Interplay between Mast Cells and Regulatory T Cells in Immune-Mediated Cholangiopathies
Abstract
1. Introduction
2. Treg Defects in Immune-Mediated Cholangiopathies
3. Hepatic Mast Cells and Their Roles in Immune-Mediated Cholangiopathies
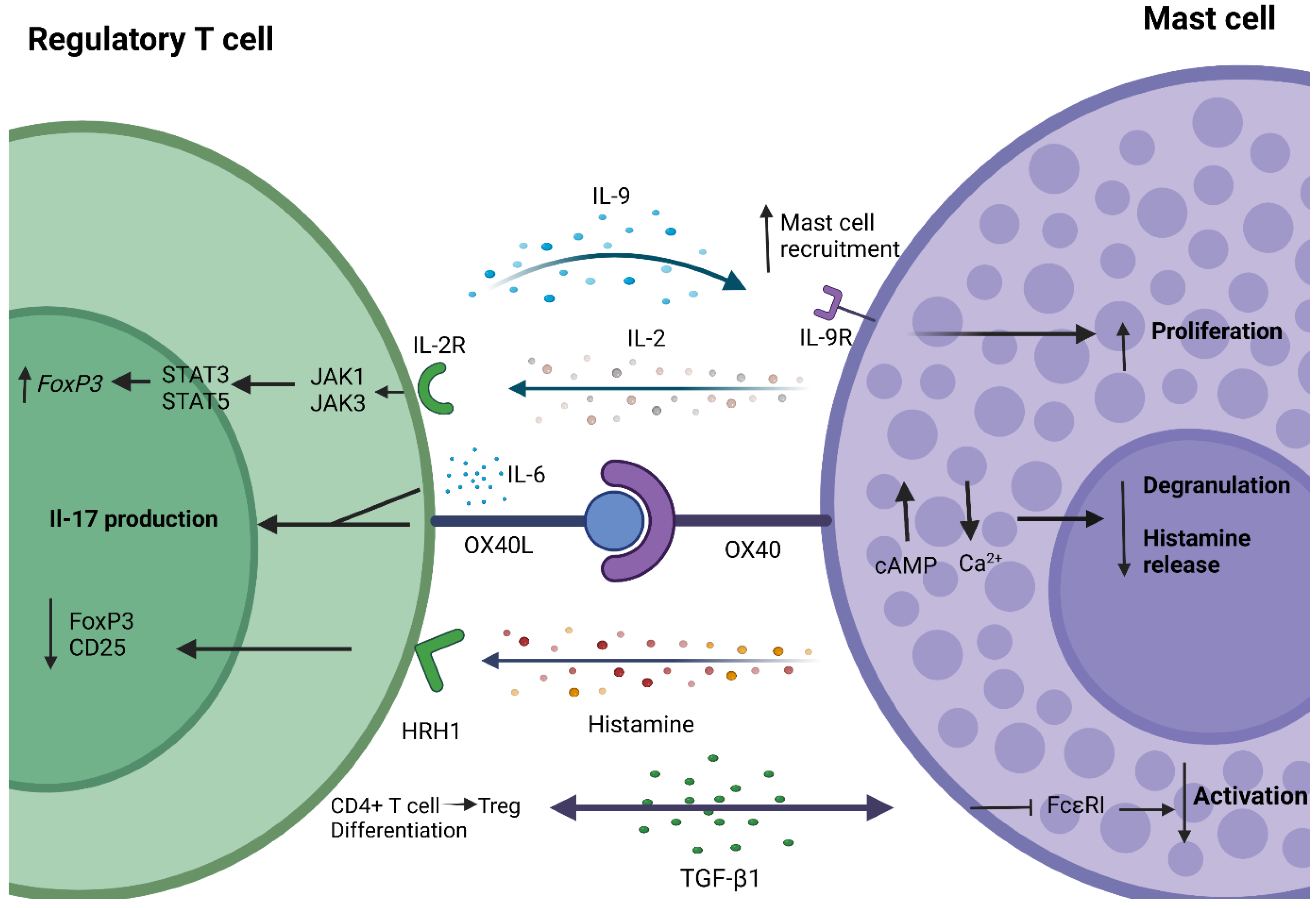
4. Crosstalk between Mast Cells and Regulatory T Cells
5. Conclusions and Final Remarks
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Acknowledgments
Conflicts of Interest
References
- Banales, J.M.; Huebert, R.C.; Karlsen, T.; Strazzabosco, M.; LaRusso, N.F.; Gores, G.J. Cholangiocyte Pathobiology. Nat. Rev. Gastroenterol. Hepatol. 2019, 16, 269–281. [Google Scholar] [CrossRef]
- Lazaridis, K.N.; LaRusso, N.F. The Cholangiopathies. Mayo Clin. Proc. 2015, 90, 791–800. [Google Scholar] [CrossRef]
- Tam, P.K.H.; Yiu, R.S.; Lendahl, U.; Andersson, E.R. Cholangiopathies—Towards a Molecular Understanding. EBioMedicine 2018, 35, 381–393. [Google Scholar] [CrossRef]
- Giordano, D.; Pinto, C.; Maroni, L.; Benedetti, A.; Marzioni, M. Inflammation and the Gut-Liver Axis in the Pathophysiology of Cholangiopathies. Int. J. Mol. Sci. 2018, 19, 3003. [Google Scholar] [CrossRef]
- Ronca, V.; Mancuso, C.; Milani, C.; Carbone, M.; Oo, Y.H.; Invernizzi, P. Immune System and Cholangiocytes: A Puzzling Affair in Primary Biliary Cholangitis. J. Leukoc. Biol. 2020, 108, 659–671. [Google Scholar] [CrossRef]
- Reshetnyak, V.I. Primary Biliary Cirrhosis: Clinical and Laboratory Criteria for Its Diagnosis. World J. Gastroenterol. 2015, 21, 7683. [Google Scholar] [CrossRef]
- Yamagiwa, S.; Kamimura, H.; Takamura, M.; Aoyagi, Y. Autoantibodies in Primary Biliary Cirrhosis: Recent Progress in Research on the Pathogenetic and Clinical Significance. World J. Gastroenterol. 2014, 20, 2606. [Google Scholar] [CrossRef]
- Janmohamed, A.; Trivedi, P.J. Patterns of Disease Progression and Incidence of Complications in Primary Biliary Cholangitis (PBC). Best Pract. Res. Clin. Gastroenterol. 2018, 34–35, 71–83. [Google Scholar] [CrossRef]
- Colapietro, F.; Lleo, A.; Generali, E. Antimitochondrial Antibodies: From Bench to Bedside. Clin. Rev. Allergy Immunol. 2021, 1–12. [Google Scholar] [CrossRef]
- Strazzabosco, M.; Fiorotto, R.; Cadamuro, M.; Spirli, C.; Mariotti, V.; Kaffe, E.; Scirpo, R.; Fabris, L. Pathophysiologic Implications of Innate Immunity and Autoinflammation in the Biliary Epithelium. Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2018, 1864, 1374–1379. [Google Scholar] [CrossRef]
- Eaton, J.E.; Talwalkar, J.A.; Lazaridis, K.N.; Gores, G.J.; Lindor, K.D. Pathogenesis of Primary Sclerosing Cholangitis and Advances in Diagnosis and Management. Gastroenterology 2013, 145, 521–536. [Google Scholar] [CrossRef]
- Khoshpouri, P.; Habibabadi, R.R.; Hazhirkarzar, B.; Ameli, S.; Ghadimi, M.; Ghasabeh, M.A.; Menias, C.O.; Kim, A.; Li, Z.; Kamel, I.R. Imaging Features of Primary Sclerosing Cholangitis: From Diagnosis to Liver Transplant Follow-Up. RadioGraphics 2019, 39, 1938–1964. [Google Scholar] [CrossRef]
- Zhang, H.; Leung, P.S.C.; Gershwin, M.E.; Ma, X. How the Biliary Tree Maintains Immune Tolerance? Biochim. Biophys. Acta BBA-Mol. Basis Dis. 2018, 1864, 1367–1373. [Google Scholar] [CrossRef]
- Trivedi, P.J.; Hirschfield, G.M. The Immunogenetics of Autoimmune Cholestasis. Clin. Liver Dis. 2016, 20, 15–31. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Sakaguchi, N.; Asano, M.; Itoh, M.; Toda, M. Immunologic Self-Tolerance Maintained by Activated T Cells Expressing IL-2 Receptor Alpha-Chains (CD25). Breakdown of a Single Mechanism of Self-Tolerance Causes Various Autoimmune Diseases. J. Immunol. 1995, 155, 1151–1164. [Google Scholar]
- Sakaguchi, S.; Sakaguchi, N.; Shimizu, J.; Yamazaki, S.; Sakihama, T.; Itoh, M.; Kuniyasu, Y.; Nomura, T.; Toda, M.; Takahashi, T. Immunologic Tolerance Maintained by CD25+ CD4+ Regulatory T Cells: Their Common Role in Controlling Autoimmunity, Tumor Immunity, and Transplantation Tolerance. Immunol. Rev. 2001, 182, 18–32. [Google Scholar] [CrossRef]
- Fontenot, J.D.; Gavin, M.A.; Rudensky, A.Y. Foxp3 Programs the Development and Function of CD4+CD25+ Regulatory T Cells. Nat. Immunol. 2003, 4, 330–336. [Google Scholar] [CrossRef]
- Hori, S.; Nomura, T.; Sakaguchi, S. Control of Regulatory T Cell Development by the Transcription Factor Foxp3. Science 2003, 299, 1057–1061. [Google Scholar] [CrossRef]
- Khattri, R.; Cox, T.; Yasayko, S.-A.; Ramsdell, F. An Essential Role for Scurfin in CD4+CD25+ T Regulatory Cells. Nat. Immunol. 2003, 4, 337–342. [Google Scholar] [CrossRef]
- Seddiki, N.; Santner-Nanan, B.; Martinson, J.; Zaunders, J.; Sasson, S.; Landay, A.; Solomon, M.; Selby, W.; Alexander, S.I.; Nanan, R.; et al. Expression of Interleukin (IL)-2 and IL-7 Receptors Discriminates between Human Regulatory and Activated T Cells. J. Exp. Med. 2006, 203, 1693–1700. [Google Scholar] [CrossRef]
- Terry, L.V.; Oo, Y.H. The Next Frontier of Regulatory T Cells: Promising Immunotherapy for Autoimmune Diseases and Organ Transplantations. Front. Immunol. 2020, 11, 2244. [Google Scholar] [CrossRef]
- Sakaguchi, S.; Mikami, N.; Wing, J.B.; Tanaka, A.; Ichiyama, K.; Ohkura, N. Regulatory T Cells and Human Disease. Annu. Rev. Immunol. 2020, 38, 541–566. [Google Scholar] [CrossRef]
- Wing, J.B.; Tanaka, A.; Sakaguchi, S. Human FOXP3+ Regulatory T Cell Heterogeneity and Function in Autoimmunity and Cancer. Immunity 2019, 50, 302–316. [Google Scholar] [CrossRef]
- Powell, B.R.; Buist, N.R.M.; Stenzel, P. An X-Linked Syndrome of Diarrhea, Polyendocrinopathy, and Fatal Infection in Infancy. J. Pediatrics 1982, 100, 731–737. [Google Scholar] [CrossRef]
- Bacchetta, R.; Barzaghi, F.; Roncarolo, M.-G. From IPEX Syndrome to FOXP3 Mutation: A Lesson on Immune Dysregulation. Ann. N. Y. Acad. Sci. 2018, 1417, 5–22. [Google Scholar] [CrossRef]
- Godfrey, V.L.; Wilkinson, J.E.; Rinchik, E.M.; Russell, L.B. Fatal Lymphoreticular Disease in the Scurfy (Sf) Mouse Requires T Cells That Mature in a Sf Thymic Environment: Potential Model for Thymic Education. Proc. Natl. Acad. Sci. USA 1991, 88, 5528–5532. [Google Scholar] [CrossRef]
- Longhi, M.S.; Mieli-Vergani, G.; Vergani, D. Regulatory T Cells in Autoimmune Hepatitis: An Updated Overview. J. Autoimmun. 2021, 119, 102619. [Google Scholar] [CrossRef]
- Oo, Y.H.; Adams, D.H. Regulatory T Cells and Autoimmune Hepatitis: What Happens in the Liver Stays in the Liver. J. Hepatol. 2014, 61, 973–975. [Google Scholar] [CrossRef]
- Oo, Y.H.; Weston, C.J.; Lalor, P.F.; Curbishley, S.M.; Withers, D.R.; Reynolds, G.M.; Shetty, S.; Harki, J.; Shaw, J.C.; Eksteen, B.; et al. Distinct Roles for CCR4 and CXCR3 in the Recruitment and Positioning of Regulatory T Cells in the Inflamed Human Liver. J. Immunol. 2010, 184, 2886–2898. [Google Scholar] [CrossRef]
- Peiseler, M.; Sebode, M.; Franke, B.; Wortmann, F.; Schwinge, D.; Quaas, A.; Baron, U.; Olek, S.; Wiegard, C.; Lohse, A.W.; et al. FOXP3+ Regulatory T Cells in Autoimmune Hepatitis Are Fully Functional and Not Reduced in Frequency. J. Hepatol. 2012, 57, 125–132. [Google Scholar] [CrossRef]
- Taubert, R.; Hardtke-Wolenski, M.; Noyan, F.; Wilms, A.; Baumann, A.K.; Schlue, J.; Olek, S.; Falk, C.S.; Manns, M.P.; Jaeckel, E. Intrahepatic Regulatory T Cells in Autoimmune Hepatitis Are Associated with Treatment Response and Depleted with Current Therapies. J. Hepatol. 2014, 61, 1106–1114. [Google Scholar] [CrossRef]
- Lan, R.Y.; Cheng, C.; Lian, Z.-X.; Tsuneyama, K.; Yang, G.-X.; Moritoki, Y.; Chuang, Y.-H.; Nakamura, T.; Saito, S.; Shimoda, S.; et al. Liver-Targeted and Peripheral Blood Alterations of Regulatory T Cells in Primary Biliary Cirrhosis. Hepatology 2006, 43, 729–737. [Google Scholar] [CrossRef] [PubMed]
- Chen, J.; Hou, X.; Jia, H.; Cui, G.; Wu, Z.; Wang, L.; Lu, C.; Wu, W.; Wei, Y.; Uede, T.; et al. Regulatory T Cells with a Defect in Inhibition on Co-Stimulation Deteriorated Primary Biliary Cholangitis. Oncotarget 2017, 8, 108406–108417. [Google Scholar] [CrossRef] [PubMed]
- Aoki, C.A.; Roifman, C.M.; Lian, Z.-X.; Bowlus, C.L.; Norman, G.L.; Shoenfeld, Y.; Mackay, I.R.; Eric Gershwin, M. IL-2 Receptor Alpha Deficiency and Features of Primary Biliary Cirrhosis. J. Autoimmun. 2006, 27, 50–53. [Google Scholar] [CrossRef] [PubMed]
- Wakabayashi, K.; Lian, Z.-X.; Moritoki, Y.; Lan, R.Y.; Tsuneyama, K.; Chuang, Y.-H.; Yang, G.-X.; Ridgway, W.; Ueno, Y.; Ansari, A.A.; et al. IL-2 Receptor A−/− Mice and the Development of Primary Biliary Cirrhosis. Hepatology 2006, 44, 1240–1249. [Google Scholar] [CrossRef] [PubMed]
- Zhang, W.; Sharma, R.; Ju, S.-T.; He, X.-S.; Tao, Y.; Tsuneyama, K.; Tian, Z.; Lian, Z.-X.; Fu, S.M.; Gershwin, M.E. Deficiency in Regulatory T Cells Results in Development of Antimitochondrial Antibodies and Autoimmune Cholangitis. Hepatology 2009, 49, 545–552. [Google Scholar] [CrossRef] [PubMed]
- Yang, C.-Y.; Ma, X.; Tsuneyama, K.; Huang, S.; Takahashi, T.; Chalasani, N.P.; Bowlus, C.L.; Yang, G.-X.; Leung, P.S.C.; Ansari, A.A.; et al. IL-12/Th1 and IL-23/Th17 Biliary Microenvironment in Primary Biliary Cirrhosis: Implications for Therapy. Hepatology 2014, 59, 1944–1953. [Google Scholar] [CrossRef]
- Cichoż-Lach, H.; Grywalska, E.; Michalak, A.; Kowalik, A.; Mielnik, M.; Roliński, J. Deviations in Peripheral Blood Cell Populations Are Associated with the Stage of Primary Biliary Cholangitis and Presence of Itching. Arch. Immunol. Ther. Exp. 2018, 66, 443–452. [Google Scholar] [CrossRef]
- Jiang, T.; Zhang, H.; Wen, Y.; Yin, Y.; Yang, L.; Yang, J.; Lan, T.; Tang, C.; Yu, J.; Tai, W.; et al. 5-Aza-2-Deoxycytidine Alleviates the Progression of Primary Biliary Cholangitis by Suppressing the FoxP3 Methylation and Promoting the Treg/Th17 Balance. Int. Immunopharmacol. 2021, 96, 107820. [Google Scholar] [CrossRef]
- Rong, G.; Zhou, Y.; Xiong, Y.; Zhou, L.; Geng, H.; Jiang, T.; Zhu, Y.; Lu, H.; Zhang, S.; Wang, P.; et al. Imbalance between T Helper Type 17 and T Regulatory Cells in Patients with Primary Biliary Cirrhosis: The Serum Cytokine Profile and Peripheral Cell Population. Clin. Exp. Immunol. 2009, 156, 217–225. [Google Scholar] [CrossRef]
- Beringer, A.; Miossec, P. IL-17 and IL-17-Producing Cells and Liver Diseases, with Focus on Autoimmune Liver Diseases. Autoimmun. Rev. 2018, 17, 1176–1185. [Google Scholar] [CrossRef] [PubMed]
- Floess, S.; Freyer, J.; Siewert, C.; Baron, U.; Olek, S.; Polansky, J.; Schlawe, K.; Chang, H.-D.; Bopp, T.; Schmitt, E.; et al. Epigenetic Control of the Foxp3 Locus in Regulatory T Cells. PLoS Biol. 2007, 5, e38. [Google Scholar] [CrossRef] [PubMed]
- Helmin, K.A.; Morales-Nebreda, L.; Torres Acosta, M.A.; Anekalla, K.R.; Chen, S.-Y.; Abdala-Valencia, H.; Politanska, Y.; Cheresh, P.; Akbarpour, M.; Steinert, E.M.; et al. Maintenance DNA Methylation Is Essential for Regulatory T Cell Development and Stability of Suppressive Function. J. Clin. Investig. 2020, 130, 6571–6587. [Google Scholar] [CrossRef] [PubMed]
- Wang, D.; Zhang, H.; Liang, J.; Gu, Z.; Zhou, Q.; Fan, X.; Hou, Y.; Sun, L. CD4+CD25+ but Not CD4+Foxp3+ T Cells as a Regulatory Subset in Primary Biliary Cirrhosis. Cell. Mol. Immunol. 2010, 7, 485–490. [Google Scholar] [CrossRef] [PubMed]
- Collison, L.W.; Workman, C.J.; Kuo, T.T.; Boyd, K.; Wang, Y.; Vignali, K.M.; Cross, R.; Sehy, D.; Blumberg, R.S.; Vignali, D.A.A. The Inhibitory Cytokine IL-35 Contributes to Regulatory T-Cell Function. Nature 2007, 450, 566–569. [Google Scholar] [CrossRef] [PubMed]
- Li, T.; Huang, Y.; Liu, P.; Liu, Y.; Guo, J.; Zhang, W.; Gu, M.; Qian, C.; Deng, A. Lower Plasma Levels of IL-35 in Patients with Primary Biliary Cirrhosis. Tohoku J. Exp. Med. 2018, 244, 123–131. [Google Scholar] [CrossRef]
- Liaskou, E.; Patel, S.R.; Webb, G.; Bagkou Dimakou, D.; Akiror, S.; Krishna, M.; Mells, G.; Jones, D.E.; Bowman, S.J.; Barone, F.; et al. Increased Sensitivity of Treg Cells from Patients with PBC to Low Dose IL-12 Drives Their Differentiation into IFN-γ Secreting Cells. J. Autoimmun. 2018, 94, 143–155. [Google Scholar] [CrossRef]
- Oertelt, S.; Lian, Z.-X.; Cheng, C.-M.; Chuang, Y.-H.; Padgett, K.A.; He, X.-S.; Ridgway, W.M.; Ansari, A.A.; Coppel, R.L.; Li, M.O.; et al. Anti-Mitochondrial Antibodies and Primary Biliary Cirrhosis in TGF-β Receptor II Dominant-Negative Mice. J. Immunol. 2006, 177, 1655–1660. [Google Scholar] [CrossRef]
- Huang, W.; Kachapati, K.; Adams, D.; Wu, Y.; Leung, P.S.C.; Yang, G.-X.; Zhang, W.; Ansari, A.A.; Flavell, R.A.; Gershwin, M.E.; et al. Murine Autoimmune Cholangitis Requires Two Hits: Cytotoxic KLRG1+ CD8 Effector Cells and Defective T Regulatory Cells. J. Autoimmun. 2014, 50, 123–134. [Google Scholar] [CrossRef]
- Wang, Y.-H.; Yang, W.; Yang, J.-B.; Jia, Y.-J.; Tang, W.; Gershwin, M.E.; Ridgway, W.M.; Lian, Z.-X. Systems Biologic Analysis of T Regulatory Cells Genetic Pathways in Murine Primary Biliary Cirrhosis. J. Autoimmun. 2015, 59, 26–37. [Google Scholar] [CrossRef]
- Tanaka, H.; Zhang, W.; Yang, G.-X.; Ando, Y.; Tomiyama, T.; Tsuneyama, K.; Leung, P.; Coppel, R.L.; Ansari, A.A.; Lian, Z.X.; et al. Successful Immunotherapy of Autoimmune Cholangitis by Adoptive Transfer of Forkhead Box Protein 3+ Regulatory T Cells. Clin. Exp. Immunol. 2014, 178, 253–261. [Google Scholar] [CrossRef] [PubMed]
- Bernuzzi, F.; Fenoglio, D.; Battaglia, F.; Fravega, M.; Gershwin, M.E.; Indiveri, F.; Ansari, A.A.; Podda, M.; Invernizzi, P.; Filaci, G. Phenotypical and Functional Alterations of CD8 Regulatory T Cells in Primary Biliary Cirrhosis. J. Autoimmun. 2010, 35, 176–180. [Google Scholar] [CrossRef] [PubMed]
- Sebode, M.; Peiseler, M.; Franke, B.; Schwinge, D.; Schoknecht, T.; Wortmann, F.; Quaas, A.; Petersen, B.-S.; Ellinghaus, E.; Baron, U.; et al. Reduced FOXP3+ Regulatory T Cells in Patients with Primary Sclerosing Cholangitis Are Associated with IL2RA Gene Polymorphisms. J. Hepatol. 2014, 60, 1010–1016. [Google Scholar] [CrossRef] [PubMed]
- Schwinge, D.; von Haxthausen, F.; Quaas, A.; Carambia, A.; Otto, B.; Glaser, F.; Höh, B.; Thiele, N.; Schoknecht, T.; Huber, S.; et al. Dysfunction of Hepatic Regulatory T Cells in Experimental Sclerosing Cholangitis Is Related to IL-12 Signaling. J. Hepatol. 2017, 66, 798–805. [Google Scholar] [CrossRef] [PubMed]
- Taylor, A.E.; Carey, A.N.; Kudira, R.; Lages, C.S.; Shi, T.; Lam, S.; Karns, R.; Simmons, J.; Shanmukhappa, K.; Almanan, M.; et al. Interleukin 2 Promotes Hepatic Regulatory T Cell Responses and Protects From Biliary Fibrosis in Murine Sclerosing Cholangitis. Hepatology 2018, 68, 1905–1921. [Google Scholar] [CrossRef]
- Jeffery, H.C.; Braitch, M.K.; Brown, S.; Oo, Y.H. Clinical Potential of Regulatory T Cell Therapy in Liver Diseases: An Overview and Current Perspectives. Front. Immunol. 2016, 7, 334. [Google Scholar] [CrossRef]
- Krystel-Whittemore, M.; Dileepan, K.N.; Wood, J.G. Mast Cell: A Multi-Functional Master Cell. Front. Immunol. 2016, 6, 620. [Google Scholar] [CrossRef]
- Reber, L.L.; Sibilano, R.; Mukai, K.; Galli, S.J. Potential Effector and Immunoregulatory Functions of Mast Cells in Mucosal Immunity. Mucosal Immunol. 2015, 8, 444–463. [Google Scholar] [CrossRef]
- Gri, G.; Frossi, B.; D’Inca, F.; Danelli, L.; Betto, E.; Mion, F.; Sibilano, R.; Pucillo, C. Mast Cell: An Emerging Partner in Immune Interaction. Front. Immunol. 2012, 3, 120. [Google Scholar] [CrossRef]
- Shimizu, Y.; Sakai, K.; Miura, T.; Narita, T.; Tsukagoshi, H.; Satoh, Y.; Ishikawa, S.; Morishita, Y.; Takai, S.; Miyazaki, M.; et al. Characterization of ‘Adult-Type’ Mast Cells Derived from Human Bone Marrow CD34+ Cells Cultured in the Presence of Stem Cell Factor and Interleukin-6. Interleukin-4 Is Not Required for Constitutive Expression of CD54, FcεRIα and Chymase, and CD13 Expressi. Clin. Exp. Allergy 2002, 32, 872–880. [Google Scholar] [CrossRef]
- Gurish, M.F.; Austen, K.F. Developmental Origin and Functional Specialization of Mast Cell Subsets. Immunity 2012, 37, 25–33. [Google Scholar] [CrossRef]
- Moon, T.C.; St Laurent, C.D.; Morris, K.E.; Marcet, C.; Yoshimura, T.; Sekar, Y.; Befus, A.D. Advances in Mast Cell Biology: New Understanding of Heterogeneity and Function. Mucosal Immunol. 2010, 3, 111–128. [Google Scholar] [CrossRef]
- Elieh Ali Komi, D.; Shafaghat, F.; Kovanen, P.T.; Meri, S. Mast Cells and Complement System: Ancient Interactions between Components of Innate Immunity. Allergy 2020, 75, 2818–2828. [Google Scholar] [CrossRef]
- Agier, J.; Pastwińska, J.; Brzezińska-Błaszczyk, E. An Overview of Mast Cell Pattern Recognition Receptors. Inflamm. Res. 2018, 67, 737–746. [Google Scholar] [CrossRef]
- Xu, H.; Shi, X.; Li, X.; Zou, J.; Zhou, C.; Liu, W.; Shao, H.; Chen, H.; Shi, L. Neurotransmitter and Neuropeptide Regulation of Mast Cell Function: A Systematic Review. J. Neuroinflamm. 2020, 17, 356. [Google Scholar] [CrossRef]
- Galli, S.J.; Starkl, P.; Marichal, T.; Tsai, M. Mast Cells and IgE in Defense against Venoms: Possible “Good Side” of Allergy? Allergol. Int. 2016, 65, 3–15. [Google Scholar] [CrossRef]
- Paivandy, A.; Pejler, G. Novel Strategies to Target Mast Cells in Disease. J. Innate Immun. 2021, 13, 131–147. [Google Scholar] [CrossRef]
- Bulfone-Paus, S.; Bahri, R. Mast Cells as Regulators of T Cell Responses. Front. Immunol. 2015, 6, 394. [Google Scholar] [CrossRef]
- Galli, S.J.; Nakae, S.; Tsai, M. Mast Cells in the Development of Adaptive Immune Responses. Nat. Immunol. 2005, 6, 135–142. [Google Scholar] [CrossRef]
- Jones, H.; Hargrove, L.; Kennedy, L.; Meng, F.; Graf-Eaton, A.; Owens, J.; Alpini, G.; Johnson, C.; Bernuzzi, F.; Demieville, J.; et al. Inhibition of Mast Cell-Secreted Histamine Decreases Biliary Proliferation and Fibrosis in Primary Sclerosing Cholangitis Mdr2−/− Mice. Hepatology 2016, 64, 1202–1216. [Google Scholar] [CrossRef]
- Nakamura, A.; Yamazaki, K.; Suzuki, K.; Sato, S. Increased Portal Tract Infiltration of Mast Cells and Eosinophils in Primary Biliary Cirrhosis. Am. J. Gastroenterol. 1997, 92, 2245–2249. [Google Scholar] [PubMed]
- Farrell, D.J.; Hines, J.E.; Walls, A.F.; Kelly, P.J.; Bennett, M.K.; Burt, A.D. Intrahepatic Mast Cells in Chronic Liver Diseases. Hepatology 1995, 22, 1175–1181. [Google Scholar] [CrossRef] [PubMed]
- Jarido, V.; Kennedy, L.; Hargrove, L.; Demieville, J.; Thomson, J.; Stephenson, K.; Francis, H. The Emerging Role of Mast Cells in Liver Disease. Am. J. Physiol. -Gastrointest. Liver Physiol. 2017, 313, G89–G101. [Google Scholar] [CrossRef] [PubMed]
- Koda, W.; Harada, K.; Tsuneyama, K.; Kono, N.; Sasaki, M.; Matsui, O.; Nakanuma, Y. Evidence of the Participation of Peribiliary Mast Cells in Regulation of the Peribiliary Vascular Plexus Along the Intrahepatic Biliary Tree. Lab. Investig. 2000, 80, 1007–1017. [Google Scholar] [CrossRef] [PubMed]
- Mittal, A.; Sagi, V.; Gupta, M.; Gupta, K. Mast Cell Neural Interactions in Health and Disease. Front. Cell. Neurosci. 2019, 13, 110. [Google Scholar] [CrossRef] [PubMed]
- Siiskonen, H.; Harvima, I. Mast Cells and Sensory Nerves Contribute to Neurogenic Inflammation and Pruritus in Chronic Skin Inflammation. Front. Cell. Neurosci. 2019, 13, 422. [Google Scholar] [CrossRef]
- Mizuno, K.; Ueno, Y. Autonomic Nervous System and the Liver. Hepatol. Res. 2017, 47, 160–165. [Google Scholar] [CrossRef]
- Matsunaga, Y.; Kawasaki, H.; Terada, T. Stromal Mast Cells and Nerve Fibers in Various Chronic Liver Diseases: Relevance to Hepatic Fibrosis. Am. J. Gastroenterol. 1999, 94, 1923–1932. [Google Scholar] [CrossRef]
- Satomura, K.; Yin, M.; Shimizu, S.; Kato, Y.; Nagano, T.; Komeichi, H.; Ohsuga, M.; Katsuta, Y.; Aramaki, T.; Omoto, Y. Increased Chymase in Livers with Autoimmune Disease: Colocalization with Fibrosis. J. Nippon Med. Sch. 2003, 70, 490–495. [Google Scholar] [CrossRef][Green Version]
- Ishii, M.; Iwai, M.; Harada, Y.; Morikawa, T.; Okanoue, T.; Kishikawa, T.; Tsuchihashi, Y.; Hanai, K.; Arizono, N. A Role of Mast Cells for Hepatic Fibrosis in Primary Sclerosing Cholangitis. Hepatol. Res. 2005, 31, 127–131. [Google Scholar] [CrossRef]
- Tsuneyama, K.; Kono, N.; Yamashiro, M.; Kouda, W.; Sabit, A.; Sasaki, M.; Nakanuma, Y. Aberrant Expression of Stem Cell Factor on Biliary Epithelial Cells and Peribiliary Infiltration of C-Kit-Expressing Mast Cells in Hepatolithiasis and Primary Sclerosing Cholangitis: A Possible Contribution to Bile Duct Fibrosis. J. Pathol. 1999, 189, 609–614. [Google Scholar] [CrossRef]
- Weiskirchen, R.; Meurer, S.K.; Liedtke, C.; Huber, M. Mast Cells in Liver Fibrogenesis. Cells 2019, 8, 1429. [Google Scholar] [CrossRef] [PubMed]
- Gittlen, S.D.; Schulman, E.S.; Maddrey, W.C. Raised Histamine Concentrations in Chronic Cholestatic Liver Disease. Gut 1990, 31, 96–99. [Google Scholar] [CrossRef] [PubMed]
- Abe, M.; Yokoyama, Y.; Amano, H.; Matsushima, Y.; Kan, C.; Ishikawa, O. Effect of Activated Human Mast Cells and Mast Cell-Derived Mediators on Proliferation, Type I Collagen Production and Glycosaminoglycans Synthesis by Human Dermal Fibroblasts. Eur. J. Dermatol. 2002, 12, 340–346. [Google Scholar] [PubMed]
- González, M.I.; Vannan, D.T.; Eksteen, B.; Flores-Sotelo, I.; Reyes, J.L. Mast Cells in Immune-Mediated Cholangitis and Cholangiocarcinoma. Cells 2022, 11, 375. [Google Scholar] [CrossRef] [PubMed]
- Reber, L.L.; Marichal, T.; Galli, S.J. New Models for Analyzing Mast Cell Functions in Vivo. Trends Immunol. 2012, 33, 613–625. [Google Scholar] [CrossRef]
- Kyritsi, K.; Kennedy, L.; Meadows, V.; Hargrove, L.; Demieville, J.; Pham, L.; Sybenga, A.; Kundu, D.; Cerritos, K.; Meng, F.; et al. Mast Cells Induce Ductular Reaction Mimicking Liver Injury in Mice Through Mast Cell–Derived Transforming Growth Factor Beta 1 Signaling. Hepatology 2021, 73, 2397–2410. [Google Scholar] [CrossRef]
- Hargrove, L.; Kennedy, L.; Demieville, J.; Jones, H.; Meng, F.; DeMorrow, S.; Karstens, W.; Madeka, T.; Greene, J.; Francis, H. Bile Duct Ligation-Induced Biliary Hyperplasia, Hepatic Injury, and Fibrosis Are Reduced in Mast Cell-Deficient Kit W-Sh Mice. Hepatology 2017, 65, 1991–2004. [Google Scholar] [CrossRef]
- Meng, F.; Kennedy, L.; Hargrove, L.; Demieville, J.; Jones, H.; Madeka, T.; Karstens, A.; Chappell, K.; Alpini, G.; Sybenga, A.; et al. Ursodeoxycholate Inhibits Mast Cell Activation and Reverses Biliary Injury and Fibrosis in Mdr2−/− Mice and Human Primary Sclerosing Cholangitis. Lab. Investig. 2018, 98, 1465–1477. [Google Scholar] [CrossRef]
- Meadows, V.; Kennedy, L.; Ekser, B.; Kyritsi, K.; Kundu, D.; Zhou, T.; Chen, L.; Pham, L.; Wu, N.; Demieville, J.; et al. Mast Cells Regulate Ductular Reaction and Intestinal Inflammation in Cholestasis Through Farnesoid X Receptor Signaling. Hepatology 2021, 74, 2684–2698. [Google Scholar] [CrossRef]
- Kambayashi, T.; Allenspach, E.J.; Chang, J.T.; Zou, T.; Shoag, J.E.; Reiner, S.L.; Caton, A.J.; Koretzky, G.A. Inducible MHC Class II Expression by Mast Cells Supports Effector and Regulatory T Cell Activation. J. Immunol. 2009, 182, 4686–4695. [Google Scholar] [CrossRef] [PubMed]
- Lu, L.-F.; Lind, E.F.; Gondek, D.C.; Bennett, K.A.; Gleeson, M.W.; Pino-Lagos, K.; Scott, Z.A.; Coyle, A.J.; Reed, J.L.; van Snick, J.; et al. Mast Cells Are Essential Intermediaries in Regulatory T-Cell Tolerance. Nature 2006, 442, 997–1002. [Google Scholar] [CrossRef] [PubMed]
- Eller, K.; Wolf, D.; Huber, J.M.; Metz, M.; Mayer, G.; McKenzie, A.N.J.; Maurer, M.; Rosenkranz, A.R.; Wolf, A.M. IL-9 Production by Regulatory T Cells Recruits Mast Cells That Are Essential for Regulatory T Cell-Induced Immune Suppression. J. Immunol. 2011, 186, 83–91. [Google Scholar] [CrossRef] [PubMed]
- Sibilano, R.; Frossi, B.; Suzuki, R.; D’Incà, F.; Gri, G.; Piconese, S.; Colombo, M.P.; Rivera, J.; Pucillo, C.E. Modulation of FcεRI-Dependent Mast Cell Response by OX40L via Fyn, PI3K, and RhoA. J. Allergy Clin. Immunol. 2012, 130, 751–760. [Google Scholar] [CrossRef]
- Valzasina, B.; Guiducci, C.; Dislich, H.; Killeen, N.; Weinberg, A.D.; Colombo, M.P. Triggering of OX40 (CD134) on CD4+CD25+ T Cells Blocks Their Inhibitory Activity: A Novel Regulatory Role for OX40 and Its Comparison with GITR. Blood 2005, 105, 2845–2851. [Google Scholar] [CrossRef]
- Webb, G.J.; Hirschfield, G.M.; Lane, P.J.L. OX40, OX40L and Autoimmunity: A Comprehensive Review. Clin. Rev. Allergy Immunol. 2016, 50, 312–332. [Google Scholar] [CrossRef]
- Gri, G.; Piconese, S.; Frossi, B.; Manfroi, V.; Merluzzi, S.; Tripodo, C.; Viola, A.; Odom, S.; Rivera, J.; Colombo, M.P.; et al. CD4+CD25+ Regulatory T Cells Suppress Mast Cell Degranulation and Allergic Responses through OX40-OX40L Interaction. Immunity 2008, 29, 771–781. [Google Scholar] [CrossRef]
- Piconese, S.; Gri, G.; Tripodo, C.; Musio, S.; Gorzanelli, A.; Frossi, B.; Pedotti, R.; Pucillo, C.E.; Colombo, M.P. Mast Cells Counteract Regulatory T-Cell Suppression through Interleukin-6 and OX40/OX40L Axis toward Th17-Cell Differentiation. Blood 2009, 114, 2639–2648. [Google Scholar] [CrossRef]
- Behfarjam, F.; Nasseri-Moghaddam, S.; Jadali, Z. Enhanced Th17 Responses in Patients with Autoimmune Hepatitis. Middle East J. Dig. Dis. 2019, 11, 98–103. [Google Scholar] [CrossRef]
- Drescher, H.K.; Bartsch, L.M.; Weiskirchen, S.; Weiskirchen, R. Intrahepatic TH17/TReg Cells in Homeostasis and Disease—It’s All About the Balance. Front. Pharmacol. 2020, 11, 1598. [Google Scholar] [CrossRef]
- Harada, K.; Shimoda, S.; Sato, Y.; Isse, K.; Ikeda, H.; Nakanuma, Y. Periductal Interleukin-17 Production in Association with Biliary Innate Immunity Contributes to the Pathogenesis of Cholangiopathy in Primary Biliary Cirrhosis. Clin. Exp. Immunol. 2009, 157, 261–270. [Google Scholar] [CrossRef] [PubMed]
- Johnson, C.; Han, Y.; Hughart, N.; McCarra, J.; Alpini, G.; Meng, F. Interleukin-6 and Its Receptor, Key Players in Hepatobiliary Inflammation and Cancer. Transl. Gastrointest. Cancer 2012, 1, 58–70. [Google Scholar] [CrossRef] [PubMed]
- Ganeshan, K.; Bryce, P.J. Regulatory T Cells Enhance Mast Cell Production of IL-6 via Surface-Bound TGF-β. J. Immunol. 2012, 188, 594–603. [Google Scholar] [CrossRef] [PubMed]
- Chinen, T.; Kannan, A.K.; Levine, A.G.; Fan, X.; Klein, U.; Zheng, Y.; Gasteiger, G.; Feng, Y.; Fontenot, J.D.; Rudensky, A.Y. An Essential Role for the IL-2 Receptor in Treg Cell Function. Nat. Immunol. 2016, 17, 1322–1333. [Google Scholar] [CrossRef] [PubMed]
- Morita, H.; Arae, K.; Unno, H.; Miyauchi, K.; Toyama, S.; Nambu, A.; Oboki, K.; Ohno, T.; Motomura, K.; Matsuda, A.; et al. An Interleukin-33-Mast Cell-Interleukin-2 Axis Suppresses Papain-Induced Allergic Inflammation by Promoting Regulatory T Cell Numbers. Immunity 2015, 43, 175–186. [Google Scholar] [CrossRef] [PubMed]
- Salamon, P.; Shefler, I.; Moshkovits, I.; Munitz, A.; Horwitz Klotzman, D.; Mekori, Y.A.; Hershko, A.Y. IL-33 and IgE Stimulate Mast Cell Production of IL-2 and Regulatory T Cell Expansion in Allergic Dermatitis. Clin. Exp. Allergy 2017, 47, 1409–1416. [Google Scholar] [CrossRef]
- Takasato, Y.; Kurashima, Y.; Kiuchi, M.; Hirahara, K.; Murasaki, S.; Arai, F.; Izawa, K.; Kaitani, A.; Shimada, K.; Saito, Y.; et al. Orally Desensitized Mast Cells Form a Regulatory Network with Treg Cells for the Control of Food Allergy. Mucosal Immunol. 2021, 14, 640–651. [Google Scholar] [CrossRef]
- Ang, W.X.G.; Church, A.M.; Kulis, M.; Choi, H.W.; Burks, A.W.; Abraham, S.N. Mast Cell Desensitization Inhibits Calcium Flux and Aberrantly Remodels Actin. J. Clin. Investig. 2016, 126, 4103–4118. [Google Scholar] [CrossRef]
- Hershko, A.Y.; Suzuki, R.; Charles, N.; Alvarez-Errico, D.; Sargent, J.L.; Laurence, A.; Rivera, J. Mast Cell Interleukin-2 Production Contributes to Suppression of Chronic Allergic Dermatitis. Immunity 2011, 35, 562–571. [Google Scholar] [CrossRef]
- Forward, N.A.; Furlong, S.J.; Yang, Y.; Lin, T.-J.; Hoskin, D.W. Mast Cells Down-Regulate CD4+CD25+ T Regulatory Cell Suppressor Function via Histamine H1 Receptor Interaction. J. Immunol. 2009, 183, 3014–3022. [Google Scholar] [CrossRef]
- de la Rosa, M.; Rutz, S.; Dorninger, H.; Scheffold, A. Interleukin-2 Is Essential for CD4+CD25+ Regulatory T Cell Function. Eur. J. Immunol. 2004, 34, 2480–2488. [Google Scholar] [CrossRef] [PubMed]
- Wolf, A.M.; Wolf, D.; McKenzie, A.; Maurer, M.; Rosenkranz, A.R.; Eller, K. IL-9 Production by Regulatory T Cells Recruits Mast Cells That Are Essential for Regulatory T Cell-Induced Immune-Suppression. Blood 2010, 116, 2782. [Google Scholar] [CrossRef]
- Zhao, Y.-B.; Yang, S.-H.; Shen, J.; Deng, K.; Li, Q.; Wang, Y.; Cui, W.; Ye, H. Interaction between Regulatory T Cells and Mast Cells via IL-9 and TGF-Β Production. Oncol. Lett. 2020, 20, 160. [Google Scholar] [CrossRef]
- Nakano, T.; Lai, C.-Y.; Goto, S.; Hsu, L.-W.; Kawamoto, S.; Ono, K.; Chen, K.-D.; Lin, C.-C.; Chiu, K.-W.; Wang, C.-C.; et al. Immunological and Regenerative Aspects of Hepatic Mast Cells in Liver Allograft Rejection and Tolerance. PLoS ONE 2012, 7, e37202. [Google Scholar] [CrossRef]
- Matsuzawa, S.; Sakashita, K.; Kinoshita, T.; Ito, S.; Yamashita, T.; Koike, K. IL-9 Enhances the Growth of Human Mast Cell Progenitors Under Stimulation with Stem Cell Factor. J. Immunol. 2003, 170, 3461–3467. [Google Scholar] [CrossRef]
- Turner, J.A.; Stephen-Victor, E.; Wang, S.; Rivas, M.N.; Abdel-Gadir, A.; Harb, H.; Cui, Y.; Fanny, M.; Charbonnier, L.-M.; Fong, J.J.H.; et al. Regulatory T Cell-Derived TGF-Β1 Controls Multiple Checkpoints Governing Allergy and Autoimmunity. Immunity 2020, 53, 1202–1214.e6. [Google Scholar] [CrossRef]
- Zhang, W.; Wu, K.; He, W.; Gao, Y.; Huang, W.; Lin, X.; Cai, L.; Fang, Z.; Zhou, Q.; Luo, Z.; et al. Transforming Growth Factor Beta 1 Plays an Important Role in Inducing CD4+CD25+forhead Box P3+ Regulatory T Cells by Mast Cells. Clin. Exp. Immunol. 2010, 161, 490–496. [Google Scholar] [CrossRef]
- Gao, Y.; Xu, B.; Zhang, P.; He, Y.; Liang, X.; Liu, J.; Li, J. TNF-α Regulates Mast Cell Functions by Inhibiting Cell Degranulation. Cell. Physiol. Biochem. 2017, 44, 751–762. [Google Scholar] [CrossRef]
- Nagano, T.; Yamamoto, K.; Matsumoto, S.; Okamoto, R.; Tagashira, M.; Ibuki, N.; Matsumura, S.; Yabushita, K.; Okano, N.; Tsuji, T. Cytokine Profile in the Liver of Primary Biliary Cirrhosis. J. Clin. Immunol. 1999, 19, 422–427. [Google Scholar] [CrossRef]
- Neuman, M.; Angula, P.; Malkiewicz, I.; Jorgensen, R.; Shear, N.; Dickson, E.R.; Haber, J.; Katz, G.; Lindor, K. Tumor Necrosis Factor-α and Transforming Growth Factor-β Reflect Severity of Liver Damage in Primary Biliary Cirrhosis. J. Gastroenterol. Hepatol. 2002, 17, 196–202. [Google Scholar] [CrossRef]
- Liu, W.-X. Chymase Inhibitor TY-51469 in Therapy of Inflammatory Bowel Disease. World J. Gastroenterol. 2016, 22, 1826. [Google Scholar] [CrossRef] [PubMed]
- Pejler, G. Novel Insight into the in Vivo Function of Mast Cell Chymase: Lessons from Knockouts and Inhibitors. J. Innate Immun. 2020, 12, 357–372. [Google Scholar] [CrossRef] [PubMed]
- Magnusson, S.E.; Pejler, G.; Kleinau, S.; Åbrink, M. Mast Cell Chymase Contributes to the Antibody Response and the Severity of Autoimmune Arthritis. FASEB J. 2009, 23, 875–882. [Google Scholar] [CrossRef] [PubMed]
- Desbiens, L.; Lapointe, C.; Gharagozloo, M.; Mahmoud, S.; Pejler, G.; Gris, D.; D’Orléans-Juste, P. Significant Contribution of Mouse Mast Cell Protease 4 in Early Phases of Experimental Autoimmune Encephalomyelitis. Mediat. Inflamm. 2016, 2016, 9797021. [Google Scholar] [CrossRef]
- Galli, S.J.; Borregaard, N.; Wynn, T.A. Phenotypic and Functional Plasticity of Cells of Innate Immunity: Macrophages, Mast Cells and Neutrophils. Nat. Immunol. 2011, 12, 1035–1044. [Google Scholar] [CrossRef]
- Gaudenzio, N.; Laurent, C.; Valitutti, S.; Espinosa, E. Human Mast Cells Drive Memory CD4+ T Cells toward an Inflammatory IL-22+ Phenotype. J. Allergy Clin. Immunol. 2013, 131, 1400–1407.e11. [Google Scholar] [CrossRef]

Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Krajewska, N.M.; Fiancette, R.; Oo, Y.H. Interplay between Mast Cells and Regulatory T Cells in Immune-Mediated Cholangiopathies. Int. J. Mol. Sci. 2022, 23, 5872. https://doi.org/10.3390/ijms23115872
Krajewska NM, Fiancette R, Oo YH. Interplay between Mast Cells and Regulatory T Cells in Immune-Mediated Cholangiopathies. International Journal of Molecular Sciences. 2022; 23(11):5872. https://doi.org/10.3390/ijms23115872
Chicago/Turabian StyleKrajewska, Natalia M., Rémi Fiancette, and Ye H. Oo. 2022. "Interplay between Mast Cells and Regulatory T Cells in Immune-Mediated Cholangiopathies" International Journal of Molecular Sciences 23, no. 11: 5872. https://doi.org/10.3390/ijms23115872
APA StyleKrajewska, N. M., Fiancette, R., & Oo, Y. H. (2022). Interplay between Mast Cells and Regulatory T Cells in Immune-Mediated Cholangiopathies. International Journal of Molecular Sciences, 23(11), 5872. https://doi.org/10.3390/ijms23115872





