Advanced Glycations End Products in the Skin as Biomarkers of Cardiovascular Risk in Type 2 Diabetes
Abstract
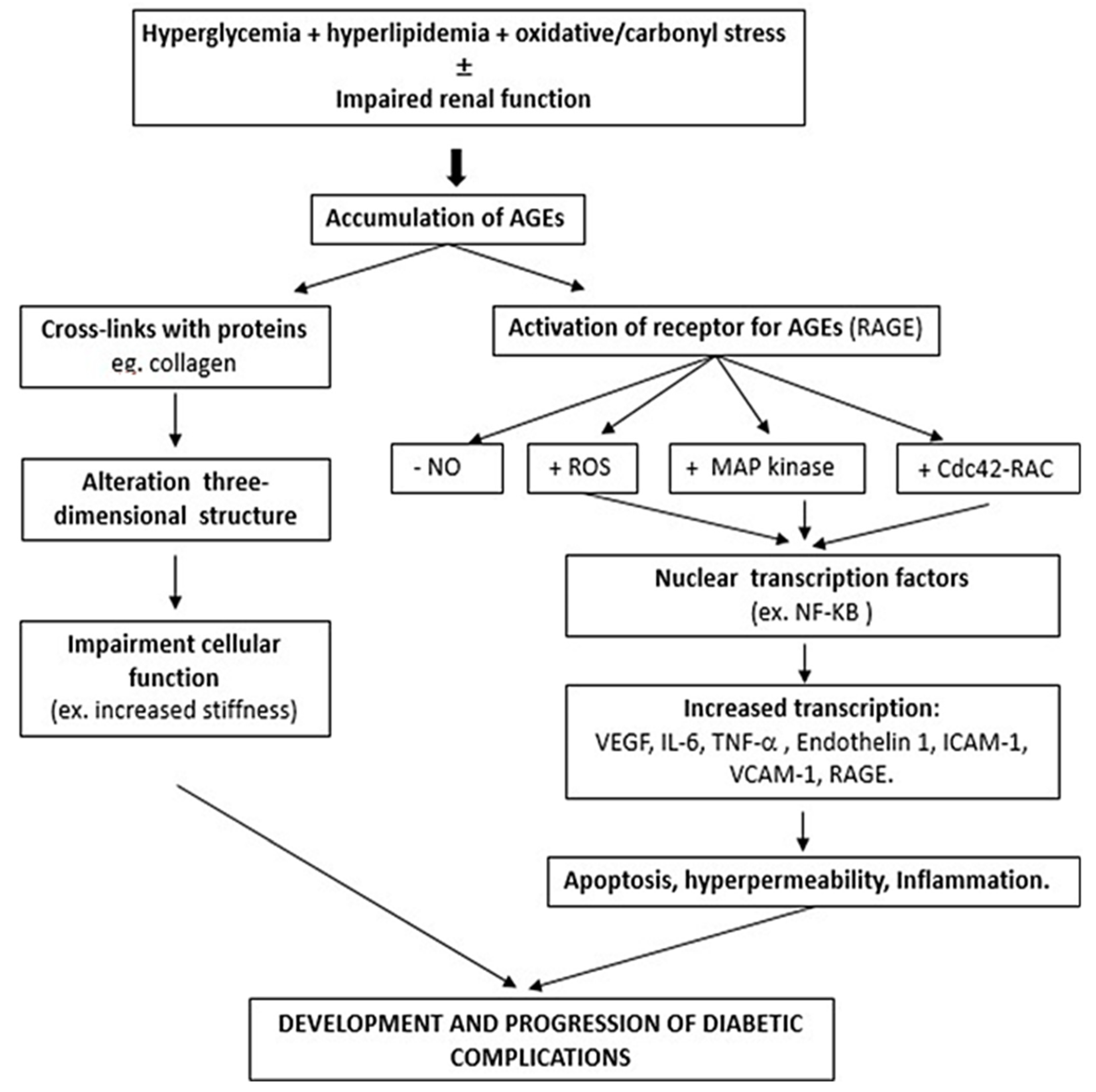
:1. Introduction
2. Formation of AGEs and Physiopathology
3. Assessment of AGEs
4. SAF and Diabetic Microvascular Complications
5. SAF and Diabetic Macrovascular Complications
5.1. SAF and Subclinical Cardiovascular Disease
5.2. SAF and Cardiovascular Disease and Mortality
6. Conclusions
Author Contributions
Funding
Institutional Review Board Statement
Informed Consent Statement
Data Availability Statement
Conflicts of Interest
References
- Fox, C.S.; Pencina, M.J.; Meigs, J.B.; Vasan, R.S.; Levitzky, Y.S.; D’Agostino, R.B. Trends in the Incidence of Type 2 Diabetes Mellitus from the 1970s to the 1990s: The Framingham Heart Study. Circulation 2006, 113, 2914–2918. [Google Scholar] [CrossRef] [PubMed]
- Demographic and Geographic Outline, n.d. Available online: https://www.diabetesatlas.org/en/sections/demographic-and-geographic-outline.html (accessed on 15 March 2022).
- Hird, T.R.; Chen, L.; Islam, R.M.; Pavkov, M.E.; Gregg, E.; Tabesh, M.; Koye, D.; Barr, E.L.; Shaw, J.E.; Magliano, D.J.; et al. 1593-P: Time Trends in Diabetes Incidence and Obesity Prevalence in Six Countries. Diabetes 2019, 68, 1593. [Google Scholar] [CrossRef]
- UK Prospective Diabetes Study (UKPDS) Group. Effect of Intensive Blood-Glucose Control with Metformin on Complications in Overweight Patients with Type 2 Diabetes (UKPDS 34). Lancet Lond. Engl. 1998, 352, 854–865. [Google Scholar] [CrossRef]
- Booth, G.L.; Kapral, M.K.; Fung, K.; Tu, J.V. Relation between Age and Cardiovascular Disease in Men and Women with Diabetes Compared with Non-Diabetic People: A Population-Based Retrospective Cohort Study. Lancet Lond. Engl. 2006, 368, 29–36. [Google Scholar] [CrossRef]
- American Diabetes Association. 9. Cardiovascular Disease and Risk Management: Standards of Medical Care in Diabetes—2018. Diabetes Care 2017, 41, S86–S104. [Google Scholar] [CrossRef] [Green Version]
- Ahern, J.; Grove, N.; Strand, T.; Wesche, J.; Seibert, C.; Brenneman, A.T.; Tamborlane, W.V. The Impact of the Trial Coordinator in the Diabetes Control and Complications Trial (DCCT). The DCCT Research Group. Diabetes Educ. 1993, 19, 509–512. [Google Scholar] [CrossRef]
- Turner, R.C. The U.K. Prospective Diabetes Study. A Review. Diabetes Care 1998, 21 (Suppl. S3), C35–C38. [Google Scholar] [CrossRef]
- Fox, C.S.; Golden, S.H.; Anderson, C.; Bray, G.A.; Burke, L.E.; de Boer, I.H.; Deedwania, P.; Eckel, R.H.; Ershow, A.G.; Fradkin, J.; et al. Update on Prevention of Cardiovascular Disease in Adults With Type 2 Diabetes Mellitus in Light of Recent Evidence: A Scientific Statement From the American Heart Association and the American Diabetes Association. Diabetes Care 2015, 38, 1777–1803. [Google Scholar] [CrossRef] [Green Version]
- Low Wang, C.C.; Hess, C.N.; Hiatt, W.R.; Goldfine, A.B. Atherosclerotic Cardiovascular Disease and Heart Failure in Type 2 Diabetes—Mechanisms, Management, and Clinical Considerations. Circulation 2016, 133, 2459–2502. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Graaff, R.; Oomen, P.H.N.; Links, T.P.; Jager, J.J.; Alderson, N.L.; Thorpe, S.R.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Simple Non-Invasive Assessment of Advanced Glycation Endproduct Accumulation. Diabetologia 2004, 47, 1324–1330. [Google Scholar] [CrossRef] [Green Version]
- Mulder, D.J.; Water, T.V.D.; Lutgers, H.L.; Graaff, R.; Gans, R.O.; Zijlstra, F.; Smit, A.J. Skin Autofluorescence, a Novel Marker for Glycemic and Oxidative Stress-Derived Advanced Glycation Endproducts: An Overview of Current Clinical Studies, Evidence, and Limitations. Diabetes Technol. Ther. 2006, 8, 523–535. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Tessier, F.J. The Maillard Reaction in the Human Body. The Main Discoveries and Factors That Affect Glycation. Pathol. Biol. (Paris) 2010, 58, 214–219. [Google Scholar] [CrossRef] [PubMed]
- Brownlee, M. Glycation Products and the Pathogenesis of Diabetic Complications. Diabetes Care 1992, 15, 1835–1843. [Google Scholar] [CrossRef] [PubMed]
- Vlassara, H.; Palace, M.R. Diabetes and Advanced Glycation Endproducts. J. Intern. Med. 2002, 251, 87–101. [Google Scholar] [CrossRef]
- Charonis, A.S.; Tsilbary, E.C. Structural and Functional Changes of Laminin and Type IV Collagen after Nonenzymatic Glycation. Diabetes 1992, 41 (Suppl. S2), 49–51. [Google Scholar] [CrossRef]
- McRobert, E.A.; Gallicchio, M.; Jerums, G.; Cooper, M.E.; Bach, L.A. The Amino-Terminal Domains of the Ezrin, Radixin, and Moesin (ERM) Proteins Bind Advanced Glycation End Products, an Interaction That May Play a Role in the Development of Diabetic Complications. J. Biol. Chem. 2003, 278, 25783–25789. [Google Scholar] [CrossRef] [Green Version]
- Goldin, A.; Beckman, J.A.; Schmidt, A.M.; Creager, M.A. Advanced Glycation End Products: Sparking the Development of Diabetic Vascular Injury. Circulation 2006, 114, 597–605. [Google Scholar] [CrossRef] [Green Version]
- Yan, S.D.; Schmidt, A.M.; Anderson, G.M.; Zhang, J.; Brett, J.; Zou, Y.S.; Pinsky, D.; Stern, D. Enhanced Cellular Oxidant Stress by the Interaction of Advanced Glycation End Products with Their Receptors/Binding Proteins. J. Biol. Chem. 1994, 269, 9889–9897. [Google Scholar] [CrossRef]
- Louvet-Vallée, S. ERM Proteins: From Cellular Architecture to Cell Signaling. Biol. Cell 2000, 92, 305–316. [Google Scholar] [CrossRef]
- Simó-Servat, O.; Ramos, H.; Bogdanov, P.; García-Ramírez, M.; Huerta, J.; Hernández, C.; Simó, R. ERM Complex, a Therapeutic Target for Vascular Leakage Induced by Diabetes. Curr. Med. Chem. 2021, 29, 2189–2199. [Google Scholar] [CrossRef]
- Suliman, M.E.; Stenvinkel, P.; Jogestrand, T.; Maruyama, Y.; Qureshi, A.R.; Bárány, P.; Heimbürger, O.; Lindholm, B. Plasma Pentosidine and Total Homocysteine Levels in Relation to Change in Common Carotid Intima-Media Area in the First Year of Dialysis Therapy. Clin. Nephrol. 2006, 66, 418–425. [Google Scholar] [CrossRef] [PubMed]
- Saulnier, P.-J.; Wheelock, K.M.; Howell, S.; Weil, E.J.; Tanamas, S.K.; Knowler, W.C.; Lemley, K.V.; Mauer, M.; Yee, B.; Nelson, R.G.; et al. Advanced Glycation End Products Predict Loss of Renal Function and Correlate With Lesions of Diabetic Kidney Disease in American Indians With Type 2 Diabetes. Diabetes 2016, 65, 3744–3753. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Simó-Servat, O.; Simó, R.; Hernández, C. Circulating Biomarkers of Diabetic Retinopathy: An Overview Based on Physiopathology. J. Diabetes Res. 2016, 2016, 5263798. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, E.; Betriu, À.; Yeramian, A.; Fernández, E.; Purroy, F.; Sánchez-de-la-Torre, M.; Pamplona, R.; Miquel, E.; Kerkeni, M.; Hernández, C.; et al. Skin Autofluorescence Measurement in Subclinical Atheromatous Disease: Results from the ILERVAS Project. J. Atheroscler. Thromb. 2019, 26, 879–889. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Busch, M.; Franke, S.; Müller, A.; Wolf, M.; Gerth, J.; Ott, U.; Niwa, T.; Stein, G. Potential Cardiovascular Risk Factors in Chronic Kidney Disease: AGEs, Total Homocysteine and Metabolites, and the C-Reactive Protein. Kidney Int. 2004, 66, 338–347. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Hangai, M.; Takebe, N.; Honma, H.; Sasaki, A.; Chida, A.; Nakano, R.; Togashi, H.; Nakagawa, R.; Oda, T.; Matsui, M.; et al. Association of Advanced Glycation End Products with Coronary Artery Calcification in Japanese Subjects with Type 2 Diabetes as Assessed by Skin Autofluorescence. J. Atheroscler. Thromb. 2016, 23, 1178–1187. [Google Scholar] [CrossRef] [Green Version]
- van Waateringe, R.P.; Fokkens, B.T.; Slagter, S.N.; van der Klauw, M.M.; van Vliet-Ostaptchouk, J.V.; Graaff, R.; Paterson, A.D.; Smit, A.J.; Lutgers, H.L.; Wolffenbuttel, B.H.R. Skin Autofluorescence Predicts Incident Type 2 Diabetes, Cardiovascular Disease and Mortality in the General Population. Diabetologia 2019, 62, 269–280. [Google Scholar] [CrossRef] [Green Version]
- Meerwaldt, R.; Links, T.; Zeebregts, C.; Tio, R.; Hillebrands, J.-L.; Smit, A. The Clinical Relevance of Assessing Advanced Glycation Endproducts Accumulation in Diabetes. Cardiovasc. Diabetol. 2008, 7, 29. [Google Scholar] [CrossRef] [Green Version]
- Peppa, M.; Vlassara, H. Advanced Glycation End Products and Diabetic Complications: A General Overview. Horm. Athens Greece 2005, 4, 28–37. [Google Scholar] [CrossRef]
- Uribarri, J.; Woodruff, S.; Goodman, S.; Cai, W.; Chen, X.; Pyzik, R.; Yong, A.; Striker, G.E.; Vlassara, H. Advanced Glycation End Products in Foods and a Practical Guide to Their Reduction in the Diet. J. Am. Diet. Assoc. 2010, 110, 911–916. [Google Scholar] [CrossRef] [Green Version]
- Sánchez, E.; Betriu, À.; Salas-Salvadó, J.; Pamplona, R.; Barbé, F.; Purroy, F.; Farràs, C.; Fernández, E.; López-Cano, C.; Mizab, C.; et al. Mediterranean Diet, Physical Activity and Subcutaneous Advanced Glycation End-Products’ Accumulation: A Cross-Sectional Analysis in the ILERVAS Project. Eur. J. Nutr. 2020, 59, 1233–1242. [Google Scholar] [CrossRef] [PubMed]
- Monnier, V.M.; Vishwanath, V.; Frank, K.E.; Elmets, C.A.; Dauchot, P.; Kohn, R.R. Relation between Complications of Type I Diabetes Mellitus and Collagen-Linked Fluorescence. N. Engl. J. Med. 1986, 314, 403–408. [Google Scholar] [CrossRef] [PubMed]
- Genuth, S.; Sun, W.; Cleary, P.; Sell, D.R.; Dahms, W.; Malone, J.; Sivitz, W.; Monnier, V.M.; DCCT Skin Collagen Ancillary Study Group. Glycation and Carboxymethyllysine Levels in Skin Collagen Predict the Risk of Future 10-Year Progression of Diabetic Retinopathy and Nephropathy in the Diabetes Control and Complications Trial and Epidemiology of Diabetes Interventions and Complications Participants with Type 1 Diabetes. Diabetes 2005, 54, 3103–3111. [Google Scholar] [CrossRef] [Green Version]
- Lutgers, H.L.; Graaff, R.; Links, T.P.; Ubink-Veltmaat, L.J.; Bilo, H.J.; Gans, R.O.; Smit, A.J. Skin Autofluorescence as a Noninvasive Marker of Vascular Damage in Patients With Type 2 Diabetes. Diabetes Care 2006, 29, 2654–2659. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Noordzij, M.J.; Mulder, D.J.; Oomen, P.H.N.; Brouwer, T.; Jager, J.; Castro Cabezas, M.; Lefrandt, J.D.; Smit, A.J. Skin Autofluorescence and Risk of Micro- and Macrovascular Complications in Patients with Type 2 Diabetes Mellitus-a Multi-Centre Study: Skin Autofluorescence and Risk of Micro- and Macrovascular Complications. Diabet. Med. 2012, 29, 1556–1561. [Google Scholar] [CrossRef] [PubMed]
- Temma, J.; Matsuhisa, M.; Horie, T.; Kuroda, A.; Mori, H.; Tamaki, M.; Endo, I.; Aihara, K.; Abe, M.; Matsumoto, T. Non-Invasive Measurement of Skin Autofluorescence as a Beneficial Surrogate Marker for Atherosclerosis in Patients with Type 2 Diabetes. J. Med. Investig. 2015, 62, 126–129. [Google Scholar] [CrossRef] [Green Version]
- Hirano, T.; Iesato, Y.; Toriyama, Y.; Imai, A.; Chiba, D.; Murata, T. Correlation between Diabetic Retinopathy Severity and Elevated Skin Autofluorescence as a Marker of Advanced Glycation End-Product Accumulation in Type 2 Diabetic Patients. J. Diabetes Complicat. 2014, 28, 729–734. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Links, T.P.; Graaff, R.; Hoogenberg, K.; Lefrandt, J.D.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Increased Accumulation of Skin Advanced Glycation End-Products Precedes and Correlates with Clinical Manifestation of Diabetic Neuropathy. Diabetologia 2005, 48, 1637–1644. [Google Scholar] [CrossRef] [Green Version]
- Tanaka, K.; Tani, Y.; Asai, J.; Nemoto, F.; Kusano, Y.; Suzuki, H.; Hayashi, Y.; Asahi, K.; Nakayama, M.; Miyata, T.; et al. Skin Autofluorescence Is Associated with Severity of Vascular Complications in Japanese Patients with Type 2 Diabetes: Skin Advanced Glycation End Products and Diabetic Vascular Complications. Diabetes Med. 2012, 29, 492–500. [Google Scholar] [CrossRef]
- Bentata, R.; Cougnard-Grégoire, A.; Delyfer, M.N.; Delcourt, C.; Blanco, L.; Pupier, E.; Rougier, M.B.; Rajaobelina, K.; Hugo, M.; Korobelnik, J.F.; et al. Skin Autofluorescence, Renal Insufficiency and Retinopathy in Patients with Type 2 Diabetes. J. Diabetes Complicat. 2017, 31, 619–623. [Google Scholar] [CrossRef]
- Meerwaldt, R.; Lutgers, H.L.; Links, T.P.; Graaff, R.; Baynes, J.W.; Gans, R.O.B.; Smit, A.J. Skin Autofluorescence Is a Strong Predictor of Cardiac Mortality in Diabetes. Diabetes Care 2007, 30, 107–112. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Gerrits, E.G.; Lutgers, H.L.; Kleefstra, N.; Graaff, R.; Groenier, K.H.; Smit, A.J.; Gans, R.O.; Bilo, H.J. Skin Autofluorescence: A Tool to Identify Type 2 Diabetic Patients at Risk for Developing Microvascular Complications. Diabetes Care 2008, 31, 517–521. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Wang, C.-C.; Shen, M.-Y.; Chang, K.-C.; Wang, G.-J.; Liu, S.-H.; Chang, C.-T. Skin Autofluorescence Is Associated with Rapid Renal Function Decline in Subjects at Increased Risk of Coronary Artery Disease. PLoS ONE 2019, 14, e0217203. [Google Scholar] [CrossRef] [PubMed]
- Hosseini, M.S.; Razavi, Z.; Ehsani, A.H.; Firooz, A.; Afazeli, S. Clinical Significance of Non-Invasive Skin Autofluorescence Measurement in Patients with Diabetes: A Systematic Review and Meta-Analysis. eClinicalMedicine 2021, 42, 101194. [Google Scholar] [CrossRef]
- Yoshioka, K. Skin Autofluorescence Is a Noninvasive Surrogate Marker for Diabetic Microvascular Complications and Carotid Intima–Media Thickness in Japanese Patients with Type 2 Diabetes: A Cross-Sectional Study. Diabetes Ther. 2018, 9, 75–85. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Zhou, L.L.; Cao, W.; Xie, C.; Tian, J.; Zhou, Z.; Zhou, Q.; Zhu, P.; Li, A.; Liu, Y.; Miyata, T.; et al. The Receptor of Advanced Glycation End Products Plays a Central Role in Advanced Oxidation Protein Products-Induced Podocyte Apoptosis. Kidney Int. 2012, 82, 759–770. [Google Scholar] [CrossRef] [Green Version]
- Shardlow, A.; McIntyre, N.J.; Kolhe, N.V.; Nellums, L.B.; Fluck, R.J.; McIntyre, C.W.; Taal, M.W. The Association of Skin Autofluorescence with Cardiovascular Events and All-Cause Mortality in Persons with Chronic Kidney Disease Stage 3: A Prospective Cohort Study. PLoS Med. 2020, 17, e1003163. [Google Scholar] [CrossRef]
- Siriopol, D.; Hogas, S.; Veisa, G.; Mititiuc, I.; Volovat, C.; Apetrii, M.; Onofriescu, M.; Busila, I.; Oleniuc, M.; Covic, A. Tissue Advanced Glycation End Products (AGEs), Measured by Skin Autofluorescence, Predict Mortality in Peritoneal Dialysis. Int. Urol. Nephrol. 2015, 47, 563–569. [Google Scholar] [CrossRef] [PubMed]
- Furuya, F.; Shimura, H.; Takahashi, K.; Akiyama, D.; Motosugi, A.; Ikegishi, Y.; Haraguchi, K.; Kobayashi, T. Skin Autofluorescence Is a Predictor of Cardiovascular Disease in Chronic Kidney Disease Patients: Skin Autofluorescence in HD Patients. Ther. Apher. Dial. 2015, 19, 40–44. [Google Scholar] [CrossRef]
- Ando, R.; Ueda, S.; Yamagishi, S.; Miyazaki, H.; Kaida, Y.; Kaifu, K.; Yokoro, M.; Nakayama, Y.; Obara, N.; Fukami, K.; et al. Involvement of Advanced Glycation End Product-Induced Asymmetric Dimethylarginine Generation in Endothelial Dysfunction. Diab. Vasc. Dis. Res. 2013, 10, 436–441. [Google Scholar] [CrossRef] [Green Version]
- Chabroux, S.; Canouï-Poitrine, F.; Reffet, S.; Mills-Joncour, G.; Morelon, E.; Colin, C.; Thivolet, C. Advanced Glycation End Products Assessed by Skin Autofluorescence in Type 1 Diabetics Are Associated with Nephropathy, but Not Retinopathy. Diabetes Metab. 2010, 36, 152–157. [Google Scholar] [CrossRef] [PubMed]
- Sugisawa, E.; Miura, J.; Iwamoto, Y.; Uchigata, Y. Skin Autofluorescence Reflects Integration of Past Long-Term Glycemic Control in Patients with Type 1 Diabetes. Diabetes Care 2013, 36, 2339–2345. [Google Scholar] [CrossRef] [Green Version]
- Yasuda, M.; Shimura, M.; Kunikata, H.; Kanazawa, H.; Yasuda, K.; Tanaka, Y.; Konno, H.; Takahashi, M.; Kokubun, T.; Maruyama, K.; et al. Relationship of Skin Autofluorescence to Severity of Retinopathy in Type 2 Diabetes. Curr. Eye Res. 2015, 40, 338–345. [Google Scholar] [CrossRef] [PubMed]
- Takayanagi, Y.; Yamanaka, M.; Fujihara, J.; Matsuoka, Y.; Gohto, Y.; Obana, A.; Tanito, M. Evaluation of Relevance between Advanced Glycation End Products and Diabetic Retinopathy Stages Using Skin Autofluorescence. Antioxidants 2020, 9, 1100. [Google Scholar] [CrossRef]
- Santos, G.S.P.; Prazeres, P.H.D.M.; Mintz, A.; Birbrair, A. Role of Pericytes in the Retina. Eye Lond. Engl. 2018, 32, 483–486. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Lin, W.-J.; Ma, X.-F.; Hao, M.; Zhou, H.-R.; Yu, X.-Y.; Shao, N.; Gao, X.-Y.; Kuang, H.-Y. Liraglutide Attenuates the Migration of Retinal Pericytes Induced by Advanced Glycation End Products. Peptides 2018, 105, 7–13. [Google Scholar] [CrossRef]
- Park, D.Y.; Lee, J.; Kim, J.; Kim, K.; Hong, S.; Han, S.; Kubota, Y.; Augustin, H.G.; Ding, L.; Kim, J.W.; et al. Plastic Roles of Pericytes in the Blood-Retinal Barrier. Nat. Commun. 2017, 8, 15296. [Google Scholar] [CrossRef]
- Ogura, S.; Kurata, K.; Hattori, Y.; Takase, H.; Ishiguro-Oonuma, T.; Hwang, Y.; Ahn, S.; Park, I.; Ikeda, W.; Kusuhara, S.; et al. Sustained Inflammation after Pericyte Depletion Induces Irreversible Blood-Retina Barrier Breakdown. JCI Insight 2017, 2, e90905. [Google Scholar] [CrossRef] [Green Version]
- Tao, D.; Ni, N.; Zhang, T.; Li, C.; Sun, Q.; Wang, L.; Mei, Y. Accumulation of Advanced Glycation End Products Potentiate Human Retinal Capillary Endothelial Cells Mediated Diabetic Retinopathy. Mol. Med. Rep. 2019, 20, 3719–3727. [Google Scholar] [CrossRef]
- Lu, M.; Kuroki, M.; Amano, S.; Tolentino, M.; Keough, K.; Kim, I.; Bucala, R.; Adamis, A.P. Advanced Glycation End Products Increase Retinal Vascular Endothelial Growth Factor Expression. J. Clin. Investig. 1998, 101, 1219–1224. [Google Scholar] [CrossRef]
- Papachristou, S.; Pafili, K.; Papanas, N. Skin AGEs and Diabetic Neuropathy. BMC Endocr. Disord. 2021, 21, 28. [Google Scholar] [CrossRef] [PubMed]
- Wan, L.; Qin, G.; Yan, W.; Sun, T. Skin Autofluorescence Is Associated with Diabetic Peripheral Neuropathy in Chinese Patients with Type 2 Diabetes: A Cross-Sectional Study. Genet. Test. Mol. Biomark. 2019, 23, 387–392. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Rajaobelina, K.; Farges, B.; Nov, S.; Maury, E.; Cephise-Velayoudom, F.L.; Gin, H.; Helmer, C.; Rigalleau, V. Skin Autofluorescence and Peripheral Neuropathy Four Years Later in Type 1 Diabetes. Diabetes Metab. Res. Rev. 2017, 33, e2832. [Google Scholar] [CrossRef] [PubMed] [Green Version]
- Stirban, A.O.; Bondor, C.I.; Florea, B.; Veresiu, I.A.; Gavan, N.A. Skin Autofluorescence: Correlation with Measures of Diabetic Sensorimotor Neuropathy. J. Diabetes Complicat. 2018, 32, 851–856. [Google Scholar] [CrossRef]
- Vouillarmet, J.; Maucort-Boulch, D.; Michon, P.; Thivolet, C. Advanced Glycation End Products Assessed by Skin Autofluorescence: A New Marker of Diabetic Foot Ulceration. Diabetes Technol. Ther. 2013, 15, 601–605. [Google Scholar] [CrossRef]
- Pyörälä, K. Diabetes and Coronary Artery Disease: What a Coincidence? J. Cardiovasc. Pharmacol. 1990, 16 (Suppl. S9), S8–S14. [Google Scholar] [CrossRef]
- Lithner, F.; Asplund, K.; Eriksson, S.; Hägg, E.; Strand, T.; Wester, P.O. Clinical Characteristics in Diabetic Stroke Patients. Diabete Metab. 1988, 14, 15–19. [Google Scholar]
- Ruderman, N.B.; Haudenschild, C. Diabetes as an Atherogenic Factor. Prog. Cardiovasc. Dis. 1984, 26, 373–412. [Google Scholar] [CrossRef]
- Stevens, R.J.; Kothari, V.; Adler, A.I.; Stratton, I.M.; United Kingdom Prospective Diabetes Study (UKPDS) Group. The UKPDS Risk Engine: A Model for the Risk of Coronary Heart Disease in Type II Diabetes (UKPDS 56). Clin. Sci. 2001, 101, 671–679. [Google Scholar] [CrossRef]
- Lutgers, H.L.; Gerrits, E.G.; Graaff, R.; Links, T.P.; Sluiter, W.J.; Gans, R.O.; Bilo, H.J.; Smit, A.J. Skin Autofluorescence Provides Additional Information to the UK Prospective Diabetes Study (UKPDS) Risk Score for the Estimation of Cardiovascular Prognosis in Type 2 Diabetes Mellitus. Diabetologia 2009, 52, 789–797. [Google Scholar] [CrossRef] [Green Version]
- Saz-Lara, A.; Álvarez-Bueno, C.; Martínez-Vizcaíno, V.; Notario-Pacheco, B.; Sequí-Dominguez, I.; Cavero-Redondo, I. Are Advanced Glycation End Products in Skin Associated with Vascular Dysfunction Markers? A Meta-Analysis. Int. J. Environ. Res. Public. Health 2020, 17, 6936. [Google Scholar] [CrossRef]
- Cavero-Redondo, I.; Soriano-Cano, A.; Álvarez-Bueno, C.; Cunha, P.G.; Martínez-Hortelano, J.A.; Garrido-Miguel, M.; Berlanga-Macías, C.; Martínez-Vizcaíno, V. Skin Autofluorescence–Indicated Advanced Glycation End Products as Predictors of Cardiovascular and All-Cause Mortality in High-Risk Subjects: A Systematic Review and Meta-analysis. J. Am. Heart Assoc. 2018, 7, e009833. [Google Scholar] [CrossRef] [Green Version]
- Smit, A.J.; Lutgers, H.L. The Clinical Relevance of Advanced Glycation Endproducts (AGE) and Recent Developments in Pharmaceutics to Reduce AGE Accumulation. Curr. Med. Chem. 2004, 11, 2767–2784. [Google Scholar] [CrossRef] [Green Version]
- Quehenberger, P.; Bierhaus, A.; Fasching, P.; Muellner, C.; Klevesath, M.; Hong, M.; Stier, G.; Sattler, M.; Schleicher, E.; Speiser, W.; et al. Endothelin 1 Transcription Is Controlled by Nuclear Factor-KappaB in AGE-Stimulated Cultured Endothelial Cells. Diabetes 2000, 49, 1561–1570. [Google Scholar] [CrossRef] [Green Version]
- Sanders, D.B.; Kelley, T.; Larson, D. The Role of Nitric Oxide Synthase/Nitric Oxide in Vascular Smooth Muscle Control. Perfusion 2000, 15, 97–104. [Google Scholar] [CrossRef]
- Henning, R.J. Type-2 Diabetes Mellitus and Cardiovascular Disease. Future Cardiol. 2018, 14, 491–509. [Google Scholar] [CrossRef]
- Striker, L.J.; Striker, G.E. Administration of AGEs In Vivo Induces Extracellular Matrix Gene Expression. Nephrol. Dial. Transplant. 1996, 11 (Suppl. S5), 62–65. [Google Scholar] [CrossRef]
- Petrova, R.; Yamamoto, Y.; Muraki, K.; Yonekura, H.; Sakurai, S.; Watanabe, T.; Li, H.; Takeuchi, M.; Makita, Z.; Kato, I.; et al. Advanced Glycation Endproduct-Induced Calcium Handling Impairment in Mouse Cardiac Myocytes. J. Mol. Cell. Cardiol. 2002, 34, 1425–1431. [Google Scholar] [CrossRef]
- Fujino, Y.; Attizzani, G.F.; Tahara, S.; Wang, W.; Takagi, K.; Naganuma, T.; Yabushita, H.; Tanaka, K.; Sato, T.; Watanabe, Y.; et al. Association of Skin Autofluorescence with Plaque Vulnerability Evaluated by Optical Coherence Tomography in Patients with Cardiovascular Disease. Atherosclerosis 2018, 274, 47–53. [Google Scholar] [CrossRef]
- Ninomiya, H.; Katakami, N.; Sato, I.; Osawa, S.; Yamamoto, Y.; Takahara, M.; Kawamori, D.; Matsuoka, T.; Shimomura, I. Association between Subclinical Atherosclerosis Markers and the Level of Accumulated Advanced Glycation End-Products in the Skin of Patients with Diabetes. J. Atheroscler. Thromb. 2018, 25, 1274–1284. [Google Scholar] [CrossRef] [Green Version]
- Osawa, S.; Katakami, N.; Sato, I.; Ninomiya, H.; Omori, K.; Yamamoto, Y.; Takahara, M.; Miyashita, K.; Sakamoto, F.; Kawamori, D.; et al. Skin Autofluorescence Is Associated with Vascular Complications in Patients with Type 2 Diabetes. J. Diabetes Complicat. 2018, 32, 839–844. [Google Scholar] [CrossRef]
- Jujić, A.; Östling, G.; Persson, M.; Engström, G.; Nilsson, P.M.; Melander, O.; Magnusson, M. Skin Autofluorescence as a Measure of Advanced Glycation End Product Levels Is Associated with Carotid Atherosclerotic Plaque Burden in an Elderly Population. Diab. Vasc. Dis. Res. 2019, 16, 466–473. [Google Scholar] [CrossRef]
- Birukov, A.; Cuadrat, R.; Polemiti, E.; Eichelmann, F.; Schulze, M.B. Advanced Glycation End-Products, Measured as Skin Autofluorescence, Associate with Vascular Stiffness in Diabetic, Pre-Diabetic and Normoglycemic Individuals: A Cross-Sectional Study. Cardiovasc. Diabetol. 2021, 20, 110. [Google Scholar] [CrossRef]
- Planas, A.; Simó-Servat, O.; Bañeras, J.; Sánchez, M.; García, E.; Ortiz, Á.M.; Ruiz-Meana, M.; Hernández, C.; Ferreira-González, I.; Simó, R. Usefulness of Skin Advanced Glycation End Products to Predict Coronary Artery Calcium Score in Patients with Type 2 Diabetes. Acta Diabetol. 2021, 58, 1403–1412. [Google Scholar] [CrossRef]
- Ying, L.; Shen, Y.; Zhang, Y.; Wang, Y.; Liu, Y.; Yin, J.; Wang, Y.; Yin, J.; Zhu, W.; Bao, Y.; et al. Association of Advanced Glycation End Products With Lower-Extremity Atherosclerotic Disease in Type 2 Diabetes Mellitus. Front. Cardiovasc. Med. 2021, 8, 696156. [Google Scholar] [CrossRef]
- Yamagishi, S. Potential Clinical Utility of Advanced Glycation End Product Cross-Link Breakers in Age- and Diabetes-Associated Disorders. Rejuvenation Res. 2012, 15, 564–572. [Google Scholar] [CrossRef]
- Bierhaus, A.; Hofmann, M.A.; Ziegler, R.; Nawroth, P.P. AGEs and Their Interaction with AGE-Receptors in Vascular Disease and Diabetes Mellitus. I. The AGE Concept. Cardiovasc. Res. 1998, 37, 586–600. [Google Scholar] [CrossRef] [Green Version]
- Heitzer, T.; Schlinzig, T.; Krohn, K.; Meinertz, T.; Münzel, T. Endothelial Dysfunction, Oxidative Stress, and Risk of Cardiovascular Events in Patients with Coronary Artery Disease. Circulation 2001, 104, 2673–2678. [Google Scholar] [CrossRef] [Green Version]
- de Vos, L.C.; Boersema, J.; Mulder, D.J.; Smit, A.J.; Zeebregts, C.J.; Lefrandt, J.D. Skin Autofluorescence as a Measure of Advanced Glycation End Products Deposition Predicts 5-Year Amputation in Patients with Peripheral Artery Disease. Arterioscler. Thromb. Vasc. Biol. 2015, 35, 1532–1537. [Google Scholar] [CrossRef] [Green Version]
- Yozgatli, K.; Lefrandt, J.D.; Noordzij, M.J.; Oomen, P.H.N.; Brouwer, T.; Jager, J.; Castro Cabezas, M.; Smit, A.J. Accumulation of Advanced Glycation End Products Is Associated with Macrovascular Events and Glycaemic Control with Microvascular Complications in Type 2 Diabetes Mellitus. Diabet. Med. 2018, 35, 1242–1248. [Google Scholar] [CrossRef]
- Kunimoto, M.; Yokoyama, M.; Shimada, K.; Matsubara, T.; Aikawa, T.; Ouchi, S.; Fukao, K.; Miyazaki, T.; Fujiwara, K.; Abulimiti, A.; et al. Relationship between Skin Autofluorescence Levels and Clinical Events in Patients with Heart Failure Undergoing Cardiac Rehabilitation. Cardiovasc. Diabetol. 2021, 20, 208. [Google Scholar] [CrossRef]
- Boersma, H.E.; van Waateringe, R.P.; van der Klauw, M.M.; Graaff, R.; Paterson, A.D.; Smit, A.J.; Wolffenbuttel, B.H.R. Skin Autofluorescence Predicts New Cardiovascular Disease and Mortality in People with Type 2 Diabetes. BMC Endocr. Disord. 2021, 21, 14. [Google Scholar] [CrossRef]
- Planas, A.; Simó-Servat, O.; Hernández, C.; Ortiz-Zúñiga, Á.; Marsal, J.R.; Herance, J.R.; Ferreira-González, I.; Simó, R. Diabetic Retinopathy and Skin Tissue Advanced Glycation End Products Are Biomarkers of Cardiovascular Events in Type 2 Diabetic Patients. J. Pers. Med. 2021, 11, 1344. [Google Scholar] [CrossRef]
- Mulder, D.J.; van Haelst, P.L.; Graaff, R.; Gans, R.O.; Zijlstra, F.; Smit, A.J. Skin Autofluorescence Is Elevated in Acute Myocardial Infarction and Is Associated with the One-Year Incidence of Major Adverse Cardiac Events. Neth. Heart J. 2009, 17, 162–168. [Google Scholar] [CrossRef] [Green Version]
- Chen, Q.; Huang, Q.; Liu, W.; Zhou, X. Advanced Glycation End Products via Skin Autofluorescence as a New Biomarker for Major Adverse Cardiovascular Events: A Meta-Analysis of Prospective Studies. Nutr. Metab. Cardiovasc. Dis. 2022, 32, 1083–1092. [Google Scholar] [CrossRef]
| First Author (Year) | Participants and Diabetes Type | Measurement | Main Findings |
|---|---|---|---|
| Temma (2015) [37] | 61 T2D | C-IMT | SAF well correlated with the degree of max-IMT of the carotid artery. |
| Hangai (2016) [27] | 122 T2D | baPWV; C-IMT; CACs | SAF positively correlated with CACs. Stronger with CACs than either PWV or IMT. |
| Fujino (2018) [80] | 108 (50% T2D) | Coronary plaques assessed by OCT. | SAF positively associated with more vulnerable and calcified plaques. |
| Ninomiya (2018) [81] | 140 (T1D and T2D) | Subclinical atherosclerosis: FMV, IMT, baPWV | SAF is an independent determinant of brachial FMD (indicator of endothelial dysfunction), and SAF is associated with IMT and baPWV (markers of early-stage atherosclerosis). |
| Yoshioka (2018) [46] | 162 T2D and 42 controls | C-IMT | SAF was an independent determinant of max-IMT (early-stage atherosclerosis). |
| Osawa (2018) [82] | 193 T2D and 24 controls | C-IMT, ankle-brachial index, baPWV | SAF was significantly associated with C-IMT and baPWV but was not an independent determinant of C-IMT and baPWV after adjustment for confounders. |
| Jujić (2019) [83] | 496 (10% T2D) | Carotid ultrasound. (TPA) | SAF is associated with the degree of atherosclerosis. A 1 SD increment in SAF is associated with increased atherosclerotic burden (TPA). |
| Sánchez (2019) [25] | 2568 (non-diabetic subjects) | TPA (vascular carotid and femoral ultrasound) | SAF is associated with increased atherosclerotic burden (the presence of plaque, number of affected territories, and TPA). |
| Birukov (2021) [84] | 1348 (T2D and non-diabetic subjects) | Vascular stiffness: carotid-femoral and aortic PWV and brachial and aortic augmentation indices. | SAF is positively associated with measures of arterial stiffness, independent of potential cardiometabolic confounders and glycemic status. |
| Planas (2021) [85] | 156 T2D and 52 non-diabetic subjects. | Coronary atherosclerosis assessed by CACs. | SAF is a good and independent predictor of CACs ≥ 400. |
| Ying (2021) [86] | 1013 T2D | LEAD (color doppler ultrasonography). | SAF is associated with the presence of lower extremity atherosclerosis. |
| First Author (Year) | Participants and Diabetes Type | Outcome | Follow Up | Main Findings |
|---|---|---|---|---|
| Meerwaldt (2007) [42] | 69 T2D, 48 T1D, and 43 controls | CV mortality | 5 years | SAF strongly associated with CV mortality. OR 2.9 CI 95% 1.3–4.4 for T2D, and OR 2.0 CI 95% 1.3–2.7 for T1D. |
| Tanaka (2011) [40] | 130 T2D | Ancient macrovascular complications | Cross sectional | SAF associated with macrovascular complications (OR 7.25 CI 95% 2.22–23.7). |
| Noordzij (2012) [36] | 563 T2D | Ancient macrovascular complications | Cross sectional | SAF was associated with macrovascular complications. |
| De Vos (2015) [90] | 267 (10% T2D) | New amputations in patients with PAD | 5.3 years | SAF predicts amputations in patients with PAD independent of diabetes. HR 2.72 (CI 95% 1.38–1.539) per unit of SAF for amputation. |
| Furuya (2015) [50] | 64 subjects with CKD in hemodialysis (56.3% subjects with diabetes) | New CV events | 3 years | SAF is significantly associated with incidence of new CV event OR 2.96 CI 95% 1.26–8.16 |
| Siriopol (2015) [49] | 304 dialysis subjects (18.4% diabetic subjects) | CV mortality, sepsis-related mortality, other causes of mortality | 2.5 years | SAF is associated in all-cause (HR 2.09 CI 95% 1.24–3.59) and sepsis-related mortality (HR 3.44 CI 95% 1.59–7.42). |
| Yozgatli (2018) [91] | 563 T2D | New CV events and microvascular complications | 5 years | SAF is a significant predictor of fatal and non-fatal CV events (HR 1.53 CI 95% 1.24–1.48 per unit of SAF in the development of CV events. |
| Kunimoto (2021) [92] | 204 subjects with heart failure and CVD (30% T2D) | Major CV event (all cause of mortality + unplanned hospitalization for heart failure) | 1.6 years | Higher SAF levels are significantly and independently associated with major CV events. SAF was associated with major CV adverse event (OR 2 CI 95% 1.41–2.78, p < 0.01). |
| Boersma (2021) [93] | 1318 T2D 1031 new T2D | New CV events | 3.7 years | SAF is significantly and independently associated with the new CV event and mortality in people with T2D (OR 2.59 CI 95% 2.1–3.2). |
| Planas (2021) [94] | 187 T2D and 57 controls | First CV event | 4.35 years | Higher values of SAF are predictors of new CV events (HR 4.68 CI 95% 1.83–11.96). |
Publisher’s Note: MDPI stays neutral with regard to jurisdictional claims in published maps and institutional affiliations. |
© 2022 by the authors. Licensee MDPI, Basel, Switzerland. This article is an open access article distributed under the terms and conditions of the Creative Commons Attribution (CC BY) license (https://creativecommons.org/licenses/by/4.0/).
Share and Cite
Planas, A.; Simó-Servat, O.; Hernández, C.; Simó, R. Advanced Glycations End Products in the Skin as Biomarkers of Cardiovascular Risk in Type 2 Diabetes. Int. J. Mol. Sci. 2022, 23, 6234. https://doi.org/10.3390/ijms23116234
Planas A, Simó-Servat O, Hernández C, Simó R. Advanced Glycations End Products in the Skin as Biomarkers of Cardiovascular Risk in Type 2 Diabetes. International Journal of Molecular Sciences. 2022; 23(11):6234. https://doi.org/10.3390/ijms23116234
Chicago/Turabian StylePlanas, Alejandra, Olga Simó-Servat, Cristina Hernández, and Rafael Simó. 2022. "Advanced Glycations End Products in the Skin as Biomarkers of Cardiovascular Risk in Type 2 Diabetes" International Journal of Molecular Sciences 23, no. 11: 6234. https://doi.org/10.3390/ijms23116234






