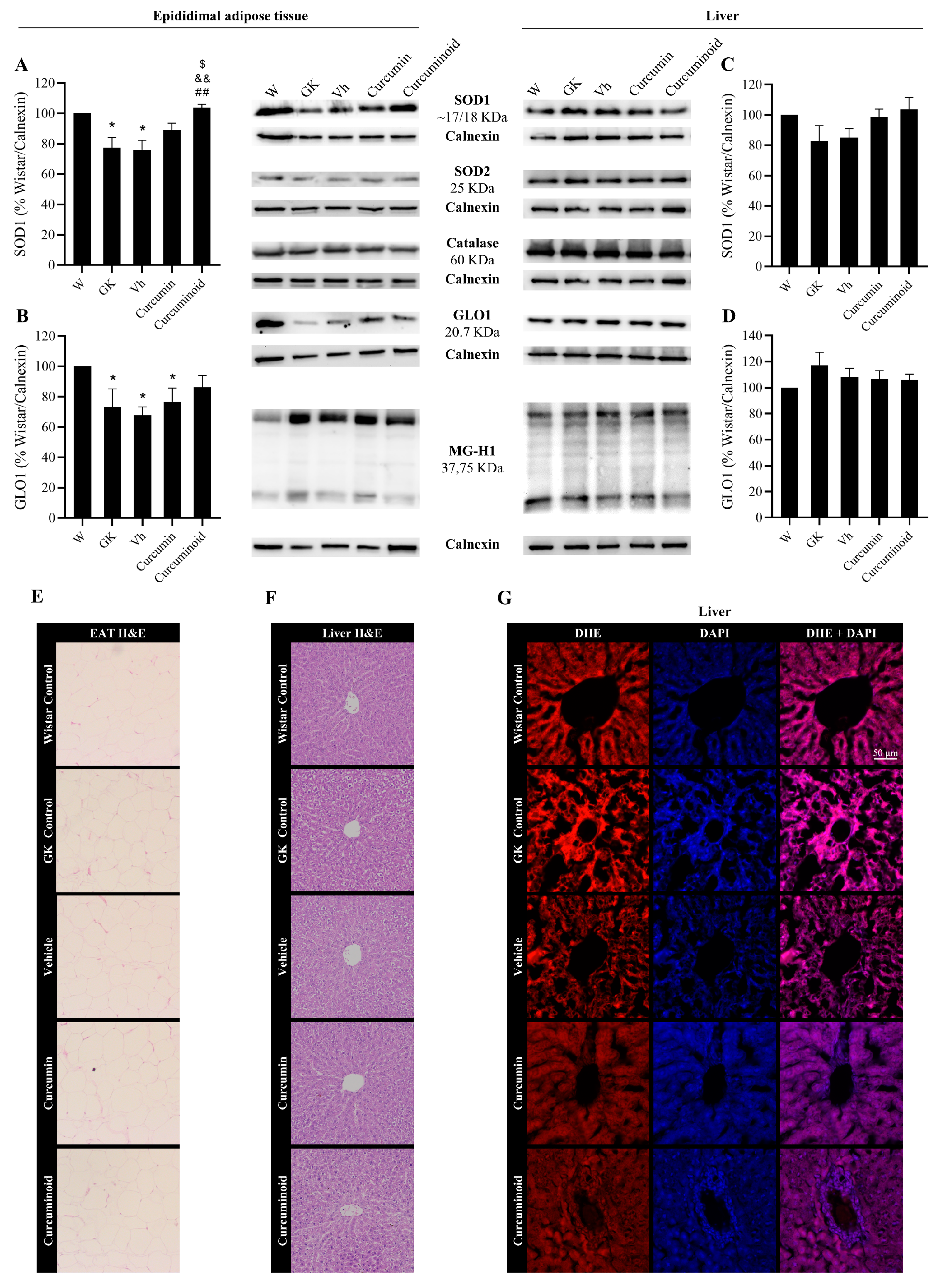
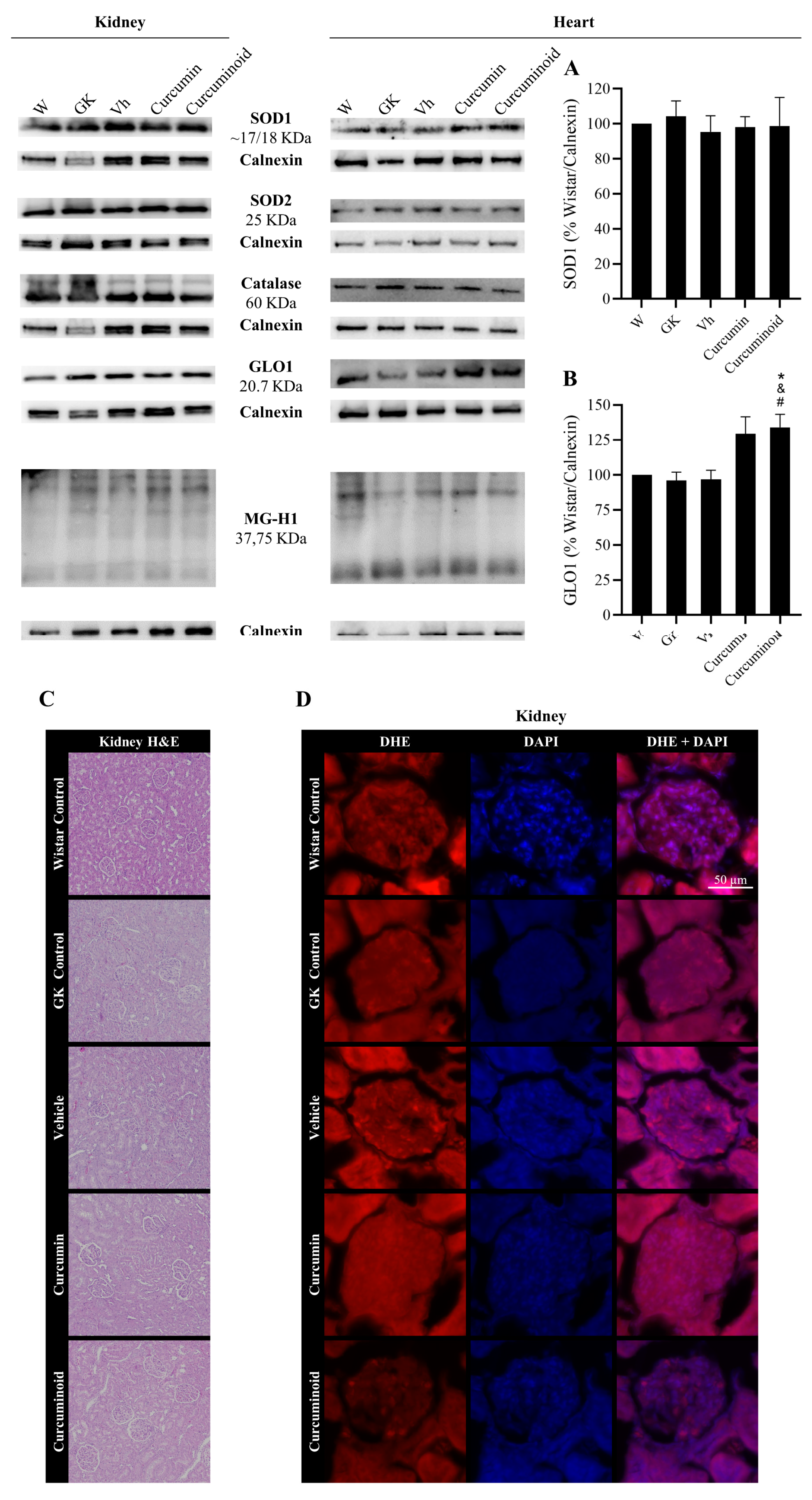
2.4. The Curcuminoid Modulates Antioxidant Systems in Epididymal Adipose Tissue and the Heart
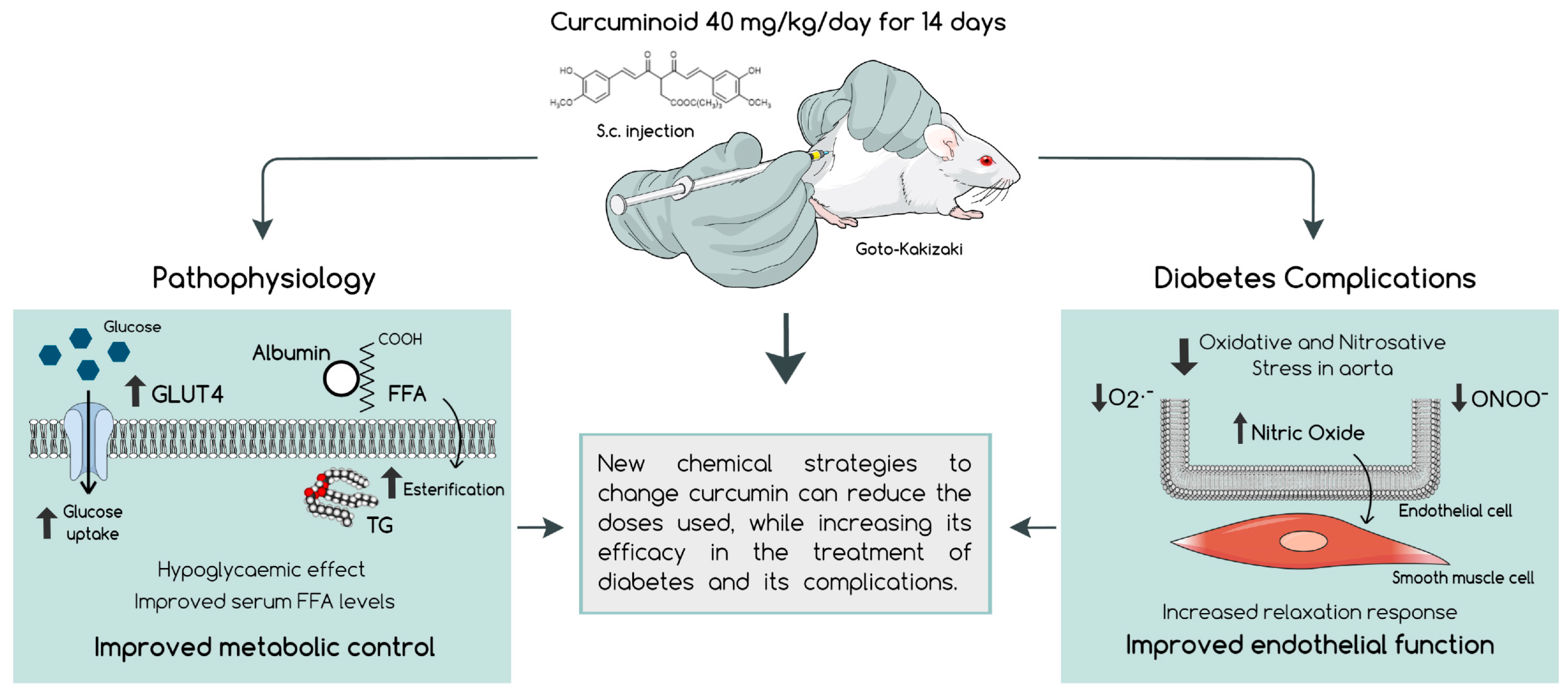
3. Discussion
4. Materials and Methods
4.1. Chemicals and Antibodies
4.2. Chemical Synthesis of Curcumin (2) and Curcuminoid (3)
4.3. Cell Culture
4.4. In Vitro Cell Viability in Cos-7
4.5. Griess Reagent Method for NO Detectionin RAW 264.7
4.6. In Vitro Antioxidant Activity
4.6.1. 2,2′-Azino-bis(3-Ethylbenzothiazoline-6-Sulfonic Acid) (ABTS) Enzymatic Assay
4.6.2. Thiobarbituric Acid-Reactive Substances (TBARS) Assay
4.7. Animal Maintenance
4.8. Experimental Groups
4.9. In Vivo Procedures and Sample Collection
4.10. Functional Studies
4.11. Western Blotting
4.12. Histological Colorimetric Assays
4.13. Assessment of Aortic Immunofluorescence
4.14. Detection of Superoxide Anion
4.15. Statistical Analysis
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- American Diabetes Association. 2. Classification and Diagnosis of Diabetes: Standards of Medical Care in Diabetes-2019. Diabetes Care 2019, 42, S13–S28. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Giacco, F.; Brownlee, M. Oxidative Stress and Diabetic Complications. Circ. Res. 2010, 107, 1058–1070. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- DeFronzo, R.A.; Ferrannini, E.; Groop, L.; Henry, R.R.; Herman, W.H.; Holst, J.J.; Hu, F.B.; Kahn, C.R.; Raz, I.; Shulman, G.I.; et al. Type 2 Diabetes Mellitus. Nat. Rev. Dis. Primers 2015, 1, 15019. [Google Scholar] [CrossRef]
- Clinical Development Plan: Curcumin. J. Cell. Biochem. 1996, 26, 72–85.
- Oliveira, S.; Monteiro-Alfredo, T.; Silva, S.; Matafome, P. Curcumin Derivatives for Type 2 Diabetes Management and Prevention of Complications. Arch. Pharm. Res. 2020, 43, 567–581. [Google Scholar] [CrossRef]
- Thota, R.N.; Dias, C.B.; Abbott, K.A.; Acharya, S.H.; Garg, M.L. Curcumin Alleviates Postprandial Glycaemic Response in Healthy Subjects: A Cross-over, Randomized Controlled Study. Sci. Rep. 2018, 8, 13679. [Google Scholar] [CrossRef] [PubMed]
- Adibian, M.; Hodaei, H.; Nikpayam, O.; Sohrab, G.; Hekmatdoost, A.; Hedayati, M. The Effects of Curcumin Supplementation on High-Sensitivity C-Reactive Protein, Serum Adiponectin, and Lipid Profile in Patients with Type 2 Diabetes: A Randomized, Double-Blind, Placebo-Controlled Trial. Phyther. Res. 2019, 33, 1374–1383. [Google Scholar] [CrossRef] [Green Version]
- Hodaei, H.; Adibian, M.; Nikpayam, O.; Hedayati, M.; Sohrab, G. The Effect of Curcumin Supplementation on Anthropometric Indices, Insulin Resistance and Oxidative Stress in Patients with Type 2 Diabetes: A Randomized, Double-Blind Clinical Trial. Diabetol. Metab. Syndr. 2019, 11, 41. [Google Scholar] [CrossRef] [Green Version]
- Santos-Parker, J.R.; Strahler, T.R.; Bassett, C.J.; Bispham, N.Z.; Chonchol, M.B.; Seals, D.R. Curcumin Supplementation Improves Vascular Endothelial Function in Healthy Middle-Aged and Older Adults by Increasing Nitric Oxide Bioavailability and Reducing Oxidative Stress. Aging 2017, 9, 187–208. [Google Scholar] [CrossRef] [Green Version]
- Ding, L.; Li, J.; Song, B.; Xiao, X.; Zhang, B.; Qi, M.; Huang, W.; Yang, L.; Wang, Z. Curcumin Rescues High Fat Diet-Induced Obesity and Insulin Sensitivity in Mice through Regulating SREBP Pathway. Toxicol. Appl. Pharmacol. 2016, 304, 99–109. [Google Scholar] [CrossRef]
- Kato, M.; Nishikawa, S.; Ikehata, A.; Dochi, K.; Tani, T.; Takahashi, T.; Imaizumi, A.; Tsuda, T. Curcumin Improves Glucose Tolerance via Stimulation of Glucagon-like Peptide-1 Secretion. Mol. Nutr. Food Res. 2017, 61, 1600471. [Google Scholar] [CrossRef] [PubMed]
- Pan, Y.; Zhao, D.; Yu, N.; An, T.; Miao, J.; Mo, F.; Gu, Y.; Zhang, D.; Gao, S.; Jiang, G. Curcumin Improves Glycolipid Metabolism through Regulating Peroxisome Proliferator Activated Receptor γ Signalling Pathway in High-Fat Diet-Induced Obese Mice and 3t3-L1 Adipocytes. R. Soc. Open Sci. 2017, 4, 170917. [Google Scholar] [CrossRef] [Green Version]
- Su, L.Q.; Wang, Y.D.; Chi, H.Y. Effect of Curcumin on Glucose and Lipid Metabolism, FFAs and TNF-α in Serum of Type 2 Diabetes Mellitus Rat Models. Saudi J. Biol. Sci. 2017, 24, 1776–1780. [Google Scholar] [CrossRef]
- Linnenkamp, U.; Greiner, G.G.; Haastert, B.; Adamczewski, H.; Kaltheuner, M.; Weber, D.; Icks, A. Postpartum screening of women with GDM in specialised practices: Data from 12,991 women in the GestDiab register. Diabet. Med. 2022, 26, e14861. [Google Scholar] [CrossRef] [PubMed]
- Li, Z.; Cheng, Y.; Wang, D.; Chen, H.; Chen, H.; Ming, W.; Wang, Z. Incidence Rate of Type 2 Diabetes Mellitus after Gestational Diabetes Mellitus: A Systematic Review and Meta-Analysis of 170,139 Women. J. Diabetes Res. 2020, 2020, 3076463. [Google Scholar] [CrossRef]
- Vounzoulaki, E.; Khunti, K.; Abner, S.; Tan, B.K.; Davies, M.J.; Gillies, C.L. Progression to type 2 diabetes in women with a known history of gestational diabetes: Systematic review and meta-analysis. BMJ 2020, 369, m13761. [Google Scholar]
- Lu, X.; Wu, F.; Jiang, M.; Sun, X.; Tian, G. Curcumin ameliorates gestational diabetes in mice partly through activating AMPK. Pharm. Biol. 2019, 57, 250–254. [Google Scholar] [CrossRef] [Green Version]
- Wu, Y.; Wang, F.; Reece, E.A.; Yang, P. Curcumin Ameliorates High Glucose-Induced Neural Tube Defects by Suppressing Cellular Stress and Apoptosis. Am. J. Obstet. Gynecol. 2015, 212, e801–e808. [Google Scholar] [CrossRef] [Green Version]
- Basak, S.; Srinivas, V.; Mallepogu, A.; Duttaroy, A.K. Curcumin Stimulates Angiogenesis through VEGF and Expression of HLA-G in First-Trimester Human Placental Trophoblasts. Cell Biol. Int. 2020, 44, 1237–1251. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Qi, L.; Jiang, J.; Zhang, J.; Zhang, L.; Wang, T. Curcumin Protects Human Trophoblast HTR8/SVneo Cells from H2O2-Induced Oxidative Stress by Activating Nrf2 Signaling Pathway. Antioxidants 2020, 9, 121. [Google Scholar] [CrossRef] [Green Version]
- Tossetta, G.; Fantone, S.; Giannubilo, S.R.; Marzioni, D. The Multifaced Actions of Curcumin in Pregnancy Outcome. Antioxidants 2021, 10, 126. [Google Scholar] [CrossRef] [PubMed]
- Anand, P.; Kunnumakkara, A.B.; Newman, R.A.; Aggarwal, B.B. Bioavailability of Curcumin: Problems and Promises. Mol. Pharm. 2007, 4, 807–818. [Google Scholar] [CrossRef] [PubMed]
- Ferrari, E.; Pignedoli, F.; Imbriano, C.; Marverti, G.; Basile, V.; Venturi, E.; Saladini, M. Newly Synthesized Curcumin Derivatives: Crosstalk between Chemico-Physical Properties and Biological Activity. J. Med. Chem. 2011, 54, 8066–8077. [Google Scholar] [CrossRef]
- Gutierres, V.O.; Campos, M.L.; Arcaro, C.A.; Assis, R.P.; Baldan-Cimatti, H.M.; Peccinini, R.G.; Paula-Gomes, S.; Kettelhut, I.C.; Baviera, A.M.; Brunetti, I.L. Curcumin Pharmacokinetic and Pharmacodynamic Evidences in Streptozotocin-Diabetic Rats Support the Antidiabetic Activity to Be via Metabolite(S). Evid.-Based Complement. Altern. Med. 2015, 2015, 678218. [Google Scholar] [CrossRef] [Green Version]
- Maithilikarpagaselvi, N.; Sridhar, M.G.; Swaminathan, R.P.; Zachariah, B. Curcumin Prevents Inflammatory Response, Oxidative Stress and Insulin Resistance in High Fructose Fed Male Wistar Rats: Potential Role of Serine Kinases. Chem. Biol. Interact. 2016, 244, 187–194. [Google Scholar] [CrossRef]
- Zaheri, Z.; Fahremand, F.; Rezvani, M.E.; Karimollah, A.; Moradi, A. Curcumin exerts beneficial role on insulin resistance through modulation of SOCS3 and Rac-1 pathways in type 2 diabetic rats. J. Funct. Foods 2019, 60, 103430. [Google Scholar] [CrossRef]
- Perugini, J.; Mercurio, E.D.; Tossetta, G.; Severi, I.; Monaco, F.; Reguzzoni, M.; Tomasetti, M.; Dani, C.; Cinti, S.; Giodano, A. Biological Effects of Ciliary Neurotrophic Factor on hMADS Adipocytes. Front. Endocrinol. 2019, 10, 768. [Google Scholar] [CrossRef] [Green Version]
- Hu, W.; Lv, J.; Han, M.; Yang, Z.; Li, T.; Jiang, S.; Yang, Y. STAT3: The art of multi-tasking of metabolic and immune functions in obesity. Prog. Lipid Res. 2018, 70, 17–28. [Google Scholar] [CrossRef]
- El-moselhy, M.A.; Taye, A.; Sharkawi, S.S.; El-sisi, S.F.I.I.; Ahmed, A.F. The Antihyperglycemic Effect of Curcumin in High Fat Diet Fed Rats. Role of TNF-α and Free Fatty Acids. Food Chem. Toxicol. 2011, 49, 1129–1140. [Google Scholar] [CrossRef] [PubMed]
- Matafome, P.; Seiça, R. Function and Dysfunction of Adipose Tissue. Adv. Neurobiol. 2017, 19, 3–31. [Google Scholar]
- Brownlee, M. Biochemistry and Molecular Cell Biology of Diabetic Complications. Nature 2001, 414, 813–820. [Google Scholar] [CrossRef] [PubMed]
- Szczesny-Malysiak, E.; Stojak, M.; Campagna, R.; Grosicki, M.; Jamrozik, M.; Kaczara, P.; Chlopicki, S. Bardoxolone Methyl Displays Detrimental Effects on Endothelial Bioenergetics, Suppresses Endothelial ET-1 Release, and Increases Endothelial Permeability in Human Microvascular Endothelium. Oxidative Med. Cell. Longev. 2020, 2020, 4678252. [Google Scholar] [CrossRef]
- Campagna, R.; Mateuszuk, Ł.; Wojnar-Lason, K.; Kaczara, P.; Tworzydło, A.; Kij, A.; Bujok, R.; Mlynarski, J.; Wang, Y.; Sartini, D.; et al. Nicotinamide N-methyltransferase in endothelium protects against oxidant stress-induced endothelial injury. BBA Mol. Cell Res. 2021, 1868, 119082. [Google Scholar] [CrossRef] [PubMed]
- Orasanu, G.; Plutzky, J. The Pathologic Continuum of Diabetic Vascular Disease. J. Am. Coll. Cardiol. 2009, 53, S35–S42. [Google Scholar] [CrossRef] [Green Version]
- Rodrigues, T.; Matafome, P.; Seiça, R. A Vascular Piece in the Puzzle of Adipose Tissue Dysfunction: Mechanisms and Consequences. Arch. Physiol. Biochem. 2014, 120, 1–11. [Google Scholar] [CrossRef]
- Monteiro-Alfredo, T.; Caramelo, B.; Arbeláez, D.; Amaro, A.; Barra, C.; Silva, D.; Oliveira, S.; Seiça, R.; Matafome, P. Distinct Impact of Natural Sugars from Fruit Juices and Added Sugars on Caloric Intake, Body Weight, Glycaemia, Oxidative Stress and Glycation in Diabetic Rats. Nutrients 2021, 13, 2956. [Google Scholar] [CrossRef]
- Monteiro-Alfredo, T.; Oliveira, S.; Amaro, A.; Rosendo-Silva, D.; Antunes, K.; Pires, A.S.; Teixo, R.; Abrantes, A.M.; Botelho, M.F.; Castelo-Branco, M.; et al. Hypoglycaemic and Antioxidant Properties of Acrocomia aculeata (Jacq.) Lodd Ex Mart. Extract Are Associated with Better Vascular Function of Type 2 Diabetic Rats. Nutrients 2021, 13, 2856. [Google Scholar] [CrossRef]
- Ferrari, E.; Saladini, M.; Pignedoli, F.; Spagnolo, F.; Benassi, R. Solvent Effect on Keto-Enol Tautomerism in a New β-Diketone: A Comparison between Experimental Data and Different Theoretical Approaches. New J. Chem. 2011, 35, 2840–2847. [Google Scholar] [CrossRef]
- Pabon, H.J.J. A Synthesis of Curcumin and Related Compounds. Recl. Trav. Chim. Pays-Bas 1964, 83, 379–386. [Google Scholar] [CrossRef]
- Monteiro-Alfredo, T.; Matafome, P.; Iacia, B.P.; Antunes, K.Á.; Dos Santos, J.M.; Da Silva Melo Da Cunha, J.; Oliveira, S.; Oliveira, A.S.; Campos, J.F.; Magalhães, M.; et al. Acrocomia Aculeata (Jacq.) Lodd. Ex Mart. Leaves Increase SIRT1 Levels and Improve Stress Resistance. Oxidative Med. Cell. Longev. 2020, 2020, 5238650. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Maciel, E.; Neves, B.M.; Martins, J.; Colombo, S.; Cruz, M.T.; Domingues, P.; Domingues, M.R.M. Oxidized Phosphatidylserine Mitigates LPS-Triggered Macrophage Inflammatory Status through Modulation of JNK and NF-KB Signaling Cascades. Cell. Signal. 2019, 61, 30–38. [Google Scholar] [CrossRef] [PubMed]
- Re, R.; Pellegrini, N.; Proteggente, A.; Pannala, A.; Yang, M.; Rice-Evans, C. Antioxidant Activity Applying an Improved ABTS Radical Cation Decolorization Assay. Free Radic. Biol. Med. 1999, 26, 1231–1237. [Google Scholar] [CrossRef]
- Cabral, C.; Poças, J.; Gonçalves, M.J.; Cavaleiro, C.; Cruz, M.T.; Salgueiro, L. Ridolfia Segetum (L.) Moris (Apiaceae) from Portugal: A Source of Safe Antioxidant and Anti-Inflammatory Essential Oil. Ind. Crops Prod. 2015, 65, 56–61. [Google Scholar] [CrossRef] [Green Version]
- Neervannan, S. Preclinical Formulations for Discovery and Toxicology: Physicochemical Challenges. Expert Opin. Drug Metab. Toxicol. 2006, 2, 715–731. [Google Scholar] [CrossRef] [PubMed]
- Rodrigues, T.; Matafome, P.; Sereno, J.; Almeida, J.; Castelhano, J.; Gamas, L.; Neves, C.; Gonçalves, S.; Carvalho, C.; Arslanagic, A.; et al. Methylglyoxal-Induced Glycation Changes Adipose Tissue Vascular Architecture, Flow and Expansion, Leading to Insulin Resistance. Sci. Rep. 2017, 7, 1698. [Google Scholar] [CrossRef] [Green Version]
- Marques, C.; Gonçalves, A.; Pereira, P.M.R.; Almeida, D.; Martins, B.; Fontes-Ribeiro, C.; Reis, F.; Fernandes, R. The Dipeptidyl Peptidase 4 Inhibitor Sitagliptin Improves Oxidative Stress and Ameliorates Glomerular Lesions in a Rat Model of Type 1 Diabetes. Life Sci. 2019, 234, 116738. [Google Scholar] [CrossRef]

| Groups | Emax (%) | a/n |
|---|---|---|
| W | 51.15 ± 4.52 | 14/5 |
| GK | 44.16 ± 3.11 | 17/9 |
| Vh | 46.02 ± 3.48 | 11/4 |
| Curcumin (2) | 62.22 ± 5.09 &&# | 12/4 |
| Curcuminoid (3) | 56.25 ± 2.46 & | 11/4 |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Oliveira, S.; Monteiro-Alfredo, T.; Henriques, R.; Ribeiro, C.F.; Seiça, R.; Cruz, T.; Cabral, C.; Fernandes, R.; Piedade, F.; Robalo, M.P.; et al. Improvement of Glycaemia and Endothelial Function by a New Low-Dose Curcuminoid in an Animal Model of Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 5652. https://doi.org/10.3390/ijms23105652
Oliveira S, Monteiro-Alfredo T, Henriques R, Ribeiro CF, Seiça R, Cruz T, Cabral C, Fernandes R, Piedade F, Robalo MP, et al. Improvement of Glycaemia and Endothelial Function by a New Low-Dose Curcuminoid in an Animal Model of Type 2 Diabetes. International Journal of Molecular Sciences. 2022; 23(10):5652. https://doi.org/10.3390/ijms23105652
Chicago/Turabian StyleOliveira, Sara, Tamaeh Monteiro-Alfredo, Rita Henriques, Carlos Fontes Ribeiro, Raquel Seiça, Teresa Cruz, Célia Cabral, Rosa Fernandes, Fátima Piedade, Maria Paula Robalo, and et al. 2022. "Improvement of Glycaemia and Endothelial Function by a New Low-Dose Curcuminoid in an Animal Model of Type 2 Diabetes" International Journal of Molecular Sciences 23, no. 10: 5652. https://doi.org/10.3390/ijms23105652
APA StyleOliveira, S., Monteiro-Alfredo, T., Henriques, R., Ribeiro, C. F., Seiça, R., Cruz, T., Cabral, C., Fernandes, R., Piedade, F., Robalo, M. P., Matafome, P., & Silva, S. (2022). Improvement of Glycaemia and Endothelial Function by a New Low-Dose Curcuminoid in an Animal Model of Type 2 Diabetes. International Journal of Molecular Sciences, 23(10), 5652. https://doi.org/10.3390/ijms23105652










